Abstract
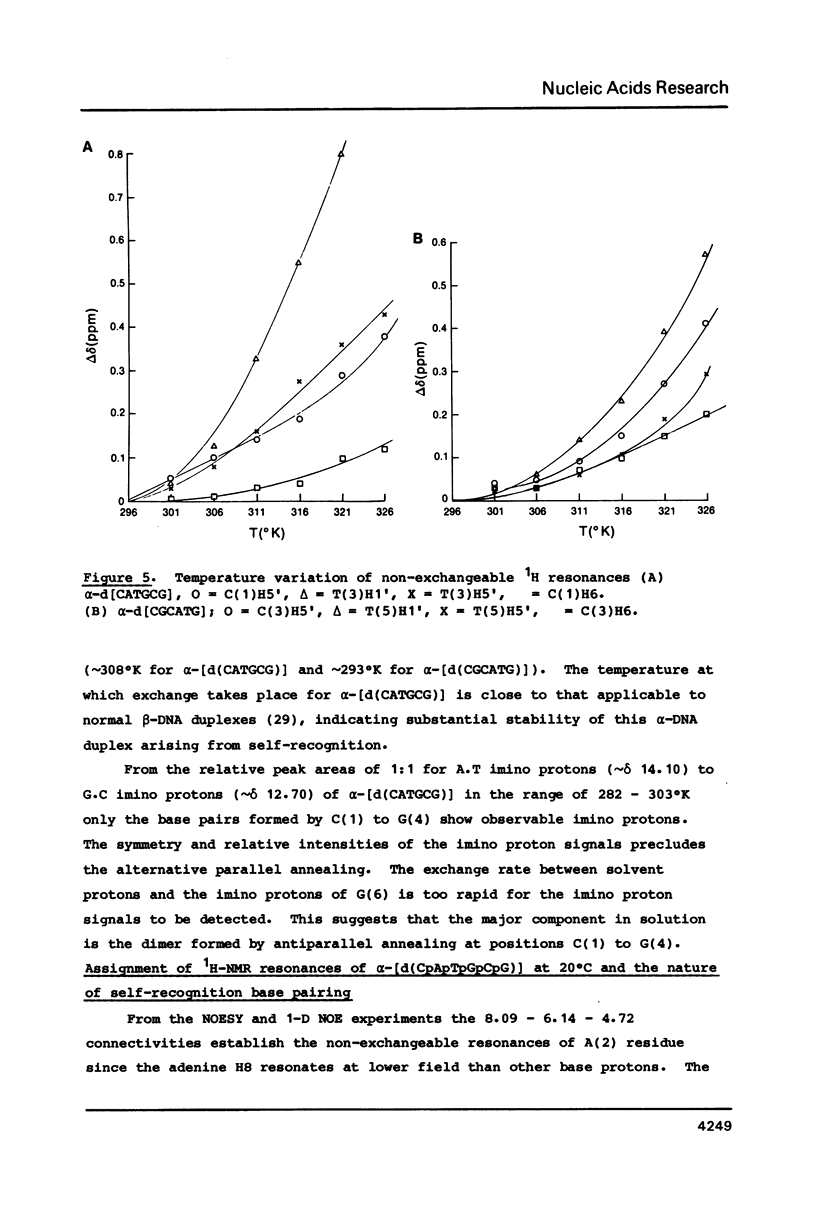
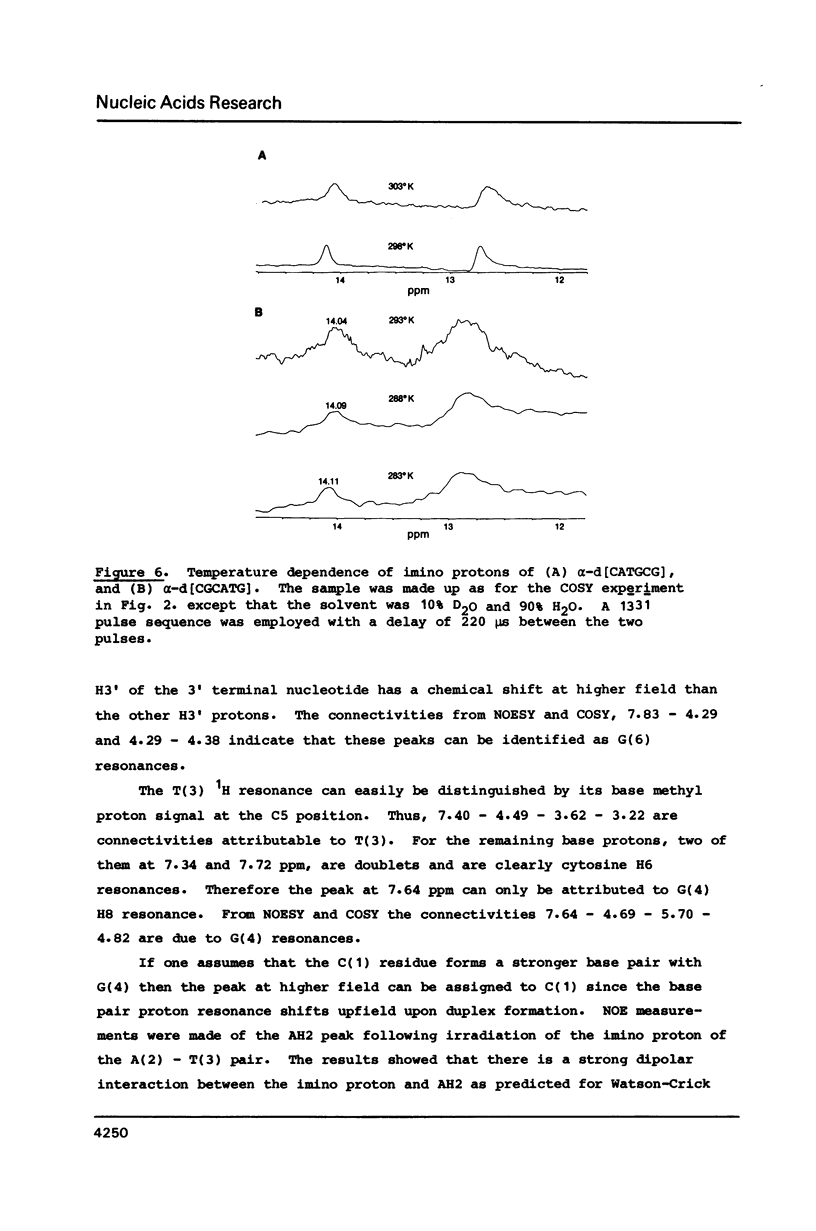
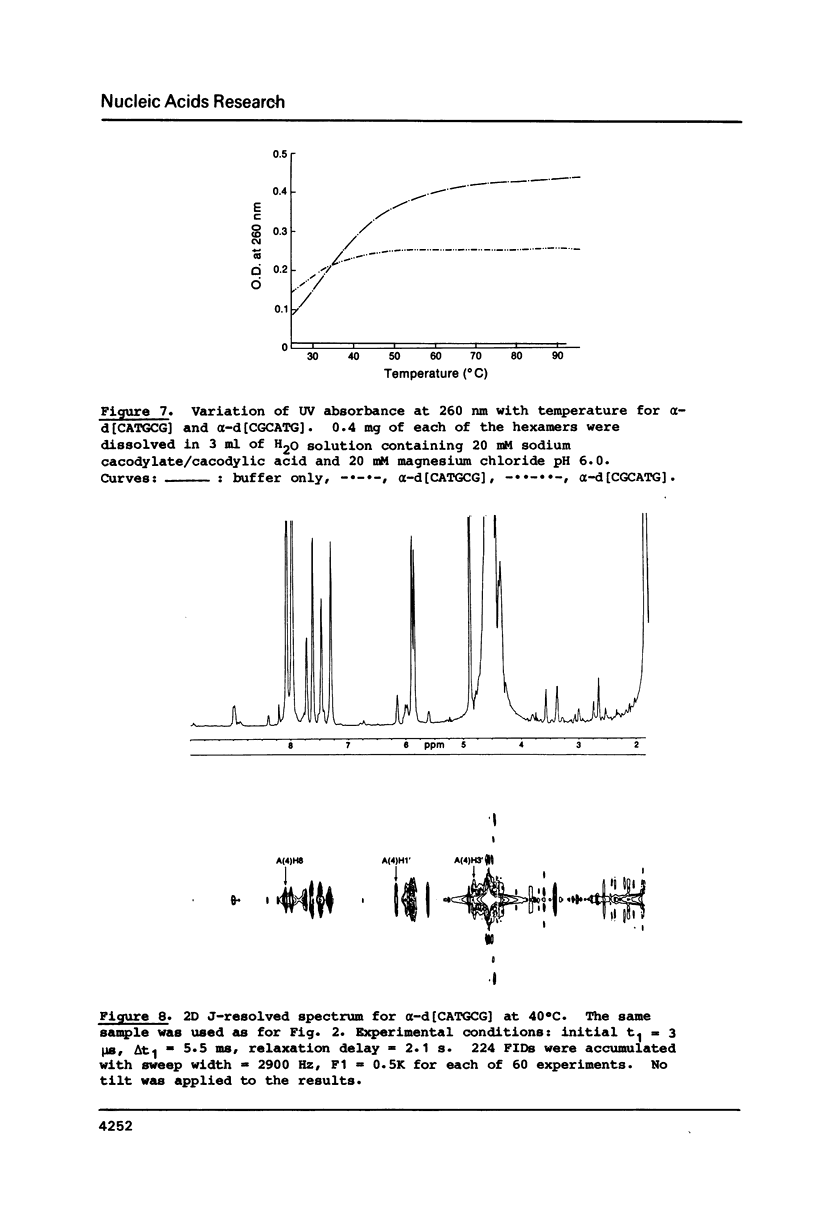
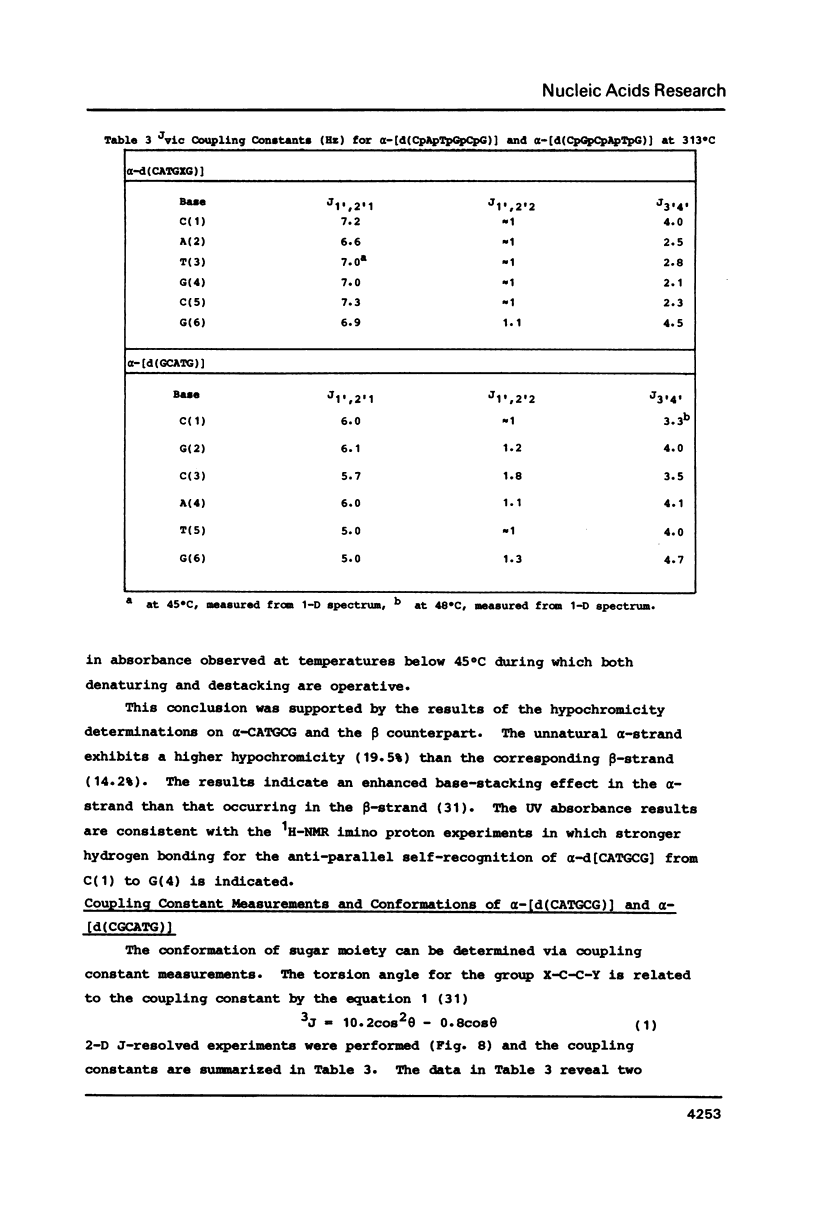
High field 2-D-1H-NMR techniques permitted the assignment of all non-exchangeable protons of the unnatural deoxyribonucleotides alpha-[d(CpApTpGpCpG)] and alpha-[d(CpGpCpApTpG)]. 1-D and 2-D NOESY experiments show strong H6H8-H4' dipolar interactions for all nucleotides in both sequences. These data, together with COSY and J-resolved spectra, indicate that these two alpha-oligomers adopt 3'-exo conformations of the sugar moieties in solution with anti conformations of the glycosyl linkages. Both 1H-NMR data, and hypochromocity comparison of alpha-CATGCG and beta-CATGCG, demonstrate a higher degree of base stacking in the case of the alpha-sequence. The UV hyperchromicity at 260 nm, and symmetry considerations in the imino proton NMR experiments reveal antiparallel self-recognition and duplex annealing at positions 1-4 for alpha-[d(CATGCG)] and positions 3-6 for alpha-[d(CGCATG)]. The temperature variation of the imino proton NMR signals suggests that the hydrogen bonding in self-recognition is comparable in strength with that in a beta-DNA duplex, and NOE data are in accord with Watson-Crick rather than Hoogsteen base pairing.