Abstract
Background
Congenital diseases of the urinary tract are frequently observed in infants. Such diseases present a number of developmental anomalies such as hydroureter and hydronephrosis. Although some genetically-modified mouse models of growth factor signaling genes reproduce urinary phenotypes, the pathogenic mechanisms remain obscure. Previous studies suggest that a portion of the cells in the external genitalia and bladder are derived from peri-cloacal mesenchymal cells that receive Hedgehog (Hh) signaling in the early developmental stages. We hypothesized that defects in such progenitor cells, which give rise to urinary tract tissues, may be a cause of such diseases.
Methodology/Principal Findings
To elucidate the pathogenic mechanisms of upper urinary tract malformations, we analyzed a series of Sonic hedgehog (Shh) deficient mice. Shh−/− displayed hydroureter and hydronephrosis phenotypes and reduced expression of several developmental markers. In addition, we suggested that Shh modulation at an early embryonic stage is responsible for such phenotypes by analyzing the Shh conditional mutants. Tissue contribution assays of Hh-responsive cells revealed that peri-cloacal mesenchymal cells, which received Hh signal secreted from cloacal epithelium, could contribute to the ureteral mesenchyme. Gain- and loss-of-functional mutants for Hh signaling revealed a correlation between Hh signaling and Bone morphogenetic protein (Bmp) signaling. Finally, a conditional ablation of Bmp receptor type IA (BmprIA) gene was examined in Hh-responsive cell lineages. This system thus made it possible to analyze the primary functions of the growth factor signaling relay. The defective Hh-to-Bmp signaling relay resulted in severe urinary tract phenotypes with a decrease in the number of Hh-responsive cells.
Conclusions/Significance
This study identified the essential embryonic stages for the pathogenesis of urinary tract phenotypes. These results suggested that Hh-responsive mesenchymal Bmp signaling maintains the population of peri-cloacal mesenchyme cells, which is essential for the development of the ureter and the upper urinary tract.
Introduction
The urinary system is composed of several highly divergent organs, including the kidneys, ureters, bladder and urethra. Such organs are essential for the transfer of urine from the renal pelvis toward the bladder. Previous studies have suggested that growth factors, such as Sonic hedgehog (Shh) and Bone morphogenetic proteins (Bmps) are essential for the formation of the urinary tract [1]–[9]. The loss of such signaling in genetically-engineered mice phenocopied human urinary diseases, such as congenital anomalies of the kidney and urinary tract (CAKUT) or the VACTERL syndrome [1], [10]–[14]. The above diseases are characterized by abnormalities in the upper urinary tract. In particular, CAKUT includes a number of developmental anomalies at the level of the kidney (e.g., hydronephrosis, hypoplasia, dysplasia, duplex kidney), ureter (e.g., hydroureter), and bladder (e.g., ectopic ureteral orifice) [15]–[17]. Despite recent advances in both the prenatal diagnosis and early surgical intervention, CAKUT still remains the primary cause of kidney failure in infants (1 in 500 live births) [18]–[20].
The Shh, a Hedgehog (Hh) family ligand, controls cell fate, cell proliferation, differentiation and tissue patterning during embryogenesis [21]–[25]. The Shh gene is expressed in the cloacal epithelia and its signaling has vital roles for the regulation of tissue lineage within the embryonic urinary tracts such as the bladder and the external genitalia [2], [26]–[30]. The SHH protein was located in the distinct urinary tract epithelia of human embryos, in the urothelium of the nascent bladder and in the kidney medullary collecting ducts [31]. In addition, we previously suggested that the bladder mesenchyme and dorsal (upper) external genitalia are derived from the peri-cloacal mesenchyme (PCM) exposed to Hh signaling [29], [32]. However, the contribution of the PCM cells to the upper urinary tract after exposure to epithelial Hh signaling remains to be elucidated. Bmp signaling genes are also expressed in the urinary tract and are essential for urinary tract development. In fact, several mouse mutants for Bmp genes display various abnormalities including kidney and urinary tract anomalies [1], [33], [34]. BMP4 is known to be expressed in the nascent mesenchyme of the urogenital sinus and smooth muscle region in the human embryo [31]. Several mouse studies have suggested that biological functions of Bmp4 during urinary tract formation are considered to prevent cell death and promote the metanephric mesenchymal growth and the ureteral smooth muscle formation [1], [33], [35], [36]. The study of growth factor mediated morphogenesis requires analyses for the roles of signaling relays among different growth factor pathways. However, the spatiotemporally regulated functions of such signaling relays are unclear. In particular, it has been speculated that epithelially derived Hh signals are interpreted at the level of the surrounding mesenchyme [2], [27], [37]–[39]. Moreover, analyses of the signaling modulation in Hh signal responsive mesenchyme have not been performed.
The current study used a novel genetic analysis, the conditional ablation of Bmp receptor type IA (BmprIA) gene in Hh-responsive cell lineages. Utilizing Hh-responsive gene modulation at the level of the mesenchyme allows an analysis of the primary functions of the growth factor signaling relay. The study also revealed the cellular origin of the mesenchymal cells in the upper urinary tract by tissue contribution analyses of Hh-responsive cell lineages. Moreover, loss- and gain-of-functional analyses of Hh signaling suggested an essential correlation between Hh and Bmp signaling during urinary tract formation. In addition, the critical time-window leading to the upper urinary phenotypes including hydroureter was suggested by conditional mutation of Shh and BmprIA genes. These analyses suggest Hh-responsive mesenchymal Bmp signaling maintains the population of the PCM cells, which is essential for the development of the ureter and the upper urinary tract.
Materials and Methods
Mouse Strains and Embryos
The Shhneo, Shhflox, BmprIAflox, Gli1CreERT2, ShhCreERT2, Rosa26-SmoM2 (R26SmoM2), Rosa26R (R26RLacZ) and Rosa26-eYFP (R26RYFP) alleles have been described previously [21], [23], [40]–[45]. All experimental procedures and protocols were approved by the Committees on the Animal Research at Wakayama Medical University and at Kumamoto University (Permit Number: 519 in Wakayama Medical University and A23-066, A23-069, A23-070 and A23-071 in Kumamoto University).
Histological Analyses
Mouse embryos were fixed overnight in 4% paraformaldehyde (PFA/PBS), dehydrated through methanol, embedded in paraffin, and 8 µm serial sections were prepared. Hematoxylin and Eosin (HE) staining was processed by standard procedures [46]. X-gal staining was carried out as described previously [29], [47]. X-gal stained samples were embedded in paraffin and sectioned. Immunohistochemical analyses were performed by standard procedures using the antibodies (Ab): anti-GFP Ab (1∶400, Abcam), anti-GFP Ab (1∶100, Roche), anti-smooth muscle myosin (SMM) Ab (1∶200, Biomedical Technology), anti-phosphorylated-Smad1/5/8 (pSmad) Ab (1∶300, Cell Signaling), anti-Pax2 Ab (1∶200, Zymed), anti-Uroplakin3 (UPIII) Ab (1∶200, Progen), anti-alpha smooth muscle actin (SMA) Ab (1∶400, Sigma). Signal amplification for pSmad staining was performed using the appropriate ABC kit (Vector Laboratories) and the immunocomplexes were detected with DAB staining. Staining for GFP, SMM, Pax2, UPIII and SMA was visualized using Alexa Fluor 488 or 546 IgG against the primary antibodies (1∶300, Molecular Probes). Nuclear counterstaining was performed using Hoechst33342 (Sigma).
In situ Hybridization for Gene Expression Analyses
Whole-mount and section in situ hybridizations for gene expression analyses were performed as previously described [26], [46]. The antisense riboprobe templates have been described previously: Shh (kindly provided by C. Shukunami, Kyoto University, Japan), Gli1, [48], Pax2 [49], Tbx18 (kindly provided by T. Suzuki, Nagoya University, Japan).
Tamoxifen Administration for Conditional Gene Recombination
Noon of the day of a vaginal plug was designated as E0.5. A 20 mg/ml stock solution of tamoxifen (Sigma) was prepared in corn oil [29]. The pregnant females received 2 mg of tamoxifen per 40 g maternal body weight using a gavage needle at the indicated time points.
Genetic Tissue Lineage Analysis Utilizing the Rosa26 Reporter Mouse Strains
Tissue lineage analyses were conducted by utilizing Gli1CreERT2/+; R26RLacZ/+ and Gli1CreERT2/+; R26RYFP/+ mice. The Gli1CreERT2/+ male mice were crossed with R26RLacZ/LacZ or R26RYFP/YFP Cre indicator female mice [42], [44], [45]. Mouse embryos were processed for X-gal or immunohistochemical staining.
