Abstract
We measured physiological functionalities, including antihypertensive angiotensin I-converting enzyme inhibitory activity and immun-stimulating β-glucan content for sixty kinds of Makgeolli that is commercially available from the market. As a result, we selected R-12 commercial raw Makgeolli, with a high content of immuno-stimulating β-glucan, and R-14 commercial raw Makgeolli, exhibiting high antihypertensive activity. Due to the similarities in their overall physicochemical properties and raw materials used for fermentation, we compared the microbial flora in order to investigate the reason for the differences in their functionalities. Nested PCR and denaturing gradient gel electrophoresis for yeasts and bacteria were performed for analysis of microbial diversity of two different kinds of Makgeolli (i.e., R-12, R-14), which showed immuno-stimulating β-glucan content and exhibited a very high level of antihypertensive activity, respectively. Analysis of the 18S rDNA amplicon revealed a major presence of the yeast strain Pichia burtonii in every Makgeolli sample. Analysis of the 16S rDNA amplicon revealed a predominance of lactic acid bacteria, and the most frequent lactic acid bacteria were Lactobacillus ingluviei, L. fermentum, and L. harbinensis, and Lactobacillus sp. Among these, L. harbinensis was detected only in R-12 and L. ingluviei was found only in R-14. Different functionalities from the individual commercially available Makgeolli may be attributed to actions of different microbial flora during fermentation.
Keywords: Commercial Makgeolli, Denaturing gradient gel electrophoresis, Lactic acid bacteria, Microbial diversity, Yeasts
Recently, public demand for traditional Korean wine or Makgeolli has shown a significant increase due to a growing perception of superiority of its nutraceuticals and physiological functionalities. The main ingredient of Makgeolli is cooked rice, which is mixed with nuruk (koji), containing amylolytic fungi, ethanol-producing yeast, and lactic acid bacteria, to initiate the alcohol fermentation process [1]. After fermenting for a period of one week, water is usually added to dilute the Makgeolli, so that its final alcohol content is approximately 6% to 7%, with a pH level around 3.4 to 4.0, since organic acids, including succinic acid and lactic acid, are produced by the actions of the yeast and lactic acid bacteria [2, 3].
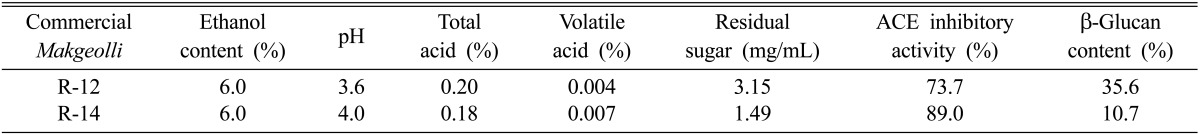
Of particular interest, antihypertensive angiotensin I-converting enzyme (ACE) inhibitory activities have been found in several alcoholic beverages, including sake and its by-products, as well as traditional Korean wine [4, 5]. β-glucans, polysaccharide of D-glucose linked by β type glycosidic bonds, are known to activate the immune system [6]. In addition, consumption of β-glucan has been reported to result in reduction of blood cholesterol concentration [7]. However, no attempt has yet been made to determine the β-glucan contents of Makgeolli. We determined the physiological functionalities, including antihypertensive ACE inhibitory activity and β-glucan content, in 60 different kinds of commercially available Makgeolli obtained from all over the country. According to the results, R-12 raw Makgeolli showed high β-glucan content (35.6%), but moderate ACE inhibitory activity (73.7%). In contrast, R-14 showed very high ACE inhibitory activity (89.0%), but moderate β-glucan content (10.7%). It is interesting to note that, although rice was used as raw material for both R-12 and R-14, each exhibited totally different functionalities. Table 1 provides a summary of the overall physicochemical properties and functionalities of these Makgeolli. The ethanol content of the raw Makgeolli was 6.0%, with similar total acid contents of 0.20% (R-12) and 0.18% (R-14). Therefore, using PCR-denaturing gradient gel electrophoresis (PCR-DGGE) analysis, we attempted to compare the differences between highly β-glucan-containing R-12 commercial raw Makgeolli and antihypertensive R-14 commercial raw Makgeolli, in terms of microbial diversity, according to the methods described in a previous paper [8].
Table 1.
Physicochemical properties and functionalities of bioactive Makgeolli used in this study
ACE, antihypertensive angiotensin I-converting enzyme.
