Abstract
One of the grand challenges in neuroengineering is to stimulate regeneration after central nervous system (CNS) or peripheral nervous system (PNS) injury to restore function. The state of the art today is that PNS injuries heal to a limited extent, whereas CNS injuries are largely intractable to regeneration. In this context, we examine the underlying biochemical and cellular constraints on endogenous healing of neural tissues. Identification and characterization of endogenous “rate-limiting” processes that constrain regeneration would allow one to craft solutions to overcome critical impediments for accelerated healing. It is increasingly evident that biochemical pathways triggered by the nature and duration of injury-triggered inflammatory response may determine the endogenous constraints and subsequently determine regenerative fate. In this paper, critical endogenous constraints of PNS and CNS regeneration are identified, and the effects of modulating the phenotypes of immune cells on neuronal regeneration are discussed.
Keywords: Central nervous system (CNS), immunomodulation, nerve regeneration, peripheral nervous system (PNS)
I. Introduction
Physiological healing, which incorporates the removal of necrotic tissue and its clearance, results in two possible outcomes: 1) “regeneration,” which is complete replacement of injured tissue with new fully functional tissue, and 2) “scarring,” which is the partial repair of injured tissue with limited or no functionality. The intriguing question that arises is—what determines these outcomes? Generally, after injury of a tissue, several biological pathways become activated and typically local cells undergo changes in phenotype, which consequently determine the physiological healing state of the tissue [1]. Regeneration occurs when this cellular and tissue response acts in concert to facilitate restoration of function. Conversely, scarring occurs when endogenous constraints, which mainly evolve to preserve the more “critical” functions, prevent this orchestration of regenerative healing [1]–[3].
Regeneration may be defined as the capacity of a tissue to regrow after injury and to restore its original function. Consequently, the regenerative capacity is the probability of regeneration occurring in an organ/tissue for a given injury [4]. Generally, the regenerative capacity of any organ or tissue is limited by endogenous constraints for repair that arise when the injury exceeds the threshold of regenerative capacity of the affected tissue. Although complex tissues retain the capacity for endogenous regeneration to some extent, regeneration is typically constrained by rate-limiting biochemical or cellular processes. Rate-limiting biochemical or cellular processes may be thought of as “knobs,” which when turned, help increase or decrease the regenerative capacity of specific tissues. Hence, the critical question in the context of regeneration is: Is it possible to modulate healing pathway of complex tissues or to turn the “knobs” to facilitate alleviation of endogenous constraints and promote regeneration? And if indeed, is this possible, are there some “master knobs” that tip the balance favoring regeneration and away from scarring? To answer these questions, it is necessary to carefully examine both endogenous constraints and healing pathways.
II. Endogenous Constraints and the Role of Inflammation
There are a number of endogenous constraints, which determine regenerative capacity of a tissue, and these vary greatly by species, age, pathophysiological state, tissue type, and the extent of injury. For instance, injured fetal tissues, in contrast to adult tissues, can occasionally be completely regenerated [2], [5]–[7] indicating their greater regenerative capacity. Although the loss of regenerative capacity from young to old is intuitively accepted to be true [8], the differences in the regenerative capacity between age-matched individuals are less well understood.
In the recent years, there is increasing evidence to suggest that inflammation [9]–[12] and the biochemical pathways [13], [14] triggered by the nature and duration of the initial inflammatory response may determine the healing outcomes [15]–[18]. Therefore, it is reasonable to assume that regeneration versus scarring responses may be modulated if mechanisms to influence this inflammatory cascade postinjury existed.