Results
Requirement for Sonic Hedgehog Signaling during Urinary Tract Formation
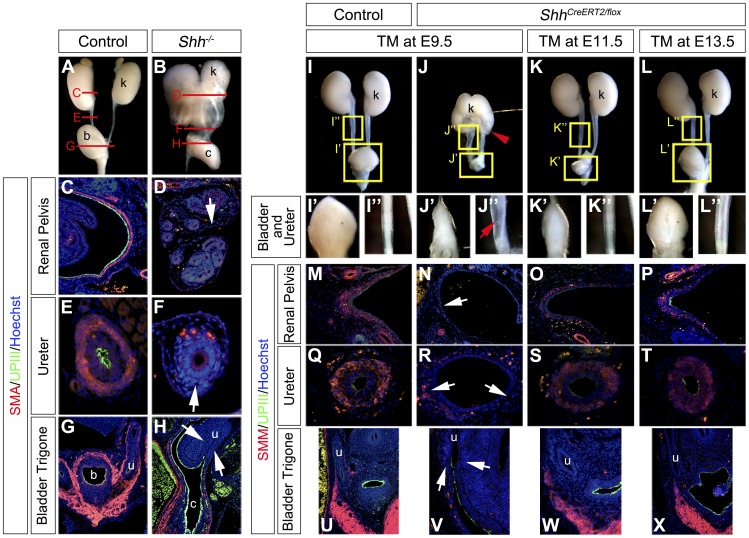
Mouse mutants for the Sonic hedgehog (Shh) gene display several abnormalities in the urogenital organs [2], [26], [28]–[30]. The urinary specimens of Shh conventional mutants (Shh−/−) displayed prominent hydroureter and hydronephrosis phenotypes (Fig. 1A,B). Shh−/− embryos also displayed a fusion of the bilateral kidneys at low frequency (Fig. 1B) [2]. This horseshoe-like kidney phenotype is considered to be caused by Shh derived from the notochord [50]. In addition, prominent ureter dilation and bladder hypoplasia were observed (Fig. 1B; data not shown). The mutant ureters were not only dilated but also displayed a severely reduced length longitudinally between the kidney and the bladder (Fig. 1B). We next performed expression analyses for several tissue differentiation markers. The ureter, renal pelvis and bladder regions develop several tissues such as transitional epithelia, connective tissues and smooth muscles. Smooth muscles of the ureter have been suggested as important for regulating proper peristaltic movement for the urine transport. One of the causes for hydronephrosis and hydroureter is considered to be derived from abnormal smooth muscle formation of the urinary outflow tract in the ureters, renal pelvis and bladder trigone [51]. The expression of alpha smooth muscle actin (SMA: red), one of the smooth muscle cell differentiation markers, was investigated by immunohistochemical analyses (Fig. 1C–H). Its prominent expression was detected in the ureter and in the renal pelvis mesenchyme of the control embryos at E18.5 (Fig. 1C,E). In contrast, a small number of SMA positive cells were observed as dotted signals in the mesenchymal layer around the urothelium of Shh−/− embryos at E18.5 (Fig. 1D,F; white arrow). The expression in the bladder wall was also reduced in such mutants (Fig. 1G,H). These samples were co-stained with SMA (red) and Uroplakin3 (UPIII: green), which is a family of transmembrane proteins selectively expressed in the ureter epithelium and required for its water-impermeable properties [52], [53]. A reduced UPIII expression was observed in the renal pelvis and the ureter of Shh−/− embryos (Fig. 1C–F). However, the expression of UPIII in the bladder urothelium was similarly detected in Shh−/− embryos (Fig. 1G,H).
Figure 1. Urinary tract malformation and reduction of marker expression in Sonic hedgehog (Shh) mutant embryos.
Gross morphology of the urinary tracts in the control (A) and Shh−/− (B) embryos at E18.5. Red lines in A and B indicate positions of transverse sections in C–H. Immunohistochemistry for the expression of alpha smooth muscle actin (SMA: red) and Uroplakin3 (UPIII: green) in transverse sections of the renal pelvis (C, D), the ureter (E, F) and the bladder trigone regions (G, H) at E18.5. White arrows in D, F and H indicate reduced expressions of these markers. Gross morphology of the urinary tracts of ShhCreERT2/flox embryos at E16.5, which were treated with TM at E9.5 (J, J’, J”), E11.5 (K, K’, K”) or E13.5 (L, L’, L”). The control embryo was treated with TM at E9.5 (I, I’, I”). I’–L’ and I”–L” indicate higher magnification views of yellow boxes in I–L. E9.5-TM treated embryos displayed severe phenotypes in the kidney, the bladder and the ureter (J, J’, J”). A red arrowhead in J indicates a hydronephrosis phenotype. A red arrow in J” indicates the hydroureter phenotype. Immunohistochemistry for the expression of smooth muscle myosin (SMM: red) and UPIII (green) in transverse sections of the renal pelvis (M–P), the ureter (Q–T) and the bladder trigone region (U–X). White arrows indicate reduced expressions of differentiation markers in mutants with E9.5-TM treatment. b: bladder, c: cloaca, k: kidney, u: ureter.
We also analyzed the expression patterns of several Hh signaling genes (Fig. S1). The Shh was expressed prominently in the cloaca and the bladder epithelium in E10.5-E13.5 embryos (Fig. S1B,F,J). A faint Shh expression was detected around the early kidney and the ureter from E11.5 onward (Fig. S1D,E,H,I; red arrowheads) [2]. The prominent expression of Gli1 gene, a readout for Hh signaling, was observed in most of the mesenchymal layers adjacent to the Shh-positive cloacal epithelia and the presumptive ureters (Fig. S1K–T).
The upper urinary tract phenotypes of Shh−/− embryos were observed from E12.5 onward. We next assessed the expression of Pax2 gene, a developmental gene in the urinary tracts. Pax2 is expressed both in the ureteric bud and the condensed mesenchyme surrounding it in the metanephros at E11.5 [54], [55]. The expression of Pax2 was detectable in both the control and Shh mutant urinary tracts (Fig. S2A–H). Its expression was not significantly altered in the metanephric mesenchyme, ureteric bud epithelium, ureter and early kidney region of Shh−/− embryos at E10.5, E11.5 and E12.5 (Fig. S2B,D,F,H). We also analyzed the expression of Tbx18 gene, which is expressed in the smooth muscle progenitor cells of the urinary tract and known as regulating their differentiation [56]–[58]. Tbx18 expression was decreased in the mesenchyme around the nephric duct of Shh−/− embryos in comparison to the control at E10.5 and E11.5 (Fig. S2I–L; red arrows). The significantly reduced Tbx18 expression was observed in the mutant mesenchyme around the ureter and the early kidney at E12.5 (Fig. S2N,P; red arrows).
The Conditional Inactivation of Shh Gene Led to Malformations in the Upper Urinary Tract
The prominent urinary phenotypes of Shh−/− embryos suggest the essential functions of Shh signaling for the upper urinary tract formation (Fig. 1A–H). To examine the temporal necessity of Hh signaling for the urinary tract formation, we analyzed the urinary tract phenotypes of Shh conditional mutants. These experiments utilized Shh flox (Shhflox) allele and ShhCreERT2 allele, which is a tamoxifen (TM) inducible form of Cre recombinase expressing strain [23], [41], [59]–[61]. Pregnant female mice were treated orally with TM. The ShhCreERT2/flox embryos exhibited upper urinary tract hypoplasia, kidney and ureter defects at E16.5. The urinary tracts of ShhCreERT2/flox embryos reproduced the milder phenotypes of Shh−/− embryos by E9.5-TM treatment (Fig. 1J–J”). We further examined ShhCreERT2/flox embryos with TM treatment at E11.5 and E13.5 (Fig. 1K–L”). These ShhCreERT2/flox embryos exhibited slight hypoplasia of the urinary tracts (Fig. 1K–L”). The cell differentiation status of these ShhCreERT2/flox embryos was examined by smooth muscle myosin (SMM) and UPIII expression. The SMM expression was reduced in the renal pelvis, ureter and bladder trigone region in E9.5-TM treated mutants (Fig. 1N,R,V; white arrows). A small number of SMM positive cells were observed in the bladder wall (Fig. 1V). Such mutants also showed reduced UPIII expression in the dilated renal pelvis and in the ureter (Fig. 1N,R). However, its expression in the bladder urothelium was not altered in the mutants (Fig. 1V). Mutants with later-staged TM treatment, such as E13.5-TM treated mutants, showed weak expression of these markers in the renal pelvis, ureter and bladder wall (Fig. 1O,P,S,T,W,X). These results suggested that urinary tract phenotypes are caused by defective Shh signaling at early embryonic stages. Moreover, we confirmed the reduced Shh signaling in E9.5-TM treated mutants by Gli1 expression analyses (Fig. S3). Its expression in mutants was decreased in the ventral bladder mesenchyme (Fig. S3B,D; red arrowheads), but not in the bladder trigone region and the ureter at E14.5 (Fig. S3D,F). These results suggested that ureteral Shh was not affected in mutants with E9.5-TM treatment.
