Abstract
During vertebrate lens development, the anterior, ectoderm-derived lens vesicle cells differentiate into a monolayer of epithelial cells that retain proliferative potential. Subsequently, they exit the cell cycle and give rise to posterior lens fiber cells that form the lens body. In the present study, we demonstrate that the transcription factor GATA-3 is expressed in the posterior lens fiber cells during embryogenesis, and that GATA-3-deficiency impairs lens development. Interestingly, expression of E-cadherin, a premature lens vesicle marker, is abnormally prolonged in the posterior region of Gata3 homozygous mutant lenses. Furthermore, expression of γ-crystallin, a differentiation marker for fiber cells, is reduced. This suppressed differentiation is accompanied by an abnormal cellular proliferation, as well as with diminished levels of the cell-cycle inhibitors Cdkn1b/p27 and Cdkn1c/p57 and increased Ccnd2/cyclin D2 abundance. Thus, these observations suggest that GATA-3 is essential for lens cells differentiation and proper cell cycle control.
Keywords: GATA-3, crystallin, lens fiber, differentiation, cell cycle, apoptosis
Introduction
During vertebrate lens development, a group of head ectoderm cells thickens and forms the lens placode in response to inductive signals from the underlying optic vesicle at embryonic day (e) 9.5 of mouse embryogenesis (Muthukkaruppan, 1965; McAvoy, 1980; Lovicu and McAvoy, 2005; Medina-Martinez and Jamerich, 2007). By e11.5, the lens placode, through invagination, develops into a lens vesicle in which the primary lens fiber cells in the posterior region eventually exit the cell cycle to elongate toward the anterior wall. Three days later, this elongation is complete, and the fully differentiated fiber cells come into contact with the monolayer of cuboidal lens epithelial cells at the anterior of the eye. Throughout most of life, cell proliferation occurs preferentially in a subset of the epithelial cells located near the equatorial zone. After undergoing cell division, they withdraw from the cell cycle and move posteriorly to differentiate into secondary lens fiber cells (McAvoy, 1980; Piatigorsky, 1981; Lovicu and McAvoy, 2005; Medina-Martinez and Jamerich, 2007). As fiber cells differentiate, they rapidly increase in length and volume and accumulate high levels of crystallins, the proteins that account for the transparency and high refractive index of the lens (Piatigorsky, 1981; Lovicu and McAvoy, 2005; Andley, 2007). After completing elongation, fiber cells partially fuse with their neighbors and degrade all membrane bound organelles, including the nuclei.
The cessation of cell proliferation requires the expression of cyclin-dependent kinase (CDK) inhibitors (CKIs). Two families of CKIs have been identified. The Cip/Kip family contains Cdkn1a/p21, Cdkn1b/p27 and Cdkn1c/p57, which inhibit all kinases involved in the G1/S transition. The INK4a family, comprised of Cdkn2b/p15, Cdkn2a/p16, Cdkn2c/p18, and Cdkn2d/p19, specifically inhibit Cdk4 and Cdk6, blocking entry into the cell cycle (Harper and Elledge, 1996; Sherr and Roberts, 1995; Nakayama and Nakayama, 1998). Cdkn1b/p27 and Cdkn1c/p57 are co-expressed during murine lens development, especially in the equatorial zone of the fetal lens (Zhang et al., 1998; Nagahama et al., 2001). The withdrawal of lens fiber cells from the cell cycle largely depends on the expression of Cdkn1b/p27 and Cdkn1c/p57 because in mice that lack these genes, fiber cells continue to proliferate and cause incomplete lens fiber elongation. Eventually these fiber cells undergo apoptosis in Cdkn1b/p27−/− and Cdkn1c/p57+/−m (m, denoting maternal active Cdkn1c/p57 allele) compound mutant mice (Zhang et al., 1998). The other cell cycle regulators involved in lens differentiation are the D-type cyclins. All three D-type cyclins are expressed during lens differentiation, with Ccnd2/Cyclin D2 being the most highly expressed cyclin in the posterior region (Zhang et al., 1998). Down-regulation of Ccnd2/Cyclin D2 in the postmitotic lens fiber cell is required for the maintenance of the postmitotic state (Gomez et al., 1999).
Several genes have been identified that play important roles in the development of the lens. Gata3 encodes a transcription factor containing two steroid hormone receptor-like zinc fingers that serve as a DNA binding domain, a motif which is highly conserved amongst all six members (GATA-1 to -6) (Patient and McGhee, 2002). These zinc fingers bind most avidly to the consensus motif AGATCTTA(Ko and Engel, 1993). The physiological roles of GATA-3 has been revealed through the analysis of GATA-3 deficient ES cells or various germ line mutant mice: GATA-3 plays a critical role in the differentiation of T lymphocytes, hair follicles, mammary gland, renal and central nervous systems (Pandolfi et al., 1995; Ting et al., 1996; Kurek et al., 2007; Kaufman et al., 2003; Hasegawa et al., 2007; van Doorninck et al., 1999; Grote et al., 2006; Kouros-Mehr et al., 2006; Asselin-Labat et al., 2007). Moreover, GATA-3 is prominently expressed in the primary sympathetic chain and persists during the development of all sympathoadrenal (SA) lineages, including sympathetic neurons, adrenal chromaffin cells and para-aortic chromaffin cells [the Zuckerkandl organ (George et al., 1994; Lakshmanan et al., 1999; Lim et al., 2000; Moriguchi et al., 2006).
Gata3 null mutants die around e11 as a consequence of primary noradrenalin biosynthetic defect and secondary cardiac failure (Gata3−/−; Pandolfi et al., 1995; Lim et al., 2000). But they can be rescued by feeding Gata3 heterozygous intercrossed dams with synthetic catecholamine intermediates, or by restoring GATA-3 function specifically in SA lineages using the human dopamine β-hydroxylase (hDBH) promoter to direct GATA-3 transgenic expression (TghDBH-G3;Lim et al., 2000; Moriguchi et al., 2006). We and others have previously reported that GATA-3 is expressed in lens fiber cells of murine embryos (Oosterwegel et al., 1992; Lakshmanan et al., 1999), although the physiological significance of this observation is unknown. In the present study, we examined the consequences of a GATA-3 loss-of-function mutation in lens development of TghDBH-G3-rescued Gata3 null mutants. We demonstrate that Gata3 inactivation led to abnormal development of the posterior lens fiber cells, which exhibit reduced levels of the differentiation marker γ-crystallin, sustained expression of lens vesicle marker E-cadherin and the increased signal of proliferation markers, i.e., BrdU incorporation and Ki67 immunoreactivity. The abnormal proliferation of the lens fiber cells in TghDBH-G3-rescued Gata3 null mutant lenses correlates with reduced levels of Cdkn1b/p27 and Cdkn1c/p57 CKIs as well as increased Ccnd2/Cyclin D2 abundance. Subsequently, these cells succumbed to apoptotic cell death. The molecular pathway that regulates lens differentiation is intimately intertwined with normal cell cycle control, and GATA-3 plays an important role in cellular differentiation of lens fiber cells by inducing cell cycle exit as a part of its regulatory functions.
Results
TghDBH-G3-rescued Gata3 null mutants displayed defective lens fiber cell differentiation
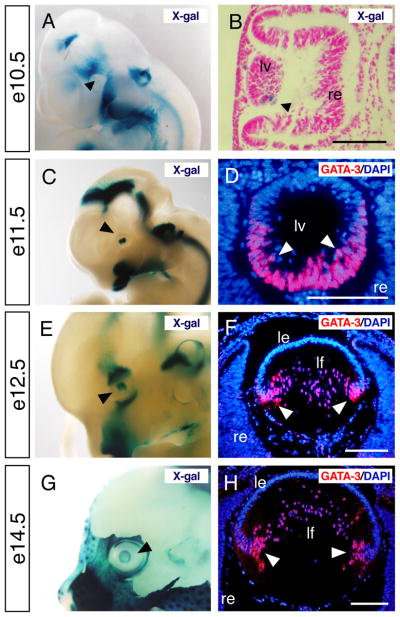
We previously reported that GATA-3 is expressed in lens fiber cells at e12.5, although its ontogeny in the mammalian lens has not been well described (Lakshmanan et al., 1999). In order to determine the precise temporal and spatial expression profiles of GATA-3 in the lens, we performed GATA-3 immunofluorescence analysis and whole-mount X-gal staining by examining Gata3lacZ knock-in heterozygous embryos (van Doorninck et al., 1999). In the developing embryonic lens, lacZ expression was first weakly observed at e10.5, then became stronger and was clearly detected in the optic vesicle at e11.5 (Fig. 1 A, B, C). Consistently, GATA-3 immunoreactivity was first specifically observed in the nucleus of e11.5 posterior primary fiber cells (Fig. 1D). By e12.5, when posterior lens fiber cells have normally begun to elongate toward the anterior wall, GATA-3 immunoreactivity was observed in the nucleus of elongating primary lens fiber cells, most prominently in the equatorial zone where fiber cell differentiation first initiates (Fig. 1E, F). GATA-3 immunoreactivity was consistently observed in the fiber cell nuclei along the equatorial zone of e14.5 embryos (Fig. 1G, H), although GATA-3 expression got decreased from e16.5 onward and was hardly observed after birth (data not shown). Importantly, GATA-3 immunoreactivity was observed in differentiated lens fiber cells, but not in proliferating lens epithelial cells of the anterior wall. Hence, GATA-3 expression is strictly confined to the differentiating lens fiber cells of the embryonic eye.