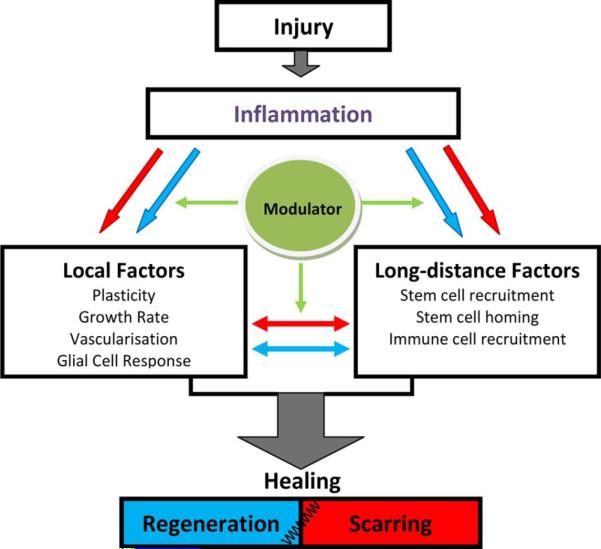
During the inflammation process, an array of complex regulatory pathways operates. Inflammatory pathways include mechanisms that regulate both “local” factors, such as the plasticity of tissue [19], [20] and its growth rate [17], and also “long-distance” factors, such as signaling mechanisms for the recruitment of circulating stem and immune cells, and their homing [21]–[24] (see Fig. 1). Inflammatory cells (e.g., macrophages and microglia) may secrete either “pro-regenerative” or “pro-scarring” chemokines and cytokines (see blue and red arrows, respectively, in Fig. 1).
Fig. 1.
Potential pathways and interactions that operate in a tissue postinjury. The sequence of events in the inflammatory pathway may determine the final outcome of healing process (regeneration or scarring) by regulating local and long-distance factors via pro-regeneration (blue arrow) and pro-scarring (red arrow) factors.
Studies on various immune cell types over the last several years have brought to light the biochemical as well as functional diversities of these immune cells, such as macrophages [25]–[29], which are extant both in normal and injured tissue [9], [30]. Macrophages as the “first responder” immune cells after injury potentially offer a valuable point of intervention, and strategically employing macrophages could direct the pathway toward predisposing the immune response to a “pro-regenerative” one.
III. Regeneration and Scarring in the Nervous System
If macrophages as well as glial cells determine differential regenerative capacity of the peripheral nervous system (PNS) and central nervous system (CNS), then evaluation and characterization of the effects of this response on the processes that thwart regeneration after PNS or CNS injury becomes critical.
A. Critical Determinants of PNS Regeneration
The PNS, unlike the CNS, has the ability to regenerate in a limited manner. Functional recovery after injuries to the PNS is dependent on factors such as 1) the rate of regeneration, which influences the time to reinnervation of target tissue, 2) the plasticity of the local peripheral nerve and the central cortical neurons in reestablishment of coherent control of target tissue, and 3) the severity of the injury, which may determine the extent to which the “pro-regenerative” glial/inflammatory responses can be modulated.
1) Rate of Regeneration
An important challenge and determining factor for functional recovery after PNS injury is the time to reinnervation [31]. Based on studies dating back to World War II, delay in repairing an injured peripheral nerve leads to feeble functional outcomes most likely due to a decrease in the ability of the end organ to be reinnervated and also atrophic changes in the regenerating support pathway of Schwann cells [32]. Although the rate of axonal regeneration is fairly constant across mammalian species (~1–4 mm/day) [32], this rate decreases with age and contributes to poor regeneration in older adults [33]. Interestingly, Fu and Gordon demonstrated that regeneration time dependency is not an intrinsic limitation of the neurons, but is controlled extrinsically by signals that originate either in glial cells such as Schwann cells, or target tissue such as muscle [34], [35]. In other words, axons maintain their regenerative capacity over time by the promoting mechanisms that stimulate their regeneration. Those promoting mechanisms may decrease over prolonged periods due to Schwann cells or target tissue dysfunction. Based on these observations, the rate of regeneration is an important rate limiter of success. This may, in turn, be determined by the quality and quantity of supporting healthy Schwann cells.
2) Plasticity
Plasticity may be defined as the ability of the PNS to sprout and make new connections with target tissues such as muscle. Plasticity may be governed by the presence of inhibitory proteoglycans, such as chondroitin sulfate proteoglycans (CSPGs) found in the basal lamina in the PNS [36], [37]. Second, plasticity is also dependent on the ability of the axons to “sprout” multiple neuritis and axonal branches to increase the probability of appropriate target finding and innervation. Neurotrophins such as neurotrophin-3 (NT-3) and nerve growth factor may play an important role in enabling this activity [38], [39]. Lastly, plasticity is dependent on the end organ receiving regenerating nerves and its ability to be innervated in a functional manner as nonspecific connections lead to misdirected regeneration [32].