Hedgehog Signal Responsive Cells can Contribute to the Upper Urinary Tract from the Early Peri-cloacal Mesenchyme
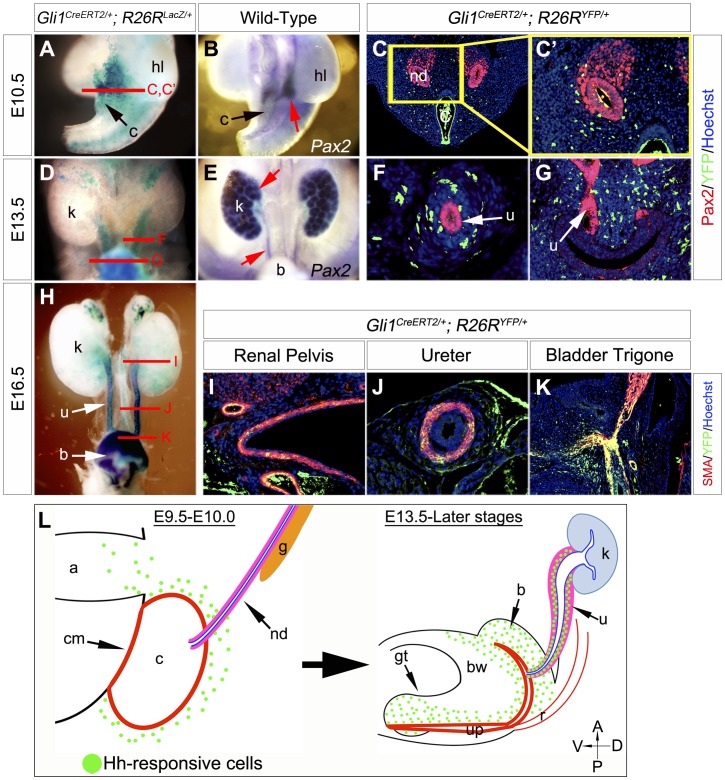
Our previous results suggested that the mesenchymal precursors for multiple urogenital structures, such as the bladder and external genitalia, are derived from the peri-cloacal mesenchyme (PCM) [29], [32]. The current study suggested that Shh signal at early embryonic stages is essential for the development of the upper urinary tract (Fig. 1). This suggests some of the Hh-responsive cells may contribute to the developing upper urinary tract. Examination of such a cell lineage is essential for understanding the pathogenic mechanisms of human congenital malformations. Therefore, genetic cell lineage analyses for Hh signal responsive cells were conducted by utilizing the Gli1CreERT2/+; R26RLacZ/+ and Gli1CreERT2/+; R26RYFP/+ systems. These strains are effective for analyzing the tissue lineage of Hh-responsive cells in many developmental contexts, such as the limb, central nervous and urogenital systems [29], [42], [62], [63]. Following TM treatment at E9.5, Gli1CreERT2/+; R26RLacZ/+ mice were harvested and X-gal stained at E10.5, E13.5 and E16.5 (Fig. 2A,D,H). Most of the Hh-responsive cells in the urinary tracts were observed in the mesenchyme of the bladder, ureter and renal pelvis in such embryos. The ureteric bud began to form from the nephric duct at E10.5 (assessed by Pax2 expression; Fig. 2B; red arrow). We also co-stained with YFP and Pax2 in Gli1CreERT2/+; R26RYFP/+ mice (Fig. 2C,C’,F,G). A relatively small number of Hh-responsive cells (indicated by the YFP expression) were observed in the metanephric mesenchyme at E10.5 (Fig. 2C,C’). Pax2 was expressed in the ureter epithelium and early kidney at E13.5 (Fig. 2E; red arrows). Hh-responsive cells are located in the mesenchyme around the ureter and the bladder at E13.5 (Fig. 2F,G). At E16.5, some of Hh-responsive cells also expressed alpha smooth muscle actin (SMA; Fig. 2I–K). The labeled cells were distributed broadly in the mesenchyme including SMA positive smooth muscle layers in the renal pelvis and the ureter (Fig. 2I,J). Such cells in the bladder were localized in the mesenchyme and smooth muscle layers (Fig. 2K). Of note, most of labeled cells were observed in smooth muscle layers in the bladder trigone region (Fig. 2K).
Figure 2. The contribution of Hh signal responsive cells to the upper urinary tracts.
Hh signal responsive cell contribution assays were performed by utilizing the Gli1CreERT2 system. Urinary tracts of embryos were analyzed at E10.5 (A–C, C’), E13.5 (D–G) or E16.5 (H–K) following TM treatment at E9.5. Gross morphology of Gli1CreERT2/+; R26RLacZ/+ embryos with whole-mount X-gal staining (A, D, H). Red lines in A, D and H indicate corresponding areas for sections in C, C’, F, G and I–K. The expression of Pax2 gene was analyzed at E10.5 and E13.5 to visualize the early urinary tract (B, E; red arrows). Pax2 (red) and Hh-responsive cells (YFP: green) were co-stained in Gli1CreERT2/+; R26RYFP/+ embryos (C, C’, F, G). A yellow box in C indicates magnified area in C’. At E13.5, urinary tissues were analyzed at the ureter (F) and bladder trigone (G) levels. Immunohistochemistry for the expression of SMA and YFP was performed in the transverse sections of the renal pelvis (I), ureter (J) and bladder trigone (K) region of Gli1CreERT2/+; R26RYFP/+ embryos at E16.5. Summary of the Hh-responsive cell lineage analysis (L). The early Hh-responsive cells labeled by the Gli1CreERT2 system are located in the peri-cloacal mesenchyme (L: left side). Part of such cells can contribute to the ureteral mesenchymal region in the late embryonic stage (L: right side). The Gli1CreERT2-labeled Hh-responsive cells are depicted as green dots. a: allantois, b: bladder, bw: body wall, c: cloaca, cm: cloacal membrane, g: gonad, gt: genital tubercle, hl: hindlimb, k: kidney, nd: nephric duct, r: rectum, u: ureter, up: urethral plate.
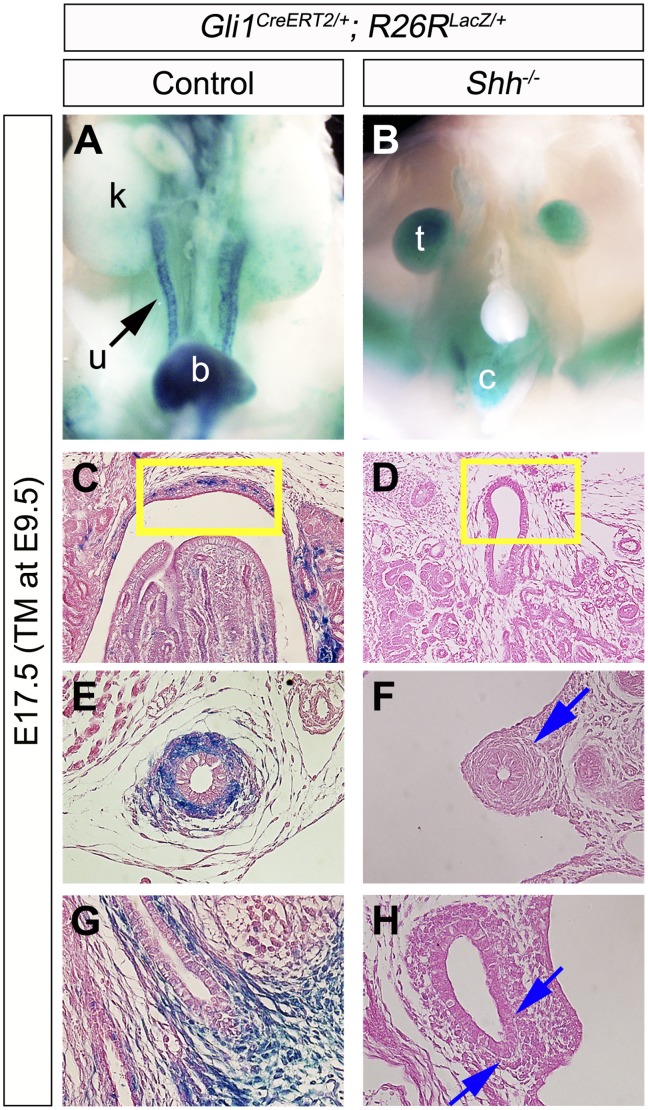
The contribution of Hh-responsive cells was also examined in the Shh−/− background (Fig. 3A–H). We analyzed the population of LacZ positive cells in Gli1CreERT2/+; R26RLacZ/+ and Shh−/−; Gli1CreERT2/+; R26RLacZ/+ embryos at E17.5 after E9.5-TM treatment. Hh-responsive cells were not detected in the upper urinary tract of such embryos under the current experimental conditions (Fig. 3B,D,F,H). These results suggest that Shh signaling plays an essential role in the early Hh-responsive cells around the PCM region, which thus contribute to several upper urogenital tracts.
