Abstract
The root of Hibiscus syriacus (Malvaceae) has been used for treatment of fungal diseases such as tinea pedis (athlete's foot). In this study, we investigated the antifungal constituent of the root of Hibiscus syriacus Ggoma, which was produced by a mutation breeding using gamma ray irradiation, and compared the antifungal activity of H. syriacus Ggoma and its parent type. According to the results, the methanolic extract of H. syriacus Ggoma exhibited four times higher antifungal activity than its parent type against Trichophyton mentagrophytes. Following purification through various column chromatographies, the antifungal substance was identified as nonanoic acid on the basis of spectroscopic analysis.
Keywords: Antifungal compound, Hibiscus syriacus Ggoma, Nonanoic acid
Antifungal agents have been used for treatment of fungal infections. The root bark of Hibiscus syriacus (Malvaceae), which is widely distributed over East Asia, has been used as an antifungal agent for treatment of athlete's foot [1, 2]. Previous studies of the chemical constituents of the root of H. syriacus have reported on hibispeptins A and B [3], triterpene caffeates [4], and syriacusins A-C [5] as antioxidants; however, no studies on antifungal substances have been reported. Recently, a new H. syriacus mutant, designated as H. syriacus Ggoma, was produced by a mutation breeding using gamma ray irradiation and has been grown as an ornamental plant for approximately four years [6]. This study has been conducted for comparison of the antifungal activity of the root extracts of H. syriacus Ggoma and its parent type, and an antifungal constituent from the root of H. syriacus has been isolated by repeated column chromatography and identified by extensive use of spectroscopic methods.
Hibiscus syriacus and its mutant, H. syriacus Ggoma were cultivated at the Herbal garden, Advanced Radiation Technology Institute-Jeongeup, Korea Atomic Energy Research Institute, Korea, and their roots were collected in July 2007.
For comparison of the antifungal activity of H. syriacus and its mutant, H. syriacus Ggoma, extraction of their ground roots (56 g for each) was performed twice using methanol. A dermatophyte, Trichophyton mentagrophytes, was used for estimation of their antifungal activities by the conventional paper disk (Advantec, 8 mm in diameter) method. In brief, paper disks containing 50 µg samples were placed on an agar plate inoculated with the test organism. Assessment of antibiotic activity was performed by measuring the diameter of the zone of inhibition after incubation for five days at 27℃. According to the results, the methanolic extract of H. syriacus Ggoma exhibited four times higher activity than that of its parent type. This finding indicates that a mutation breeding using gamma ray irradiation can result in significant variation in metabolism and can be an efficient method for achievement of high production of valuable plant materials.
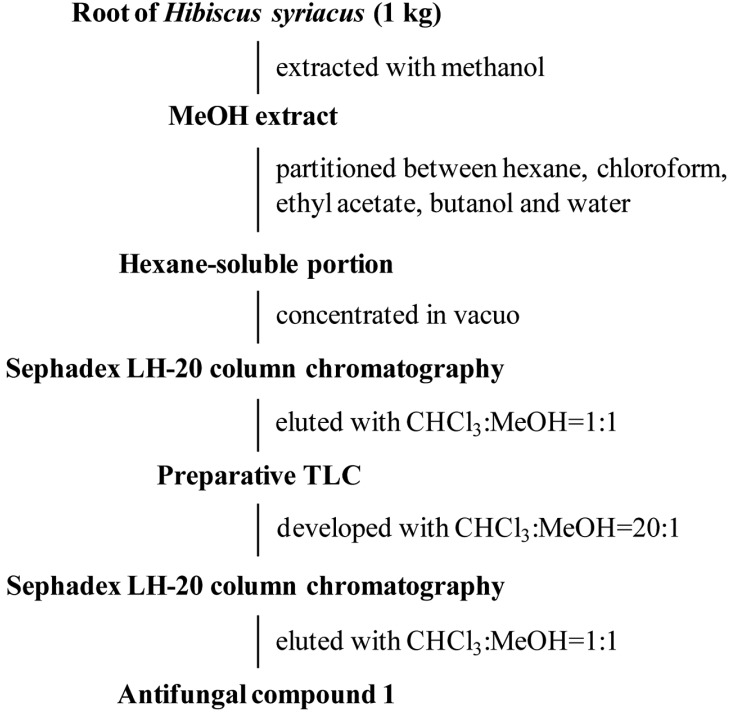
The root bark of H. syriacus has been used as an antifungal agent for treatment of athlete's foot; however, the compound responsible for this activity remains unclear. Therefore, we investigated the antifungal constituents and their productivity in H. syriacus and its mutant, H. syriacus Ggoma. For isolation of antifungal substance, roots of H. syriacus and its mutant, H. syriacus Ggoma (1 kg for each) were ground and extracted twice using methanol for 24 hr. The methanolic extracts were combined and concentrated under reduced pressure. The concentrate was dissolved in water, followed by consecutive partitioning with hexane, chloroform, ethyl acetate, and butanol. The hexane-soluble portion, which exhibited potent antifungal activity, was subjected to a Sephadex LH-20 column eluted with chloroform : methanol (1 : 1, v/v). Preparative thin layer chromatography (chloroform : methanol = 20 : 1) was performed for purification of an antifungal fraction, followed by Sephadex LH-20 column chromatography eluted with chloroform : methanol (1 : 1, v/v) to provide compound 1 (Fig. 1).