3) Degree of Injury
As with any tissue, the degree of injury has the potential to overwhelm the regenerative capability. In the case of PNS injury, this translates to the gap length across which regeneration needs to occur. Coaptation of the two nerve stumps is usually used to repair short distance nerve defects, but when larger nerve gaps exist (15 mm or longer), the current clinical gold standard is to use an autograft [40].
B. Critical Determinants of CNS Regeneration
As is the case with PNS repair, adult CNS regeneration is hostage to the biochemical and cellular processes triggered by neural injury. Functional recovery is dependent on survival of the injured neuronal cell body, regeneration of the damaged axon, remyelination, and functional synapse formation [41]. However, contrary to the neurons in the PNS, severed CNS axons fail to regenerate beyond the lesion site [42]. The difference between regenerative capabilities of these two systems stems from the differential glial response after injury, resulting in vastly different degrees of “permissiveness” of the injury environment to regenerating axons [43].
Hence, nonpermissive regenerative environment after injury stemming from the astroglial scar tissue and inhibitory adult CNS myelin represent formidable barriers to CNS regeneration.
1) Astroglial Scar Tissue
After CNS injury, microglia, oligodendrocyte precursors, meningial cells, and astrocytes are recruited to the lesion site and form an astroglial scar tissue that “walls off” the CNS lesion [3]. Although some of these events have beneficial effects (like isolating the injury site, minimizing the area of inflammation, and cellular degeneration), many astrocytes in the lesion site become hypertrophic and adopt a “reactive” phenotype, releasing inhibitory extracellular matrix (ECM) molecules such as CSPGs [3]. CSPGs are also present in perineural nets that stabilize CNS synapses and limit plasticity in the CNS. Overcoming astroglial scar tissue represents a grand challenge as it negatively impacts regeneration and limits the integration of implanted electrodes or transplanted engineered constructs into the CNS.
2) Myelin-Associated Inhibition and Wallerian Degeneration
Myelin, a paracrystalline array of lipid-rich glial or Schwann cell plasma membranes, supports rapid conduction of nerve impulses by providing a high-resistance, low-capacitance sheath around large axons [44]. After injury in the CNS, immobilized CNS myelin inhibits axon outgrowth by varied mechanisms [45]. Wallerian degeneration, the process in which damaged cells are removed and recycled by glial cells, is slower in CNS in comparison to PNS. The rate of Wallerian degeneration is different in these two systems because of some distinct differences in the glial clearance responses after PNS and CNS injuries. The PNS is more efficient at clearing myelin debris in comparison to the CNS, and Schwann cells are the primary cause of this difference [46]. Schwann cells in the PNS, in contrast to their CNS counterparts, oligodendrocytes, do not require axon signals to survive. In their developmental stages, oligodendrocytes that fail to make contact to an axon and those that receive any axon signals undergo apoptosis [47]. Therefore, unlike Schwann cells, oligodendrocytes fail to clean up the myelin sheaths and their debris. Moreover, oligodendrocytes fail to recruit macrophages for debris removal [46].
3) Blood-Brain Barrier
Another important determinant in CNS regeneration is the blood-brain barrier (BBB). The BBB is the separating system of circulating blood and cerebrospinal fluid in the CNS. One of the main roles of the BBB was considered to be the separation of the CNS from the systemic immune system. Although studies show that resident CNS macrophages (microglia) present within the CNS actively interact with peripheral immune cells [48], the CNS is largely thought to be limited in its capacity to deliver antigens to local lymph nodes and cause T-cell activation [46]. In an uninjured tissue, antigens are taken up by antigen presenting cells (dendritic cells) and, subsequently, transported to the lymph nodes. Alternatively, soluble antigens can drain into the lymph nodes. In contrast, in the CNS, dendritic cells are not thought to be present in normal parenchymal tissue or perivascular space, although they are present in the meninges and choroids plexus [49].
Moreover, in contrast to the PNS, the barrier disruption in the CNS is limited to just the site of injury, whereas in the PNS, the permeability increases throughout the distal stump. The decreased permeability in the CNS could also explain the difference in the number of infiltrated macrophage to the site of injury [46].
C. Common Determinants of PNS and CNS Regeneration
Besides the aforementioned rate-determining processes in the PNS and CNS, there are some other critical determinants of regenerative fate that are common in both systems.
