Dab2 binds EH domain proteins. This interaction is required for integrin β1 but not TfnR endocytosis. β1 and TfnR do not colocalize, even though their adaptors sort to the same pits. The data suggest that Dab2 selectively drives β1 endocytosis. It is proposed that specific cargo–adaptor–EH domain protein complexes are needed for efficient endocytosis.
Abstract
Endocytic adaptor proteins facilitate cargo recruitment and clathrin-coated pit nucleation. The prototypical clathrin adaptor AP2 mediates cargo recruitment, maturation, and scission of the pit by binding cargo, clathrin, and accessory proteins, including the Eps-homology (EH) domain proteins Eps15 and intersectin. However, clathrin-mediated endocytosis of some cargoes proceeds efficiently in AP2-depleted cells. We found that Dab2, another endocytic adaptor, also binds to Eps15 and intersectin. Depletion of EH domain proteins altered the number and size of clathrin structures and impaired the endocytosis of the Dab2- and AP2-dependent cargoes, integrin β1 and transferrin receptor, respectively. To test the importance of Dab2 binding to EH domain proteins for endocytosis, we mutated the EH domain–binding sites. This mutant localized to clathrin structures with integrin β1, AP2, and reduced amounts of Eps15. Of interest, although integrin β1 endocytosis was impaired, transferrin receptor internalization was unaffected. Surprisingly, whereas clathrin structures contain both Dab2 and AP2, integrin β1 and transferrin localize in separate pits. These data suggest that Dab2-mediated recruitment of EH domain proteins selectively drives the internalization of the Dab2 cargo, integrin β1. We propose that adaptors may need to be bound to their cargo to regulate EH domain proteins and internalize efficiently.
INTRODUCTION
Many plasma membrane receptors and essential macromolecules are internalized by clathrin-mediated endocytosis (CME). Receptors associate with endocytic adaptors, which coassemble with clathrin to form clathrin-coated pits (CCPs). The endocytic adaptors bind to phosphatidylinositol (4,5)-bisphosphate (PtdIns(4,5)P2), clathrin, and internalization signals within the cytoplasmic tails of their cargoes, thus linking cargoes to clathrin and the membrane. Subsequent recruitment of an extensive network of proteins regulates invagination of the pit and internalization. The prototypical clathrin adaptor AP2 is a tetrameric complex comprising a core of small (σ2), medium (μ2), and large (α and β2) subunits (Pearse, 1988; Kirchhausen, 1999; Traub, 2003). The μ2 subunit recognizes PtdIns(4,5)P2 and the YxxΦ sorting signal in the transferrin receptor (TfnR; Ohno et al., 1995; Rohde et al., 2002). The σ2 subunit binds to the acidic dileucine internalization signal, [DE]XXXL[LI] (Chaudhuri et al., 2007). The α and β2 subunits bind to endocytic accessory proteins that regulate assembly and budding of CCPs, including scaffolding proteins Eps15 and Itsn, membrane-bending/curvature-sensing proteins epsin, endophilin, and FCHO1/2, the phosphoinositide phosphatase synaptojanin, and dynamin, a GTPase implicated in vesicle fission (Praefcke et al., 2004; Schmid et al., 2006; Henne et al., 2010). Thus AP2 may be thought of as a hub for protein–protein interactions that sequentially and cooperatively regulate two key processes in endocytosis: first, the recruitment of cargo and clathrin to form CCPs and, second, the invagination and eventual budding of the CCP to form a clathrin-coated vesicle.
Some cargoes use alternative, monomeric adaptor proteins known as clathrin-associated sorting proteins (CLASPs; Traub, 2009). Dab2 is one such CLASP. It contains a phosphotyrosine-binding domain that recognizes PtdIns(4,5)P2 and the FxNPxY internalization motif found in Dab2 cargo low-density lipoprotein (LDL) receptor and β-integrin (Morris and Cooper, 2001; Mishra et al., 2002; Keyel et al., 2006; Maurer and Cooper, 2006; Chao and Kunz, 2009; Ezratty et al., 2009; Teckchandani et al., 2009). Dab2 binds clathrin as well as cargoes, and is thus well suited to recruit cargoes to CCPs. Furthermore, Dab2 can function in AP2-deficient cells (Motley et al., 2003; Keyel et al., 2006; Maurer and Cooper, 2006; Cihil et al., 2012), implying that Dab2 may also bind accessory proteins that regulate CCP invagination and budding. Indeed, Dab2 contains binding sites for FCHO2, myosin VI, CIN85, and Grb2 (Xu et al., 1998; Morris et al., 2002; Kowanetz et al., 2003; Mulkearns and Cooper, 2012). Dab2 also contains asparagine-proline-phenylalanine (NPF) motifs that potentially bind Eps-homology (EH) domains (Salcini et al., 1997; de Beer et al., 1998).
CCPs contain the EH domain proteins Eps15, Eps15R, and intersectin1 and 2 (Itsn1 and 2), which are important for endocytosis (Miliaras and Wendland, 2004; Henne et al., 2007; van Bergen En Henegouwen, 2009). These EH domain proteins provide binding sites for numerous other accessory proteins, including dynamin, synaptojanin, stonin, synaptotagmin, SHIP2, FCHO2, and epsin (Sengar et al., 1999; Miliaras and Wendland, 2004; Henne et al., 2010). Thus EH domain proteins could assemble many of the same endocytic accessory proteins as are recruited by AP2.
In this article we report that Dab2 binds to Eps15 and Itsn. Depleting cells of Eps15, Itsn, and their close relatives inhibited endocytosis of both Dab2-dependent and Dab2-independent cargoes and affected clathrin assembly, causing an increase in size of large clathrin-coated structures (CCSs), probably plaques. To test the role of Dab2–EH domain protein interactions under conditions in which the size distribution of clathrin structures was not affected, we reconstituted Dab2-deficient cells with a mutant Dab2 that cannot bind EH domain proteins. Clathrin structures reconstituted with this mutant recruited integrin β1 normally but contained less Eps15 and internalized integrin β1 inefficiently, suggesting that the Dab2–EH domain interaction is important for Dab2-dependent endocytosis. Surprisingly, transferrin receptor endocytosis was unaffected. Integrin β1 and TfnR do not colocalize on the cell surface, even though most clathrin structures contain both Dab2 and AP2. We propose that cargo regulates adaptor-dependent EH domain protein recruitment or activation for efficient internalization.
RESULTS
The Dab2 NPF sequences mediate binding to endocytic EH domain proteins
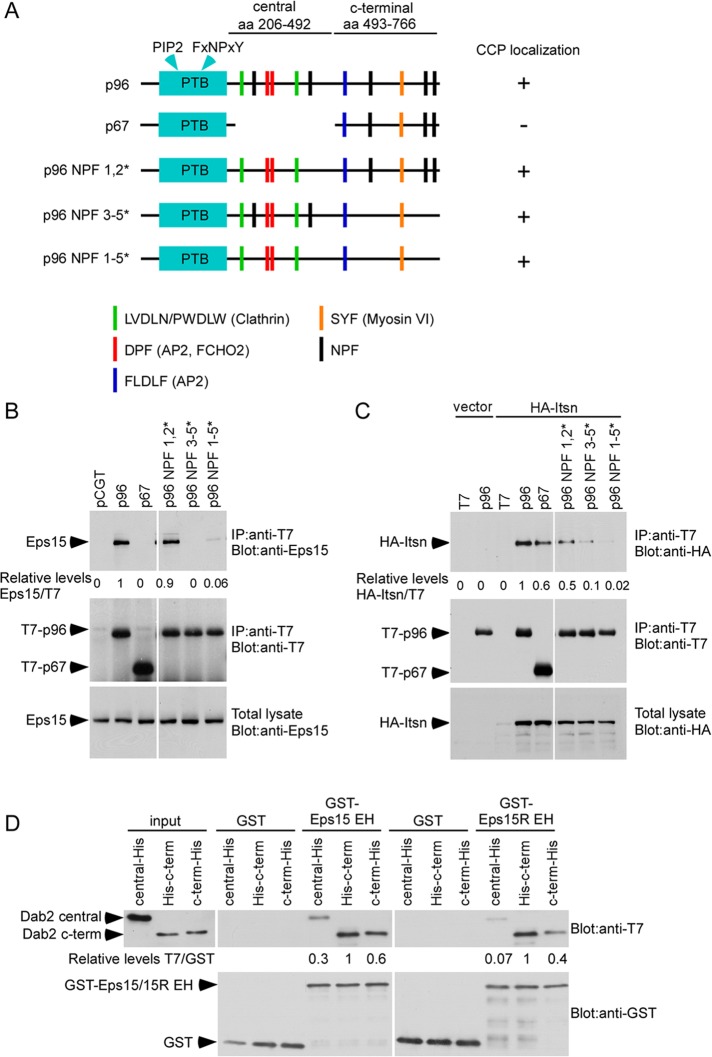
The major endocytic splice form of Dab2, p96, contains five NPF sequences (Figure 1A), suggesting that it might interact with the endocytic EH domain–containing proteins Eps15 and Itsn. We tested for coimmunoprecipitation of T7-tagged Dab2 p96 with hemagglutinin (HA)-tagged Itsn and endogenous Eps15. Dab2 p96 coimmunoprecipitated with both Eps15 and HA-Itsn (Figure 1, B and C) and localized to clathrin structures that also contained Eps15 and HA-Itsn (Supplemental Figure S1A). Moreover, endogenous Eps15 and Dab2 coimmunoprecipitated (Supplemental Figure S1B). Another splice form of Dab2, p67, does not associate with CCPs (Supplemental Figure S2; Morris and Cooper, 2001). p67 did not bind to Eps15 and bound only weakly to HA-Itsn (Figure 1, B and C). To test whether the NPF sequences in Dab2 were required for binding Eps15 and Itsn, we made point mutations in p96. Point mutations in all five NPFs completely blocked Dab2 interaction with both Eps15 and Itsn (Figure 1, B and C). Mutating the three C-terminal NPFs strongly inhibited Eps15 binding and partially inhibited Itsn binding, whereas mutating the two central NPFs, which are absent from p67, did not inhibit binding of either Eps15 or Itsn (Figure 1, B and C). These mutant forms of p96 were all localized in CCSs (Supplemental Figure S2). This suggests that the Dab2 NPF sequences are not required for Dab2 localization to CCSs but are required for binding Eps15 and Itsn. In addition, the C-terminal NPF sequences play predominant roles in these interactions.