Figure 3. The contribution of Hh-responsive cells in Shh mutant urinary tracts.
Hh-responsive cell contribution assay was performed by utilizing the Gli1CreERT2; R26RLacZ/+ system. Urinary organs of embryos were analyzed at E17.5 following TM treatment at E9.5 (A–H). Gross morphology of X-gal stained embryos (A, B). A weak LacZ signal was detected in Shh−/−; Gli1CreERT2/+; R26RLacZ/+embryos in comparison to control embryos in the renal pelvis (yellow boxes in C, D), the ureter (E, F) and the bladder trigone region (G, H). Blue arrows indicate reduced LacZ signals in the urinary tracts of Shh−/−; Gli1CreERT2/+; R26RLacZ/+ embryos. b: bladder, c: cloaca, k: kidney, t: testis, u: ureter.
Moreover, we performed Hh-responsive cell contribution analyses at E18.5 embryos subsequent to TM administration at E11.5, E12.5 or E13.5. Few labeled cells were observed in the renal pelvis and the ureter region after TM treatment at E11.5 (Fig. S4A,D,G). In contrast to the administration of TM at E11.5, some LacZ positive cells contributed to the ureteral subluminal mesenchyme following the treatment at E12.5 or E13.5 (Fig. S4H,I). The embryos with TM treatment at E12.5 or E13.5 displayed weaker LacZ signals in the bladder mesenchyme than embryos with TM treatment at E11.5 (Fig. S4J–L). These results may be attributed to the difference of TM treatment timing and the tissues where Hh ligands emanated from. The Hh ligands are likely derived from the cloacal epithelia at the early embryonic stages. In fact, gene expression analyses revealed that Shh was not observed in the nephric duct at E10.5 (Fig. S1A,B). Additionally, Hh ligands are thought to be derived from the urothelia (bladder, ureter and renal pelvis epithelia) during the late embryonic stages (Fig. S1C–J).
Constitutive Activation of Hh Signaling Induced Aberrant Smooth Muscle Development and Augmented Bmp Signaling
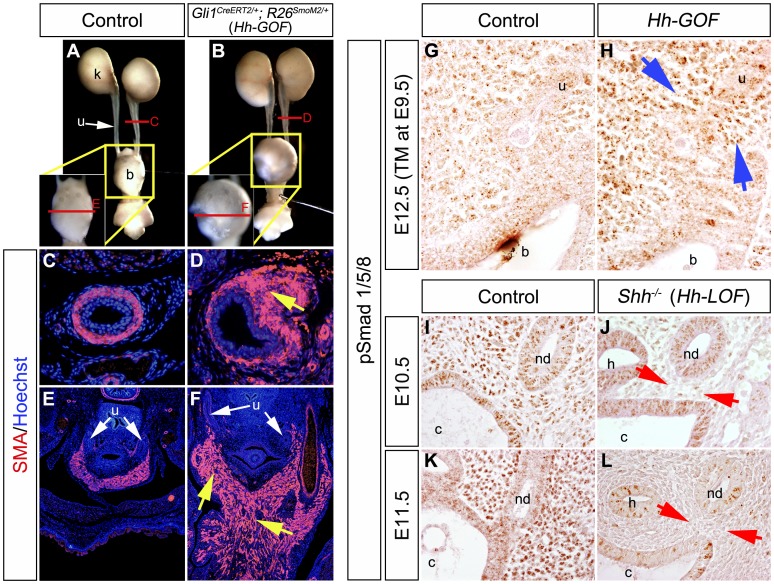
Shh null mutants displayed urogenital abnormalities and a reduced expression of SMA (Fig. 1A–H). Shh conditional mutants also displayed urogenital abnormalities such as hydronephrosis, hydroureter and bladder hypoplasia by a temporal gene modulation (Fig. 1J–J”). To further examine the functions of Hh signaling during urogenital tract formation, we performed Hh signal gain-of-function (Hh-GOF) experiments. Mice carrying a constitutively activated form of Smoothened (R26SmoM2) and Gli1CreERT2 alleles were utilized [40], [64]. The Gli1CreERT2/+; R26SmoM2/+ (Hh-GOF mutants) displayed mesenchymal hyperplasia of the urinary tracts at E17.5 after E9.5-TM treatment (Fig. 4A–F). The ventral bladder wall was hyperplastic in comparison to the control and SMA expression domain was expanded in the mutant bladder wall (Fig. 4A,B,E,F). The width of the ureter smooth muscles was also expanded (Fig. 4C,D). The renal pelvis in Hh-GOF mutants did not display prominent phenotypes (data not shown). We then analyzed the activity of other growth factor signals in such mutants. The Hh-GOF mutants displayed augmented phosphorylated-Smad1/5/8 (pSmad) expression mainly in the Gli1CreERT2-activated mesenchyme at E12.5 (Fig. 4G,H; blue arrows). We also analyzed the expression of pSmad in Shh−/− embryos (Hh signal loss-of-function mutants: Hh-LOF mutants). Its expression was significantly decreased in the mutant PCM at E10.5 and E11.5 (Fig. 4I–L; red arrows). The pSmad expression in mutant epithelial layers was retained at E10.5, while it was reduced at E11.5 (Fig. 4J,L). Such significant differences of pSmad expression in the mesenchyme of both Hh-GOF and Hh-LOF mutants were also confirmed statistically (Figure S5). These results suggested that the activity of Bmp signaling may be regulated by the Hh signal during urinary tract formation.
Figure 4. Constitutive activation of Hh signaling led to hyperplasia of urinary smooth muscles and augmented expression of phosphorylated-Smad1/5/8 in the urogenital mesenchyme.
The gain-of-function experiments in Hh-responsive cells were performed by utilizing Gli1CreERT2; R26SmoM2 (Hh-GOF) mice. Gross morphology of urinary tracts in control and mutant embryos at E17.5 after E9.5-TM treatment (A, B). Locations of the magnified bladder regions were indicated by yellow boxes in A and B. Levels of transverse sections in C–F are indicated by red lines in A and B. Immunohistochemistry for the expression of SMA (red) in transverse sections of the ureter (C, D) and the bladder trigone (E, F) regions. Yellow arrows indicate the abnormally expanded urinary smooth muscle layers. Immunohistochemistry for phosphorylated-Smad1/5/8 (pSmad) expression was also performed in Hh-GOF mutants with TM treatment at E9.5 (G, H). The Hh-GOF mutants displayed augmented pSmad expression in peri-cloacal mesenchyme at E12.5 (blue arrows). The Shh−/− (Hh signal loss-of-function mutants: Hh-LOF mutants) displayed a reduction of the pSmad expression at E10.5 and E11.5 (I–L: red arrows). b: bladder, c: cloaca, h: hindgut, nd: nephric duct, k: kidney, u: ureter.
