Abstract
During the search for neuraminidase inhibitors from medicinal fungi, we found that the culture broth of Phellinus linteus exhibited potent inhibitory activity. Solvent partition, Sephadex LH-20 column chromatography, and high-performance liquid chromatography (HPLC) were performed for purification of two active substances from the culture broth. According to 1H NMR measurements and comparison of HPLC retention times with those of authentic compounds, their chemical structures were identified as hispidin and hypholomine B. Compounds (hispidin) 1 and 2 (hypholomine B) inhibited neuraminidase, with IC50 values of 13.1 and 0.03 µM, respectively.
Keywords: Hispidin, Hypholomine B, Neuraminidase inhibitor, Phellinus linteus
Mushrooms are nutritionally functional foods and important sources of physiologically beneficial medicines. They produce a large variety of secondary metabolites with unique chemical structures and interesting biological activities. Phellinus linteus is cultivated as a folk medicine in Korea and is known to exhibit various biological activities, such as anticancer, antioxidative, anti-angiogenic, anti-inflammatory and anti-viral effects [1-5]. However, except for polysaccharides, a limited number of bioactive metabolites have been reported from P. linteus.
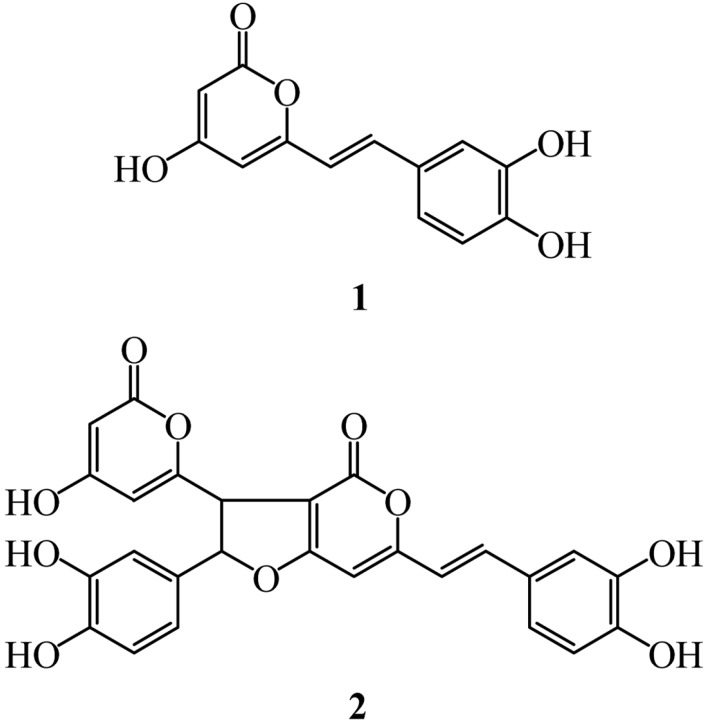
Neuraminidases, called sialidases, catalyze hydrolysis of terminal neuraminic-acid residues from newly formed virions and from host cell receptors [6], and are therefore involved in release of progeny virus from infected cells. Specifically, cleavage of the α-ketosidic bond linking a terminal neuraminic-acid residue to an adjacent oligosaccharide moiety by neuraminidase, which is also important for trafficking of virus in the mucus layer of the respiratory tract, allowing the virus access to underlying epithelial cells, has been reported [7]. Therefore, neuraminidase that plays an important role in viral proliferation is a drug target for the prevention of the spread of influenza infection. Neuraminidase inhibitors, such as zanamivir administered by inhalation and oseltamivir administered orally, have been used for combating influenza infection [8]. However, the long-term efficacy of these drugs is often limited by toxicity and the almost inevitable selection of drug-resistant viral mutants [9]. In our ongoing effort to identify bioactive substances from fungi, primarily medicinal fungi [10, 11], two neuraminidase inhibitors have been isolated from the culture broth of P. linteus (Fig. 1). This paper describes the isolation, structure determination, and neuraminidase inhibitory activity of these compounds.