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asseline U., Delarue M., Lancelot G., Toulmé F., Thuong N. T., Montenay-Garestier T., Hélène C. Nucleic acid-binding molecules with high affinity and base sequence specificity: intercalating agents covalently linked to oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3297–3301. doi: 10.1073/pnas.81.11.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseline U., Nguyen T. T., Hélène C. Oligonucleotides covalently linked to intercalating agents. Influence of positively charged substituents on binding to complementary sequences. J Biol Chem. 1985 Jul 25;260(15):8936–8941. [PubMed] [Google Scholar]
- Asseline U., Toulme F., Thuong N. T., Delarue M., Montenay-Garestier T., Hélène C. Oligodeoxynucleotides covalently linked to intercalating dyes as base sequence-specific ligands. Influence of dye attachment site. EMBO J. 1984 Apr;3(4):795–800. doi: 10.1002/j.1460-2075.1984.tb01887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigon J., Leupin W., Denny W. A., Kearns D. R. Two-dimensional proton nuclear magnetic resonance investigation of the synthetic deoxyribonucleic acid decamer d(ATATCGATAT)2. Biochemistry. 1983 Dec 6;22(25):5943–5951. doi: 10.1021/bi00294a038. [DOI] [PubMed] [Google Scholar]
- Fried M. G., Bloomfield V. A. DNA gelation in concentrated solutions. Biopolymers. 1984 Nov;23(11 Pt 1):2141–2155. doi: 10.1002/bip.360231104. [DOI] [PubMed] [Google Scholar]
- Guittet E., Piveteau D., Lallemand J. Y., Huyn-Dinh T., Igolen J. Nuclear magnetic resonance study of d-TGGCCA in solution. Nucleic Acids Res. 1984 Jul 25;12(14):5927–5941. doi: 10.1093/nar/12.14.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg R. P., Dervan P. B. Cleavage of DNA with methidiumpropyl-EDTA-iron(II): reaction conditions and product analyses. Biochemistry. 1984 Aug 14;23(17):3934–3945. doi: 10.1021/bi00312a022. [DOI] [PubMed] [Google Scholar]
- Härd T., Kearns D. R. Association of short DNA fragments: steady state fluorescence polarization study. Biopolymers. 1986 Aug;25(8):1519–1529. doi: 10.1002/bip.360250810. [DOI] [PubMed] [Google Scholar]
- Kondo N. S., Holmes H. M., Stempel L. M., Ts'o O. P. Influence of the phosphodiester linkage (3'-5', 2'-5', and 5'-5') on the conformation of dinucleoside monophosphate. Biochemistry. 1970 Sep 1;9(18):3479–3498. doi: 10.1021/bi00820a002. [DOI] [PubMed] [Google Scholar]