FIGURE 1:
The Dab2 NPFs are required for binding Eps15 and Itsn. (A) Drawing of T7-p96, T7-p67, or T7-p96 NPF mutants showing binding sites. The Dab2 central and C-terminal fragments used for in vitro binding are also shown. (B, C) HEK 293 cells were transfected with control vector or T7-p96, T7-p67, or T7-p96 NPF mutants and HA-Itsn. Cell lysates were immunoprecipitated with anti-T7 antibody and immunoblotted with either anti-Eps15 (B) or anti-HA (C). Representative blots from one of four independent experiments are shown. Pixel intensities were measured by ImageJ. (D) Binding of purified Dab2 and Eps15/15R EH domain. Purified, bacterially grown T7-mDab2-206-492-His (central fragment), T7-mDab2-493-766-His (C-terminus of Dab2), and T7-His-mDab2-493-766 (C-terminus of Dab2 with N-terminal tag) were mixed with purified, glutathione-Sepharose–bound GST-EH domain of Eps15 or Eps15R. Pixel intensities were measured by ImageJ.
We tested whether Dab2 binds Eps15 directly, by use of purified histidine (His)-tagged fragments of Dab2 and glutathione S-transferase (GST)–tagged EH domains of Eps15 in vitro (Figure 1D). The Eps15 EH domains bound more strongly to a Dab2 fragment containing the three C-terminal NPFs than to a central fragment containing the two central NPFs (Figure 1D), consistent with the coimmunoprecipitation results (Figure 1, B and C). Previous studies suggested that some EH domains recognize NPFXCOOH or NPFXXCOOH (Paoluzi et al., 1998; Yamabhai et al., 1998). The fifth NPF of Dab2 lies in the sequence NPFACOOH. This may contribute to the stronger binding of the C-terminal region of Dab2 to Eps15 because placing an epitope tag after the fifth NPF sequence slightly inhibited binding (Figure 1D). Similar results were obtained with EH domains from Eps15R (Figure 1D).
Taken together, these results suggest that Eps15 and Itsn bind to Dab2 p96 in cells, probably directly, principally via the C-terminal three NPF sequences. They do not bind significantly to the nonendocytic Dab2 p67 splice form, even though the relevant NPF sequences are present, possibly because p67 is not in coated pits.
EH domain proteins regulate integrin β1 and TfnR endocytosis
We tested whether endocytic EH domain proteins are important for Dab2-dependent and Dab-independent endocytosis using small interfering RNA (siRNA). We first evaluated which endocytic EH domain proteins are expressed in HeLa cells, using antibodies and reverse transcription (RT)-PCR (Supplemental Figure S3). We detected Eps15, Eps15R, Itsn1S, and Itsn2 but not the neuronal form, Itsn1L (Hussain et al., 1999). We found that all four gene products could be depleted using a mixture of siRNAs (Supplemental Figure S3). We call cells depleted of all four EH domain proteins “EH-depleted” or “EH-deficient” cells.
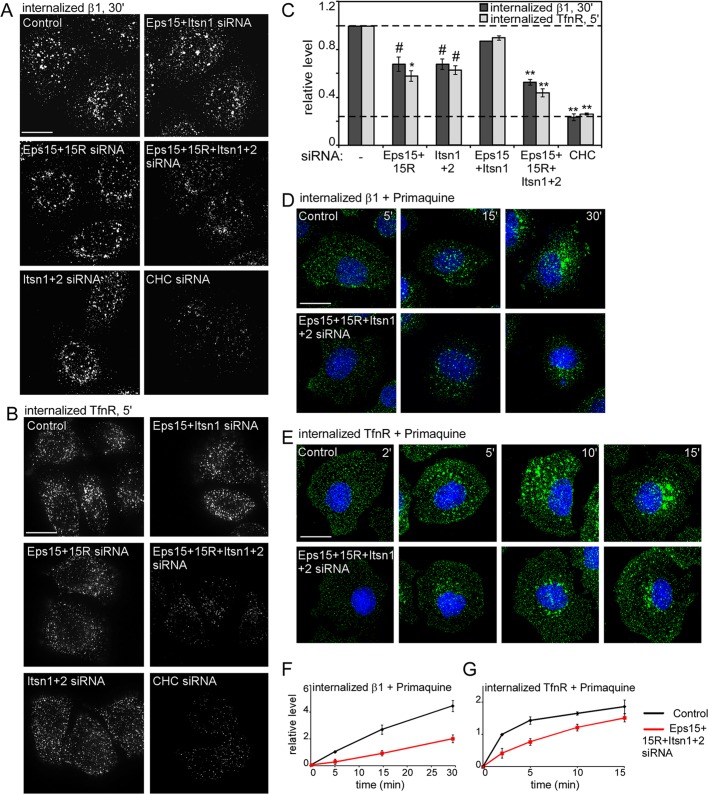
We measured endocytosis of integrin β1, a Dab2-dependent cargo, and TfnR, a Dab2-independent cargo, in EH-deficient cells. Cell surface receptors were labeled at 4°C with antibodies to the extracellular domain of integrin β1 or TfnR. Cells were then warmed to 37°C to allow internalization. Surface antibody was removed by a low-pH wash, and internalized antibody was detected by indirect immunofluorescence. Approximately 40% less integrin β1 and TfnR was detected inside Eps15+15R– and Itsn1+2–depleted cells (Figure 2, A–C). Depleting all four EH domain proteins showed a further decrease in internalized integrin β1 and TfnR levels, although this was not as low as with clathrin depletion (Figure 2, A–C). To confirm that the decreased content of internalized receptor was due to defective internalization and not accelerated recycling, we followed the uptake of integrin β1 and TfnR in the presence of Primaquine, an inhibitor of recycling. Control cells internalized both receptors more rapidly than did EH-deficient cells (Figure 2, D–G). Taken together, the results suggest that Eps15/15R and Itsn1/2 play partially overlapping roles in CME of Dab2-dependent and Dab2-independent cargoes.
FIGURE 2:
EH domain proteins regulate integrin β1 and TfnR endocytosis. (A–C) Control and siRNA-treated HeLa cells were incubated with anti–integrin β1 (A) or anti-TfnR antibody (B) for 30 min at 4°C, followed by warming at 37°C for 30 (β1) or 5 min (TfnR). (D–G) Control and EH domain–deficient HeLa cells were incubated with anti–integrin β1 (D) or anti-TfnR antibody (E) for 30 min at 4°C and then warmed to 37°C for the indicated times in the presence of 2 μM Primaquine. Surface antibody was removed by acid stripping, and internalized antibody was detected. (A, B, D, E) Z-projections of the entire cell. (C, F, G) Pixel intensities measured by ImageJ. Mean values and standard errors of internalized receptor after normalizing with surface receptor at time = 0 are shown. Approximately 15 cells/treatment from three independent experiments were analyzed. #p < 0.05, *p < 0.01, **p < 0.001 by t test. Bar, 10 μm.