One such factor is the number of available local stem or progenitor cells in the immediate vicinity of the site of injury. Besides resident neural stem cells [50], stem cells can be either transplanted [51] or endogenously recruited to the site of injury [52]. However, in spite of encouraging data, which indicate that replacement of cells is promising [53]–[55], the functional impact and reliability have been underwhelming. Moreover, despite intrinsic plasticity of endogenous stem cells, they are incapable of providing complete recovery in severe trauma [40].
Another rate limiter is the ability of an injured tissue to form blood vessels (vascularization and angiogenesis). Angiogenesis is a normal and vital process in growth and development, as well as in wound healing. Vascular endothelial growth factor and the fibroblast growth factors are two long-known effective factors in vessel formation. There are many other critical growth factors involved in the physiological regulation of blood vessel formation [30]. These factors should be very carefully orchestrated in terms of time, space, and dose so as to form a functioning vascular network [56]. Enhancement in neurogenesis by increasing vascularization has been reported [57].
IV. New Insights Into Regeneration: Immune System as The Key Determinant of Regeneration
There is evidence that secretory products of immune cells are capable of affecting most of the critical determinants of regeneration in the CNS and PNS that makes the immune system the key determinant of regenerative fate. Therefore, the inflammatory cascade postinjury and the role of the immune system has the potential of being the lynchpin upon which regeneration versus scarring responses are determined.
Considering the complexity of the biochemical and cellular responses to neural injury, targeting an individual point in the cascade may or may not be the most efficient approach to bias the response to regeneration versus scarring. In this context, the inflammatory response as well as the resulting immune reaction to the injury represent a promising point of intervention and could potentially represent one of the “master knobs” for eliciting regeneration. Before discussing the effects of different inflammatory responses, a brief introduction to microglia and macrophages as the most studied and diverse inflammatory cells will be provided.
A. Macrophage and Microglia
Macrophages are present in all tissues with the different resident names: osteoclasts in bone, histocytes in connective tissue, Kupffer cells in liver, and microglia in the CNS. They migrate as monocytes into the tissue in a steady-state fashion or in response to an inflammation. Although macrophages have been known as the professional phagocytes and the executers of the innate immunity, recent studies illustrate their homeostatic as well as regenerative roles [26], [28], [29]. In fact, remarkable plasticity of macrophages makes them capable of effectively responding to the different environmental signals by changing their phenotype and physiology. Mosser and Edwards suggest that there exist many shades of activation of macrophages, resulting in a spectrum of macrophage population rather than a few distinct groups [29]. However, there is consensus that macrophage phenotypes roughly fall into three categories: classically activated, wound healing, and regulatory (see Table I) [16], [26], [28], [29].
TABLE I.
Three Categories of Macrophage Phenotypes
| Activation state | Classically activated | Wound-healing | Regulatory |
|---|---|---|---|
| Function | Tissue defense, Pro-inflammatory cytokine production, NO production | Tissue repair, Anti-inflammatory cytokine production, ECM reconstruction | Immunosuppression, Apoptotic cell uptake |
| Activating signal | IFN-γ, TNF | IL-4, IL-13 | TGF-β, IL-10, apoptotic cells |
| Secretory Products | ↑TNF, ↑IL-12, IL-6, IL-1b, MCP-1 | ↑IL-1RA, IL-10, IGF-1 | ↑IL-10, TNF, IL-6 |
↑= unregulated
Classically activated macrophages become activated by injury-triggered endogenous inflammatory signals like Th1 cytokine interferon gamma (IFN-γ), or by exogeneous inflammatory signals like lipopolysaccharide (LPS) [58]. These cells are prototypical immune effector cells, which kill pathogens by production of oxygen, nitrogen radicals as well as phagocytosis. Classically activated macrophages are beneficial for the survival of the organism. However, resolving the injury and restoring normal tissue homeostasis requires an innate immune response that supports replacement of lost and damaged cells and restructuring of the damaged ECM. Wound-healing macrophages represent a second class of macrophages that help in the tissue repair by producing antiinflammatory cytokines, which mediate angiogenesis as well as ECM deposition. One of the well-established activating signals of this phenotype is interleukin-4 (IL-4). Moreover, the induction of arginase in these cells may lead to polyamine and proline biosynthesis, promoting cell growth and collagen formation. The third broad category of macrophages is regulatory macrophages that are elicited by exposure of macrophages to apoptotic cells and are associated with the robust suppression of the innate immune response. This phenotype allows them to engulf apoptotic cells without inducing a classical innate immune response [15], [26], [28]. Furthermore, these phenotypes can sequentially change their functional phenotype in response to signals in their respective microenvironments [58], [59].