Temporal Inactivation of the BmprIA Gene in Hh-responsive Cells Resulted in Severe Hydroureter and Hydronephrosis Phenotypes; a Genetic Analysis of the Hh and Bmp Signaling Relay
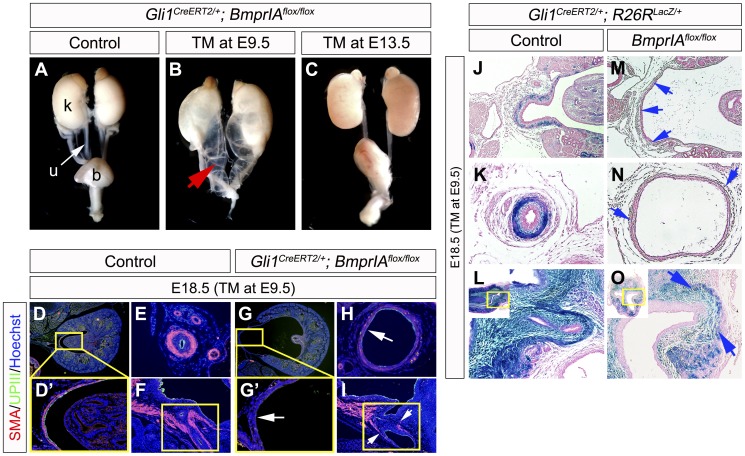
The current study suggested that Bmp signaling is linked with Hh signaling, based on the augmentation and the reduction of phosphorylated-Smad1/5/8 (pSmad) activities in Hh-GOF and Hh-LOF mutants (Fig. 4G–L). We next examined Gli1CreERT2/+; BmprIAflox/flox embryos to further investigate the possible relationship between Hh and Bmp signaling during upper urinary tract formation. We confirmed no significant alteration of Gli1 expression in Gli1CreERT2/+; BmprIAflox/flox embryos at E12.5 with E9.5-TM treatment (Figure S6). Therefore, such allelic combination introduces the mutation of BmprIA in Hh-responsive cells and which is effective for analyzing the Hh-to-Bmp signaling relay in PCM cells. Embryos were treated once with TM at E9.5-E13.5 and the phenotypes were examined at E18.5. The Gli1CreERT2/+; BmprIAflox/flox embryos with TM treatment at E9.5 displayed severe phenotypes in the urinary tract such as hydroureter and hydronephrosis (Fig. 5B; red arrow). The mutant renal pelvis and ureter were widely dilated and the renal papilla was hypoplastic. These structures were surrounded by poorly differentiated smooth muscle cells (Fig. 5G,G’,H; white arrows). In addition, a reduction of UPIII expression was also observed in the renal pelvis region (Fig. 5D’,G’). The differentiation of urinary smooth muscle cells was also impaired in the bladder trigone of such mutants (Fig. 5F,I; white arrows). In contrast, mutants with TM treatment at E13.5 showed milder hydroureter-like phenotypes than E9.5-TM treated mutants (Fig. 5C). These results suggested that the Hh-to-Bmp signaling relay in the early PCM is essential for urinary tract development. Further experiments examined conditional inactivation of the Bmp4 gene, which is a major ligand for BmprIA. However, Gli1CreERT2/+; Bmp4flox/flox embryos did not display prominent phenotypes under the current experimental conditions (data not shown).
Figure 5. Conditional mutation of BmprIA gene resulted in urogenital organ defects and reduction in the number of Hh-responsive cells.
BmprIA gene was mutated in Hh-responsive cells by utilizing Gli1CreERT2/+; BmprIAflox/flox mice. TM administration dependent hydronephrosis and hydroureter phenotypes (red arrow) were observed at E18.5 (A–C). The Gli1CreERT2/+; BmprIAflox/flox embryos displayed the reduced expression of SMA and UPIII in the renal pelvis (D, D’, G, G’), the ureter (E, H) and the bladder trigone region (F, I; yellow boxes). Locations of D’ and G’ were indicated in yellow boxes in D and G. White arrows indicate defective differentiation of cells based on marker expression. Hh-responsive cell contribution assays in BmprIAflox/flox conditional mutants were performed using the Gli1CreERT2/+; R26RLacZ/+ system (J–O). The X-gal stained urinary organs from the control (J–L) and BmprIAflox/flox; Gli1CreERT2/+; R26RLacZ/+ embryos (M–O) following the treatment with TM at E9.5. A reduced number of LacZ positive cells in the renal pelvis (J, M; blue arrows), the ureter (K, N; blue arrows) and the bladder trigone (L, O; blue arrows) were observed in BmprIAflox/flox; Gli1CreERT2/+; R26RLacZ/+ embryos. b: bladder, k: kidney, u: ureter.
Furthermore, the contribution of Hh-responsive cells was analyzed in Gli1CreERT2/+; BmprIAflox/flox embryos (Fig. 5J–O). The LacZ-labeled cells were observed in the mesenchyme of the renal pelvis, the ureter and the bladder trigone of control embryos following TM administration at E9.5 (Fig. 5J–L). On the other hand, few LacZ positive cells were observed in the renal pelvis and the ureter in BmprIA mutants (Fig. 5M,N; blue arrows). The LacZ positive cells were observed in the mesenchyme of the mutant bladder, but it was also reduced in number compared with the control embryos (Fig. 5L,O). These results suggested that Bmp signaling may therefore maintain the Hh-responsive cell population.
Discussion
Recent advances of genetic techniques in animal models allow an analysis of the complex processes of embryonic development [65]–[67]. Genetic analyses of human patients have advanced greatly in recent years [68], [69]. Despite such advantages, the mechanisms for normal development and the pathogenic mechanisms for embryonic urinary systems remain obscure. Mesenchymal development in response to epithelially derived growth factors remains to be investigated. The current study showed the requirement of Hh signaling for the processes of urinary tract formation including its patterning and smooth muscle formation by analyzing a series of Shh mutant mice. Analysis of Hh-responsive cell contribution revealed that the progenitor cells for the mesenchyme of urinary organs are initially located in the peri-cloacal mesenchyme (PCM). A portion of those cells contribute to the urogenital mesenchyme and differentiate into urinary smooth muscles in response to Hh signaling. The conditional ablation of BmprIA gene in Hh signal responsive cell lineages suggested that the Hh-to-Bmp signaling relay in the PCM cells is essential for urogenital development. The current findings suggest new insights for the cellular origin and molecular requirements during urinary tract formation. The current results implicate the possible causative factors of urinary malformations such as CAKUT, one of the primary causes of kidney failure in infants.
The Significance of Cloacal Hh Signaling during Urinary Tract Formation
Hedgehog signaling plays essential functions during urogenital development [26], [29], [30], [70]–[73]. Shh mutants displayed various abnormalities in the urinary tract such as the bladder agenesis, hydroureter and hydronephrosis [2], [6].
The current analysis of tamoxifen (TM) inducible conditional Shh mutants revealed the essential embryonic stages of Shh signaling for such urinary organ patterning and urinary smooth muscle differentiation. The time-controlled TM administration resulted in selective inactivation of the Shh gene in temporally CreERT2 activated tissues. Previous reports suggest the recombination event occurs within 6–12 hours and continues for up to 36 hours after TM administration [74], [75]. The Shh gene modulation in early embryonic stages (E9.5-E10.0) led to the urinary tract phenotypes such as hydroureter and hydronephrosis. In such stages, Shh gene is expressed in the cloacal epithelium and not in the epithelium of the nephric ducts. In addition, the ureteric bud is not yet formed in such stages. Accordingly, the Shh gene in the cloaca is selectively ablated in the urogenital tracts of such mutants. The current results also showed that Hh signal responsive cells in the peri-cloacal mesenchyme contribute to the urinary tract mesenchyme. These observations suggest that Hh signaling from the cloacal epithelia to the peri-cloacal mesenchyme plays an indispensable role for urinary tract development.
On the other hand, Shh was expressed broadly in the urothelia in later embryonic stages. Although the current ShhCreERT2/flox mice with TM treatment at later stages (e.g., E13.5) did not display prominent phenotypes, the Hoxb7-Cre; Shhflox/− mice, which are conditional mutants of the Shh gene in the mesonephric duct from E9.5, show delayed differentiation of ureteral smooth muscle, hydroureter and hydronephrosis phenotypes [2]. This suggests the necessity of early ureteral Hh signaling for proper urinary tract formation. These observations suggest cloaca derived and early ureter derived Hh signals would be critical for the Hh-signal-related urinary abnormalities.
In the current study, we showed that the reduction of Uroplakin3 (UPIII) expression in Shh conventional and conditional mutants. UPIII is a differentiation marker for urothelia and is known to be required for the water-impermeable properties of the urinary tract [52], [53]. Hence, the urothelial structures of these Shh mutants are suggested as rather immature. The reduction in the UPIII expression has also been reported to be observed in Tbx18 mutants [56]. A reduction of the Tbx18 expression was also demonstrated in Shh mutants. The reduction of the UPIII expression is likely caused by the reduction in the Tbx18 expression. Both Shh and Tbx18 are considered to function in the mesenchyme and the mutants for each gene display defects in the smooth muscle formation [2], [56]–[58]. The ShhCreERT2/flox embryos with E9.5-TM treatment still show Gli1 expression in the ureteral mesenchyme, suggesting the presence of ureteral Shh signaling. Hence, the effect of such ureteral Shh seemed not affecting to the UPIII expression. From these observations, we speculate that the reduced UPIII expression may be attributed to indirect effects from the mesenchyme such as smooth muscles. Further analyses would illuminate the mechanisms for the urothelial maturation related with Hh signaling.
Functions of the Hh-to-BMP Signaling Relay in Regulating the Urinary Tract Formation
The current results suggested that PCM cells can contribute to the urogenital mesenchyme and some of those cells differentiate into smooth muscle cells in later embryonic stages. We revealed that Hh signaling secreted from the cloacal epithelium is one of growth factors acting on PCM cells. In addition, we suggested its downstream signaling and assessed the function for urinary tract formation.
The current study found that the expression of phosphorylated-Smad1/5/8 (pSmad), an indicator of Bmp signaling activity, was decreased in Shh deficient (Hh signal loss-of-function: Hh-LOF) embryos. The reduced expression was mainly observed in the mesenchyme, but not in the epithelial layer at E10.5. In contrast, Hh signal gain-of-function mutants (Hh-GOF: Gli1CreERT2/+; R26SmoM2/+) showed the augmented pSmad signal mainly in the PCM at E12.5.