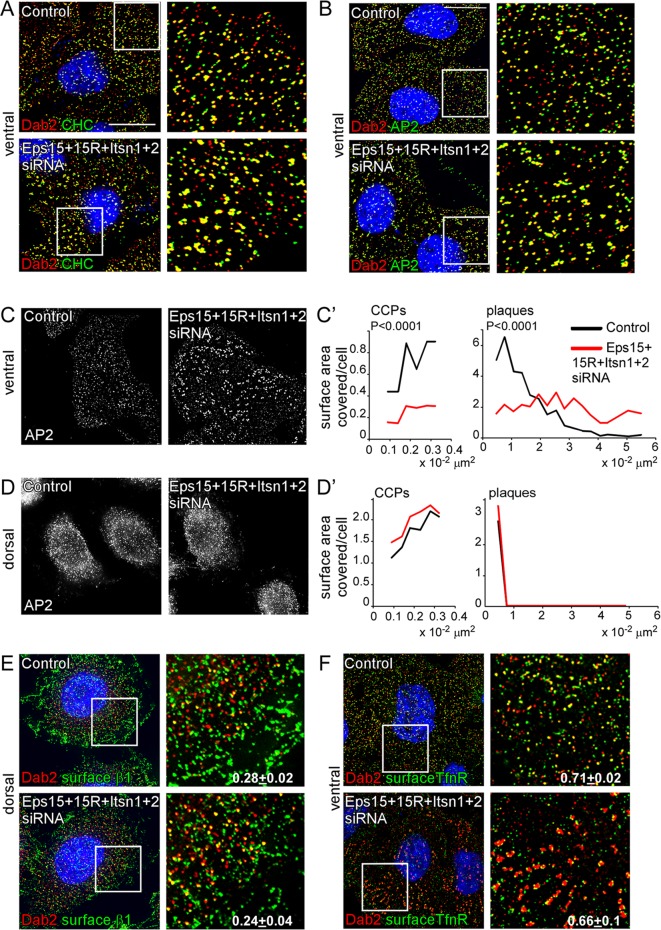
To determine whether EH domain proteins regulate CCS nucleation or assembly, we analyzed clathrin structures in EH-deficient HeLa cells by immunofluorescence using antibodies to clathrin, AP2, and Dab2. We designate CCSs with diameter 100–200 nm as CCPs and CCSs with diameters >200 nm as plaques (Saffarian et al., 2009). As reported, the ventral surface of HeLa cells has CCPs and plaques, whereas the dorsal surface has only CCPs (Figure 3, C–D′, and Table 1; Saffarian et al., 2009). Clathrin, AP2, and Dab2 were still recruited to pits and plaques in EH-depleted cells (Figure 3, A and B). The number and size of CCPs on the dorsal surface and the size of CCPs on the ventral surface were unchanged (Table 1). However, EH-depleted cells had decreased numbers of CCPs and plaques on the ventral surface, and the median plaque size significantly increased (Figure 3, A–D, and Table 1). The larger plaques were particularly strongly affected (Figure 3C′ and Table 1). The overall result of EH domain–protein deficiency is that cell surface area occupied by CCPs was decreased, whereas area occupied by large plaques was dramatically increased (Figure 3C′).
FIGURE 3:
Effect of EH domain protein depletion on clathrin, AP2, Dab2, and receptor localization. Control and EH domain–deficient HeLa cells grown on collagen IV–coated coverslips were fixed, permeabilized, and stained with anti-Dab2 and anti-CHC (A) or anti-AP2 (B) to detect clathrin-coated structures. (C) AP2 staining alone on the ventral and dorsal surface. AP2 staining was used to calculate the surface area covered by CCPs (size, 0.009–0.032 μm2, which corresponds to structures with diameters 107–202 nm) or plaques (size, >0.032 μm2) by ImageJ. Approximately 2000 puncta/treatment from three independent experiments were used. p values were calculated using the Mann–Whitney test, a nonparametric test. (E, F) Cells were stained with either anti–integrin β1 (E) or anti-TfnR (F) antibody before permeabilizing to detect surface receptor. Shown are 0.2-μm sections of the ventral or dorsal surface. The white boxes indicate the enlarged images shown in the insets. The fraction of surface integrin β1 or TfnR that colocalized with Dab2 was measured by ImageJ (Manders colocalization coefficient). In all cases random colocalization, obtained by flipping the red channel (Dab2), was <0.08. Approximately 10 cells/treatment from three separate experiments were analyzed. Bar, 10 μm.
TABLE 1:
Effect of EH domain protein depletion on size and number of CCPs and clathrin plaques.
| Parameter | Control | siRNA Eps15+15R | siRNA Itsn1+2 | siRNA Eps15+15R+Itsn1+2 |
|---|---|---|---|---|
| CCPs, ventral | ||||
| Number/cell | 221 ± 50 | 110 ± 8# | 106 ± 18# | 78 ± 17# |
| Median diameter (nm) | 151 | 151 | 156 | 151 |
| Mean intensity | 46 ± 3 | 47 ± 1 | 47 ± 1 | 47 ± 1 |
| Plaques, ventral | ||||
| Number/cell | 335 ± 18 | 286 ± 35 | 240 ± 48 | 186 ± 20** |
| Median diameter (nm) | 310 | 383*** | 327 | 429*** |
| Mean intensity | 66 ± 5 | 77 ± 3 | 68 ± 3 | 74 ± 1 |
| CCPs, dorsal | ||||
| Number/cell | 610 ± 23 | 641 ± 19 | 626 ± 16 | 715 ± 39 |
| Median diameter (nm) | 151 | 151 | 151 | 151 |
| Mean intensity | 48 ± 1 | 45 ± 1 | 45 ± 1 | 48 ± 1 |
Control and siRNA-treated HeLa cells grown on collagen IV–coated coverslips were fixed, permeabilized, and stained with anti-AP2 to detect clathrin-coated structures. CCP, structures with diameter ∼100–200 nm; plaques, structures with diameter >217 nm. EH domain protein depletion significantly decreased the numbers of AP2-containing pits and plaques. We analyzed 4000 structures for each condition, from three experiments. ImageJ was used to obtain measurements.
#p < 0.05; **p < 0.001, by t test. The median size of pits on both surfaces was unaltered. However, there was a significant increase in the median size of plaques on the ventral surface of the cell. ***p < 0.001 by the Mann–Whitney test. This test was used because the data follow a nonparametric distribution.
Changes in CCP and plaque number and plaque size could conceivably contribute to decreased endocytosis of integrin β1 and TfnR in EH-deficient cells. We tested whether recruitment of receptors to CCPs and plaques was affected in EH-deficient cells by immunofluorescence. Dab2 colocalizes with integrin β1 predominantly on the dorsal surface of HeLa cells (Teckchandani et al., 2009), and this colocalization was not altered when EH domain proteins were depleted (Figure 3E). Although Dab2 is not an adaptor for TfnR internalization, TfnR, AP2, and Dab2 are found together in many clathrin structures (Keyel et al., 2006). The fraction of TfnR colocalizing with Dab2 was also unaffected when EH domain proteins were depleted (Figure 3F). Therefore EH domain proteins are not needed for recruitment of Dab2-dependent or Dab-independent cargoes to CCSs. These results suggest that EH domain proteins regulate CCP/plaque nucleation, growth, and internalization but not the collection of Dab2-dependent or Dab2-independent cargoes into CCPs and plaques.
Internalization of Dab2-dependent cargo requires Dab2 binding to EH domain proteins
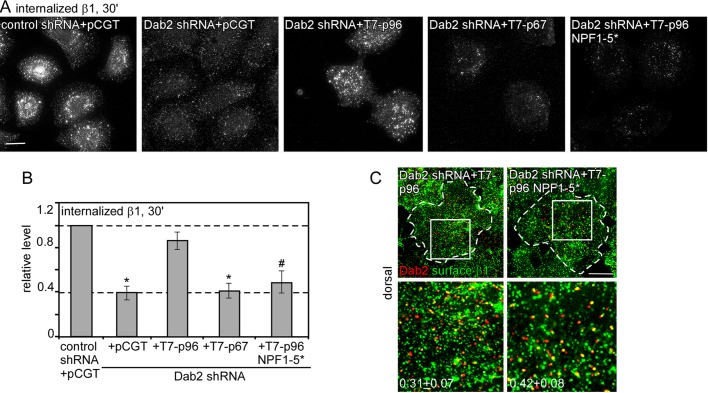
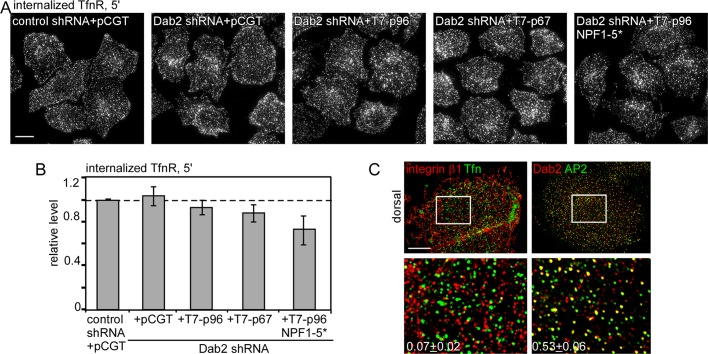
To determine the importance of Dab2 binding to EH domain proteins in Dab2-mediated endocytosis without the complication of altered CCP/plaque nucleation and growth, we used the p96 NPF1-5* mutant, which does not bind EH domain proteins (Figure 1, B and C). Dab2-deficient HeLa cells were reconstituted with wild-type or mutant Dab2. We first confirmed that the number and size of CCSs were not altered (Table 2). We then measured the uptake of integrin β1 (Figure 4, A and B). As expected, integrin β1 internalization was inhibited in Dab2-deficient cells and rescued by p96 but not p67 or vector (Teckchandani et al., 2009). Of interest, p96 NPF1-5* did not rescue integrin β1 endocytosis (Figure 4, A and B), even though it localized correctly and recruited integrin β1 to CCSs (Supplemental Figures S2 and 4C). These results suggest that Dab2-mediated integrin β1 internalization requires binding of Dab2 to EH-domain proteins.