Fig. 1. Optic expression of GATA-3 during embryogenesis.
A–F: Gata3lacZ knock-in heterozygotes at e10.5, e11.5, e12.5 and e14.5 were stained with X-gal and photographed in whole-mounts (A, C, E and G) and section (B). Black arrowheads in each panel indicate lacZ-positive lens. GATA-3 immunoreactivity was specifically observed in the posterior part of the lens vesicle in e11.5 embryos and in lens fiber cells from e12.5 onward (white arrowheads in D, F and H). le, lens epithelium; lf, lens fiber; lv, lens vesicle; re, retina. Scale bars = 100 μm.
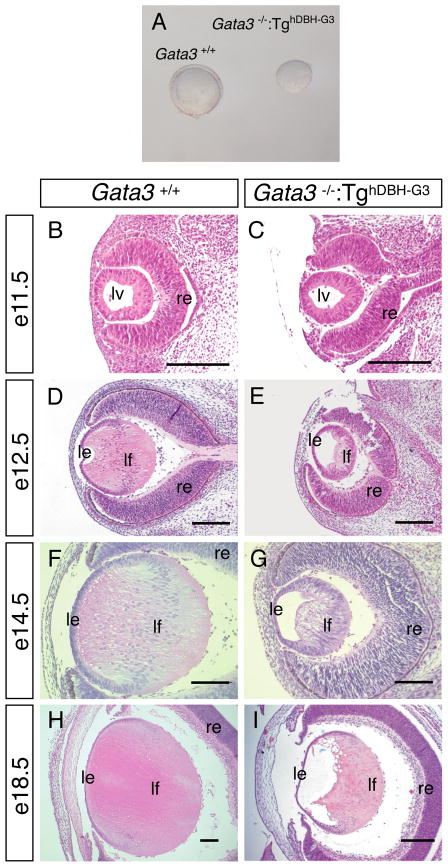
Next, we examined the biological consequences, if any, of Gata3 loss-of-function mutation on lens development, using TghDBH-G3–rescued Gata3 null mutant mice (Moriguchi et al., 2006). Intriguingly, lenses dissected from e16.5 TghDBH-G3-rescued Gata3 null mutant mice were smaller and appeared densely opaque when compared to the lenses of wild type littermates (Fig. 2A). Although the initial formation of the lens vesicle was not affected at e11.5 (Fig. 2B, C), histological analyses of e12.5–e18.5 TghDBH-G3-rescued Gata3 null mutants showed that lens development was disrupted later in development. In the mutants, the lens fiber cells appeared as shortened spindle-shaped cells that failed to extend to the anterior of the lens (Fig. 2E, G). By e18.5, a lumen remained visible in the embryonic eye (Fig. 2E, G, I). And moreover, the degradation of fiber cell nuclei, a marker for terminal differentiation of secondary fiber cells, does not take place in the mutants at E18.5 (Fig. 2 H, I). This contrasted starkly with the normal lens development in the control embryo, in which the lumen at e12.5 gradually disappeared by e14.5. By e18.5 the secondary lens fiber cells elongated to fill the cavity and properly degraded their nuclei (Fig. 2D, F, H). In keeping with the spatial expression pattern of GATA-3, the anterior epithelial cell layer was essentially unaffected in the TghDBH-G3-rescued Gata3 null mutant lens. Hence, these data indicate that GATA-3 is essential for the differentiation of the lens fiber cells from e12.5 onwards.
Figure 2. Defective lens development in rescued Gata3−/−:TghDBH-G3 embryos.
A: Lenses dissected from e16.5 Gata3−/−:TghDBH-G3 embryos are smaller than wild type. B–I: Sagittal sections of e11.5 (B, C), e12.5 (D, E), e14.5 (F, G) and e18.5 (H, I) Gata3+/+ and Gata3−/−:TghDBH-G3 embryos were stained with hematoxylin and eosin. In e11.5 Gata3 mutant embryos, the lens vesicle appeared to be normal when compared to wild type (B and C). From e12.5 onward, GATA-3-deficient lenses were smaller and contained a visible lumen as the lens fiber cells failed to elongate to fill the cavity during late embryogenesis. Note that the lens fiber cells of e18.5 Gata3−/−:TghDBH-G3 embryos remained highly nucleated (I) in contrast to the wild type lens fiber cells (H). lv, lens vesicle, le, lens epithelium; lf, lens fiber; re, retina. Scale bars = 100 μm.
Crystallin expression in TghDBH-G3-rescued Gata3 null mutant lens
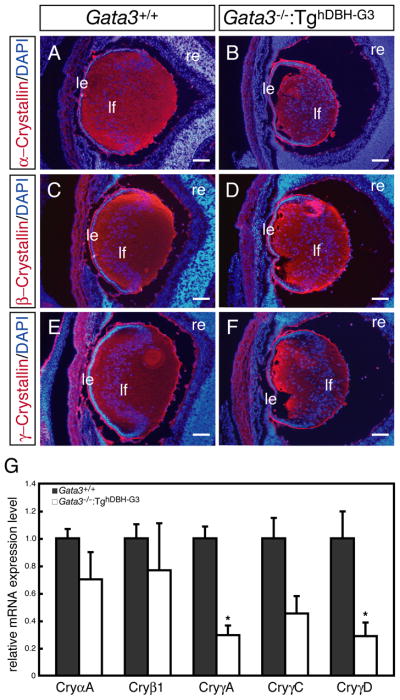
The opacity of the GATA-3-deficient lens led us to examine crystallin expression using pan anti-α-, pan anti-β- or pan anti-γ-crystallin monoclonal antibodies (Sawada et al., 1993). Unexpectedly, the anti-crystallin staining in the Gata3-deficient lens hardly differed from that of wild type lens (Fig. 3A–F). However, DAPI nuclear staining showed that there was increased nuclear density and disorganized alignment of the mutant lens fiber cells (Fig. 3A–F).
Figure 3. γ–crystallin gene expression is reduced in the e16.5 Gata3−/−:TghDBH-G3 lens.
A–F: Pan anti-α-,β- and γ-crystallin immunofluorescent staining of sagittal sections from e16.5 wild type and Gata3 mutant lenses did not reveal any qualitative differences. DAPI nuclear staining showed an increase in nuclear density as well as a disorganized alignment of the mutant lens fiber cells. le, lens epithelium; lf, lens fiber; re, retina. Scale bars: 100 μm. G: mRNA levels of each crystallin gene (normalized to Hprt mRNA) in both sides of the lens of individual e16.5 Gata3−/−:TghDBH-G3 (n=7) and wild type embryos (n=6) was assessed by qRT-PCR. Data are presented as mean±s.e.m. The statistical significance of differences between Gata3+/+ and Gata3−/−:TghDBH-G3 are indicated (*P<0.05; Student’s t-test).
We then examined the mRNA level of each crystallin subtype in e16.5 embryonic lenses using quantitative real-time RT-PCR assay (qRT-PCR). α-crystallins are normally expressed in both lens epithelial and fiber cells, whereas members of the β- and γ-crystallin families are expressed more abundantly in the fiber cells (McAvoy, 1978; Murer-Orlando et al., 1987; Goring et al., 1992; Andley, 2007). As anticipated, crystallin mRNA accumulation was moderately to severely reduced in the Gata3 mutant lenses (Fig. 3G). Interestingly, γ-crystallin (γ-A, C, and D) expression, which is most abundantly expressed in fiber cells (Murer-Orlando et al., 1987; Goring et al., 1992; Andley, 2007), was more significantly affected than the other types of crystallins (Fig. 3G). Hence, the presence of GATA-3 activity is essential for normal γ-crystallin expression.