Regarding the correlation between Hh and Bmp signaling, several possibilities have been considered. Such possibilities could be (1) the regulation of the Bmp ligand expression by Hh signaling, (2) the regulation of the Bmp receptor expression by Hh signaling and (3) the modulation of the Bmp receptor activity and also the subsequent Bmp signaling by Hh signaling. Assuming that Hh signal activates the expression of Bmp ligands, the epithelial pSmad activity can also be augmented. However, an alteration of pSmad activity was mainly observed in the mesenchyme around the cloaca. In addition, we did not observe any significant phenotypes in the Gli1CreERT2/+; Bmp4floxflox embryos (data not shown). These observations may not support the possibility of number (1). However, it might be also possible that because of the mosaic induction of the Gli1CreERT2 activity, only some PCM cells lost Bmp4 expression. Hence, the effect of such Bmp4-deficient-cells may be masked by the Bmp4 proteins secreted from their neighboring cells. Further analysis is necessary to fully understand these phenomena. As for the possibility of number (2), we could not detect any alteration of the BmprIA expression in Shh mutants (data not shown). Accordingly, the current results suggest that Hh signaling likely functions as noted in number (3). Namely, it may modulate the mesenchymal Bmp signaling activity.
Several previous studies suggest an essential relationship between Hh and Bmp signaling [2], [27], [37]–[39]. However, the functions of Bmp signaling after receiving Hh signaling (Hh-to-Bmp signaling relay) remain obscure. To assess the significance of Hh-to-Bmp signaling relay, we analyzed BmprIA conditional mutant mice (Gli1CreERT2/+; BmprIAflox/flox mice). The BmprIA gene was modulated in Hh-responsive PCM cells by the administration of TM at E9.5. The Gli1CreERT2/+; BmprIAflox/flox mice displayed severe hydroureter, hydronephrosis and reduced smooth muscle differentiation in the urinary tract. The similarity of ureteral phenotypes between Shh−/− and Gli1CreERT2/+; BmprIAflox/flox mice also suggests that they are involved in the same signaling cascade in the peri-cloacal mesenchyme.
Several Bmp signaling genes are described as playing roles in the formation of the upper urogenital tract [76]–[79]. In fact, expressions of Bmp signaling genes (e.g., BMP2, BMP7 and BMPRIA) are reported to be decreased in the fibrotic renal tissue of human hydronephrosis [80]. The Bmp4 heterozygote mice display severe hydroureter and kidney hypoplasia with poorly differentiated ureteral smooth muscles [1], [81]. Bmp4 may also have multiple biological functions such as prevention of apoptosis in the metanephric mesenchyme and serving as a chemoattractant for ureteral mesenchymal cells [33]. Such functions may be diverged spatiotemporally during urinary tract formation. The current analysis revealed a reduced number of the LacZ-positive cells in the Gli1CreERT2/+; BmprIAflox/flox; R26RLacZ/+ mice in comparison to the control embryos. The mutation of the BmprIA gene in Hh-responsive mesenchyme resulted in severe hypoplasia of the mesenchymal region. These results imply a requirement of Bmp signaling for the maintenance of Hh-responsive PCM cell lineages.
In spite of the recent advances in the clinical care of the fetus, the mechanisms of urinary tract malformations such as CAKUT remain to be elucidated [18]–[20]. The current work revealed the possible influences of the cloaca, a transient embryonic cavity, to the pathogenesis of urinary tract abnormalities. The study revealed the unique contribution of the immature PCM to differentiated urinary tract mesenchyme and such contribution of PCM cells was regulated by the Hh and Bmp signaling receptions. These results may therefore help to elucidate the pathogenesis of urinary tract abnormalities.
Supporting Information
The expression of Shh and Gli1 genes at whole-mount and sections of the urinary tract at E10.5 (A, B, K, L), E12.5 (C–F, M-P) and E13.5 (G–J, Q–T). Red lines in A, C, G, K, M and Q indicate locations of the transverse sections in B, D–F, H–J, L, N–P and R–T. The Shh was expressed in the epithelia of the cloaca and the ureter (A–J). Red arrowheads indicate the expression of Shh in ureteral epithelia at E12.5 and E13.5. The Gli1 was expressed in mesenchymal cells surrounding the cloaca at E10.5 (L). Its expression was observed in the urinary tract mesenchyme at E12.5 and E13.5 (N–P, R–T). b: bladder, c: cloaca, g: gonad, k: kidney, nd: nephric duct, u: ureter.
(TIF)
The transverse sections of the urinary tract at E10.5, E11.5 and E12.5 (A–P). Expression of Pax2 in control (A, C, E, G) and Shh−/− (B, D, F, H) embryos. Expression of Tbx18 in control (I, K, M, O) and Shh−/− (J, L, N, P) embryos. Red arrows indicate a reduced expression of the Tbx18 gene around the nephric duct and the ureter. c: cloaca, nd: nephric duct, u: ureter, ub: ureteric bud.
(TIF)
The expression of Gli1 in the control (A, C, E) and ShhCreERT2/flox (B, D, F) embryos at E14.5 with E9.5-TM treatment. Transverse sections of the bladder (A, B), bladder trigone (C, D) and ureter (E, F). Red arrowheads indicate the reduced Gli1 expression.
(TIF)
The contribution assays of Hh-responsive cells utilizing the Gli1CreERT2/+; R26RLacZ/+ system. Gross morphology and sections of X-gal stained urinary organs at E18.5 subsequent to TM treatment at E11.5 (A, D, G, J), E12.5 (B, E, H, K) and E13.5 (C, F, I, L). Yellow lines in A–C indicate the levels of the transverse sections in D–L. Transverse sections of the renal pelvis (D–F; yellow boxes), ureter (G–I) and bladder trigone (J–L; yellow boxes) regions. Blue arrows in G–I indicate weak activity of LacZ. b: bladder, k: kidney, u: ureter.
(TIF)
Quantitative analysis on ratios of pSmad positive cells between wild-type and mutants. The cell number of pSmad positive and negative cells in the defined three areas of 4 different sections was counted and ratios of pSmad positive cells were compared. Data were analyzed using the Student’s t-test or Welch’s t-test followed by the F-test. The ratio of pSmad positive cells was significantly reduced in Shh−/− embryos at E11.5 (A: Wild-Type: 0.718±0.05, n = 12, Shh−/−: 0.499±0.06, n = 12; P<0.001). The ratio of pSmad positive cells was significantly increased in Gli1CreERT2/+; R26SmoM2/+ (Hh-GOF) mice at E12.5 (B: Wild-Type: 0.627±0.118, n = 12, Hh-GOF: 0.776±0.05, n = 12; P<0.05).
(TIF)
The Gli1 expression in the control and Gli1CreERT2/+; BmprIAflox/flox embryos at E12.5 with E9.5-TM treatment. Its expression was not significantly altered in the mutant bladder trigone (A, B) and ureter (C, D) mesenchyme.
(TIF)
Acknowledgments
We would like to thank to Drs. Alexandra L. Joyner, Yuji Mishina, Richard R. Behringer and Cathy Mendelsohn for their invaluable supports. We would also thank Drs. Pierre Chambon, Laurence S. Baskin, Shigeaki Kato, Ryuichi Nishinakamura, Gerald R. Cunha, Tsutomu Ogata, Chisa Shukunami, Chi-Chung Hui, Jun Motoyama, Takayuki Suzuki, Chin Chiang, Brian D. Harfe, Walter Birchmeier, Mark Lewandoski and Andrew P. McMahon for encouragement and suggestions. We would also like to express our appreciation to Sawako Fujikawa, Shiho Miyaji and Tomiko I. Iba for their valuable assistance.