TABLE 2:
Effect of Dab2 on the size, number, and composition of CCSs.
| Parameter | Control + pCGT | Dab2 shRNA + pCGT | Dab2 shRNA + T7-p96 | Dab2 shRNA + T7-p96NPF 1-5* |
|---|---|---|---|---|
| Median diameter of CCSs (nm) | 242 | 224 | 240 | 252 |
| Number of CCSs | 588 ± 41 | 521 ± 31 | 557 ± 63 | 569 ± 59 |
| Relative level clathrin/CCS | 1 | 0.91 | 1.03 | 1.04 |
| Relative level Eps15/CCS | 1 | 1.07 | 0.96 | 0.76*** |
| Relative level AP2/CCS | 1 | 1.74*** | 1.23 | 1.09 |
Control and Dab2-deficient HeLa cells reexpressing vector alone, T7-p96, or T7-p96 NPF1-5* and clathrin light chain (LCa)-EGFP were grown on collagen-coated coverslips and fixed, permeabilized, and stained with anti-Eps15 or anti-AP2 antibody. Clathrin structures on the ventral surface were characterized. No difference in the median size, number of CCSs, or clathrin content (area × mean intensity) was detected. However, the Eps15 content per clathrin structure was decreased in cells expressing the T7-p96 NPF1-5* mutant, and the AP2 content was increased in Dab2-deficient cells. The Eps15 content in clathrin structures was obtained by multiplying binary images of clathrin and Eps15 (to obtain area of “colocalized” Eps15), and superimposing onto the raw Eps15 image (to obtain intensity).
***p < 0.001 by the Mann–Whitney test. This test was used because the data follow a nonparametric distribution. Approximately 2000 structures were analyzed for each condition. ImageJ was used to obtain measurements.
FIGURE 4:
Disruption of the Dab2–EH domain protein interaction slows Dab2-dependent integrin β1 internalization. (A–C) Control and Dab2-deficient HeLa cells reexpressing vector alone or T7-p96, T7-p67, or T7-p96 NPF 1-5* were incubated with anti–integrin β1 antibody for 30 min at 4°C, followed by warming at 37°C for 30 min. (A, B) Surface antibody was removed by acid stripping, and internalized antibody was detected. (A) Z-projections of the entire cell showing internalized integrin β1 antibody. (B) Pixel intensities were measured by ImageJ. Mean values and standard errors are shown for ∼30 cells/treatment from three independent experiments. #p < 0.05, *p < 0.01, by t test. (C) Cells were stained with anti–integrin β1 antibody before permeabilizing to detect surface receptor. Z-projections of the dorsal surface are shown. The white boxes indicate the enlarged images shown in the insets. Bar, 10 μm. The fraction of surface integrin β1 that colocalized with Dab2 in T7-p96 or T7-p96 NPF 1-5*–expressing cells was calculated using ImageJ (Manders colocalization coefficient). In all cases random colocalization, obtained by flipping the red channel (Dab2), was <0.07. Approximately five cells/treatment were analyzed.
We tested whether reduced integrin β1 internalization by p96 NPF1-5* correlates with reduced recruitment of EH domain proteins to CCSs. We measured Eps15 and AP2 levels in CCSs in control, Dab2-depleted, wild-type p96 rescued and p96 NPF1-5* reconstituted cells. Similar amounts of Eps15 were recruited to CCSs in control and Dab2-depleted cells, but ∼25% less Eps15 (p < 0.001) was recruited to CCSs in cells reconstituted with p96 NPF1-5* (Table 2). AP2 content was similar in CCSs of control cells and cells reconstituted with either wild-type p96 or p96 NPF1-5* but was increased ∼75% (p < 0.001) in cells depleted of Dab2 (Table 2). This suggests that Dab2 normally competes with AP2 for incorporation into CCSs. When Dab2 is absent, EH domain proteins can be recruited by AP2 (Praefcke et al., 2004), but when p96 NPF1-5* replaces wild-type Dab2, fewer EH domain proteins are recruited.
Because p96 NPF1-5* recruits but does not internalize integrin β1 (Figure 4, A–C), we suspected that CCS invagination or budding is defective. This would be consistent with a report that CCP half-life is increased when Eps15 or Itsn is depleted from BSC1 cells (Mettlen et al., 2009). Therefore we used live microscopy and automated tracking software to measure CCS lifetimes in HeLa cells transfected with enhanced green fluorescent protein (EGFP)–tagged clathrin light chain (Jaqaman et al., 2008; Loerke et al., 2009). CCSs on the ventral surface of control HeLa cells exhibited a broad range of lifetimes, with a preponderance of short-lived structures (Supplemental Figure S4). These may correspond to early-aborting CCPs observed in BSC1 cells (Loerke et al., 2009). When Dab2 was absent, there was a noticeable decrease in the proportion of structures with short lives (Supplemental Figure S4). The defect was rescued by reexpressing wild-type p96 but not mutant p96 NPF1-5* (Supplemental Figure S4 and Supplemental Videos S1–S4). Thus removing Dab2 or replacing Dab2 with a mutant that cannot bind EH domain proteins reduces the number of short-lived CCSs. These results suggest that the linkage of wild-type Dab2 to EH domain proteins has a unique role in determining whether short-lived clathrin structures grow or disappear.
Because CCSs reconstituted with the Dab2 NPF1-5* mutant have less Eps15 (Table 2) and altered dynamics (Supplemental Figure S4), we wondered whether all CME was impaired. To test this, we measured TfnR endocytosis. As expected, TfnR internalization was unaltered in Dab2-deficient, p96-expressing or p67-expressing cells (Figure 5, A and B; Maurer and Cooper, 2006; Teckchandani et al., 2009). Surprisingly, TfnR endocytosis was also unaffected in p96 NPF1-5*–reexpressing cells. This suggests that enough Eps15 is recruited to CCSs to support internalization of a Dab2-independent cargo. Given that Dab2 and AP2 extensively colocalize in CCSs (Supplemental Figures S2 and S5C; Morris and Cooper, 2001; Keyel et al., 2006), why is integrin β1 not cointernalized with TfnR? We considered the possibility that integrin β1 and TfnR may sort to separate CCSs. Previous reports showed that different receptors may localize to distinct CCP subsets (Cao et al., 1998; Mundell et al., 2006; Puthenveedu and von Zastrow, 2006; Leonard et al., 2008). We performed immunofluorescence for surface integrin β1 and Tfn. Both integrin β1 and Tfn puncta were widely distributed over both surfaces (Figures 3F, 4C, and 5C; Teckchandani et al., 2009). However, very few integrin β1 puncta contained Tfn (Figure 5C). Thus p96 NPF1-5* may affect internalization of CCSs containing integrin β1 but have no effect on structures containing TfnR.
FIGURE 5:
Disruption of the Dab2-EH domain protein interaction does not affect TfnR endocytosis. (A, B) Control and Dab2-deficient HeLa cells reexpressing vector alone or T7-p96, T7-p67, or T7-p96 NPF 1-5* were incubated with anti-TfnR antibody for 30 min at 4°C, followed by warming at 37°C for 5 min. (A, B) Surface antibody was removed by acid stripping, and internalized antibody was detected. (A) Z-projections of the entire cell showing internalized TfnR antibody. (B) Pixel intensities were measured by ImageJ. Mean values and standard errors are shown for ∼30 cells/treatment from three independent experiments. #p < 0.05, *p < 0.01, by t test. (C) Control cells were incubated with anti–integrin β1 antibody and Alexa Fluor 488–conjugated human Tfn to detect surface β1 and TfnR or permeabilized and stained with anti-Dab2 and anti-AP2. Z-projections of the dorsal surface are shown. The white boxes indicate the enlarged images shown in the insets. Bar, 10 μm. The fraction of CCSs that contain both receptors integrin β1 and Tfn or both adaptors Dab2 and AP2 is shown. Random colocalization, obtained by flipping the red channel, was <0.03.
