Abstract
Purpose
To study choroidal thickness and its topographic profile in normal eyes using 3D OCT-1000 spectral domain optical coherence tomography and the correlation with age and refractive error.
Methods
Fifty-seven eyes (45 individuals) with no visual complaints or ocular disease underwent horizontal and vertical line scanning using 3D OCT-1000. The definition of choroidal thickness was the vertical distance between the posterior edge of the hyper-reflective retinal pigment epithelium and the choroid/sclera junction. Choroidal thickness was measured in the subfoveal area at 500 µm intervals from the fovea to 2,500 µm in the nasal, temporal, superior, and inferior regions. The spherical equivalent refractive error was measured by autorefractometry. Statistical analysis was used to confirm the correlations of choroidal thickness with age and refraction error.
Results
The mean age of the 45 participants (57 eyes) was 45.28 years. Detailed visualization of the choroid for measuring its thickness was possible in 63.3% of eyes. The mean subfoveal choroidal thickness was found to be 270.8 µm (standard deviation [SD], ±51 µm), in horizontal scanning and 275.0 µm (SD, ±49 µm) in vertical scanning. The temporal choroidal thickness was greater than any 500 µm interval in corresponding locations, and there was no significant difference between the superior and inferior choroid as far as 2,000 µm from the fovea. Age and refractive error were associated with subfoveal choroidal thickness in terms of regression (p < 0.05).
Conclusions
Choroidal thickness in normal Korean eyes can be measured using 3D OCT-1000 with high resolution line scanning. The topographical profile of choroidal thickness varies depending on its location. Age and refractive error are essential factors for interpretation of choroidal thickness.
Keywords: Choroidal thickness, Spectral domain optical coherence tomography
The choroid is the connective tissue-containing vascular layer between the retina and the sclera that provides oxygen and nourishment to the outer retina. It is associated with the pathophysiology of many diseases affecting the retina, such as age-related macular degeneration, polypoidal choroidal vasculopathy, central serous chorioretinopathy, and high myopia-related chorioretinal atrophies [1]. Because choroidal change has a major role in the development and progression of these diseases, choroidal thickness, which reflects choroidal change, provides useful information to clinicians.
Adequate measurement of choroidal thickness using spectral domain optical coherence tomography (SD-OCT) is possible by introduction of an enhanced depth imaging technique and by averaging several B-scan signals from the same position [2,3]. Using a long-wavelength light source is another approach to visualize the posterior choroid and sclera [1,4]. Recent studies in a healthy population report a range of choroidal thickness from 270 to 350 µm. A negative correlation between choroidal thickness and age was previously reported [1-3,5]. Choroidal thinning is prominent in highly myopic eyes, and refractive error affects choroidal thickness [6].
Previous studies of normal choroidal thickness profiles utilized Heidelberg Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany), Cirrus HD-OCT (Carl Zeiss Meditec Inc., Dublin, CA, USA), or high-penetration OCT with long-wavelength (prototype). Moreover, normal choroidal thickness was usually reported in Western populations [2-5]. 3D OCT-1000 (Topcon, Tokyo, Japan) is another commercially available SD-OCT that supports 'choroid mode' optimized for choroidal and scleral imaging. However, the choroidal visualization ability of 3D OCT-1000 has not been reported. We therefore investigated the choroidal thickness profile based on location using 3D OCT-1000 in healthy Korean individuals and its correlations with age and refractive error.
Materials and Methods
Fifty-seven eyes of 45 healthy volunteers with no visual complaints or history of ocular disease and who visited the healthcare clinic of Hanyang University Hospital from March 2010 to August 2010 underwent high resolution 6 mm single line scanning using 3D OCT-1000, and spherical equivalent refractive error was measured by autorefractometer (KW-1500; Kowa, Tokyo, Japan). High myopic and hyperopic refractive errors greater than -6.0 or +6.0 diopters were excluded from this study. Also, any retinal or choroidal abnormalities in SD-OCT scans were excluded. This study adhered to the tenets of the Declaration of Helsinki, and informed consent was obtained from the participants before participation in the study.