In the nervous system, although other glial cells (like astrocyte [60]) and neurons [61] may also play an immune role, the primary cells involved in the generation of innate immune response are microglia cells. It has been demonstrated that microglia, as the CNS resident macrophages, are also capa ble of transiting between the three activation states previously described upon receiving an appropriate activating signal (see Table I) [15], [16].
Generally, a pro-regenerative bias in activation of these populations in the inflammatory cascade at the appropriate time and location will lead to regeneration, and any inappropriate triggering of pro-scarring macrophages/microglia/glial cells will lead to scarring. For example, in cancer, macrophages inappropriately switch from the classically activated to regulatory phenotype [29], or in autoimmune diseases, such as Alzehimer's or multiple sclerosis (MS), microglia cells become chronically inflammatory (coexistence of wound healing and classically activated phenotypes) in the brain [15], [16], [62]. Interestingly, many parasitic organisms also alternate the macrophage activation state to the wound healing as a means to enhance their survival within cells or tissues [63].
B. Harnessing the Immune System to Enhance Nerve Regeneration
Regenerative biochemical cascade is sometimes halted by lack of critical component, such as resident pluripotent cells, or weak “homing” signals to recruit circulating stem cells, or poor permissivity to regeneration at the site of injury (e.g., gliotic scar). Although the precise effect of each phenotype of microglia (see Fig. 1) is not completely understood, there is strong evidence to support the notion that macrophages can modulate regeneration in the nervous system. The Schwartz laboratory has demonstrated that appropriate activation of microglia by IL-4 or IFN-γ differentially induces neurogenesis as well as oligodendrogenesis from adult stem cells [17]. This data suggests that both neurogenesis and oligodendrogenesis of adult neural progenitor cells in the mice are blocked by classically activated microglia cells. However, this process can be altered to be “pro-regenerative” by wound healing associated cytokines (IL-4) in combination with low levels of IFN-γ. Therefore, controlled levels of appropriate cytokines can overcome the inhibition of neurogenesis in an inflamed brain. These studies also suggest that the duration, combination, and order of biochemical signals after injury ultimately determine the outcomes [64].
It has also been shown that exposing microglia cells to low concentrations of classically activated cytokine IFN-γ (5 ng/mL) enables them to buffer the excitatory neurotransmitter glutamate (a common player in neurodegenerative diseases) and subsequently afford protection to neural tissue [65].
Schwartz group has demonstrated that the cytotoxic effects of microglia exposure to large amounts of IFN-γ (>50 ng/mL) is a consequence of upregulated neurotoxic cytokine, tumor necrosis factor-alpha (TNFα) [19], which is a secretory product of classically activated macrophages. However, treatment of the macrophages with TNFα during Wallerian degeneration significantly reduced their phagocytic capacity as well as their ability to ingest myelin debris, and in turn, their regenerative capacity [66].
On the other hand, it has also been demonstrated that lack of TNFα will significantly delay remyelination, which is an important regenerative step in the autoimmune disease such as MS [20]. In addition, the neuroprotective effect of TNFα might be indirectly controlled by astrocytes. Expression of the brain-derived neurotrophic factor by cultured astrocytes is usually elevated by increasing the amount of TNFα [67]. IL-1β, a proinflammatory neurotoxic cytokine, has also been shown to have a similar paradoxical effect in promoting regeneration [67]. Thus, although many cytokines such as TNFα and IL-1β are traditionally thought to be neurotoxic, their role is context dependent as their beneficial function was demonstrated in animals deficient in these cytokines [62]. Therefore, macrophages/microglia phenotype has a regulatory effect on the inhibitory environment of CNS as a critical determinant of regeneration.
Additionally, microglia exposure to IL-4 stimulates production of insulin-like growth factor (IGF-1) [29] (see Table I), supports neurogenesis [69] and oligodendrogenesis [70], as well as ameliorating the age-related decline of those regenerative process [71].