Fungal analysis in the functional Makgeolli
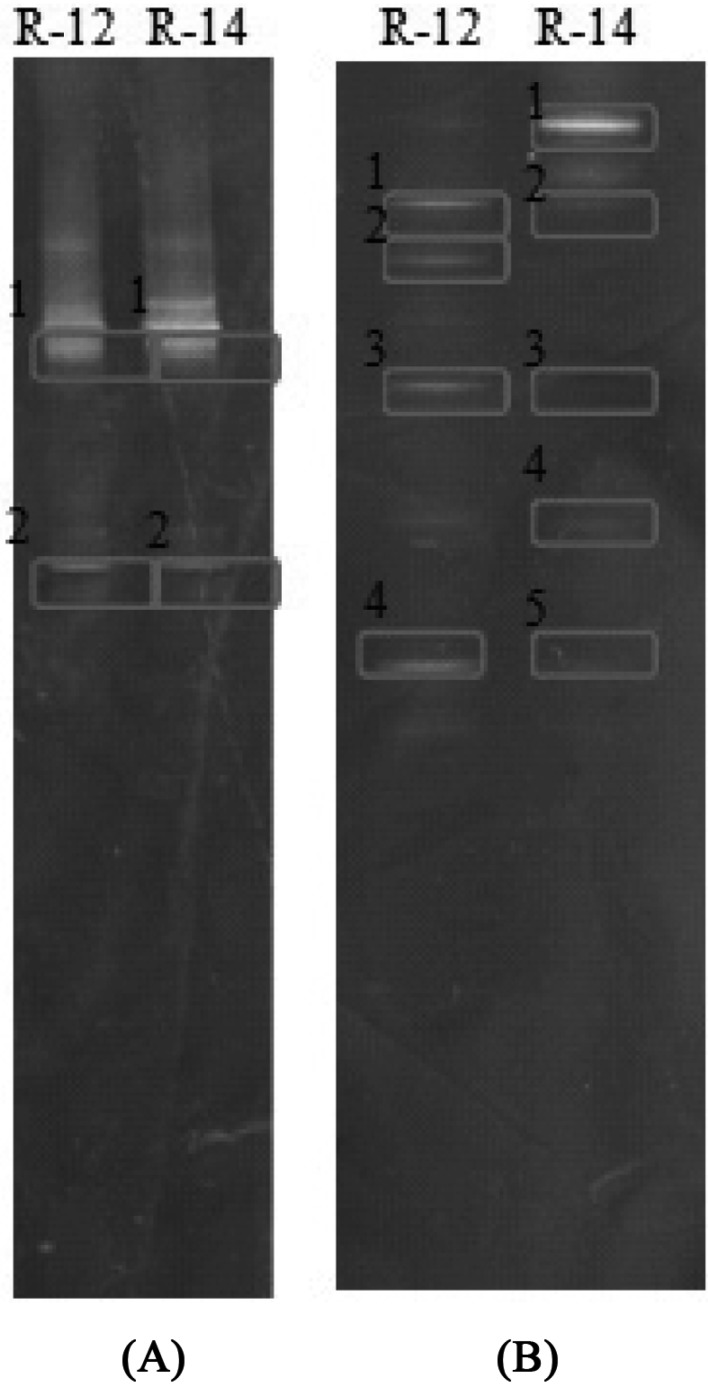
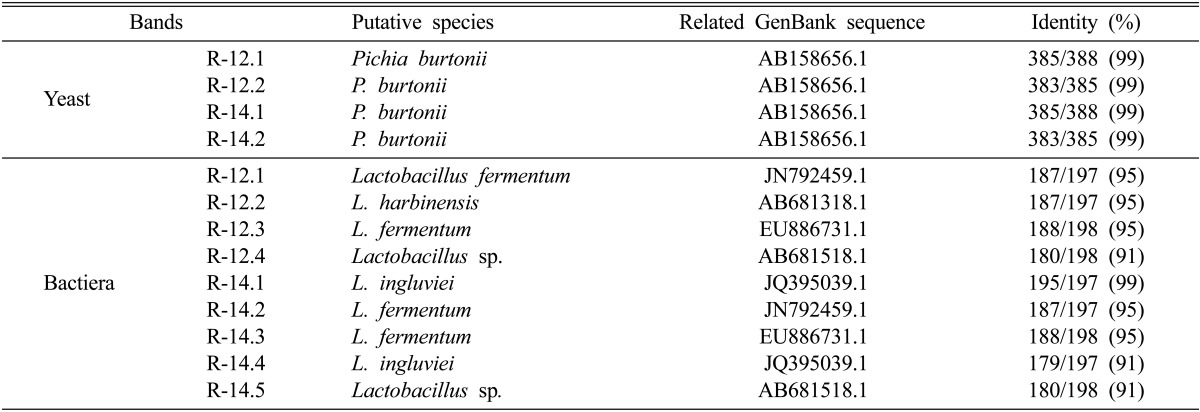
To investigate fungal diversity of the two types of functional Makgeolli, nested PCR-DGGE was performed for analysis of the V3 region of 18S rDNA [9-13]. As shown in Fig. 1A, two clear 18S rDNA bands of the two types of Makgeolli were observed on the DGGE gel. Bands that formed on the gel were recovered and analysis was performed for identification. DNA sequencing and subsequent BLAST analysis confirmed that every DNA band originates from the Pichia burtonii (Table 2). Of particular interest, we detected no other fungi or yeasts in the two types of Makgeolli. Alcoholic fermentation is typically carried out by complex microorganisms derived from yeast and bacteria from nuruk or koji and yeast seed cultures [14]. Some papers have reported on discovery of P. burtonii in murcha in Nepal, Bhutan, and the Himalayan regions of India; regi in Indonesia; loog-pang in Thailand; bubod in the Philippines; and Chinese yeast in Taiwan as a starter for rice wine-making [15, 16]. However, this is the first report to demonstrate the exclusive presence of P. burtonii, without Saccharomyces cerevisiae, in antihypertensive R-14 Makgeolli and high β-glucan-containing R-12 Makgeolli, which were used in this study.