Fig. 1.
Structures of compounds 1 (hispidin) and 2 (hypholomine B).
A strain of P. linteus, which was obtained from the Rural Development Administration (RDA) in Korea, was grown in potato dextrose broth medium at 28℃ for 10 days. Extraction of three liters of the culture broth was performed using ethyl acetate, with vigorous shaking. Neuraminidase inhibitory activity was observed in the ethyl acetate-soluble portion. Following concentration of the ethyl acetate-soluble portion under reduced pressure, the concentrate was subjected to a Sephadex LH-20 (Pharmacia, Uppsala, Sweden) column eluted with methanol to give two active fractions. A Sephadex LH-20 column with 70% aqueous methanol was used for chromatography of one fraction, followed by purification with preparative reversed-phase high-performance liquid chromatography (HPLC) with 60% aqueous methanol with 0.04% trifluoroacetic acid to give compound 1. Sephadex LH-20 column chromatography, with 70% aqueous methanol, was performed for purification of the other fraction, followed by preparative reversed-phase HPLC using the same solvent used for compound 1 to afford compound 2.
The chemical structure of compound 1 was determined according to 1H NMR spectrum and by comparison of HPLC retention time with that of the authentic compound. The 1H NMR spectrum of compound 1 in CD3OD exhibited signals due to a 1,2,4-trisubstituted benzene at δ 7.01 (d, J = 1.5 Hz), 6.93 (dd, J = 8.3, 1.5 Hz) and 6.76 (d, J = 8.3 Hz), two olefinic methines attributable to a trans-1,2-disubstituted double bond unit at δ 7.29 (d, J = 16.0 Hz) and 6.58 (d, J = 16.0 Hz), and a sp2 singlet methine at δ 6.09. These spectroscopic data were well matched with those of hispidin, which was previously isolated by our group as an antioxidant [12]. Thus, analytical HPLC equipped with a reversed-phase C18 column (150 × 4.6 mm i.d.) was performed for comparison of retention time of compound 1 with that of authentic hispidin. A linear gradient using water acidified with 0.04% trifluoroacetic acid (v/v) and methanol, at a flow rate of 1 mL/min, was used, starting at 10% aqueous methanol and reaching 100% methanol within a period of 30 min. Consequently, the retention time of compound 1 was the same as that of hispidin. Thus, compound 1 was identified as hispidin, a ubiquitous compound in genera Phellinus and Inonotus [12].
The structure of compound 2 was assigned using the same methods employed for compound 1. The 1H NMR spectrum of compound 2 in CD3OD exhibited signals due to two 1,2,4-trisubstituted benzenes at δ 7.03 (d, J = 2.0 Hz), 6.93 (dd, J = 8.3, 2.0 Hz), 6.72 (d, J = 8.3Hz) and 6.79 (d, J = 2.0 Hz), 6.78 (d, J = 8.3 Hz), 6.70 (dd, J = 8.3, 2.0 Hz), two olefinic methines attributable to a trans-1,2-disubstituted double bond unit at δ 7.35 (d, J = 16.1 Hz) and 6.63 (d, J = 16.1 Hz), two sp3 methines at δ 5.76 (d, J = 6.4 Hz) and 4.30 (d, J = 6.4 Hz), and two sp2 singlet methines at δ 6.36 and 6.08. Based on findings from an extensive literature survey, we observed that 1H NMR spectrum was in good agreement with that of hypholomine B [12]. Thus, analytical HPLC equipped with a reversed-phase C18 column (150 × 4.6 mm i.d.), using the same solvent used for compound 1, was performed for comparison of the retention time of compound 2 with that of authentic hypholomine B. Consequently, the retention time for compound 2 was the same as that of hypholomine B, revealing that compound 2 was identical to hypholomine B.