Aberrant proliferation and apoptosis in TghDBH-G3-rescued Gata3 null mutant lens
In normal lens development, a precise transition from actively proliferating epithelial cells to terminally differentiating, non-proliferating fiber cells occurs in the equatorial zone of the lens. Our analyses indicated that GATA-3-deficient fiber cells lacked characteristics of fully differentiated lens fiber cells, hence we examined several markers that are indicative of cell proliferation.
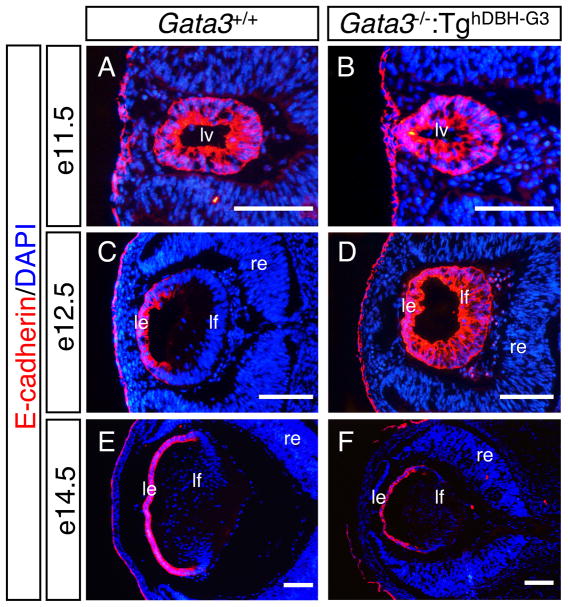
E-cadherin is a marker first expressed in lens vesicle cells in early eye development, which then becomes associated with proliferating epithelial cells from e12.5 onward (Wigle et al., 1999; Pontoriero et al., 2009). Although E-cadherin was expressed throughout the entire vesicle of e11.5 wild type and Gata3 mutant lens, the expression pattern was different after initiation of fiber cell elongation (Fig 4A, B). At e12.5, E-cadherin expression in wild type lens was restricted to the lens epithelium, whereas in Gata3 mutant lens, we observed that the E-cadherin immunoreactivity was still present throughout the lens with stronger staining in the anterior lens epithelium (Fig. 4C, D). The prolonged E-cadherin expression in the posterior lens of e12.5 Gata3 mutants was quenched two days later, as anti-E-cadherin staining was similar in e14.5 wild type and GATA-3-deficient lenses, although the size of the mutant lens never caught up to wild type control (Fig. 4E, F). These observations suggest that the GATA-3-deficient posterior lens vesicle cells failed to fully differentiate into fiber cells, so that the posterior half of the lens vesicle retained epithelial cell properties longer than their e12.5 wild type counterparts.
Figure 4. Prolonged E-cadherin expression in the GATA-3-deficient lens.
A, B: E-cadherin was expressed throughout the entire vesicle of e11.5 wild type and Gata3 mutant lens. C, E: E-cadherin expression was restricted to the anterior proliferative epithelium in e12.5 and e14.5 wild type lenses. D: In e12.5 Gata3 mutant lens, an anterioposteriorly graded expression of E-cadherin was observed. F: In e14.5 Gata3 mutant lens, E-cadherin expression pattern was normal. lv, lens vesicle; le, lens epithelium; lf, lens fiber; re, retina. Scale bars = 100 μm.
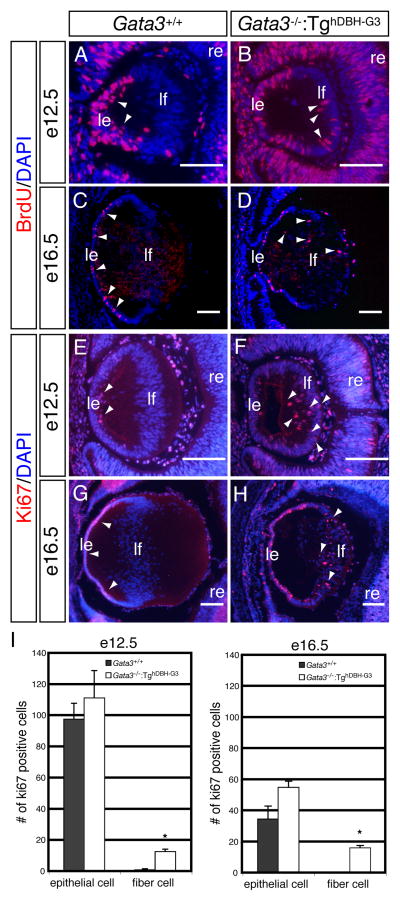
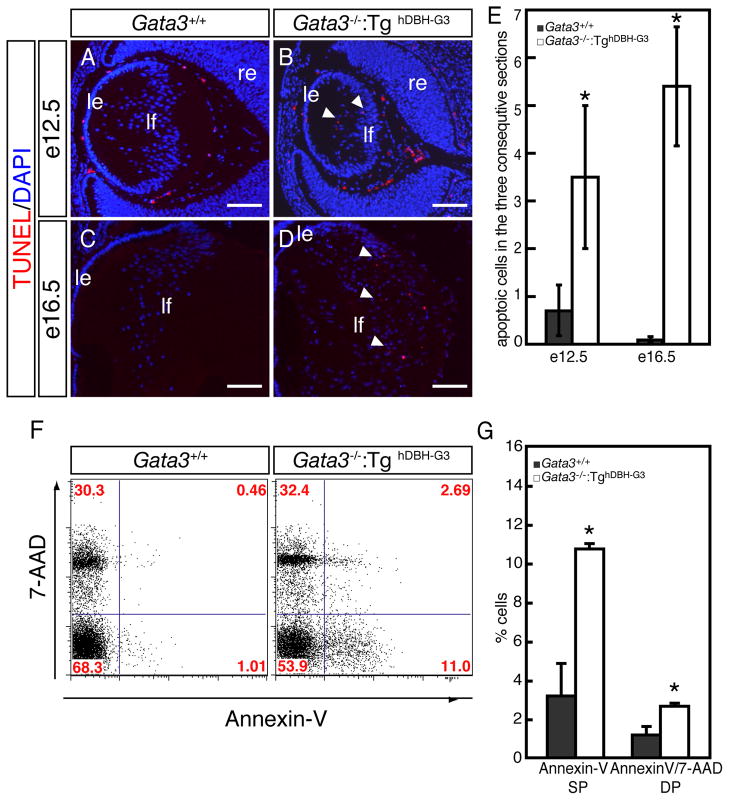
During later lens development, Ki67- or BrdU-positive proliferating cells were exclusively observed in the epithelial layer of e12.5 and e16.5 wild type embryos (arrowheads in Fig. 5A, C, E, G). However, e12.5 and e16.5 Gata3−/−:TghDBH-G3 lenses had a substantially greater number of Ki67- or BrdU-immunopositive nuclei in the posterior fiber cell zone (arrowheads in Fig. 5B, D, F, H, I). Concomitantly, we also detected increased number of programmed cell death in the posterior region of lenses from e12.5 and e16.5 Gata3−/−:TghDBH-G3 embryos, whereas TUNEL-positive nuclei were only rarely detected in the lenses of wild type control embryos (Fig. 6A–E). This conclusion was further substantiated by the flow cytometric analysis using Annexin-V as an apoptotic cell marker (van Engeland et al., 1998). Lenses from e18.5 Gata3−/−:TghDBH-G3 or wild type embryos were dispersed by trypsin treatment, and then single cell suspensions were stained with Annexin-V and 7-Amino Actinomycin D (7-AAD; nucleic acid dye) prior to being analyzed by flow cytometry. Early apoptotic cells resided in the Annexin-V-single positive fraction, viable cells were negative for both Annexin-V and 7-AAD, and late apoptotic and necrotic cells stained positively for both (Lecoeur et al., 1997; Rasola et al., 2001; van Engeland et al., 1998). As shown in Fig. 6F and G, the Annexin-V-single positive fraction (early apoptotic cells) was dramatically increased in the Gata3−/−:TghDBH-G3 lens (10.85±0.23%; n=5) in comparison to wild type lens (3.12±1.23%; n=5). Late apoptotic and necrotic cells (positive for both 7-AAD and Annexin-V) also significantly increased in the Gata3−/−:TghDBH-G3 lens (3.09±0.06%; n=5) compared to the wild type lens (1.06±0.23%; n=5; Fig. 6F, G). Hence, the GATA-3-deficient lens fiber cells display epithelial cell property as well as abnormally high proliferative and apoptotic indices.
Figure 5. Altered cellular proliferation in the lenses of Gata3−/−:TghDBH-G3 embryos.