Fig. 1.
Yeast (A) and Bacteria (B) community of Korean functional Makgeolli as revealed by PCR-denaturing gradient gel electrophoresis. Lanes corresponding to two Makgeolli samples are indicated by R-12 and R-14 at the top. Numbers on the gel represent bands that were recovered for identification. A summary of the identities of the excised 18S rDNA fragments (A) and 16S rDNA fragments (B) is shown Table 2.
Table 2.
Identification of microorganisms from Korean functional Makgeolli showing high β-glucan content (R-12) and high antihypertensive activity (R-14) by PCR-DGGE
PCR-DGGE, PCR-denaturing gradient gel electrophoresis.
Bacteria in the functional Makgeolli
Many lactic acid bacteria, including Lactobacillus paracasei, L. hilgardii, and L. arizonensis, have been identified in Makgeolli [17]. Nested PCR-DGGE analysis of the V2 region of 16S rDNA was performed for investigation of bacterial diversity in the two types of Makgeolli. Fig. 1B shows typical DGGE patterns of amplicons for 16S rDNA. Clear bands for the 16S rDNA fragments that formed on the DGGE gel were identified by direct DNA sequencing after performance of PCR for re-amplification of the excised bands. A list of identified bands is shown in Table 2. We detected L. fermentum, L. harbinensis, and Lactobacillus sp. in the R-12 and L. ingluviei, L. fermentum, and Lactobacillus sp. in the R-14 Makgeolli. DNA bands common to all Makgeolli samples were L. fermentum and Lactobacillus sp. However, L. ingluviei was found only in the antihypertensive R-14 commercial raw Makgeolli and L. harbinensis was detected only in highly β-glucan-containing R-12 commercial raw Makgeolli. The R-12.1 and R-12.3 bands were identified as L. fermentum and the R-14.1, and R-14.4 bands were identified as L. ingluvie; however, they were observed at different positions on the DGGE gel. Sequence heterogeneity between multiple copies of the 16S rDNA gene of any given strain may cause these multiple banding patterns [18]. Meanwhile, the presence of antihypertensive ACE inhibitory activity has been reported in several alcoholic beverages, including Japanese rice wine, sake, and Korean rice wine, and most ACE inhibitors are peptides that originate from raw materials during fermentation [4, 5]. In addition, findings reported from several clinical trials have suggested that consumption of milk that has been fermented with S. cerevisiae and L. helveticus may result in suppression of blood pressure in hypertensive individuals [19, 20]. This effect is attributed not to viable probiotic cells themselves but to the action of the ACE inhibitor-like peptides produced during fermentation. Based on these reports, we cannot exclude the possibility that the high level of antihypertensive biological activity found in R-14 Makgeolli may be attributed to peptides produced by the proteolytic action of microorganisms, including L. ingluviei, on the raw materials during fermentation of Makgeolli. Similarly, L. harbinensis may play a role in the high level of β-glucan detected in R-12 commercial raw Makgeolli. However, conduct of further study to determine the nature of antihypertensive ACE inhibitory activity and origin of β-glucan found in Makgeolli products will be necessary.