Fig. 1.
Procedures for isolation of an antifungal compound.
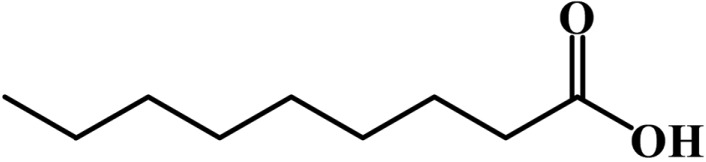
On the basis of 1H NMR, 13C NMR, and electrospray ionization (ESI) mass measurements, the structure of antifungal compound 1 was identified as nonanoic acid. The 1H NMR spectrum in CD3OD showed signals due to seven methylenes at δ 2.35 (2H, t, J = 7.2 Hz, H-2), 1.63 (2H, m, H-3), and 1.38~1.23 (10H, m, H-4, H-5, H-6, H-7, H-8) and one methyl at δ 0.88 (3H, t, J = 6.6 Hz, H-9). In the 13C NMR spectrum in CDCl3, nine carbons containing one carbonyl carbon were evident; 179.8 (C-1), 34.0 (C-2), 31.8 (C-7), 29.1 (C-4), 29.1 (C-5), 29.1 (C-6), 24.7 (C-3), 22.6 (C-8), and 14.1 (C-9). These spectral data suggested that 1 was a fatty acid, nonanoic acid (Fig. 2). This suggestion was supported by ESI-mass measurement in negative mode, which showed a quasi-molecular ion peak at m/z 157.4 [M-H]-. Nonanoic acid has been reported as an inhibitor of spore germination and mycelial growth of pathogenic fungus [7]. However, to the best of our knowledge, this is the first report on nonanoic acid as a major antifungal substance from the root of H. syriacus.
Fig. 2.
Structure of antifungal compound 1 (= nonanoic acid).
In an assessment of antifungal activity using the agar diffusion method, compound 1 (50 µg) exhibited potent antifungal activity against Trichophyton mentagrophytes, with a diameter of approximately 16 mm.
Acknowledgements
This work was supported by a grant from the KAERI and by the Agenda project of the Rural Development Administration (RDA), Republic of Korea.
References
- 1.Hsu HY, Chen YP, Shen SJ, Hsu CS, Chen CC, Chang HC. Oriental materia medica: a concise guide. Taiwan: Oriental Healing Arts Institute; 1986. pp. 503–504. [Google Scholar]
- 2.Huang KC. The pharmacology of Chinese herbs. Tokyo: CRC Press; 1993. pp. 193–194. [Google Scholar]
- 3.Yun BS, Ryoo IJ, Lee IK, Yoo ID. Hibispeptin B, a novel cyclic peptide from Hibiscus syriacus. Tetrahedron. 1998;54:15155–15160. [Google Scholar]
- 4.Yun BS, Ryoo IJ, Lee IK, Park KH, Choung DH, Han KH, Yoo ID. Two bioactive pentacyclic triterpene esters from the root bark of Hibiscus syriacus. J Nat Prod. 1999;62:764–766. doi: 10.1021/np9804637. [DOI] [PubMed] [Google Scholar]
- 5.Yoo ID, Yun BS, Lee IK, Ryoo IJ, Choung DH, Han KH. Three naphthalenes from root bark of Hibiscus syriacus. Phytochemistry. 1998;47:799–802. doi: 10.1016/s0031-9422(97)00674-2. [DOI] [PubMed] [Google Scholar]
- 6.Song HS, Park IS, Lim YT, Kim JK, Lee GJ, Kim DS, Lee SJ, Kang SY. A dwarf type new rose of Sharon variety, "Ggoma" developed by a mutation breeding. Korean J Breed. 2006;38:293–294. [Google Scholar]
- 7.Aneja M, Gianfagna TJ, Hebbar PK. Trichoderma harzianum produces nonanoic acid, an inhibitor of spore germination and mycelial growth of two cacao pathogens. Physiol Mol Plant Pathol. 2005;67:304–307. [Google Scholar]