A–H: In wild type embryos, Ki67- or BrdU-immunoreactive cells were observed exclusively in the anterior lens epithelium (arrowheads in A, C, E and G), whereas an increased number of mitotic cells were present in the lens fiber cells located in the lens posterior (arrowheads in B, D, F and H) in e12.5 and e16.5 Gata3−/−:TghDBH-G3 embryos. le, lens epithelium; lf, lens fiber; re, retina. Scale bars: 100 μm. I: Quantification of ki-67-positive cells in e12.5 and e16.5 embryonic lenses epithelial and fiber cells from wild type (n=6) and Gata3−/−:TghDBH-G3 (n=6) embryos (*P<0.05; Student’s t-test). Six lenses from six different embryos of each genotype were analyzed. Data are presented as means±s.e.m. The statistical significance of the differences between Gata3−/−:TghDBH-G3 and Gata3+/+ are indicated by (*P<0.05; Student’s t-test).
Figure 6. Increased cell death in lens fiber cells of Gata3−/−:TghDBH-G3 mutant embryos.
A–D: TUNEL assays detected an increase in the number of apoptotic cells in the posterior chamber of e12.5 and e16.5 Gata3−/−:TghDBH-G3 lenses (arrowheads). le, lens epithelium; lf, lens fiber; re, retina. Scale bars: 100 μm. E: Quantification of TUNEL-positive cells in e12.5 and e16.5 embryonic lenses of wild type (n=6) and Gata3−/−:TghDBH-G3 (n=6) embryos. Six lenses from six different embryos of each genotype were analyzed. Data are presented as means±s.e.m. The statistical significance of the differences between Gata3−/−:TghDBH-G3 and Gata3+/+ are indicated by (*P<0.05; Student’s t-test). F: Representative flow cytometric profiles of single-cell suspensions that were dissociated from lenses of e18.5 wild type or Gata3−/−:TghDBH-G3 embryos, stained with PE-conjugated Annexin-V antibody (horizontal axis) and 7-AAD (vertical axis). The percentage of cells in each quadrant is indicated. G: In the lens fiber cells from e18.5 Gata3−/−:TghDBH-G3 embryos, the Annexin-V-single positive (SP) population, representing early apoptotic cells, increased by more than three-fold (10.85±0.23% in Gata3 mutant [n=5], 3.12±1.23% in wild type control [n=5]). The 7-AAD- and Annexin-V-double positive (DP) cell population (representing late apoptotic and necrotic cells) also increased by more than 2-fold (1.06±0.23% in Gata3 mutant [n=5], 3.09±0.06% in wild type control [n=5]).
Elevated Ccnd2/Cyclin D2, diminished Cdkn1b/p27 and Cdkn1c/p57 levels in Gata3−/−:TghDBH-G3 lens fiber cells
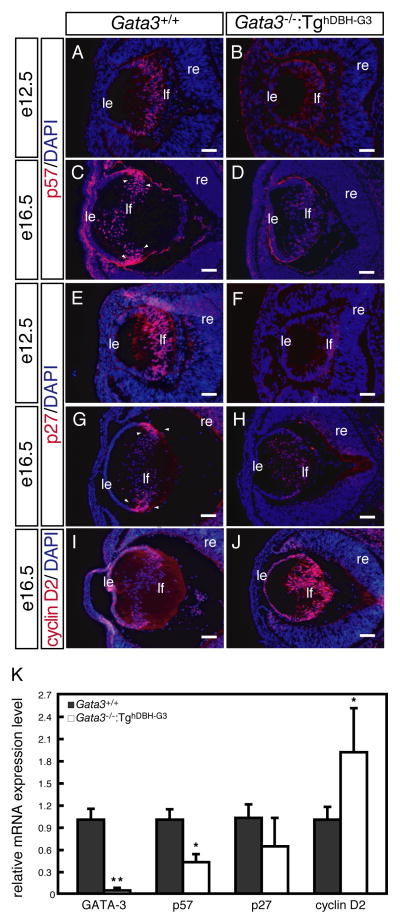
Given the abnormal accumulation of proliferative or apoptotic lens fiber cells, we next examined the expression of the cell cycle regulators Cdkn1b/p27, Cdkn1c/p57 and Ccnd2/cyclin D2 in wild type and Gata3−/−:TghDBH-G3 lenses. At e12.5, posterior lens vesicle cells begin to express Cdkn1b/p27 and Cdkn1c/p57 in the wild type lenses (Fig. 7A, E). In e16.5 wild type embryos, Cdkn1b/p27 and Cdkn1c/p57 were expressed predominantly in the equatorial zone where the epithelial cells exit cell cycle to differentiate into lens fiber cells (Fig. 7C, G). However, both Cdkn1b/p27 and Cdkn1c/p57 expression was conspicuously reduced in e12.5 and e16.5 Gata3−/−:TghDBH-G3 lenses (Fig. 7B, D, F, H). Meanwhile, anti- Ccnd2/Cyclin D2 labeled nuclei were observed in the equatorial zone of the e16.5 wild type lens (Fig. 7I), but the fiber cells in the equatorial zone of the e16.5 Gata3−/−:TghDBH-G3 lens displayed significantly more abundant Ccnd2/Cyclin D2 immunoreactivity (Fig. 7J). Indeed, mRNA quantification of isolated e16.5 embryonic lenses demonstrated that both Cdkn1b/p27 and Cdkn1c/p57 mRNA expression was suppressed and that Ccnd2/Cyclin D2 mRNA expression was activated, consistent with the immunohistochemcal observations (Fig. 7K).
Figure 7. Altered levels of cyclin dependent kinase inhibitors Cdkn1b/p27 and Cdkn1c/p57 as well as Ccnd2/Cyclin D2 in Gata3 mutant lenses.
A, C, E, G: Strong Cdkn1b/p27 and Cdkn1c/p57 immuno-reactive signals were detected in the equatorial zone of e12.5 and e16.5 wild type lenses (arrowheads). B, D, F, H: Gata3−/−:TghDBH-G3 lenses showed significantly diminished intensity of Cdkn1b/p27 and Cdkn1c/p57 labeling. I, J: In contrast, Gata3−/−:TghDBH-G3 lens displayed significant induction of Ccnd2/Cyclin D2 expression in comparison to the wild type control. le, lens epithelium; lf, lens fiber; re, retina. Scale bars = 100 μm. K: GATA-3, Cdkn1b/p27, Cdkn1c/p57 and Ccnd2/cyclin D2 mRNA levels (normalized to Hprt mRNA) in whole lenses of individual e16.5 Gata3−/−:TghDBH-G3 (n=7) and Gata3+/+ (n=6) embryos as quantified by qRT-PCR. Data are presented as mean±s.e.m. The statistical significance of the differences between Gata3−/−:TghDBH-G3 and Gata3+/+ are indicated (*P<0.05; **P<0.01; Student’s t-test).
Thus, these data indicate that in the absence of GATA-3, the lens fiber cells exhibited impaired terminal differentiation as evidenced by the abnormal lens morphology, misexpression of epithelial cell characteristics and reduced γ-crystallin levels. Instead, they remained Cdkn1b/p27 -negative, Cdkn1c/p57-negative and Ccnd2/Cyclin D2-positive and failed to properly exit the cell cycle, probably undergoing apoptotic cell death.