Acknowledgements
This study was conducted as the "Detection of physiological functionality for illustration of superiority of Makgeolli efficacy" Project (2011), supported by the Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
References
- 1.Seo MY, Lee JK, Ahn BH, Cha SK. The changes of microflora during the fermentation of Takju and Yakju. Korean J Food Sci Technol. 2005;37:61–66. [Google Scholar]
- 2.Lee SJ, Kwon YH, Kim HR, Ahn BH. Chemical and sensory characterization of Korean commercial rice wines (yakju) Food Sci Biotechnol. 2007;16:374–380. [Google Scholar]
- 3.Lee WK, Kim JR, Lee MW. Studies on the changes in free amino acids and organic acids of Takju prepared with different koji strains. Agric Chem Biotechnol. 1987;30:323–327. [Google Scholar]
- 4.Saito Y, Wanezaki K, Kawato A, Imayasu S. Structure and activity of angiotensin I converting enzyme inhibitory peptides from sake and sake lees. Biosci Biotechnol Biochem. 1994;58:1767–1771. doi: 10.1271/bbb.58.1767. [DOI] [PubMed] [Google Scholar]
- 5.Kim JH, Lee DH, Choi SY, Lee JS. Characterization of physiological functionalities in Korean traditional liquors. Korean J Food Sci Technol. 2002;34:118–122. [Google Scholar]
- 6.Miura NN, Ohno N, Aketagawa J, Tamura H, Tanaka S, Yadomae T. Blood clearance of (1-->3)-beta-D-glucan in MRL lpr/lpr mice. FEMS Immunol Med Microbiol. 1996;13:51–57. doi: 10.1111/j.1574-695X.1996.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 7.Othman RA, Moghadasian MH, Jones PJ. Cholesterol-lowering effects of oat β-glucan. Nutr Rev. 2011;69:299–309. doi: 10.1111/j.1753-4887.2011.00401.x. [DOI] [PubMed] [Google Scholar]
- 8.Min JH, Nam YG, Ju JI, Jung JH, Lee JS. Changes of Yeast and Bacterial Flora during Fermentation and Storage of Gugija-Liriope tuber Makgeolli using PCR-DGGE. Korean J Microbiol Biotechnol. 2012;40:111–116. [Google Scholar]
- 9.Sheffield VC, Cox DR, Lerman LS, Myers RM. Attachment of a 40-base-pair G+C-rich sequence (GC-clamp) to genomic DNA fragments by polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989;86:232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ercolini D. PCR-DGGE fingerprinting: novel strategies for detection of microbes in food. J Microbiol Methods. 2004;56:297–314. doi: 10.1016/j.mimet.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Thanh VN, Mai LT, Tuan DA. Microbial diversity of traditional Vietnamese alcohol fermentation starters (bahn men) as determined by PCR-mediated DGGE. Int J Food Microbiol. 2008;128:268–273. doi: 10.1016/j.ijfoodmicro.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Endo A, Okada S. Monitoring the lactic acid bacterial diversity during shochu fermentation by PCR-denaturing gradient gel electrophoresis. J Biosci Bioeng. 2005;99:216–221. doi: 10.1263/jbb.99.216. [DOI] [PubMed] [Google Scholar]
- 13.Cocolin L, Bisson LF, Mills DA. Direct profiling of the yeast dynamics in wine fermentations. FEMS Microbiol Lett. 2000;189:81–87. doi: 10.1111/j.1574-6968.2000.tb09210.x. [DOI] [PubMed] [Google Scholar]
- 14.Jo KY, Ha DM. Isolation and identification of the lactic acid bacteria from nuruk. Agric Chem Biotechnol. 1995;38:95–99. [Google Scholar]
- 15.Takeuchi A, Shimizu-Ibuka A, Nishiyama Y, Mura K, Okada S, Tokue C, Araj S. Purification and characterization of an α-amylase of Pichia burtonii isolated from the traditional starter "Murcha" in Nepal. Biosci Biotechnol Biochem. 2006;70:3019–3024. doi: 10.1271/bbb.60430. [DOI] [PubMed] [Google Scholar]
- 16.Hesseltine CW, Rogers R, Winarno FG. Microbiological studies on amylolytic oriental fermentation starters. Mycopathologia. 1988;101:141–155. [Google Scholar]
- 17.Jin J, Kim SY, Jin Q, Eom HJ, Nam NS. Diversity analysis of lactic acid bacteria in Takju, Korean rice wine. J Microbiol Biotechnol. 2008;18:1678–1682. [PubMed] [Google Scholar]
- 18.Nübel U, Engelen B, Felske A, Snaidr J, Wieshuber A, Amann RI, Ludwig W, Backhaus H. Sequence heterogeneities of genes encoding 16 rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J Bacteriol. 1996;178:5636–5643. doi: 10.1128/jb.178.19.5636-5643.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanders ME. Considerations for use of probiotic bacteria to modulate human health. J Nutr. 2000;130:384S–390S. doi: 10.1093/jn/130.2.384S. [DOI] [PubMed] [Google Scholar]
- 20.Takano T. Milk derived peptides and hypertension reduction. Int Dairy J. 1998;8:375–381. [Google Scholar]