These data show that recruitment of EH domain proteins to Dab2 is critical for endocytosis of CCSs containing the Dab2 cargo integrin β1 but not CCSs containing TfnR. Proteins besides Dab2 in integrin β1 CCSs do recruit EH domain proteins but seemingly do not mediate internalization. We propose that receptors may internalize only if they are bound to the cognate adaptor and the adaptor can recruit EH domain proteins.
DISCUSSION
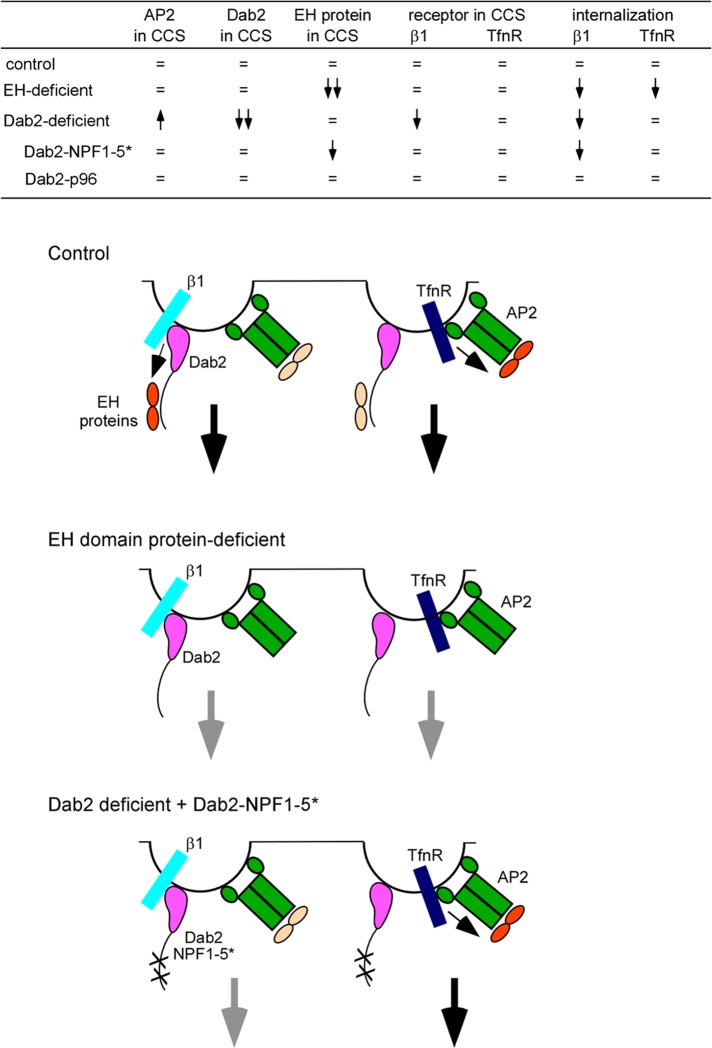
Our results are summarized in Figure 6 and suggest the following model: EH domain proteins regulate the number and size of CCSs and the internalization, but not recruitment, of Dab2-dependent and Dab2-independent cargoes, integrin β1 and TfnR. EH domain proteins are recruited to CCSs by AP2, the NPF sequences in Dab2, and other accessory proteins. If Dab2 is absent, more AP2 enters CCSs, and the content of EH domain proteins is unaffected. However, cargo internalization may depend on specific adaptor–EH domain protein interactions. A Dab2 mutant that cannot bind EH domain proteins (p96 NPF1-5*) acts as a dominant negative, slightly reducing the recruitment of EH domain proteins to CCSs and inhibiting integrin β1 internalization. Internalization of TfnR from a different set of CCSs was unaffected. We propose that specific receptor–adaptor–EH domain protein complexes are required for efficient endocytosis. In addition, the number of clathrin structures with short half-life is reduced in Dab2-deficient cells or cells reconstituted with the p96 NPF1-5* mutant. We speculate that the Dab2–EH domain protein complex may also have a role in determining whether short-lived clathrin structures grow or abort.
FIGURE 6:
Summary of results and proposed model. Top, summary of results taken from Table 2, Supplemental Figure S2, and Figures 2, 4, and 5. =, same as in control; upward arrow, increased; downward arrow, decreased. Bottom, model. Dab2 and AP2 are found together in most clathrin-coated structures. However, their receptors, integrin β1 and TfnR, do not localize to the same structures. Internalization of a clathrin structure is driven by adaptors bound to both receptor and EH domain proteins (shown in orange). Inactive EH domains are shown in pale yellow. Small black arrows indicate regulation of EH domain proteins by adaptor–receptor complexes. Large black arrows indicate endocytosis. In the absence of EH domain proteins internalization of integrin β1 and TfnR is impaired (gray arrows). When cells are reconstituted with a Dab2 mutant that does not bind EH domain proteins, integrin β1 is not internalized efficiently because this Dab2 mutant cannot bind EH domain proteins. However, TfnR endocytosis proceeds normally because its adaptor AP2 can bind EH domain proteins. The AP2–EH domain complex in integrin β1 structures is unable to drive endocytosis probably because it is not bound to receptor.
We found that Eps15 and Itsn bind to NPF sequences in Dab2 (Figure 1). Whereas NPF is the core of most EH-binding peptides, individual EH domains prefer specific surrounding sequences. For example, the second EH domain of Eps15 shows a slight preference for alanine at +1 (NPFA), and the first EH domain of Itsn is specific for NPFXCOOH or NPFXXCOOH at the C-terminus of a protein (Paoluzi et al., 1998; Yamabhai et al., 1998). The fifth NPF motif of Dab2 is the most highly conserved across evolution and fits both these requirements; it lies in the sequence NPFACOOH. This may explain why the C-terminal region of Dab2 binds Eps15 and Itsn more strongly than the central region (Figure 1, B–D). In addition, Eps15 and Itsn form heterodimers, so they may bind cooperatively or competitively (Sengar et al., 1999).
EH domain proteins in CCSs may act as scaffolds to bring in additional endocytic accessory proteins, including dynamin, synaptojanin, stonin, synaptotagmin, SHIP2, FCHO2, and epsin (Miliaras and Wendland, 2004). We found that depleting EH domain proteins from HeLa cells had no effect on CCPs on the dorsal surface but significantly decreased the number of pits and plaques on the ventral surface. The median size of plaques, but not pits, increased significantly, suggesting a role in CCS nucleation and growth (Table 1). It is possible that EH domain proteins help recruit membrane curvature–sensing proteins, like FCHO2, epsin, and endophilin, to form CCPs. In the absence of EH domain proteins, clathrin structures still form, but they tend to be flat plaques rather than curved CCPs.
Our results differ from a report that depletion of EH domain proteins inhibited all clathrin-coated structures (Henne et al., 2010). However, these authors used BSC1 cells, and the functions of EH domain proteins may depend on the cell type. Unlike HeLa cells, BSC1 cells have neither Dab2 nor plaques, so the presence of Dab2 in HeLa cells may permit CCS nucleation in the absence of EH domain proteins (Ehrlich et al., 2004; Saffarian et al., 2009; Mettlen et al., 2010). Alternatively, HeLa cells may express additional EH domain proteins besides Eps15, Eps15R, Itsn1, and Itsn2 that can also nucleate CCSs. It is possible that complete removal of the entire suite of endocytic EH domain proteins from HeLa cells would also abolish all clathrin-containing structures, as reported in BSC1 cells.
Internalization of Dab2-dependent and Dab2-independent cargoes (integrin β1 and TfnR, respectively) was inhibited in EH-depleted HeLa cells (Figure 2). Decreased internalization of TfnR may be an indirect consequence of the decreased numbers of pits and plaques and the increased plaque size on the ventral surface of the cell (Table 1 and Figure 3). Plaques have longer lifetimes (2–16 min) than CCPs (∼60 s) (Saffarian et al., 2009), so internalization from the larger structures may be inhibited. However, this explanation is unlikely to account for the decreased internalization of integrin β1, since integrin β1 is internalized from the dorsal surface, where the number and size of CCPs was unaltered (Table 1; Teckchandani et al., 2009). Therefore it is possible that EH domain proteins may directly regulate internalization of integrin β1. This conclusion is supported by use of a Dab2 mutant that does not bind EH proteins. This mutant fails to support integrin β1 internalization, but TfnR internalization is normal (Figures 4 and 5). Separate regulation may be possible because integrin β1 and TfnR do not significantly colocalize, suggesting that they internalize from different CCSs (Figure 6). This separation may have arisen because integrin β1 and TfnR follow different endocytic routes after internalization (Bleil and Bretscher, 1982; Iacopetta and Morgan, 1983; Roberts et al., 2004). Segregation of cargoes has been reported before: the EGF and Tfn receptors also sort to different CCP populations (Leonard et al., 2008), and different GPCRs (G-protein coupled receptors) localize to distinct CCP subsets (Cao et al., 1998; Mundell et al., 2006; Puthenveedu and von Zastrow, 2006). However, many other receptors are found in the same CCSs. For example, the LDL and Tfn receptors are predominantly found in the same CCSs (Keyel et al., 2006). So some receptors enter through shared pits and some through different pits.