Elevated GATA3 expression in c-Maf knock out mice
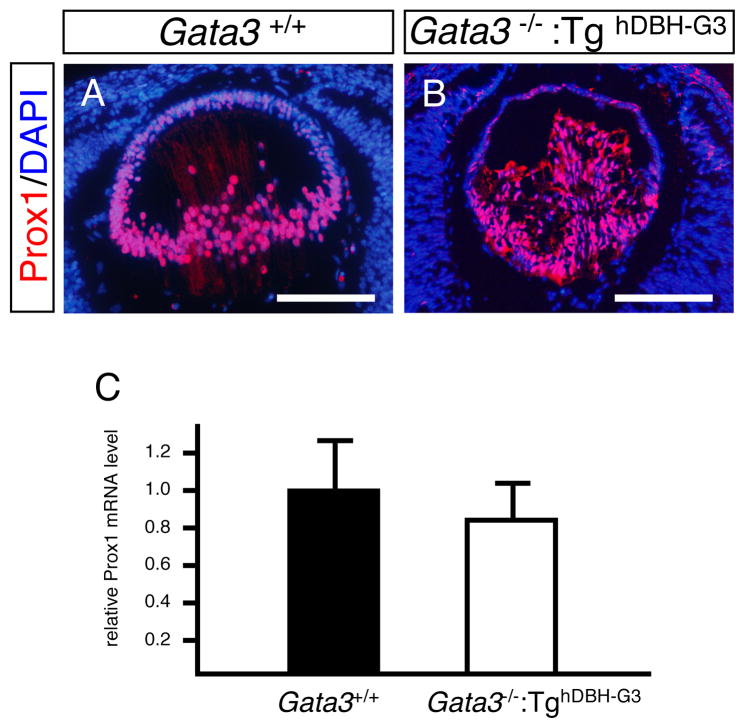
To address possible genetic programs in which GATA3 might participate during lens development, we examined the expression of several transcription factors that were previously implicated in the regulation of lens development. Prox1 is a homeobox protein that is essential for fiber cell differentiation. Prox1 deficiency in mice leads to aberrant fiber cell proliferation accompanied by suppression of Cdkn1b/p27, Cdkn1c/p57 and γ-crystallins, an alteration in expression that is similar to what is observed in the GATA3-deficient lens (Wigle et al., 1999). Indeed, immunohistochemical analysis of Prox1 demonstrated a quite similar expression pattern in the e14.5 lens to that of GATA-3, except for the epithelial expression (compare Fig. 1F and Fig. 8A). Quantitative RT-PCR performed on GATA-3-deficient e16.5 lenses showed that Prox1 mRNA level was only modestly suppressed in comparison to wild type controls (Fig. 8C), and Prox1 immunoreactivity was virtually identical in the e14.5 GATA3-deficient and wild type lens (Fig. 8A, B). Additionally, we examined the mRNA expression of Sox1, Pax6, and Foxe3, all of which are required for normal lens development and crystalline gene expression. However, the abundance of those transcription factor mRNAs was essentially unchanged in the Gata3 mutant deficient lens (Nishiguchi et al., 1998; Hogan et al., 1986; Hill et al., 1991; Matsuo et al., 1993; Medina-Martinez et al., 2005; Fig. 1S).
Figure 8. Expression of Prox1 in Gata3 mutant lenses.
A, B: Prox1 immunoreactivity was approximately equal in e16.5 Gata3−/−:TghDBH-G3 and Gata3+/+ littermate embryonic lenses. C: e16.5 Gata3−/−:TghDBH-G3 (n=7) embryonic lenses also had statistically equal levels of Prox1 mRNA in comparison with wild-type control embryos (n=6). Data are presented as mean±s.e.m (normalized to Hprt mRNA) (C).
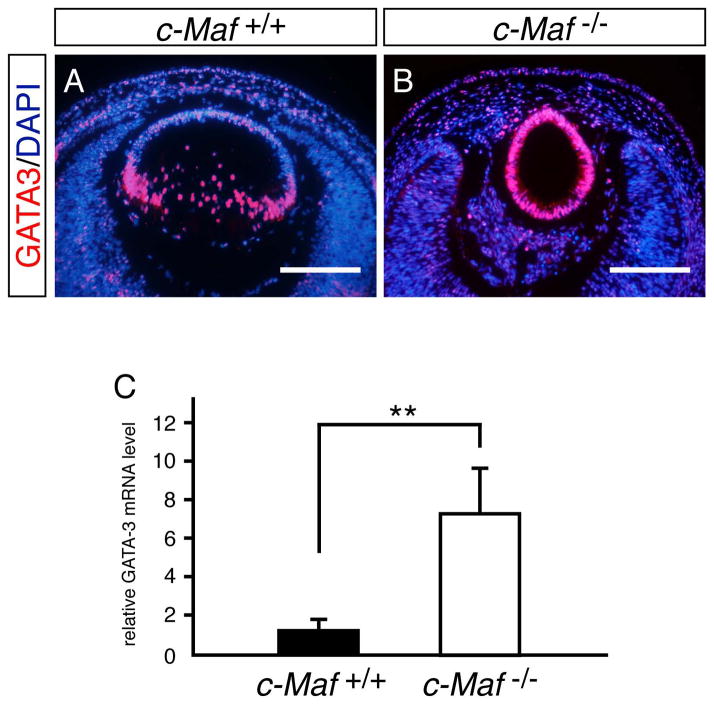
c-Maf is a basic leucine zipper transcription factor that is expressed specifically in lens fiber cells of the equatorial zone, and is essential for early lens morphogenesis as well as for crystalline gene activation (Kim et al., 1999; Kawauchi et al., 1999; Ring et al., 2000). c-Maf mRNA levels, as was the case with the previously examined factors, were unchanged in the GATA-3-deficient lens (Fig. 1S). However, given the coincident expression pattern of c-Maf and GATA-3 in lens fiber cells, we also examined GATA-3 expression in the c-Maf-deficient embryonic lens, assuming a possible regulatory interaction between those genes in the lens fiber heirarchy. Interestingly, we observed an almost 8-fold increase of GATA-3 mRNA in c-Maf-deficient e16.5 lens (Fig. 9C). In concert with the elevation in GATA-3 mRNA levels in the c-Maf mutants, increased GATA-3 immunoreactivity was recorded in all vesicle cells of the c-Maf-deficient dysplastic remnant lens at e14.5, demonstrating that GATA-3 expression is either directly or indirectly negatively regulated by c-Maf during normal lens fiber cell development (Fig. 9A, B).
Figure 9. GATA-3 expression is de-repressed in c-Maf mutant lenses.
A, B: e14.5 c-Maf mutant lenses had significantly greater GATA-3 immunoreactivity than wild type littermates. C: Realtime RT-PCR quantification of GATA-3 mRNA in e16.5 wild type and c-Maf mutant lenses (normalized to Hprt mRNA). Data are presented as mean±s.e.m. The statistical significance of the differences between c-Maf +/+ (n=5) and c-Maf−/− (n=6) are indicated (**P<0.01; Student’s t-test).
Discussion
In the present study, we demonstrated that GATA-3 expression begins in the developing lens vesicle at mid-embryogenesis (around e11.5) and continues to be expressed in fiber cells throughout embryonic lens development. Its expression is specifically restricted to fiber cells during lens morphogenesis. Consistent with its spatiotemporal expression in the developing lens, the absence of GATA-3 led to interrupted differentiation of posterior lens fiber cells from e12.5 onward, as evidenced by the diminished γ-crystallin levels and prolonged E-cadherin expression in primary lens fiber cells. There was also an increase of mitotic (BrdU- or Ki67-immunopositive) and apoptotic fiber cells in the GATA-3-depleted lens.
Cell cycle regulation by GATA factors has been reported in a variety of different tissues. Recently, it was reported that Gata2-deficient mouse embryonic neuroepithelial cells exhibited aberrant proliferation and that GATA-2 over-expression induced neural differentiation by inhibiting the proliferation of neuronal progenitors via activation of Cdkn1b/p27 expression (El Wakil et al., 2006). In erythroid cell differentiation, GATA-1 was reported to induce erythro-megakaryotic differentiation by suppressing the active cell cycle of hematopoietic progenitor cells via induction of Cdkn2a/p16 expression (Pan et al., 2005). Although it is still unclear if GATA-2 or GATA-1 directly regulates Cdkn1b/p27 or Cdkn2a/p16 expression, respectively, these reports as well as the present observations suggest that the potential cell cycle regulatory function for GATA factors in the normal differentiation process acts by activating expression of CKIs. GATA-3 has been reported to suppress abnormal proliferation of mesonephric cells as well as mammary epithelial cells, although the molecular basis for these phenomena remains elusive (Grote et al., 2006; Kouros-Mehr et al., 2006). More recently, transcriptome analysis of GATA-3 conditional deletion in hair follicles indicated that multiple cell cycle regulatory genes were altered in expression (Kurek et al., 2007). Further studies will be necessary to determine how GATA-3 functionally coordinates cell cycle regulation with normal differentiation in a variety of GATA-3-expressing tissues, including lens fiber cells.
Although the mechanistic details of how the loss of GATA-3 results in lens fiber differentiation failure remains to be elucidated, cell cycle regulators may be the potential key molecules underlying the abnormal increase of proliferating cells. In the wild type lens, the epithelial cells near the equatorial zone exit the cell cycle to give rise to fiber cells, and in the process, they initiate the expression of Cdkn1b/p27 and Cdkn1c/p57. Cdkn1b/p27 and Cdkn1c/p57 cooperatively control the cell cycle exit and the subsequent differentiation of lens fiber cells. Cdkn1b/p27 is normally dispensable for lens development due to its redundancy with Cdkn1c/p57, whereas Cdkn1b/p27−/− and Cdkn1c/p57+/−m, like the Gata3-deficient mice, exhibit significant deficiencies in cell cycle withdrawal and in the subsequent differentiation of lens fiber cells (Zhang et al., 1998; Nagahama et al., 2001). In the Gata3-deficient lens, we demonstrated that Cdkn1b/p27 and Cdkn1c/p57 immunoreactivities were dramatically suppressed in the equatorial zone, and that their mRNA abundance was reduced in lens, suggesting that GATA-3 deficiency results in the suppression of these two CKIs at the transcription level. However, we have been unable to identify conserved GATA consensus binding sites around the Cdkn1b/p27 and Cdkn1c/p57 promoters or to observe GATA-3-dependent trans-activation of a reporter gene cis-linked to a 1.6 kbp Cdkn1b/p27 or a 2.0 kbp Cdkn1c/p57 promoter in several cell lines in co-transfection experiments (data not shown), suggesting that Cdkn1b/p27 and Cdkn1c/p57 are either not direct target genes of GATA-3 or that GATA-3 regulates those genes through enhancers that lie outside of the promoter boundaries.