Macrophages also play a key role in the regeneration process. They actively participate in the cell replacement by the stem cell recruitment and homing, as well as in the resident progenitor cell differentiation and proliferation [72]. Besides the granulocyte-macrophage colony-stimulating factors (GM-CSF), it has been shown that IL-12 (upregulated by classically activated phenotype) also mobilizes hematopoietic stem cells [22], [23]. It has also been demonstrated that the monocyte chemoattractant protein-1 (MCP-1), a chemokine produced by classically activated macrophages, has an ability to recruit neural progenitors to the site of injury [21], [24]. Moreover, different phenotypes of macrophages by secreting factors like TNF, IL-1, and GMCSF can cause an increase in the production of granulocyte and monocytes (defensive immune cells) by the bone marrow, which can contribute in healing pathway in several ways [30]. For example, one type of granulocyte, basophils, can release histamine, which leads to dilation and increased permeability of capillaries close to them [30]. It has also been shown that secretory products of macrophages influence the different phase of angiogenesis both in vivo and in vitro [60], [61]. These participations also support the idea of capability of macrophages in regulating some other rate-determining factors.
Unfortunately, detailed molecular mechanisms and healing pathways of all the different phenotypes of microglia and macrophages in the nervous system are not well characterized yet; preliminary results show that a tightly controlled modulation of these cells can potentially enhance the regeneration in CNS and PNS significantly. Neuroengineering tools involving electrical stimulation, polymeric fibers, hydrogel nanoparticles, and hydrogel microparticles may all offer powerful tools to modulate these intricate inflammatory signaling fates in a manner that is spatially and temporally controlled [73]–[79].
Therefore, in order to meet the challenge of regenerating PNS and CNS nerves, it is important to explore the full spectrum of the microglial and macrophagic cell phenotypes in the inflammatory cascade, and to identify their influence on both local and long-distance critical rate limiters to endogenous regeneration, and where necessary, to use biological and engineering tools to modulate these critical phenotypes to maximize regeneration.
V. Conclusion
Hence, an important grand challenge in neuroengineering is stimulating endogenous repair of injured peripheral and central neural tissues. Specific challenges include bridging long peripheral nerve gaps and overcoming astroglial scar tissue to promote regeneration after spinal cord injury. The particular insight afforded here is the possibility that modulation of the inflammatory cascade after injury may significantly alter the course of healing in the nervous system, thus offering a critical modulation opportunity for promoting regeneration and integration of engineered materials and devices in the nervous system.
Acknowledgment
The authors would like to thank Dr. B. Pai and Dr. L. Karumbaiah in the Bellamkonda Laboratory for helpful technical and editorial discussions. The authors also thank Prof. W. Robert Taylor, Emory University, for useful discussion regarding the concept of regenerative capacity.
This work was supported by the National Institutes of Health under Grant EB006343, Grant NS44409, Grant NS65109, and Grant NS43486.
Biographies

Nassir Mokarram received the B.S. and M.S. degrees in polymer science and engineering from Amirkabir University of Technology, Tehran, Iran, in 2009. He is currently working toward the Ph.D. degree in materials science and engineering at the School of Materials Science and Engineering, Georgia Institute of Technology, Atlanta.
His research interests include regulation of immune response at the site of peripheral nerve injuries to control neuronal healing.

Ravi V. Bellamkonda received the B.S. degree in biomedical engineering from Osmania University, Hyderabad, India, in 1989, and the Ph.D. degree in medical sciences from Brown University (with P. Aebischer), Providence, RI, in 1994.
He was a Postdoctoral Researcher in the Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology (MIT), Cambridge. He was also an Assistant and Associate Professor with tenure at Case Western Reserve University, Cleveland, OH. Since 2003, he has been with Georgia Institute of Technology, Atlanta, where he is currently a GCC Distinguished Scholar, Professor of Biomedical Engineering, and Associate Vice President for Research. His laboratory focuses on uncovering the mechanisms of peripheral nerve repair, understanding the role of inhibitory scar tissue in spinal cord regeneration, designing brain–machine interfaces, designing “nanofiber-based” bridges for peripheral nerve repair, and designing and developing imaging nanoprobes that are able to, in a personalized manner, determine the degree of aggressiveness of individual tumors, and predict whether or not tumors will respond to chemotherapy. His research interests include the application of principles of regenerative medicine for the repair and regeneration of neural tissue, and in the development of novel nanocarriers for personalized medicine and cancer diagnosis and therapy.