Even though integrin β1 and TfnR appear to internalize through different CCSs, both cargoes colocalize with both adaptors Dab2 and AP2 as well as EH domain proteins. These commonalities make it difficult to explain how the Dab2 NPF mutant inhibits internalization of integrin β1 but not of TfnR. One possibility is that integrin β1 internalization requires integrin β1–Dab2–EH domain complexes and TfnR internalization requires TfnR–AP2–EH domain complexes (Figure 6). This model implies that cargo regulates EH domain protein function. Several studies suggest that cargo regulates internalization (Puthenveedu and von Zastrow, 2006; Loerke et al., 2009; Cao et al., 2010; Liu et al., 2010; Mettlen et al., 2010). Information on cargo occupancy may be relayed to the EH proteins by conformational changes in the adaptors. For example, AP2 undergoes conformation changes that enhance the affinity between AP2 and membranes after phosphorylation of AP2 by AAK1. In the new conformation, the YxxΦ sorting signal and PtdIns(4,5)P2-binding sites are both exposed. The simultaneous binding of AP2 to multiple sites on the plasma membrane stabilizes the adaptor–membrane complex such that it can mediate clathrin assembly (Ricotta et al., 2002; Honing et al., 2005). In principle, cargo–adaptor complexes could then regulate the affinity of EH domain proteins for downstream accessory proteins, thus allowing for efficient internalization. Further experiments will be needed to understand whether and how EH domain protein function may be regulated by cargoes.
Why would adaptors need to bind both cargo and EH domain proteins for efficient endocytosis? One reason may be to ensure that only clathrin structures “fully loaded” with receptors are internalized. Clathrin pits with less than their full receptor capacity may not have enough functional EH domain proteins for internalization and may need to wait to recruit additional receptors. The lifetime of productive CCPs (not including plaques) ranges from 30 to >120 s (Loerke et al., 2009). It is tempting to speculate that CCPs with longer lifetimes wait on the cell surface until they have their “full” receptor load and therefore sufficient functional EH domain proteins for internalization. Communication between receptors and EH domain proteins, mediated by endocytic adaptors like Dab2, may be important for regulating the endocytic checkpoint (Loerke et al., 2009; Mettlen et al., 2009). In the absence of Dab2 or in the absence of Dab2-associated EH domain proteins, the checkpoint may be incomplete and CCSs may escape past the checkpoint instead of being aborted, perhaps explaining the decrease in short-lifetime events (Supplemental Figure S4). However, there are other possible explanations, and more experiments will be required to determine the role of EH domain proteins in CCP dynamics and to fully understand the composition and regulation of the checkpoint.
MATERIALS AND METHODS
Cells and DNA constructs
HeLa cells and human embryonic kidney (HEK) 293 cells were cultured in DME supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin at 37°C with 5% CO2. HeLa cells stably expressing Dab2 short hairpin RNA (RNA) were previously described (Teckchandani et al., 2009) and were grown in the presence of 250 μg/ml hygromycin B.
T7-tagged mouse p96 and p67 were previously described (Maurer and Cooper, 2006). The NPF motifs were mutated to NPV by site-directed mutagenesis (TTC/TTT to GTC/GTT). shRNA-resistant p96 was created using site-directed mutagenesis using the following primers and their reverse complements: 5′CAGGGACAACACAAGCAGAGAATATGGGTCAACATTTCCTTGTCTGGCATA3′ and 5′CAACACAAGCAGAGAATATGGGTTAATATATCCTTGTCTGGCATAAAAATCATT3′. LCa-GFP was a gift from M. Von Zastrow (University of California, San Francisco, San Francisco, CA). HA-tagged Itsn was a gift from J. O'Bryan (University of Illinois, Chicago, IL).
T7-mDab2-206-492-His, T7-His-mDab2-493-766, and T7-mDab2-493-766-His were created by cloning of the Dab2 insert from GST-Dab2-206-492 (Morris and Cooper, 2001; Mulkearns and Cooper, 2012) or amino acids 493–766 of full-length protein into PET21a(+) (EMD Biosciences, Darmstadt, Germany). To create the N-terminal His fusion protein of 493–766, sequence for the His tag was included in the 5′ PCR primer, and a stop codon was introduced at the end of the Dab2 insert. GST-Eps15-EH and GST-Eps15R-EH were from S. Confalonieri (Salcini et al., 1997).
Antibodies
The following antibodies were used: mouse anti-T7 (Novagen, EMD4Biosciences, Gibbstown, NJ), mouse anti-HA.11 (Covance, Berkeley, CA), rabbit anti-Eps15 (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-Eps15 (a kind gift from S. Polo, Istituto FIRC di Oncologia Molecolare, Milan, Italy), rabbit anti-Dab2 (Santa Cruz Biotechnology), mouse anti–integrin β1, P5D2 (provided by E. Wayner, Fred Hutchinson Cancer Research Center, Seattle, WA), mouse anti-TfnR (Abcam, Cambridge, MA), mouse anti-ERK (BD Transduction Laboratories, Lexington, KY), mouse anti-clathrin (Abcam), and mouse anti-adaptin (Calbiochem, La Jolla, CA).
Protein purification and binding
GST-EH domains were purified as described (Mulkearns and Cooper, 2012). His-tagged Dab2 fragments were purified similar to the procedure in Boettner et al. (2009). DNA was introduced into NiCo21(DE3) Escherichia coli, and a 20-ml starter culture was grown overnight at 37°C. The starter was added to 200 ml LB and grown for 2 h at 30°C in the presence of protease inhibitors (20 mM benzamidine, 1 mM phenylmethylsulfonyl fluoride, 5 mM EDTA). Protein expression was induced at 22°C overnight with 300 mM isopropyl-β-d-thiogalactoside. After centrifugation, the bacterial pellet was resuspended in resuspension buffer (0.5% NP-40, 1 mM dithiothreitol [DTT], 20 mM imidazole, pH 8.0, in phosphate-buffered saline [PBS] with protease inhibitors). Lysozyme was added to a concentration of 0.5 mg/ml, and cells were lysed 15 min on ice. The lysates were then sonicated and centrifuged at 4000 × g for 20 min. Lysate was incubated for 1.5 h with Ni2+ beads (Qiagen, Valencia, CA) at 4°C. Beads were washed twice in resuspension buffer with 350 mM NaCl and 15 mM imidazole and then washed three times in resuspension buffer with 15 mM imidazole. Beads were then loaded into an empty Bio-Rad Poly-Prep column (Bio-Rad, Hercules, CA) and eluted with increasing concentrations of imidazole ranging from 50 to 200 mM in PBS with 1 mM DTT and 5% glycerol.
Equal amounts of glutathione-Sepharose–immobilized GST, GST-Eps15-EH, and GST-Eps15R-EH were mixed with eluted His-tagged Dab2 fragments overnight at 4°C in buffer A (50 mM Tris, pH 8.0, 150 mM NaCl, 1 mM EDTA, 0.5% Triton X-100). Beads were washed five times in buffer A and resuspended in 5× Laemmli buffer for SDS–PAGE.
Immunoprecipitation
For coimmunoprecipitation experiments, T7-tagged p96, p67, and p96 NPF mutants and HA-tagged Itsn were transfected into HEK 293 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's protocols. After 48 h, cells were lysed at 4°C in buffer containing 150 mM NaCl, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.4, 2 mM EDTA, 50 mM NaF, 1% Triton-X-100, and protease inhibitors, followed by centrifugation for 15 min at 14,000 × g. Lysate was rotated with primary antibody for 3 h at 4°C. Protein A + G beads were added to the lysate/antibody mix for 1 h at 4°C. After centrifugation, the protein/antibody/bead complex was washed three times in lysis buffer, resuspended in 5× Laemmli buffer, and then resolved by SDS–PAGE.
To determine whether endogenous Dab2 and Eps15 coimmunoprecipitate, lysates were prepared in the presence of a reversible cross-linking agent, dithiobis succinimidyl propionate (Pierce Biotechnology, Rockford, IL).
siRNA transfection
Knockdown experiments were performed as described previously (Maurer and Cooper, 2006). Cells were transfected with 50 pmol of a pool of four siRNA oligonucleotides specific for Eps15, Eps15R, Itsn1 (ThermoFisher Scientific, Waltham, MA), and Itsn2 (Santa Cruz Biotechnology), using Oligofectamine (Invitrogen, Carlsbad, CA) on days 1 and 3 and analyzed on day 5. The siRNA to Itsn1 and 2 knocked down both the long and short Itsn isoforms.