We examined Sox1, Foxe3, Prox1, c-Maf and Pax6 mRNA expression in e16.5 GATA-3-deficient lenses to examine potential genetic interactions between GATA-3 and each of those other known lens developmental regulators. Sox1 expression initiates in the lens vesicle at around e10 and continues to be expressed in lens fiber cells at e15.5 (Nishiguchi et al., 1999). Foxe3, Prox1 and c-Maf expression is first detected at around e9.0~e9.5 over the lens placode (Medina-Marinez et al., 2005; Wigle et al., 1999; Kawauchi et al., 1999). Foxe3 expression later becomes restricted to the anterior lens epithelium, while Prox1 and c-Maf expression are maintained in the lens fiber cells (Medina-Martinez et al., 2005; Wigle et al., 1999; Kawauchi et al., 1999). Pax6 expression is observed much earlier (in head neural ectoderm) including in the optic pit at e8.0, although from e13.5 onward Pax6 expression is down regulated in lens fiber cells (Grindley et al., 1995; Donner et al., 2007). Given those spacio-temporal expression patterns and the similarities in lens deficiencies encountered in various mutant mice, we initially expected to establish a genetic regulatory relationship between GATA-3 and Sox1 or Prox1 expression in the developing lens fiber cells. However, all of those transcriptional regulators are in general only modestly, if at all, changed in the GATA-3 deficient lens. Given the later appearance of GATA-3 expression in the e10.5 lens vesicle as well as the relatively mild lens deficiency in Gata3 mutant mice, we assumed that GATA-3 might be located at a lower position in the hierarchy, but upstream of γ-crystallin and both CKIs (Cdkn1b/p27 and Cdkn1c/p57) in the genetic program of lens development. Interestingly, GATA-3 expression is strongly activated in the remnants of the c-Maf deficient lens. This observation clearly demonstrates that GATA-3 expression is directly or indirectly negatively controlled by c-Maf in normal developing lens fiber cells, so that a c-Maf deficiency derepresses GATA-3 expression, possibly to compensate for the suppressed crystalin gene activation. Precise mapping of lens-specific Gata3 gene regulatory sequences, which are presumably located within a 2 kbp region lying 5′ to the gene (George et al., 1994; Lieuw et al., 1997), will provide additional insight into the identities of upstream regulators of GATA-3 expression in lens fiber cells.
During differentiation, mature lens fiber cells produce abundant β- and γ-crystallins (McAvoy, 1978). Of the crystalline subtypes, α-crystallins are normally expressed in both lens epithelial and fiber cells, and are first expressed at the lens vesicle stage (McAvoy, 1978; Murer-Orland et al., 1987; Goring et al., 1992; Horwitz et al., 2003). β-crystallin expression, which begins at e11 in the mouse embryo, serves as an early marker of fiber cell differentiation, whereas γ-crystallin gene activation initiates around e12.5 (Nishiguchi et al., 1998; Goring et al., 1992; Ring et al., 2000). We showed here that γA, γC and γD-crystallin expression, which are normally restricted in expression to terminally differentiated fiber cells, were more diminished than αA- and β1-crystallin in Gata3−/−:TghDBH-G3 lenses. In the Gata3 mutant lens, the fiber cell differentiation failure is associated with aberrant accumulation of mitotic posterior cells. There are two possible explanations for this observation. One is that GATA-3 primarily promotes fiber cell differentiation, i.e., activation of γ-crystallin genes as well as suppression of E-cadherin expression, so that a GATA-3 deficiency would primarily induce a fiber cell deficiency which in turn would lead to the accumulation of premature epithelial cell-like posterior cells. The other possibility is that the GATA-3 deficiency primarily but indirectly leads to transcriptional suppression of Cdkn1b/p27 and Cdkn1c/p57, which then cause the failure of cell cycle cessation during epithelial to fiber cell transition. Consequently, the posterior lens fiber cells do not fully differentiate and eventually apoptose. Although these two explanations could both be partially correct, cell differentiation and cell cycle cessation are probably tightly interwoven. It will therefore be of great interest to further define how GATA-3 functions during induction of cell differentiation as well as how it regulates cell cycle suppression during lens development.
In conclusion, we demonstrated here that GATA-3 is essential for terminal differentiation of lens fiber cells. It will be intriguing to clarify the underlying mechanisms by which the expression of CKIs and CDKs are controlled by GATA factors, and to identify other cell cycle/apoptosis-related factors which might be responsible for the increased cell death observed in the Gata3-deficient lens fiber cells. We conclude, from the data presented here, that the Gata3 mutant mouse lens may serve as another useful model for elucidating the general principles of cell cycle regulation by GATA transcription factors.
Experimental Procedures
Mice
Generation of c-Maf knock-out mice, Gata3lacZ knock-in mice, Gata3 knock-out mice (Gata3+/−), SA lineage-specific GATA-3-expressing transgenic mice (TghDBH-G3) and Gata3+/−: TghDBH-G3 compound heterozygotes were reported previously (Kawauchi et al., 1999; van Doorninck et al., 1999; Pandolfi et al., 1995; Moriguchi et al., 2006). Animals were genotyped by PCR and/or Southern blotting as previously reported (Moriguchi et al., 2006). Primers used to detect the hDBH-GATA-3 transgene are shown in Table 1. All experiments were performed according to the Guide for the Care and Use of Laboratory Animals at the University of Michigan and the University of Tsukuba.
Table 1.
Sequence of primers used in quantitative RT-PCR analyses and genotyping
| Gene | Sense primer | Antisense primer |
|---|---|---|
| hDBH-GATA-3 | AGT GAC CAG CTACAG TCG GA | GGA GAG GGG TCG TTT AAT GG |
| GATA-3 | GGT GGA CGTACT TTT TAA CAT CGA | CCC TGA CGG AGT TTC CGT AG |
| Crystallin αA | ACA ACG AGA GGC AGG ATG AC | AGG GGA CAA CCA AGG TGA G |
| Crystallin β1 | AAC TTC CAG GGC AAG AGG AT | AGA TGG GTC GGA AGG ACA T |
| Crystallin γA | CTC GTG GTA GCG CCT GTA GT | GTC GTG GTA GCG CCT GTA GT |
| Crystallin γC | TGC TGC CTC ATC CCC CAA CA | TCG CCT AAA AGA GCC AAC TT |
| Crystallin γD | CTG CTG GAT GCTCTA TGAGC | TTC CGT GAA CTC TATCAC TTG GC |
| Cdkn1c/p57 | GAG GAC CAG AAC CGC TGG GAC TT | ACT CGC TGT CCA CCT CCA TCC A |
| Cdkn1b/p27 | CGC CAT TAG CGC AAC TGA | CGG CTG CGA AGA TTA GGG |
| Ccnd1/Cyclin D1 | TCT ATC CGG CCC GAG | GAG CTT GTT CAC CAG AAG CAG |
| Ccnd2/Cyclin D2 | ACTGATGTGGATTGTCTCAAAGCCT | CCA CCA GGC ACA ATA GCA ACTACG |
| Ccnd3/Cyclin D3 | GGC TAT GAA CTA CCT GGA TCG CTA | GTA CCT AGA AGC TGC AAT TG |
| Prox1 | GCT CCAACATGC TGAAGA CC | TCATTG ATG GCT TGA CGC GC |
| Pax6 | GGA GAG AACACC AAC TCC AT | TCTGGATAA TGG GTC CTC TC |
| Foxe3 | AGT GGC AGAACAGCATCC GC | TCGAGC GTC CAG TAG TTG CC |
| Sox1 | AAG ATG CACAAC TCG GAG ATCAG | TGTAAT CCG GGT GTT CCT TCA T |
| c-Maf | CTG CCG CTT CAAGAG GGT GCA GC | TCG CGT GTCACACTC ACATG |
| HPRT | CAA ACT TTG CTT TCC CTG GT | CAA GGG CATATC CAA CAA CA |
Quantitative real-time PCR (qPCR)
Total RNA was extracted from isolated lens tissues of e16.5 wild type or mutant embryos using TRIZOL (Invitogen Corp, Carlsbad, CA). First-strand cDNA was synthesized starting with 0.5 μg of total RNA using Superscript III (Invitrogen). qRT-PCR was performed using an ABI PRISM 7700 sequence detector (PE-Applied Biosystems, Foster City, CA) with a 2X SYBR Green PCR master mix (Invitrogen), reverse transcribed cDNA and gene-specific primers as previously described (Moriguchi et al., 2006). The sequences of the primers are listed in Table 1. The data were recorded as means ± standard error of the mean. The statistical significance of differences among means of several groups was determined by Student’s t-test.