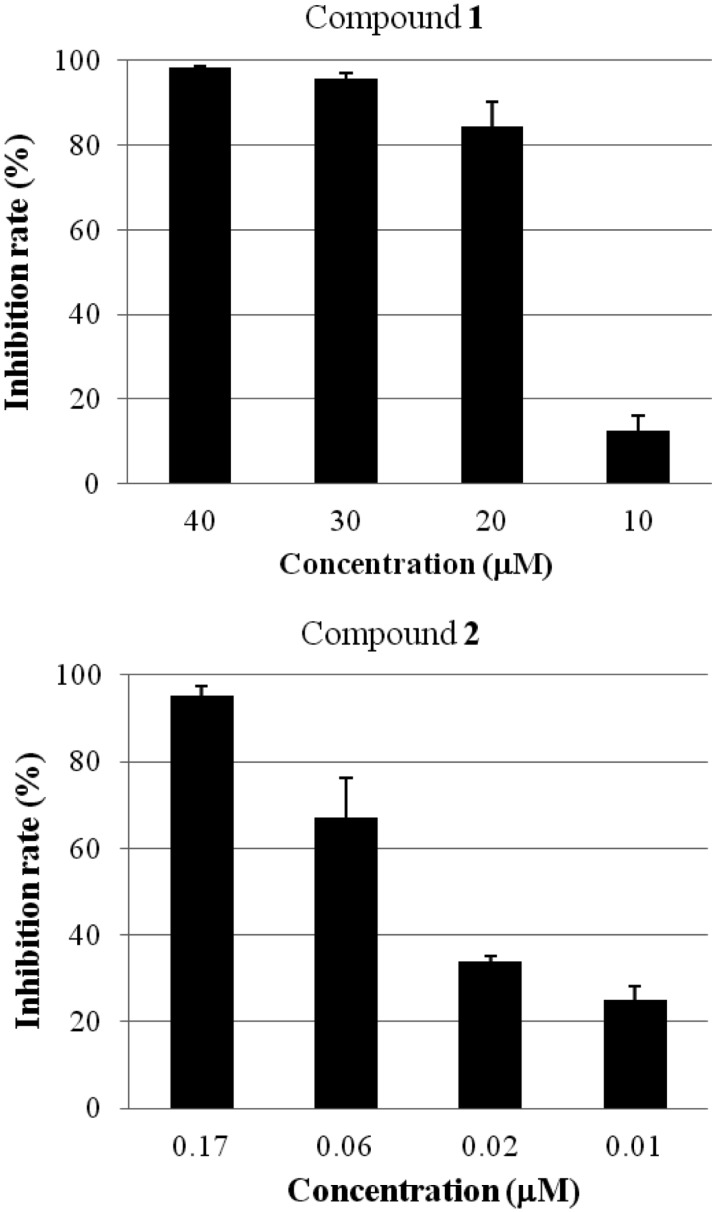
A previously reported method, with minor modifications, was used in performance of the neuraminidase inhibition assay [13]. Briefly, 50 µL neuraminidase (N2876; Sigma, St. Louis, MO, USA) solution (0.05 U/mL) was pre-mixed with 10 µL of sample solution at different concentrations in 90 µL of 50 mM sodium acetate buffer (pH 5.0) in a cuvette. Then, 4-methylumbelliferyl-α-D-N-acetylneuraminic acid sodium salt hydrate (M8639; Sigma) 0.05 mM in buffer (pH 5.0) was added (50 µL) to the mixture as a substrate in order to start the reaction at 37℃. Quantification of 4-methylumbelliferone was performed immediately by fluorometric determination using a POLARstar OPTIMA multi-detection microplate reader (BMG Labtech, Offenburg, Germany). The excitation and emission wavelengths were 355 nm and 460 nm, respectively. As a result, compounds 1 and 2 exhibited neuraminidase inhibitory activity with IC50 values of 13.1 and 0.03 µM, respectively, in a dose-dependent manner (Fig. 2). Compound 2 induced potent inhibition of neuraminidase and exhibited approximately 430-times higher activity than compound 1. To the best of our knowledge, this is the first study to report on hispidin and hypholomine B as neuraminidase inhibitors. Details regarding the mode of action of hispidin and hypholomine B for inhibition of neuraminidase are under investigation.