RT-PCR
Total RNA was extracted using an RNeasy Kit (Qiagen) according to the manufacturer's directions. Total RNA was reverse transcribed using SuperScript II reverse transcription and random primers (Invitrogen). PCR was performed using Taq polymerase and the following primers:
Eps15R: 5′-TGGGAAGATATGGGACTTGG-3′ and 5′-CCTTTTCTTCCACCCTCACA-3′
Itsn1 pan: 5′-CTCAGGAAAGGGACAAGCAG-3′ and 5′-CTGGCTTAGCTGGTTCTTGG-3′
Itsn2 pan: 5′-CTCAGAGTGGGCAGTTCCTC-3′ and 5′-CAGCTTTGGCCATGTCAGTA-3′
Actin: 5′-GCGAGAAGATGACCCAGATCATGTT-3′ and 5′-GCTTCTCCTTAATGTCACGCACGAT-3′
Antibody internalization assay
Antibody uptake assays were performed essentially as described (Roberts et al., 2001) with some modifications. Cells were plated on coverslips coated with 4 μg/ml collagen IV and then incubated with anti–integrin β1 antibody (P5D2) or anti-TfnR diluted in assay media (DME, 10 mM HEPES, pH 7.4, and 0.1% BSA) for 30 min at 4°C. After washing off of unbound antibody with cold DME, cells were warmed to 37°C in DME with 10% FBS for the appropriate time and fixed with cold Formalin for 20 min. To visualize internalized receptors, surface-bound antibody was removed by acid stripping for 5 min on ice (0.5 M NaCl and 0.2 M acetic acid) before fixing with cold Formalin. To inhibit recycling, 2 μM Primaquine (Sigma-Aldrich, St. Louis, MO) was used.
To detect surface receptors, anti–integrin β1 antibody (P5D2) or Alexa Fluor 488–conjugated human Tfn was added to cells in assay media (DME, 10 mM HEPES, pH 7.4, and 0.1% BSA) for 60 min at 4°C. After washing off of unbound antibody/Tfn with cold DME, cells were fixed with cold Formalin for 20 min.
Immunofluorescence
To visualize surface receptors, nonpermeabilized cells were fixed and stained with antibodies to extracellular epitopes. To detect intracellular proteins, cells were fixed and permeabilized with 0.1% Triton X-100 in PBS for 5 min at 25°C. Cells were washed in PBS and blocked for 30 min in 5% normal goat serum plus 2% BSA in PBS before primary antibody was added for 3–4 h at 25°C or overnight at 4°C. Coverslips were rinsed in PBS before the addition of Alexa Fluor 488 or Alexa Fluor 568 secondary antibodies, diluted 1:1000 (all from Invitrogen), for 1 h at 25°C. Secondary antibodies included goat anti-mouse, immunoglobulin G 2b isotype–specific antibodies, and goat anti-rabbit. After several PBS rinses, coverslips were mounted in ProLong Gold solution (Invitrogen).
Imaging
Fixed cells were visualized using a 100×/numerical aperture (NA) 1.4 oil objective on a DeltaVision IX70 microscope (Olympus, Tokyo, Japan). Images were recorded using fixed camera settings (IX-HLSH100; Olympus). Images were acquired and deconvolved using softWoRx (Applied Precision, Issaquah, WA), and all exposure times and image scaling were equal within an experiment. Deconvolved images from single planes corresponding to the ventral surfaces of the cells or flattened z-projections were analyzed using ImageJ (National Institutes of Health, Bethesda, MD). Figures were assembled using Photoshop (Adobe, San Jose, CA) and Canvas (Deneba, ACD Systems, Miami, FL) software.
To measure CCS lifetimes, control and Dab2 shRNA HeLa cells expressing clathrin-LCa-EGFP and pCGT, T7-p96, or T7-p96 NPF1-5* were plated in 35-mm dishes containing a glass coverslip 1 d before imaging. Images were acquired on a confocal Nikon LiveScan (Prairie Technologies [Middleton, WI] Swept Field) system (Nikon, Melville, NY), equipped with a 37°C chamber and a QuantEM 512 × 512 pixel electron-multiplying charge-coupled device camera (Photometrics, Tucson, AZ). A 100×/NA 1.4 oil objective was used. Images were recorded every 2 s for 4 min. CCS lifetimes were analyzed using the uTrack software package described in Jaqaman et al. (2008) and Loerke et al. (2009). The mean lifetime of CCPs in BSC1 cells is 39 s (Loerke et al., 2009), comparable to the median lifetimes of 34–36 s that we obtain.
Supplementary Material
Acknowledgments
We gratefully acknowledge reagents and assistance from M. Von Zastrow, J. O'Bryan, S. Polo, E. Wayner, S. Confalonieri, M. Maurer, J. Vazquez, and D. McDonald. We are grateful to Jihong Bai, James Baleja, and Roland Walter for reading the manuscript. This research was supported by National Institutes of Health Grants R01-GM66257 (to J.A.C.) and R01-CA126205 (to T.W.R.).
Abbreviations used:
- CCP
clathrin-coated pit
- CCS
clathrin-coated structure
- CHC
clathrin heavy chain
- CLASP
clathrin-associated sorting protein
- CME
clathrin-mediated endocytosis
- DAPI
4′,6-diamidino-2-phenylindole
- DTT
dithiothreitol
- EGF
epidermal growth factor
- EH
eps homology
- GFP
green fluorescent protein
- GPCR
G-protein coupled receptor
- GST
glutathione S-transferase
- HA
hemagglutinin
- HEK
human embryonic kidney
- LCa-GFP
clathrin light chain GFP
- LDLR
low-density lipoprotein receptor
- PTB
phosphotyrosine binding
- PtdIns(4,5)P2
phosphatidylinositol (4,5) bisphosphate
- TfnR
transferrin receptor
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-12-1007) on May 30, 2012.