Histological analysis, immunofluorescece and TUNEL assays
Embryos (e12.5 – e18.5) were fixed overnight in 4% paraformaldehyde at 4°C and then processed for paraffin or frozen sections. Paraffin sections (3 μm) were cut with a microtome and processed for either Hematoxylin-Eosin (HE) staining or immunohistochemistry. The following primary antibodies were used: rabbit anti-GATA-3 (Lim K.-C.. unpublished), pan anti-α-, β-, and γ-Crystallin monoclonal antibodies (the gifts of K. Kataoka), rabit anti-ProX1 (CHEMICON, CA), rabbit anti-E-cadherin (Takara Biotech, Tokyo, Japan), rabbit anti-Cdkn1b/p27, goat anti- Cdkn1c/p57 and rabbit anti-Ccnd2/Cyclin D2 (all from Santa Cruz Biotechnology, Santa Cruz, CA). For immunofluorescence staining, Alexa Fluor 488-conjugated donkey anti-goat and goat anti-rabbit IgG (Molecular Probes, Eugene, OR) or FITC-conjugated rabbit anti-mouse IgG (Zymed, San Francisco, CA) secondary antibodies were used. Whole-mount X-gal staining was performed as previously described (Lakshmanan et al., 1999).
To analyze 5-bromo-2′-deoxyuridine (BrdU) uptake, pregnant females were administered BrdU (100 μg/gram of body weight) by intraperitoneal injection. After 2h, embryos were collected and fixed overnight in 4% PFA. Sections were then stained with mouse anti-BrdU (Becton Dickinson, San Jose, CA). Ki67 is a nuclear protein expressed in all proliferating cells during late G1, S, M and G2 phases of the cell cycle (Gerdes et al., 1984; 1991). Rabbit anti-Ki67 (Novocastra Laboratories Ltd, UK) was used for detection.
Terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) assays were performed using the In Situ Apoptosis Detection Kit (Takara BIOTECH) according to the manufacturer’s instructions. For quantification, three transverse sections extending from the center of the lens of each e12.5 or e16.5 embryo were examined by TUNEL or anti-Ki67 antibody (Novocastra Laboratories Ltd, UK). The mean of the numbers of TUNEL-positive fiber cells and Ki67-immunoreactive epithelial or fiber cells on the sections was individually determined for each embryo.
Flow cytometric analysis of apoptosis in lens
Single cell suspension was prepared from e18.5 lens of each mouse genotype by treatment with 0.05% trypsin and 0.53 mM EDTA (GIBCO BRL, Gaithersburg, MD) at 37°C for 30 min, and cells were dissociated using fine-tipped pipettes. After the cells were filtered through a 35μm nylon mesh screen, they were resuspended in PBS containing 4% FCS. Apoptotic cell analysis was performed using Annexin-V:PE Apoptosis Detection Kit I (BD-Biosciences, San Jose, CA) according to the manufacturer’s instructions. Apoptotic cells were stained with Annexin-V, while necrotic cells were distinguished by staining with both Annexin-V and 7-AAD (Herault et al., 1999). FACS analysis was performed with the FACS LSR and CellQuest software (BD-Biosciences).
Supplementary Material
Realtime RT-PCR quantification of Sox1, Foxe3, Pax6 and c-Maf mRNA in e16.5 wild type and GATA-3 mutant lenses (normalized to Hprt mRNA). Data are presented as mean±s.e.m.
Acknowledgments
We thank Drs. S. Kawauchi and J. Maher for critical reading of the manuscript, T. Takeuchi for helpful discussion and K. Kataoka for providing the anti-α-, β- and γ-crystallin monoclonal antibodies. We also thank Eriko Naganuma, Naomi Kaneko and Yuko Suzuki for technical assistance. This work was supported by grants from the NIH (GM28896; J.D.E.) and from the Ministry of Education, Culture, Sports, Science and Technology of Japan (ST and TM).
References
- 1.Andley UP. Crystallins in the eye: Function and pathology. Prog Retin Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, Lindeman GJ, Visvader JE. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 3.Donner AL, Ko F, Episkopou V, Maas RL. Pax6 is misexpressed in Sox1 null lens fiber cells. Gene Expr Patterns. 2007;7:606–613. doi: 10.1016/j.modgep.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Wakil A, Francius C, Wolff A, Pleau-Varet J, Nardelli J. The GATA2 transcription factor negatively regulates the proliferation of neuronal progenitors. Development. 2006;133:2155–2165. doi: 10.1242/dev.02377. [DOI] [PubMed] [Google Scholar]
- 5.George KM, Leonard MW, Roth ME, Lieuw KH, Kioussis D, Grosveld F, Engel JD. Embryonic expression and cloning of the murine GATA-3 gene. Development. 1994;120:2673–2686. doi: 10.1242/dev.120.9.2673. [DOI] [PubMed] [Google Scholar]
- 6.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 7.Gerdes J, Li L, Schlueter C, Duchrow M, Wohlenberg C, Gerlach C, Stahmer I, Kloth S, Brandt E, Flad HD. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991;38:867–873. [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez Lahoz E, Liegeois NJ, Zhang P, Engelman JA, Horner J, Silverman A, Burde R, Roussel MF, Sherr CJ, Elledge SJ, DePinho RA. Cyclin D- and E-dependent kinases and the p57(KIP2) inhibitor: cooperative interactions in vivo. Mol Cell Biol. 1999;19:353–363. doi: 10.1128/mcb.19.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goring DR, Breitman ML, Tsui LC. Temporal regulation of six crystallin transcripts during mouse lens development. Exp Eye Res. 1992;54:785–795. doi: 10.1016/0014-4835(92)90034-p. [DOI] [PubMed] [Google Scholar]
- 10.Grote D, Souabni A, Busslinger M, Bouchard M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development. 2006;133:53–61. doi: 10.1242/dev.02184. [DOI] [PubMed] [Google Scholar]
- 11.Grindley JC, Davidson DR, Hill RE. The role of Pax-6 in eye and nasal development. Development. 1995;121:1433–1442. doi: 10.1242/dev.121.5.1433. [DOI] [PubMed] [Google Scholar]
- 12.Harper JW, Elledge SJ. Cdk inhibitors in development and cancer. Curr Opin Genet Dev. 1996;6:56–64. doi: 10.1016/s0959-437x(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa SL, Moriguchi T, Rao A, Kuroha T, Engel JD, Lim KC. Dosage-dependent rescue of definitive nephrogenesis by a distant Gata3 enhancer. Dev Biol. 2007;301:568–577. doi: 10.1016/j.ydbio.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herault O, Colombat P, Domenech J, Degenne M, Bremond JL, Sensebe L, Bernard MC, Binet C. A rapid single-laser flow cytometric method for discrimination of early apoptotic cells in a heterogenous cell population. Br J Haematol. 1999;104:530–537. doi: 10.1046/j.1365-2141.1999.01203.x. [DOI] [PubMed] [Google Scholar]
- 15.Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- 16.Hogan BL, Horsburgh G, Cohen J, Hetherington CM, Fisher G, Lyon MF. Small eyes (Sey): a homozygous lethal mutation on chromosome 2 which affects the differentiation of both lens and nasal placodes in the mouse. J Embryol Exp Morphol. 1986;97:95–110. [PubMed] [Google Scholar]
- 17.Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman CK, Zhou P, Pasolli HA, Rendl M, Bolotin D, Lim KC, Dai X, Alegre ML, Fuchs E. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev. 2003;17:2108–2122. doi: 10.1101/gad.1115203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawauchi S, Takahashi S, Nakajima O, Ogino H, Morita M, Nishizawa M, Yasuda K, Yamamoto M. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J Biol Chem. 1999;274:19254–19260. doi: 10.1074/jbc.274.27.19254. [DOI] [PubMed] [Google Scholar]