Funding Statement
This work is supported by Grant-in-Aid for Young Scientists B (23790228 and 24790292), for Scientific Research on Innovative Areas: Molecular mechanisms for establishment of sex differences (22132006), and the Global COE program Cell Fate Regulation Research and Education Unit from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. This work is also supported by National Institutes of Health Grant R01ES016597. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I (2000) Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest 105: 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu J, Carroll TJ, McMahon AP (2002) Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129: 5301–5312. [DOI] [PubMed] [Google Scholar]
- 3. Hartwig S, Bridgewater D, Di Giovanni V, Cain J, Mishina Y, et al. (2008) BMP receptor ALK3 controls collecting system development. J Am Soc Nephrol 19: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cain JE, Rosenblum ND (2011) Control of mammalian kidney development by the Hedgehog signaling pathway. Pediatr Nephrol 26: 1365–1371. [DOI] [PubMed] [Google Scholar]
- 5. Di Giovanni V, Alday A, Chi L, Mishina Y, Rosenblum ND (2011) Alk3 controls nephron number and androgen production via lineage-specific effects in intermediate mesoderm. Development 138: 2717–2727. [DOI] [PubMed] [Google Scholar]
- 6. Hu MC, Mo R, Bhella S, Wilson CW, Chuang PT, et al. (2006) GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development 133: 569–578. [DOI] [PubMed] [Google Scholar]
- 7. Mendelsohn C (2009) Using mouse models to understand normal and abnormal urogenital tract development. Organogenesis 5: 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu J, Qi X, Gong J, Yu M, Zhang F, et al. (2012) Fstl1 antagonizes BMP signaling and regulates ureter development. PLoS One 7: e32554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Omori A, Harada M, Ohta S, Villacorte M, Sugimura Y, et al. (2011) Epithelial Bmp (Bone morphogenetic protein) signaling for bulbourethral gland development: a mouse model for congenital cystic dilation. Congenit Anom (Kyoto) 51: 102–109. [DOI] [PubMed] [Google Scholar]
- 10. Kim P, Mo R, Hui Cc C (2001) Murine models of VACTERL syndrome: Role of sonic hedgehog signaling pathway. J Pediatr Surg 36: 381–384. [DOI] [PubMed] [Google Scholar]
- 11. Kim J, Kim P, Hui CC (2001) The VACTERL association: lessons from the Sonic hedgehog pathway. Clin Genet 59: 306–315. [DOI] [PubMed] [Google Scholar]
- 12. Aguinaga M, Zenteno JC, Pérez-Cano H, Morán V (2010) Sonic hedgehog mutation analysis in patients with VACTERL association. Am J Med Genet A 152A: 781–783. [DOI] [PubMed] [Google Scholar]
- 13. Martínez-Frias ML, Bermejo E, Frias JL (2001) The VACTERL association: lessons from the Sonic hedgehog pathway. Clin Genet 60: 397–398. [DOI] [PubMed] [Google Scholar]
- 14. Tabatabaeifar M, Schlingmann KP, Litwin M, Emre S, Bakkaloglu A, et al. (2009) Functional analysis of BMP4 mutations identified in pediatric CAKUT patients. Pediatr Nephrol 24: 2361–2368. [DOI] [PubMed] [Google Scholar]
- 15. Uetani N, Bertozzi K, Chagnon MJ, Hendriks W, Tremblay ML, et al. (2009) Maturation of ureter-bladder connection in mice is controlled by LAR family receptor protein tyrosine phosphatases. J Clin Invest 119: 924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schedl A (2007) Renal abnormalities and their developmental origin. Nat Rev Genet 8: 791–802. [DOI] [PubMed] [Google Scholar]
- 17. Miyazaki Y, Ichikawa I (2003) Ontogeny of congenital anomalies of the kidney and urinary tract, CAKUT. Pediatr Int 45: 598–604. [DOI] [PubMed] [Google Scholar]
- 18. Nishimura H, Yerkes E, Hohenfellner K, Miyazaki Y, Ma J, et al. (1999) Role of the angiotensin type 2 receptor gene in congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Mol Cell 3: 1–10. [DOI] [PubMed] [Google Scholar]
- 19. Daneman A, Alton DJ (1991) Radiographic manifestations of renal anomalies. Radiol Clin North Am 29: 351–363. [PubMed] [Google Scholar]
- 20. Song R, Yosypiv IV (2011) Genetics of congenital anomalies of the kidney and urinary tract. Pediatr Nephrol 26: 353–364. [DOI] [PubMed] [Google Scholar]
- 21. Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, et al. (1996) Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383: 407–413. [DOI] [PubMed] [Google Scholar]
- 22. Kraus P, Fraidenraich D, Loomis C (2001) Some distal limb structures develop in mice lacking Sonic hedgehog signaling. Mech Dev 100: 45–58. [DOI] [PubMed] [Google Scholar]
- 23. Harfe B, Scherz P, Nissim S, Tian H, McMahon A, et al. (2004) Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118: 517–528. [DOI] [PubMed] [Google Scholar]
- 24. McGlinn E, Tabin C (2006) Mechanistic insight into how Shh patterns the vertebrate limb. Curr Opin Genet Dev 16: 426–432. [DOI] [PubMed] [Google Scholar]
- 25. Zhu J, Nakamura E, Nguyen M, Bao X, Akiyama H, et al. (2008) Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev Cell 14: 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miyagawa S, Moon A, Haraguchi R, Inoue C, Harada M, et al. (2009) Dosage-dependent hedgehog signals integrated with Wnt/beta-catenin signaling regulate external genitalia formation as an appendicular program. Development 136: 3969–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bitgood MJ, McMahon AP (1995) Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol 172: 126–138. [DOI] [PubMed] [Google Scholar]
- 28. Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, et al. (2001) Unique functions of Sonic hedgehog signaling during external genitalia development. Development 128: 4241–4250. [DOI] [PubMed] [Google Scholar]
- 29. Haraguchi R, Motoyama J, Sasaki H, Satoh Y, Miyagawa S, et al. (2007) Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development 134: 525–533. [DOI] [PubMed] [Google Scholar]
- 30. Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ (2002) Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol 247: 26–46. [DOI] [PubMed] [Google Scholar]
- 31. Jenkins D, Winyard PJ, Woolf AS (2007) Immunohistochemical analysis of Sonic hedgehog signalling in normal human urinary tract development. J Anat 211: 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suzuki K, Economides A, Yanagita M, Graf D, Yamada G (2009) New horizons at the caudal embryos: coordinated urogenital/reproductive organ formation by growth factor signaling. Curr Opin Genet Dev 19: 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miyazaki Y, Oshima K, Fogo A, Ichikawa I (2003) Evidence that bone morphogenetic protein 4 has multiple biological functions during kidney and urinary tract development. Kidney Int 63: 835–844. [DOI] [PubMed] [Google Scholar]
- 34. Cain JE, Bertram JF (2006) Ureteric branching morphogenesis in BMP4 heterozygous mutant mice. J Anat 209: 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cain JE, Nion T, Jeulin D, Bertram JF (2005) Exogenous BMP-4 amplifies asymmetric ureteric branching in the developing mouse kidney in vitro. Kidney Int 67: 420–431. [DOI] [PubMed] [Google Scholar]
- 36. Wang GJ, Brenner-Anantharam A, Vaughan ED, Herzlinger D (2009) Antagonism of BMP4 signaling disrupts smooth muscle investment of the ureter and ureteropelvic junction. J Urol 181: 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ingham PW, McMahon AP (2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15: 3059–3087. [DOI] [PubMed] [Google Scholar]
- 38. McMahon AP, Ingham PW, Tabin CJ (2003) Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol 53: 1–114. [DOI] [PubMed] [Google Scholar]
- 39. Zhang Z, Song Y, Zhao X, Zhang X, Fermin C, et al. (2002) Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development 129: 4135–4146. [DOI] [PubMed] [Google Scholar]
- 40. Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP (2004) Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev 18: 937–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dassule H, Lewis P, Bei M, Maas R, McMahon A (2000) Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127: 4775–4785. [DOI] [PubMed] [Google Scholar]
- 42. Ahn S, Joyner A (2004) Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell 118: 505–516. [DOI] [PubMed] [Google Scholar]
- 43. Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR (2002) Generation of Bmpr/Alk3 conditional knockout mice. Genesis 32: 69–72. [DOI] [PubMed] [Google Scholar]
- 44. Soriano P (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71. [DOI] [PubMed] [Google Scholar]
- 45. Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, et al. (2001) Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haraguchi R, Suzuki K, Murakami R, Sakai M, Kamikawa M, et al. (2000) Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development 127: 2471–2479. [DOI] [PubMed] [Google Scholar]
- 47. Matsumaru D, Haraguchi R, Miyagawa S, Motoyama J, Nakagata N, et al. (2011) Genetic Analysis of Hedgehog Signaling in Ventral Body Wall Development and the Onset of Omphalocele Formation. PLoS ONE 6: 1307–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Motoyama J, Liu J, Mo R, Ding Q, Post M, et al. (1998) Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet 20: 54–57. [DOI] [PubMed] [Google Scholar]