Prof. Bellamkonda is a Fellow of the Institute of Physics and American Institute for Medical Biological Engineering. He is also on the editorial boards of several journals, and has won numerous awards including a EUREKA award from National Cancer Institute (NCI), National Science Foundation (NSF) CAREER Award, MIT's “Technovator” Award, and the Best Professor Award at GT/BME. His Lab. received financial support from the National Institutes of Health (National Institute of Neurological Disorders and Stroke & NCI), NSF, Ian's Friends Foundation, the Georgia Cancer Coalition, and the Wallace H. Coulter Foundation active research program.
References
- [1].Gurtner G, Werner S, Barrandon Y, Longaker M. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- [2].Ferguson M, O'Kane S. Scar–free healing: from embryonic mechanisms to adult therapeutic intervention. Philos. Trans. B. 2004;359:839. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosc. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Odelberg S. Unraveling the molecular basis for regenerative cellular plasticity. PLoS Biol. 2004;2:E232. doi: 10.1371/journal.pbio.0020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- [6].Harty M, Neff A, King M, Mescher A. Regeneration or scarring: an immunologic perspective. Dev. Dyn. 2003;226:268–279. doi: 10.1002/dvdy.10239. [DOI] [PubMed] [Google Scholar]
- [7].Nodder S, Martin P. Wound healing in embryos: a review. Anatomy Embryol. 1997;195:215–228. doi: 10.1007/s004290050041. [DOI] [PubMed] [Google Scholar]
- [8].Gurtner G, Callaghan M, Longaker M. Progress and potential for regenerative medicine. Annu. Rev. Med. 2007;58:299–312. doi: 10.1146/annurev.med.58.082405.095329. [DOI] [PubMed] [Google Scholar]
- [9].DiPietro L. Wound healing: The role of the macrophage and other immune cells. Shock. 1995;4:233. [PubMed] [Google Scholar]
- [10].Lucas S, Rothwell N, Gibson R. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006;147:S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Donnelly D, Popovich P. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wilgus T. Immune cells in the healing skin wound: Influential players at each stage of repair. Pharmacol. Res. 2008;58:112–116. doi: 10.1016/j.phrs.2008.07.009. [DOI] [PubMed] [Google Scholar]
- [13].Baum C, Arpey C. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatol. Surg. 2005;31:674–686. doi: 10.1111/j.1524-4725.2005.31612. [DOI] [PubMed] [Google Scholar]
- [14].Eubank T, Marsh C. “Cytokines and growth factors in the regulation of wound inflammation,” 2010. 2011 [Google Scholar]
- [15].Cameron B, Landreth G. Inflammation, microglia, and alzheimer's disease. Neurobiol. Dis. 2009:503–509. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Colton C. Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar A, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-[gamma] differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- [18].Mountziaris P, Mikos A. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. Part B: Rev. 2008;14:179–186. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Butovsky O, Talpalar A, Ben-Yaakov K, Schwartz M. Activation of microglia by aggregated [beta]-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-[gamma] and IL-4 render them protective. Mol. Cell. Neurosci. 2005;29:381–393. doi: 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- [20].Arnett H, Mason J, Marino M, Suzuki K, Matsushima G, Ting J. TNF promotes proliferation of oligodendrocyte progenitors and remyelination. Nat. Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- [21].Belmadani A, Tran P, Ren D, Miller R. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J. Neurosci. 2006;26:3182–3191. doi: 10.1523/JNEUROSCI.0156-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cashen A, Lazarus H, Devine S. Mobilizing stem cells from normal donors: is it possible to improve upon G-CSF? Bone Marrow Transplantation. 2007;39:577–588. doi: 10.1038/sj.bmt.1705616. [DOI] [PubMed] [Google Scholar]
- [23].To L, Haylock D, Simmons P, Juttner C. The biology and clinical uses of blood stem cells. Blood. 1997;89:2233–2258. [PubMed] [Google Scholar]