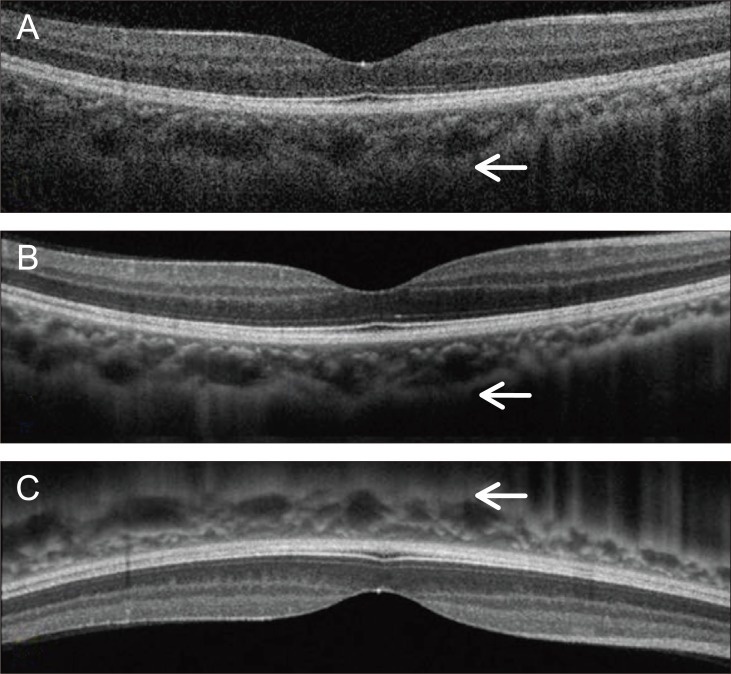
A 3D OCT-1000 Mark II (Topcon) with an 840 nm wave-length light source, 5-µm axial image resolution, and a speed of 27,000 A-scans per second was used for obtaining detailed choroidal images. The 6 mm single line scan was captured 50 times in the same position using eye tracking technology, then the software generated a high resolution averaged image of the 50 B-scans. These images were captured using 'choroid mode,' which provided a fine focus for the choroidal structure by adjusting the mirror position. For better visualization of choroidal details, the device was positioned close to the eye to produce an inverted image. Using this technique, the zero-delay line with the highest sensitivity was closer to the choroid, and more accurate image acquisition was possible in comparison with those of conventional methods (Fig. 1).
Fig. 1.
Choroidal B-scan image without averaging in the conventional method shows an obscure choroid/sclera junction (A). Averaging of 50 B-scan images on choroidal mode improves choroidal visualization, but a partially unclear choroid/sclera junction image is produced (B). The device was pushed closely to the eye to obtain an inverted image. Using this technique, the zero-delay line that had highest sensitivity was closer to the choroid, and more accurate image acquisition was possible (compare arrows) (C).
The definition of choroidal thickness was the vertical distance between the posterior edge of the hyper-reflective retinal pigment epithelium and the choroid/sclera junction. Two well-trained observers manually measured the choroidal thickness using a built-in caliper in the OCT software. Choroidal thickness was measured at the fovea and in 500 µm intervals from the fovea to a distance of 2,500 µm in the temporal, nasal, superior, and inferior regions. The average value of choroidal thickness obtained by the two observers was used for analysis.
The paired t-test was used to compare choroidal thickness at each location. The correlations with age and refractive error were calculated by univariate and multiple regression analyses. A p-value 0.05 or less was considered statistically significant. All statistics were calculated using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Twenty men and 25 women (57 eyes) having a mean age of 45.28 ± 14.8 years (range, 23 to 80 years) participated in this study. The mean refractive error was -1.34 ± 2.1 D (range, -6.0 to +3.0 D). Reliable measurements of choroidal thickness were obtainable in 63.3% of the examined eyes (57 of 90 eyes). In 33 eyes, an obscured margin between the choroid and sclera made accurate delineation impossible. There was strong inter-observer correlation in the manual measurement of choroidal thickness (r = 0.92, p < 0.001).