- 20.Kim JI, Li T, Ho IC, Grusby MJ, Glimcher LH. Requirement for the c-Maf transcription factor in crystallin gene regulation and lens development. Proc Natl Acad Sci U S A. 1999;96:3781–3785. doi: 10.1073/pnas.96.7.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko LJ, Engel JD. DNA-binding specificities of the GATA transcription factor family. Mol Cell Biol. 1993;13:4011–4022. doi: 10.1128/mcb.13.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–1055. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurek D, Garinis GA, van Doorninck JH, van der Wees J, Grosveld FG. Transcriptome and phenotypic analysis reveals Gata3-dependent signalling pathways in murine hair follicles. Development. 2007;134:261–272. doi: 10.1242/dev.02721. [DOI] [PubMed] [Google Scholar]
- 24.Lakshmanan G, Lieuw KH, Lim KC, Gu Y, Grosveld F, Engel JD, Karis A. Localization of distant urogenital system-, central nervous system-, and endocardium-specific transcriptional regulatory elements in the GATA-3 locus. Mol Cell Biol. 1999;19:1558–1568. doi: 10.1128/mcb.19.2.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecoeur H, Ledru E, Prevost MC, Gougeon ML. Strategies for phenotyping apoptotic peripheral human lymphocytes comparing ISNT, annexin-V and 7-AAD cytofluorometric staining methods. J Immunol Methods. 1997;209:111–123. doi: 10.1016/s0022-1759(97)00138-5. [DOI] [PubMed] [Google Scholar]
- 26.Lieuw KH, Li G, Zhou Y, Grosveld F, Engel JD. Temporal and spatial control of murine GATA-3 transcription by promoter-proximal regulatory elements. Dev Biol. 1997;188:1–16. doi: 10.1006/dbio.1997.8575. [DOI] [PubMed] [Google Scholar]
- 27.Lim KC, Lakshmanan G, Crawford SE, Gu Y, Grosveld F, Engel JD. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat Genet. 2000;25:209–212. doi: 10.1038/76080. [DOI] [PubMed] [Google Scholar]
- 28.Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Matsuo T, Osumi-Yamashita N, Noji S, Ohuchi H, Koyama E, Myokai F, Matsuo N, Taniguchi S, Doi H, Iseki S, Ninomiya Y, Fujiwara T, Watanabe T, Eto K. A mutation in the Pax-6 gene in rat small eye is associated with impaired migration of midbrain crest cells. Nat Genet. 1993;3:299–304. doi: 10.1038/ng0493-299. [DOI] [PubMed] [Google Scholar]
- 30.McAvoy JW. Cell division, cell elongation and distribution of α-, β- and γ-crystallins in the rat lens. J Embryol Exp Morphol. 1978;44:149–165. [PubMed] [Google Scholar]
- 31.McAvoy JW. Induction of the eye lens. Differentiation. 1980;17:137–149. doi: 10.1111/j.1432-0436.1980.tb01091.x. [DOI] [PubMed] [Google Scholar]
- 32.Medina-Martinez O, Brownell I, Amaya-Manzanares F, Hu Q, Behringer RR, Jamrich M. Severe defects in proliferation and differentiation of lens cells in Foxe3 null mice. Mol Cell Biol. 2005;25:8854–63. doi: 10.1128/MCB.25.20.8854-8863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medina-Martinez O, Jamrich M. Foxe view of lens development and disease. Development. 2007;134:1455–1463. doi: 10.1242/dev.000117. [DOI] [PubMed] [Google Scholar]
- 34.Moriguchi T, Takako N, Hamada M, Maeda A, Fujioka Y, Kuroha T, Huber RE, Hasegawa SL, Rao A, Yamamoto M, Takahashi S, Lim KC, Engel JD. Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development. 2006;133:3871–3881. doi: 10.1242/dev.02553. [DOI] [PubMed] [Google Scholar]
- 35.Murer-Orlando M, Paterson RC, Lok S, Tsui LC, Breitman ML. Differential regulation of γ-crystallin genes during mouse lens development. Dev Biol. 1987;119:260–267. doi: 10.1016/0012-1606(87)90227-2. [DOI] [PubMed] [Google Scholar]
- 36.Muthukkaruppan V. Inductive tissue interaction in the development of the mouse lens in vitro. J Exp Zool. 1965;159:269–287. doi: 10.1002/jez.1401590210. [DOI] [PubMed] [Google Scholar]
- 37.Nagahama H, Hatakeyama S, Nakayama K, Nagata M, Tomita K, Nakayama K. Spatial and temporal expression patterns of the cyclin-dependent kinase (CDK) inhibitors p27Kip1 and p57Kip2 during mouse development. Anat Embryol. 2001;203:77–87. doi: 10.1007/s004290000146. [DOI] [PubMed] [Google Scholar]
- 38.Nakayama K, Nakayama K. Cip/Kip cyclin-dependent kinase inhibitors: brakes of the cell cycle engine during development. Bioessays. 1998;20:1020–1029. doi: 10.1002/(SICI)1521-1878(199812)20:12<1020::AID-BIES8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 39.Nishiguchi S, Wood H, Kondoh H, Lovell-Badge R, Episkopou V. Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 1998;12:776–81. doi: 10.1101/gad.12.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oosterwegel M, Timmerman J, Leiden J, Clevers H. Expression of GATA-3 during lymphocyte differentiation and mouse embryogenesis. Dev Immunol. 1992;3:1–11. doi: 10.1155/1992/27903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan X, Ohneda O, Ohneda K, Lindeboom F, Iwata F, Shimizu R, Nagano M, Suwabe N, Philipsen S, Lim KC, Engel JD, Yamamoto M. Graded levels of GATA-1 expression modulate survival, proliferation, and differentiation of erythroid progenitors. J Biol Chem. 2005;280:22385–22394. doi: 10.1074/jbc.M500081200. [DOI] [PubMed] [Google Scholar]
- 42.Pandolfi PP, Roth ME, Karis A, Leonard MW, Dzierzak E, Grosveld FG, Engel JD, Lindenbaum MH. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat Genet. 1995;11:40–44. doi: 10.1038/ng0995-40. [DOI] [PubMed] [Google Scholar]
- 43.Patient RK, McGhee JD. The GATA family (vertebrates and invertebrates) Curr Opin Genet Dev. 2002;12:416–422. doi: 10.1016/s0959-437x(02)00319-2. [DOI] [PubMed] [Google Scholar]
- 44.Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 45.Pontoriero GF, Smith AN, Miller LA, Radice GL, West-Mays JA, Lang RA. Co-operative roles for E-cadherin and N-cadherin during lens vesicle separation and lens epithelial cell survival. Dev Biol. 2009;326:403–417. doi: 10.1016/j.ydbio.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rasola A, Geuna M. A flow cytometry assay simultaneously detects independent apoptotic parameters. Cytometry. 2001;45:151–157. doi: 10.1002/1097-0320(20011001)45:2<151::aid-cyto1157>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 47.Ring BZ, Cordes SP, Overbeek PA, Barsh GS. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development. 2000;12:307–317. doi: 10.1242/dev.127.2.307. [DOI] [PubMed] [Google Scholar]
- 48.Sawada K, Agata K, Yoshiki A, Eguchi G. A set of anti-crystallin monoclonal antibodies for detecting lens specificities: beta-crystallin as a specific marker for detecting lentoidogenesis in cultures of chicken lens epithelial cells. Jpn J Ophthalmol. 1993;37:355–368. [PubMed] [Google Scholar]
- 49.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 50.Ting CN, Olson MC, Barton KP, Leiden JM. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature. 1996;384:474–478. doi: 10.1038/384474a0. [DOI] [PubMed] [Google Scholar]
- 51.van Doorninck JH, van Der Wees J, Karis A, Goedknegt E, Engel JD, Coesmans M, Rutteman M, Grosveld F, De Zeeuw CI. GATA-3 is involved in the development of serotonergic neurons in the caudal raphe nuclei. J Neurosci. 1999;19:RC12. doi: 10.1523/JNEUROSCI.19-12-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 53.Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- 54.Zhang P, Wong C, DePinho RA, Harper JW, Elledge SJ. Cooperation between the Cdk inhibitors p27(KIP1) and p57(KIP2) in the control of tissue growth and development. Genes Dev. 1998;12:3162–3167. doi: 10.1101/gad.12.20.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Realtime RT-PCR quantification of Sox1, Foxe3, Pax6 and c-Maf mRNA in e16.5 wild type and GATA-3 mutant lenses (normalized to Hprt mRNA). Data are presented as mean±s.e.m.