REFERENCES
- Bleil JD, Bretscher MS. Transferrin receptor and its recycling in HeLa cells. EMBO J. 1982;1:351–355. doi: 10.1002/j.1460-2075.1982.tb01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner DR, D'Agostino JL, Torres OT, Daugherty-Clarke K, Uygur A, Reider A, Wendland B, Lemmon SK, Goode BL. The F-BAR protein Syp1 negatively regulates WASp-Arp2/3 complex activity during endocytic patch formation. Curr Biol. 2009;19:1979–1987. doi: 10.1016/j.cub.2009.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Chen J, Krueger EW, McNiven MA. SRC-mediated phosphorylation of dynamin and cortactin regulates the “constitutive” endocytosis of transferrin. Mol Cell Biol. 2010;30:781–792. doi: 10.1128/MCB.00330-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao TT, Mays RW, von Zastrow M. Regulated endocytosis of G-protein-coupled receptors by a biochemically and functionally distinct subpopulation of clathrin-coated pits. J Biol Chem. 1998;273:24592–24602. doi: 10.1074/jbc.273.38.24592. [DOI] [PubMed] [Google Scholar]
- Chao WT, Kunz J. Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. FEBS Lett. 2009;583:1337–1343. doi: 10.1016/j.febslet.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri R, Lindwasser OW, Smith WJ, Hurley JH, Bonifacino JS. Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor. J Virol. 2007;81:3877–3890. doi: 10.1128/JVI.02725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cihil KM, Ellinger P, Fellows A, Beer Stolz D, Madden DR, Swiatecka-Urban A. DAB2 facilitates AP-2 independent recruitment of CFTR to endocytic vesicles in polarized human airway epithelial cells. J Biol Chem. 2012;287:15087–15099. doi: 10.1074/jbc.M112.341875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beer T, Carter RE, Lobel-Rice KE, Sorkin A, Overduin M. Structure and Asn-Pro-Phe binding pocket of the Eps15 homology domain. Science. 1998;281:1357–1360. doi: 10.1126/science.281.5381.1357. [DOI] [PubMed] [Google Scholar]
- Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733–747. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Boucrot E, Meinecke M, Evergren E, Vallis Y, Mittal R, McMahon HT. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 2010;328:1281–1284. doi: 10.1126/science.1188462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Kent HM, Ford MG, Hegde BG, Daumke O, Butler PJ, Mittal R, Langen R, Evans PR, McMahon HT. Structure and analysis of FCHo2 F-BAR domain: a dimerizing and membrane recruitment module that effects membrane curvature. Structure. 2007;15:839–852. doi: 10.1016/j.str.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Honing S, Ricotta D, Krauss M, Spate K, Spolaore B, Motley A, Robinson M, Robinson C, Haucke V, Owen DJ. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol Cell. 2005;18:519–531. doi: 10.1016/j.molcel.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Hussain NK, Yamabhai M, Ramjaun AR, Guy AM, Baranes D, O'Bryan JP, Der CJ, Kay BK, McPherson PS. Splice variants of intersectin are components of the endocytic machinery in neurons and nonneuronal cells. J Biol Chem. 1999;274:15671–15677. doi: 10.1074/jbc.274.22.15671. [DOI] [PubMed] [Google Scholar]
- Iacopetta BJ, Morgan EH. The kinetics of transferrin endocytosis and iron uptake from transferrin in rabbit reticulocytes. J Biol Chem. 1983;258:9108–9115. [PubMed] [Google Scholar]
- Jaqaman K, Loerke D, Mettlen M, Kuwata H, Grinstein S, Schmid SL, Danuser G. Robust single-particle tracking in live-cell time-lapse sequences. Nat Methods. 2008;5:695–702. doi: 10.1038/nmeth.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyel PA, Mishra SK, Roth R, Heuser JE, Watkins SC, Traub LM. A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors. Mol Biol Cell. 2006;17:4300–4317. doi: 10.1091/mbc.E06-05-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T. Adaptors for clathrin-mediated traffic. Annu Rev Cell Dev Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- Kowanetz K, Terzic J, Dikic I. Dab2 links CIN85 with clathrin-mediated receptor internalization. FEBS Lett. 2003;554:81–87. doi: 10.1016/s0014-5793(03)01111-6. [DOI] [PubMed] [Google Scholar]
- Leonard D, Hayakawa A, Lawe D, Lambright D, Bellve KD, Standley C, Lifshitz LM, Fogarty KE, Corvera S. Sorting of EGF and transferrin at the plasma membrane and by cargo-specific signaling to EEA1-enriched endosomes. J Cell Sci. 2008;121:3445–3458. doi: 10.1242/jcs.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AP, Aguet F, Danuser G, Schmid SL. Local clustering of transferrin receptors promotes clathrin-coated pit initiation. J Cell Biol. 2010;191:1381–1393. doi: 10.1083/jcb.201008117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loerke D, Mettlen M, Yarar D, Jaqaman K, Jaqaman H, Danuser G, Schmid SL. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 2009;7:e57. doi: 10.1371/journal.pbio.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer ME, Cooper JA. The adaptor protein Dab2 sorts LDL receptors into coated pits independently of AP-2 and ARH. J Cell Sci. 2006;119:4235–4246. doi: 10.1242/jcs.03217. [DOI] [PubMed] [Google Scholar]
- Mettlen M, Loerke D, Yarar D, Danuser G, Schmid SL. Cargo- and adaptor-specific mechanisms regulate clathrin-mediated endocytosis. J Cell Biol. 2010;188:919–933. doi: 10.1083/jcb.200908078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettlen M, Stoeber M, Loerke D, Antonescu CN, Danuser G, Schmid SL. Endocytic accessory proteins are functionally distinguished by their differential effects on the maturation of clathrin-coated pits. Mol Biol Cell. 2009;20:3251–3260. doi: 10.1091/mbc.E09-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliaras NB, Wendland B. EH proteins: multivalent regulators of endocytosis (and other pathways) Cell Biochem Biophys. 2004;41:295–318. doi: 10.1385/CBB:41:2:295. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Keyel PA, Hawryluk MJ, Agostinelli NR, Watkins SC, Traub LM. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 2002;21:4915–4926. doi: 10.1093/emboj/cdf487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SM, Cooper JA. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic. 2001;2:111–123. doi: 10.1034/j.1600-0854.2001.020206.x. [DOI] [PubMed] [Google Scholar]
- Morris SM, Arden SD, Roberts RC, Kendrick-Jones J, Cooper JA, Luzio JP, Buss F. Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic. 2002;3:331–341. doi: 10.1034/j.1600-0854.2002.30503.x. [DOI] [PubMed] [Google Scholar]
- Motley A, Bright NA, Seaman MN, Robinson MS. Clathrin-mediated endocytosis in AP-2-depleted cells. J Cell Biol. 2003;162:909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkearns EE, Cooper JA. FCH domain only-2 organizes clathrin-coated structures and interacts with Disabled-2 for low-density lipoprotein receptor endocytosis. Mol Biol Cell. 2012;23:1330–1342. doi: 10.1091/mbc.E11-09-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundell SJ, Luo J, Benovic JL, Conley PB, Poole AW. Distinct clathrin-coated pits sort different G protein-coupled receptor cargo. Traffic. 2006;7:1420–1431. doi: 10.1111/j.1600-0854.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Paoluzi S, Castagnoli L, Lauro I, Salcini AE, Coda L, Fre S, Confalonieri S, Pelicci PG, Di Fiore PP, Cesareni G. Recognition specificity of individual EH domains of mammals and yeast. EMBO J. 1998;17:6541–6550. doi: 10.1093/emboj/17.22.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse BM. Receptors compete for adaptors found in plasma membrane coated pits. EMBO J. 1988;7:3331–3336. doi: 10.1002/j.1460-2075.1988.tb03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praefcke GJ, Ford MG, Schmid EM, Olesen LE, Gallop JL, Peak-Chew SY, Vallis Y, Babu MM, Mills IG, McMahon HT. Evolving nature of the AP2 alpha-appendage hub during clathrin-coated vesicle endocytosis. EMBO J. 2004;23:4371–4383. doi: 10.1038/sj.emboj.7600445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveedu MA, von Zastrow M. Cargo regulates clathrin-coated pit dynamics. Cell. 2006;127:113–124. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Ricotta D, Conner SD, Schmid SL, von Figura K, Honing S. Phosphorylation of the AP2 mu subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J Cell Biol. 2002;156:791–795. doi: 10.1083/jcb.200111068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M, Barry S, Woods A, van der Sluijs P, Norman J. PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr Biol. 2001;11:1392–1402. doi: 10.1016/s0960-9822(01)00442-0. [DOI] [PubMed] [Google Scholar]
- Roberts MS, Woods AJ, Dale TC, Van Der Sluijs P, Norman JC. Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of alpha v beta 3 and alpha 5 beta 1 integrins. Mol Cell Biol. 2004;24:1505–1515. doi: 10.1128/MCB.24.4.1505-1515.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde G, Wenzel D, Haucke V. A phosphatidylinositol (4,5)-bisphosphate binding site within mu2-adaptin regulates clathrin-mediated endocytosis. J Cell Biol. 2002;158:209–214. doi: 10.1083/jcb.200203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffarian S, Cocucci E, Kirchhausen T. Distinct dynamics of endocytic clathrin-coated pits and coated plaques. PLoS Biol. 2009;7:e1000191. doi: 10.1371/journal.pbio.1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcini AE, Confalonieri S, Doria M, Santolini E, Tassi E, Minenkova O, Cesareni G, Pelicci PG, Di Fiore PP. Binding specificity and in vivo targets of the EH domain, a novel protein-protein interaction module. Genes Dev. 1997;11:2239–2249. doi: 10.1101/gad.11.17.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid EM, Ford MG, Burtey A, Praefcke GJ, Peak-Chew SY, Mills IG, Benmerah A, McMahon HT. Role of the AP2 beta-appendage hub in recruiting partners for clathrin-coated vesicle assembly. PLoS Biol. 2006;4:e262. doi: 10.1371/journal.pbio.0040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengar AS, Wang W, Bishay J, Cohen S, Egan SE. The EH and SH3 domain Ese proteins regulate endocytosis by linking to dynamin and Eps15. EMBO J. 1999;18:1159–1171. doi: 10.1093/emboj/18.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teckchandani A, Toida N, Goodchild J, Henderson C, Watts J, Wollscheid B, Cooper JA. Quantitative proteomics identifies a Dab2/integrin module regulating cell migration. J Cell Biol. 2009;186:99–111. doi: 10.1083/jcb.200812160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2003;163:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- van Bergen En Henegouwen PM. Eps15: a multifunctional adaptor protein regulating intracellular trafficking. Cell Commun Signal. 2009;7:24. doi: 10.1186/1478-811X-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XX, Yi T, Tang B, Lambeth JD. Disabled-2 (Dab2) is an SH3 domain-binding partner of Grb2. Oncogene. 1998;16:1561–1569. doi: 10.1038/sj.onc.1201678. [DOI] [PubMed] [Google Scholar]
- Yamabhai M, Hoffman NG, Hardison NL, McPherson PS, Castagnoli L, Cesareni G, Kay BK. Intersectin, a novel adaptor protein with two Eps15 homology and five Src homology 3 domains. J Biol Chem. 1998;273:31401–31407. doi: 10.1074/jbc.273.47.31401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.