Fig. 2.
Neuraminidase inhibitory activity of compounds 1 (hispidin) and 2 (hypholomine B).
Acknowledgements
This work was supported by the Technology Development Program for Bio-industry, Ministry for Food, Agriculture, Forestry and Fisheries and, in part, by a grant from the Korea Forest Service, Republic of Korea.
References
- 1.Zhu T, Kim SH, Chen CY. A medicinal mushroom: Phellinus linteus. Curr Med Chem. 2008;15:1330–1335. doi: 10.2174/092986708784534929. [DOI] [PubMed] [Google Scholar]
- 2.Lee IK, Yun BS. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J Antibiot (Tokyo) 2011;64:349–359. doi: 10.1038/ja.2011.2. [DOI] [PubMed] [Google Scholar]
- 3.Lee YS, Kim YH, Shin EK, Kim DH, Lim SS, Lee JY, Kim JK. Anti-angiogenic activity of methanol extract of Phellinus linteus and its fractions. J Ethnopharmacol. 2010;131:56–62. doi: 10.1016/j.jep.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 4.Kim HG, Yoon DH, Lee WH, Han SK, Shrestha B, Kim CH, Lim MH, Chang W, Lim S, Choi S, et al. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-κB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophage. J Ethnopharmacol. 2007;114:307–315. doi: 10.1016/j.jep.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Ichinohe T, Ainai A, Nakamura T, Akiyama Y, Maeyama J, Odagiri T, Tashiro M, Takahashi H, Sawa H, Tamura S, et al. Induction of cross-protective immunity against influenza A virus H5N1 by an intranasal vaccine with extracts of mushroom mycelia. J Med Virol. 2010;82:128–137. doi: 10.1002/jmv.21670. [DOI] [PubMed] [Google Scholar]
- 6.von Itzstein M. The war against influenza: discovery and development of sialidase inhibitors. Nat Rev Drug Discov. 2007;6:967–974. doi: 10.1038/nrd2400. [DOI] [PubMed] [Google Scholar]
- 7.Klenk HD, Rott R. The molecular biology of influenza virus pathogenicity. Adv Virus Res. 1988;34:247–281. doi: 10.1016/S0065-3527(08)60520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Yu K, Zhu W, Jiang H. Neuraminidase pharmacophore model derived from diverse classes of inhibitors. Bioorg Med Chem Lett. 2006;16:3009–3014. doi: 10.1016/j.bmcl.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362:1733–1745. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang YW, Lee IK, Kim YS, Seok SJ, Yu SH, Yun BS. Chemical constituents of the fruiting body of Xylaria polymorpha. Mycobiology. 2009;37:207–210. doi: 10.4489/MYCO.2009.37.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee IK, Jung JY, Yeom JH, Ki DW, Lee MS, Yeo WH, Yun BS. Fomitoside K, a new lanostane triterpene glycoside from the fruiting body of Fomitopsis nigra. Mycobiology. 2012;40:76–78. doi: 10.5941/MYCO.2012.40.1.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung JY, Lee IK, Seok SJ, Lee HJ, Kim YH, Yun BS. Antioxidant polyphenols from the mycelial culture of the medicinal fungi Inonotus xeranticus and Phellinus linteus. J Appl Microbiol. 2008;104:1824–1832. doi: 10.1111/j.1365-2672.2008.03737.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim JY, Jeong HJ, Park JY, Kim YM, Park SJ, Cho JK, Park KH, Ryu YB, Lee WS. Selective and slow-binding inhibition of shikonin derivatives isolated from Lithospermum erythrorhizon on glycosyl hydrolase 33 and 34 sialidases. Bioorg Med Chem. 2012;20:1740–1748. doi: 10.1016/j.bmc.2012.01.011. [DOI] [PubMed] [Google Scholar]