- Lown J. W., Krowicki K., Bhat U. G., Skorobogaty A., Ward B., Dabrowiak J. C. Molecular recognition between oligopeptides and nucleic acids: novel imidazole-containing oligopeptides related to netropsin that exhibit altered DNA sequence specificity. Biochemistry. 1986 Nov 18;25(23):7408–7416. doi: 10.1021/bi00371a024. [DOI] [PubMed] [Google Scholar]
- Lown J. W., Sondhi S. M., Ong C. W., Skorobogaty A., Kishikawa H., Dabrowiak J. C. Deoxyribonucleic acid cleavage specificity of a series of acridine- and acodazole-iron porphyrins as functional bleomycin models. Biochemistry. 1986 Sep 9;25(18):5111–5117. doi: 10.1021/bi00366a020. [DOI] [PubMed] [Google Scholar]
- Morvan F., Rayner B., Imbach J. L., Chang D. K., Lown J. W. alpha-DNA. I. Synthesis, characterization by high field 1H-NMR, and base-pairing properties of the unnatural hexadeoxyribonucleotide alpha-[d(CpCpTpTpCpC)] with its complement beta-[d(GpGpApApGpG)]. Nucleic Acids Res. 1986 Jun 25;14(12):5019–5035. doi: 10.1093/nar/14.12.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama K., Wüthrich K. Systematic application of two-dimensional 1H nuclear-magnetic-resonance techniques for studies of proteins. 1. Combined use of spin-echo-correlated spectroscopy and J-resolved spectroscopy for the identification of complete spin systems of non-labile protons in amino-acid residues. Eur J Biochem. 1981 Feb;114(2):365–374. doi: 10.1111/j.1432-1033.1981.tb05156.x. [DOI] [PubMed] [Google Scholar]