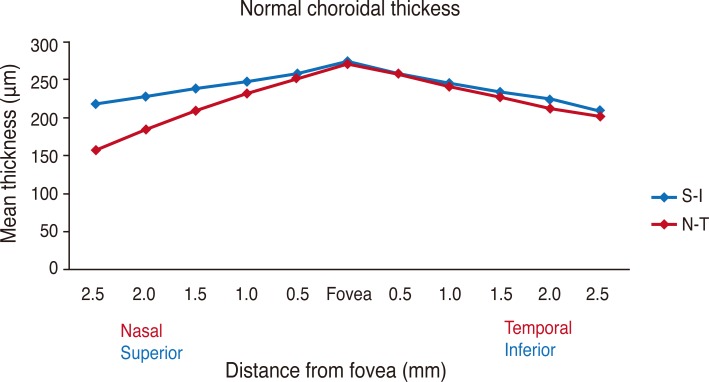
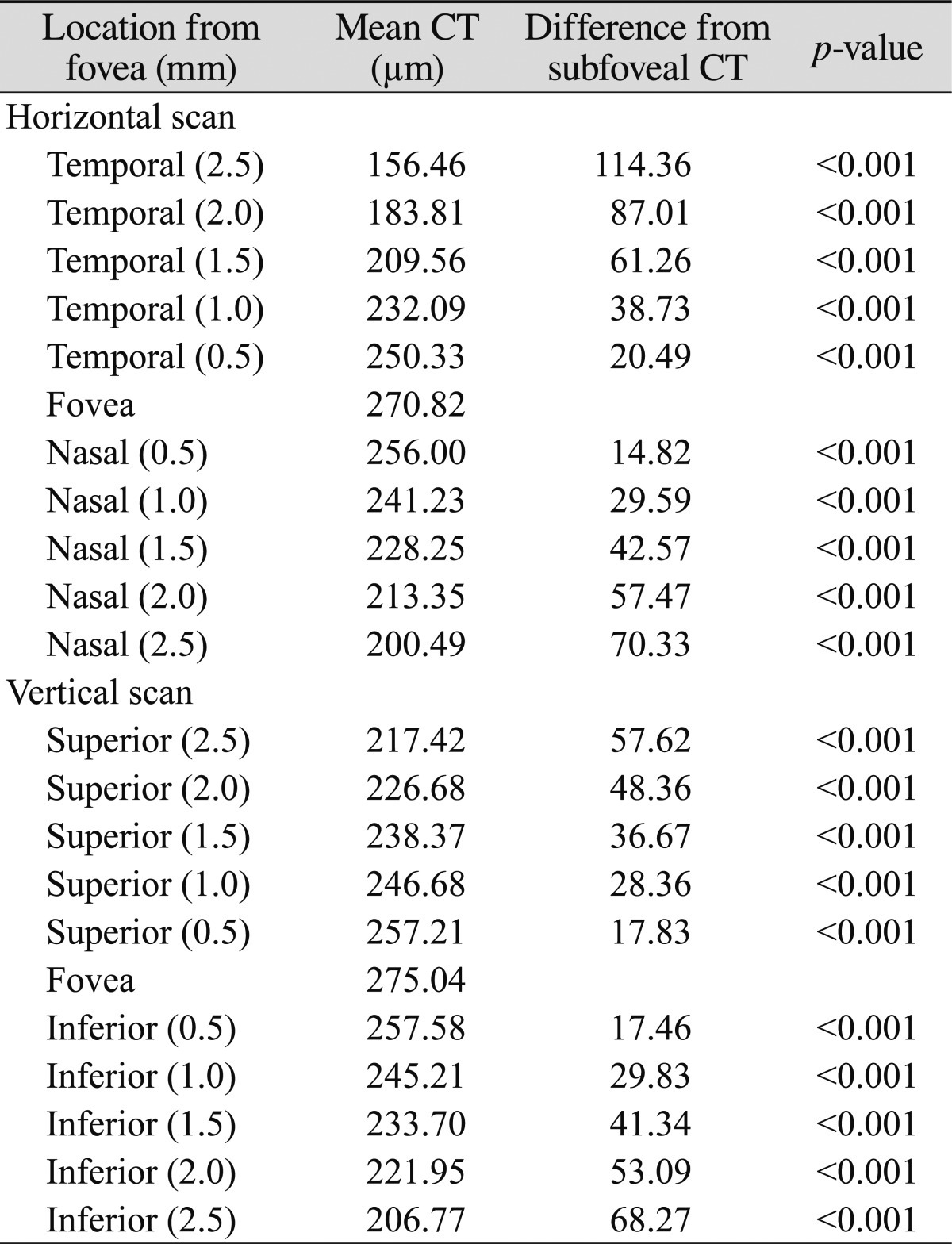
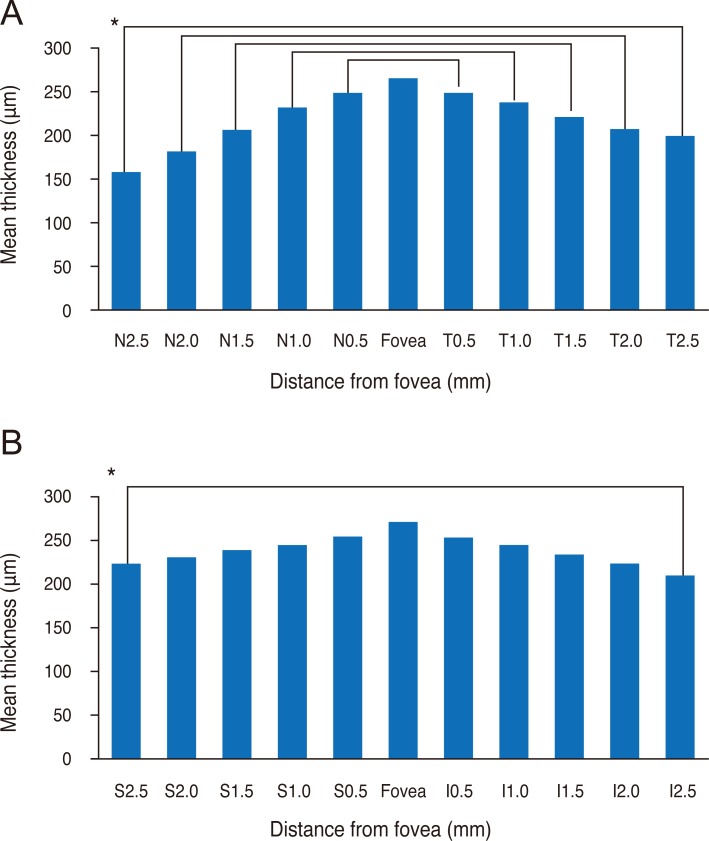
Mean subfoveal choroidal thickness was 270.82 ± 51.4 µm (range, 161 to 383 µm) horizontally and 275.04 ± 48.7 µm (range, 161 to 380 µm) vertically. There was no statistically significant difference between horizontal and vertical subfoveal choroidal thickness (paired t-test, p = 0.131) (Fig. 2). The mean choroidal thickness at each location is shown in Table 1, and subfoveal choroidal thickness was significantly greater than any other locations (p < 0.05, respectively). The temporal choroid was significantly thicker than the nasal choroid at all 500 µm intervals and corresponding locations (p < 0.05, respectively), and there was no difference in thickness between the superior and inferior choroid, except at the location 2,500 µm from the fovea (Fig. 3).
Fig. 2.
Mean choroidal thickness at each of 22 locations in healthy Korean individuals. Subfoveal choroidal thickness is significantly greater than any other locations (paired t-test, p < 0.05, respectively). There is no statistically significant difference between horizontal and vertical subfoveal choroidal thickness (paired t-test, p = 0.131). S = superior; I = inferior; N = nasal; T = temporal.