- 49. Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P (1990) Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development 109: 787–795. [DOI] [PubMed] [Google Scholar]
- 50. Tripathi P, Guo Q, Wang Y, Coussens M, Liapis H, et al. (2010) Midline signaling regulates kidney positioning but not nephrogenesis through Shh. Dev Biol 340: 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chevalier RL (1998) Pathophysiology of obstructive nephropathy in the newborn. Semin Nephrol 18: 585–593. [PubMed] [Google Scholar]
- 52. Hu P, Deng FM, Liang FX, Hu CM, Auerbach AB, et al. (2000) Ablation of uroplakin III gene results in small urothelial plaques, urothelial leakage, and vesicoureteral reflux. J Cell Biol 151: 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wu XR, Kong XP, Pellicer A, Kreibich G, Sun TT (2009) Uroplakins in urothelial biology, function, and disease. Kidney Int 75: 1153–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brophy PD, Ostrom L, Lang KM, Dressler GR (2001) Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development 128: 4747–4756. [DOI] [PubMed] [Google Scholar]
- 55. Nishinakamura R, Matsumoto Y, Nakao K, Nakamura K, Sato A, et al. (2001) Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development 128: 3105–3115. [DOI] [PubMed] [Google Scholar]
- 56. Airik R, Bussen M, Singh MK, Petry M, Kispert A (2006) Tbx18 regulates the development of the ureteral mesenchyme. J Clin Invest 116: 663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nie X, Sun J, Gordon RE, Cai CL, Xu PX (2010) SIX1 acts synergistically with TBX18 in mediating ureteral smooth muscle formation. Development 137: 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang Y, Tripathi P, Guo Q, Coussens M, Ma L, et al. (2009) Cre/lox recombination in the lower urinary tract. Genesis 47: 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Feil R, Wagner J, Metzger D, Chambon P (1997) Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun 237: 752–757. [DOI] [PubMed] [Google Scholar]
- 60. Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, et al. (1996) Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci U S A 93: 10887–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP (1998) Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol 8: 1323–1326. [DOI] [PubMed] [Google Scholar]
- 62. Ahn S, Joyner A (2005) In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 437: 894–897. [DOI] [PubMed] [Google Scholar]
- 63. Aoto K, Shikata Y, Imai H, Matsumaru D, Tokunaga T, et al. (2009) Mouse Shh is required for prechordal plate maintenance during brain and craniofacial morphogenesis. Dev Biol 327: 106–120. [DOI] [PubMed] [Google Scholar]
- 64. Mao J, Ligon KL, Rakhlin EY, Thayer SP, Bronson RT, et al. (2006) A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway. Cancer Res 66: 10171–10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wong EY, Wang XA, Mak SS, Sae-Pang JJ, Ling KW, et al. (2011) Hoxb3 negatively regulates Hoxb1 expression in mouse hindbrain patterning. Dev Biol 352: 382–392. [DOI] [PubMed] [Google Scholar]
- 66. Chan K, Qi J, Sham M (2010) Multiple coding and non-coding RNAs in the Hoxb3 locus and their spatial expression patterns during mouse embryogenesis. Biochem Biophys Res Commun 398: 153–159. [DOI] [PubMed] [Google Scholar]
- 67. Hensley MR, Hassenplug E, McPhail R, Leung YF (2012) ZeBase: an open-source relational database for zebrafish laboratories. Zebrafish 9: 44–49. [DOI] [PubMed] [Google Scholar]
- 68. Li MM, Andersson HC (2009) Clinical application of microarray-based molecular cytogenetics: an emerging new era of genomic medicine. J Pediatr 155: 311–317. [DOI] [PubMed] [Google Scholar]
- 69. Li MM, Nimmakayalu MA, Mercer D, Andersson HC, Emanuel BS (2008) Characterization of a cryptic 3.3 Mb deletion in a patient with a “balanced t(15;22) translocation” using high density oligo array CGH and gene expression arrays. Am J Med Genet A 146: 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mo R, Kim JH, Zhang J, Chiang C, Hui CC, et al. (2001) Anorectal malformations caused by defects in sonic hedgehog signaling. Am J Pathol 159: 765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Seifert AW, Bouldin CM, Choi KS, Harfe BD, Cohn MJ (2009) Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development 136: 3949–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lin C, Yin Y, Veith GM, Fisher AV, Long F, et al. (2009) Temporal and spatial dissection of Shh signaling in genital tubercle development. Development 136: 3959–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Miyagawa S, Matsumaru D, Murashima A, Omori A, Satoh Y, et al. (2011) The role of sonic hedgehog-Gli2 pathway in the masculinization of external genitalia. Endocrinology 152: 2894–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hayashi S, McMahon AP (2002) Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 244: 305–318. [DOI] [PubMed] [Google Scholar]
- 75. Joyner AL, Zervas M (2006) Genetic inducible fate mapping in mouse: establishing genetic lineages and defining genetic neuroanatomy in the nervous system. Dev Dyn 235: 2376–2385. [DOI] [PubMed] [Google Scholar]
- 76. Dudley AT, Robertson EJ (1997) Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn 208: 349–362. [DOI] [PubMed] [Google Scholar]
- 77. Godin RE, Takaesu NT, Robertson EJ, Dudley AT (1998) Regulation of BMP7 expression during kidney development. Development 125: 3473–3482. [DOI] [PubMed] [Google Scholar]
- 78. Yanagita M (2005) BMP antagonists: their roles in development and involvement in pathophysiology. Cytokine Growth Factor Rev 16: 309–317. [DOI] [PubMed] [Google Scholar]
- 79. Tanaka M, Endo S, Okuda T, Economides AN, Valenzuela DM, et al. (2008) Expression of BMP-7 and USAG-1 (a BMP antagonist) in kidney development and injury. Kidney Int 73: 181–191. [DOI] [PubMed] [Google Scholar]
- 80. Yao Y, Zhang J, Ye DF, Tan DQ, Peng JP, et al. (2011) Left-right determination factor is down-regulated in fibrotic renal tissue of human hydronephrosis. BJU Int 107: 1002–1008. [DOI] [PubMed] [Google Scholar]
- 81. Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, et al. (1997) Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol 188: 235–247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The expression of Shh and Gli1 genes at whole-mount and sections of the urinary tract at E10.5 (A, B, K, L), E12.5 (C–F, M-P) and E13.5 (G–J, Q–T). Red lines in A, C, G, K, M and Q indicate locations of the transverse sections in B, D–F, H–J, L, N–P and R–T. The Shh was expressed in the epithelia of the cloaca and the ureter (A–J). Red arrowheads indicate the expression of Shh in ureteral epithelia at E12.5 and E13.5. The Gli1 was expressed in mesenchymal cells surrounding the cloaca at E10.5 (L). Its expression was observed in the urinary tract mesenchyme at E12.5 and E13.5 (N–P, R–T). b: bladder, c: cloaca, g: gonad, k: kidney, nd: nephric duct, u: ureter.
(TIF)
The transverse sections of the urinary tract at E10.5, E11.5 and E12.5 (A–P). Expression of Pax2 in control (A, C, E, G) and Shh−/− (B, D, F, H) embryos. Expression of Tbx18 in control (I, K, M, O) and Shh−/− (J, L, N, P) embryos. Red arrows indicate a reduced expression of the Tbx18 gene around the nephric duct and the ureter. c: cloaca, nd: nephric duct, u: ureter, ub: ureteric bud.
(TIF)
The expression of Gli1 in the control (A, C, E) and ShhCreERT2/flox (B, D, F) embryos at E14.5 with E9.5-TM treatment. Transverse sections of the bladder (A, B), bladder trigone (C, D) and ureter (E, F). Red arrowheads indicate the reduced Gli1 expression.
(TIF)
The contribution assays of Hh-responsive cells utilizing the Gli1CreERT2/+; R26RLacZ/+ system. Gross morphology and sections of X-gal stained urinary organs at E18.5 subsequent to TM treatment at E11.5 (A, D, G, J), E12.5 (B, E, H, K) and E13.5 (C, F, I, L). Yellow lines in A–C indicate the levels of the transverse sections in D–L. Transverse sections of the renal pelvis (D–F; yellow boxes), ureter (G–I) and bladder trigone (J–L; yellow boxes) regions. Blue arrows in G–I indicate weak activity of LacZ. b: bladder, k: kidney, u: ureter.
(TIF)
Quantitative analysis on ratios of pSmad positive cells between wild-type and mutants. The cell number of pSmad positive and negative cells in the defined three areas of 4 different sections was counted and ratios of pSmad positive cells were compared. Data were analyzed using the Student’s t-test or Welch’s t-test followed by the F-test. The ratio of pSmad positive cells was significantly reduced in Shh−/− embryos at E11.5 (A: Wild-Type: 0.718±0.05, n = 12, Shh−/−: 0.499±0.06, n = 12; P<0.001). The ratio of pSmad positive cells was significantly increased in Gli1CreERT2/+; R26SmoM2/+ (Hh-GOF) mice at E12.5 (B: Wild-Type: 0.627±0.118, n = 12, Hh-GOF: 0.776±0.05, n = 12; P<0.05).
(TIF)
The Gli1 expression in the control and Gli1CreERT2/+; BmprIAflox/flox embryos at E12.5 with E9.5-TM treatment. Its expression was not significantly altered in the mutant bladder trigone (A, B) and ureter (C, D) mesenchyme.
(TIF)