- Pless R. C., Ts'o P. O. Duplex formation of a nonionic oligo(deoxythymidylate) analogue (heptadeoxythymidylyl-(3'-5')-deoxythymidine heptaethyl ester (d-(Tp(Et))7T)) with poly(deoxyadenylate). Evaluation of the electrostatic interaction. Biochemistry. 1977 Mar 22;16(6):1239–1250. doi: 10.1021/bi00625a033. [DOI] [PubMed] [Google Scholar]
- Sigman D. S., Graham D. R., D'Aurora V., Stern A. M. Oxygen-dependent cleavage of DNA by the 1,10-phenanthroline . cuprous complex. Inhibition of Escherichia coli DNA polymerase I. J Biol Chem. 1979 Dec 25;254(24):12269–12272. [PubMed] [Google Scholar]
- Séquin U. Nucleosides and nucleotides. Part 7. Four dithymidine monophosphates with different anomeric configurations, their synthesis and behaviour towards phosphodiesterases. Helv Chim Acta. 1974;57(1):68–81. doi: 10.1002/hlca.19740570108. [DOI] [PubMed] [Google Scholar]
- Wemmer D. E., Wand A. J., Seeman N. C., Kallenbach N. R. NMR analysis of DNA junctions: imino proton NMR studies of individual arms and intact junction. Biochemistry. 1985 Oct 8;24(21):5745–5749. doi: 10.1021/bi00342a009. [DOI] [PubMed] [Google Scholar]