Table 1.
Mean choroidal thickness profile in 500 µm intervals
CT = choroidal thickness.
Fig. 3.
(A) Temporal choroid is thicker at any corresponding location than the nasal choroid in horizontal scan (paired t-test, p < 0.05, respectively). (B) Superior choroid at 2,500 µm from the fovea is thicker than the inferior choroid (paired t-test, p < 0.05), and there is no difference in the vertical scan. *p < 0.05. S = superior; I = inferior; N = nasal; T = temporal.
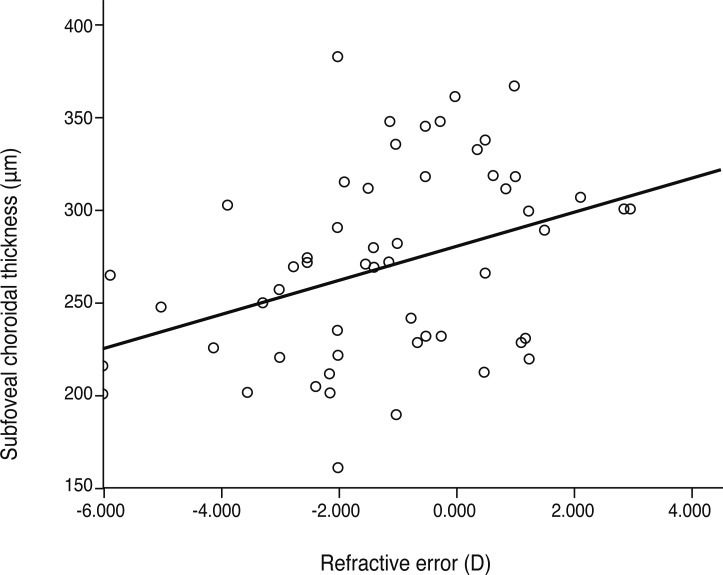
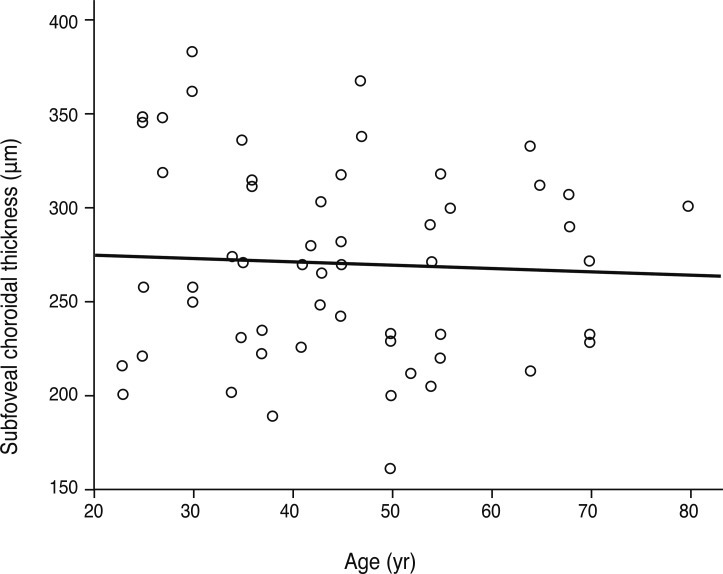
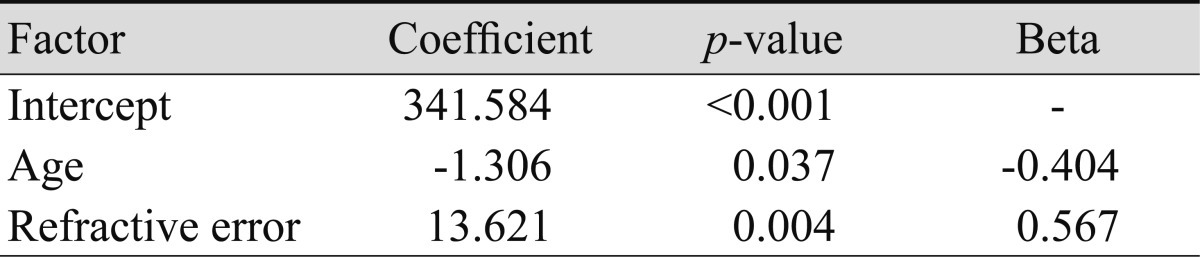
In this study, refractive error was significantly correlated with subfoveal choroidal thickness upon simple linear regression (p = 0.046, R2 = 0.101) (Fig. 4). Age was negatively correlated with choroidal thickness in previous studies, but our study showed that age was not a factor independently related to choroidal thickness (p = 0.688) (Fig. 5). By considering the influence of refractive error, age was related to choroidal thickness upon multiple regression analysis (p = 0.015, R2 = 0.202). Regression analysis showed that choroidal thickness decreased 1.31 µm for each year of age and increased 13.62 µm for each diopter of refractive error (Table 2).
Fig. 4.
Relationship between refractive error and subfoveal choroidal thickness. The plot shows a significant positive correlation in simple linear regression analysis (p = 0.046, y = 7.62x + 274.13, R2 = 0.101). D = diopters.
Fig. 5.
Relationship between age and subfoveal choroidal thickness. Age is not related to choroidal thickness in simple linear regression (p = 0.688, y = -0.188x + 279.35, R2 = 0.003). Age is negatively correlated with choroidal thickness in multiple linear regression (p = 0.015, y = 341.584 - 1.306x1 + 13.621x2, R2 = 0.202).
Table 2.
Multiple regression analysis for subfoveal choroidal thickness with regard to age and refractive error
Discussion
Previous choroidal thickness measurement research was based on Cirrus and Heidelberg OCT devices or prototype long wavelength HP-OCT. We studied choroid features using another commercially available spectral-domain OCT device, a Topcon 3D OCT-1000. The choroid and sclera are normally difficult to detect with commercially available OCT instruments using a shorter wavelength of approximately 840 nm. For better visualization of these structures, we applied an image averaging technique using choroid mode and an inverted image that is closer to the zero delay line. Detailed choroidal and scleral visualization was possible in 63.3% of examined eyes. Manjunath et al. [4] reported that reliable choroidal thickness measurement was possible in 74% of patients. The results of choroidal thickness profile indicated that the choroid was thicker at the fovea than at nasal, temporal, superior, and inferior locations. The mean subfoveal thickness was 270.82 µm on the horizontal scan and 275.04 µm on the vertical scan, which are similar findings to the 272 µm reported by Manjunath et al. [4], 287 µm by Margolis and Spaide [3], and 354 µm by Ikuno et al. [1]. In our study, superior choroidal thickness had no difference with that of the inferior choroid; however, a previous study showed the inferior choroid to be thinner than the superior choroid [1]. Differences among these studies might be due to individual variation in device characteristics such as wavelength, eye tracking method, and averaging software or to differences of participant profiles such as ethnicity, age, and refractive error.
The choroid has a rich vascular layer, the thickness of which is affected by blood flow, perfusion pressure, and intraocular pressure [7]. With aging, physiological functions of the choroid decrease, and histological evaluation shows decrement in the vascular density, overall luminal area, and diameter of the choriocapillary vessels [8-10]. Choroidal thickness decreases 1.1 µm per year according to a study using cadaver eyes [8], and we found a similar 1.31 µm reduction in choroidal thickness for each year of age using multiple regression analysis. A recent in vivo study using SD-OCT showed similar results of choroidal thickness reduction (1.4 to 1.56 µm per year) [1,3]. Because the choroid is rich in vascular tissue, in vivo imaging can more accurately reflect the actual status of the choroid with aging.
In highly myopic eyes, the choroid is three times thinner than that in normal eyes, and refractive error has a great impact on choroidal thinning in terms of regression [1,6]. Ikuno et al. [1] found a 9.3 µm increment of choroidal thickness per diopter of refractive change. We also found a correlation between choroidal thickness and refractive error in multiple regression analysis (13.6 µm/D). Although both age and refractive error affected choroidal thickness, age was not correlated to choroidal thickness in univariate regression analysis (p = 0.688). Refractive error had a greater explanatory power on choroidal thickness change than did age (beta, 0.567 vs. -0.404).
Functional and structural changes of the choroid are associated with many retinal diseases and result in increasing or decreasing choroidal thickness. Recently, in vivo choroidal thickness measurement was applied to central serous chorioretinopathy [11,12], age-related macular degeneration with pigment epithelial detachment [13], age-related choroidal atrophy [14], highly myopic eyes [6,15], and idiopathic macular hole [16] as a noninvasive method to monitor response to therapy or progression of disease in Western populations. However, there are no comparable normative data available in the Korean population. It is worthwhile to understand the normal choroidal thickness profile for accurate and reliable monitoring of choroid change.
The limitation of this study is the use of a relatively short 840 nm wavelength that cannot visualize the choroid in the presence of thick pigmented retinal pigment epithelium or lens opacity. Despite averaging using choroid mode, choroidal thickness measurement is not always possible. Another limitation is that the distributions of age and refractive error did not exactly reflect those of a normal population because the participants were a small group of volunteers. It is also difficult to directly compare the present results with those of other studies due to difference of participant profiles. Therefore, further research will be needed to investigate various ocular factors or retinal diseases and their effects on choroidal thickness in a well distributed large group study.
In our study, choroidal thickness measurement in healthy Koreans using Topcon 3D OCT-1000 was possible in most cases. The topographical profile of choroidal thickness varies depending on location. Aging and myopic shift are critical factors for interpretation of choroidal thickness, and refractive error has a major impact. Our results have some similarities and differences compared to previous studies and show the choroidal thickness measuring ability of 3D OCT-1000.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010;51:2173–2176. doi: 10.1167/iovs.09-4383. [DOI] [PubMed] [Google Scholar]
- 2.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 3.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–815. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150:325–329.e1. doi: 10.1016/j.ajo.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unterhuber A, Povazay B, Hermann B, et al. In vivo retinal optical coherence tomography at 1040 nm - enhanced penetration into the choroid. Opt Express. 2005;13:3252–3258. doi: 10.1364/opex.13.003252. [DOI] [PubMed] [Google Scholar]
- 6.Ikuno Y, Tano Y. Retinal and choroidal biometry in highly myopic eyes with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:3876–3880. doi: 10.1167/iovs.08-3325. [DOI] [PubMed] [Google Scholar]
- 7.Kiel JW, van Heuven WA. Ocular perfusion pressure and choroidal blood flow in the rabbit. Invest Ophthalmol Vis Sci. 1995;36:579–585. [PubMed] [Google Scholar]
- 8.Ramrattan RS, van der Schaft TL, Mooy CM, et al. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–2864. [PubMed] [Google Scholar]
- 9.Feeney-Burns L, Burns RP, Gao CL. Age-related macular changes in humans over 90 years old. Am J Ophthalmol. 1990;109:265–278. doi: 10.1016/s0002-9394(14)74549-0. [DOI] [PubMed] [Google Scholar]
- 10.Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976;60:324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010;117:1792–1799. doi: 10.1016/j.ophtha.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469–1473. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 13.Spaide RF. Enhanced depth imaging optical coherence tomography of retinal pigment epithelial detachment in age-related macular degeneration. Am J Ophthalmol. 2009;147:644–652. doi: 10.1016/j.ajo.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009;147:801–810. doi: 10.1016/j.ajo.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara T, Imamura Y, Margolis R, et al. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148:445–450. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Reibaldi M, Boscia F, Avitabile T, et al. Enhanced depth imaging optical coherence tomography of the choroid in idiopathic macular hole: a cross-sectional prospective study. Am J Ophthalmol. 2011;151:112–117.e2. doi: 10.1016/j.ajo.2010.07.004. [DOI] [PubMed] [Google Scholar]