- [24].Yan Y, Sailor K, Lang B, Park S, Vemuganti R, Dempsey R. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J. Cerebral Blood Flow Metab. 2006;27:1213–1224. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- [25].Mills C, Kincaid K, Alt J, Heilman M, Hill A. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- [26].Mosser D. The many faces of macrophage activation. J. Leukocyte Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- [27].Gordon S, Taylor P. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- [28].Edwards J, Zhang X, Frauwirth K, Mosser D. Biochemical and functional characterization of three activated macrophage populations. J. Leukocyte Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mosser D, Edwards J. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- [31].Krarup C, Archibald S, Madison R. Factors that influence peripheral nerve regeneration: an electrophysiological study of the monkey median nerve. Ann. Neurol. 2002;51:69–81. doi: 10.1002/ana.10054. [DOI] [PubMed] [Google Scholar]
- [32].Höke A, Brushart T. Introduction to special issue: Challenges and opportunities for regeneration in the peripheral nervous system. Exp. Neurol. 2009 doi: 10.1016/j.expneurol.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Verdú E, Ceballos D, Vilches J, Navarro X. Influence of aging on peripheral nerve function and regeneration. J. Peripheral Nerv. Syst. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- [34].Fu S, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged axotomy. J. Neurosci. 1995;15:3876–3885. doi: 10.1523/JNEUROSCI.15-05-03876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fu S, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged denervation. J. Neurosci. 1995;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Groves M, McKeon R, Werner E, Nagarsheth M, Meador W, English A. Axon regeneration in peripheral nerves is enhanced by proteoglycan degradation. Exp. Neurol. 2005;195:278–292. doi: 10.1016/j.expneurol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- [37].Zuo J, Hernandez Y, Muir D. Chondroitin sulfate proteoglycan with neurite-inhibiting activity is up-regulated following peripheral nerve injury. J. Neurobiol. 1998;34:41–54. [PubMed] [Google Scholar]
- [38].Patel M, McNamara J. Selective enhancement of axonal branching of cultured dentate gyrus neurons by neurotrophic factors. Neuroscience. 1995;69:763–770. doi: 10.1016/0306-4522(95)00281-m. [DOI] [PubMed] [Google Scholar]
- [39].Kennedy T, Tessier-Lavigne M. Guidance and induction of branch formation in developing axons by target-derived diffusible factors. Curr. Opin. Neurobiol. 1995;5:83–90. doi: 10.1016/0959-4388(95)80091-3. [DOI] [PubMed] [Google Scholar]
- [40].Bellamkonda R. Peripheral nerve regeneration: An opinion on channels, scaffolds and anisotropy. Biomaterials. 2006;27:3515–3518. doi: 10.1016/j.biomaterials.2006.02.030. [DOI] [PubMed] [Google Scholar]
- [41].Horner P, Gage F. Regenerating the damaged central nervous system. Nature. 2000;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- [42].Richardson P, McGuinness U, Aguayo A. Axons from CNS neurones regenerate into PNS grafts. 1980 doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- [43].David S, Aguayo A. Axonal elongation into peripheral nervous system” bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- [44].Siegel G. Basic Neurochemistry. Little Brown and Company; Boston: 1981. [Google Scholar]
- [45].Schwab M, Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J. Neurosci. 1985;5:2415–23. doi: 10.1523/JNEUROSCI.05-09-02415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vargas M, Barres B. Why is Wallerian degeneration in the CNS so slow? 2007 doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- [47].Barres B, Jacobson M, Schmid R, Sendtner M, Raff M. Does oligodendrocyte survival depend on axons? Curr. Biol. 1993;3:489–497. doi: 10.1016/0960-9822(93)90039-q. [DOI] [PubMed] [Google Scholar]
- [48].Carson M, Doose J, Melchior B, Schmid C, Ploix C. CNS immune privilege: hiding in plain sight. Immunol. Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Galea I, Bechmann I, Perry V. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- [50].Gage F. Mammalian neural stem cells. Science. 2000;287:1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- [51].Gaiano N, Fishell G. Transplantation as a tool to study progenitors within the vertebrate nervous system. J. Neurobiol. 1998;36:152–161. [PubMed] [Google Scholar]
- [52].Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- [53].McDonald J, Liu X, Qu Y, Liu S, Mickey S, Turetsky D, Gottlieb D, Choi D. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat. Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- [54].Vescovi A, Parati E, Gritti A, Poulin P, Ferrario M, Wanke E, Frölichsthal-Schoeller P, Cova L, Arcellana-Panlilio M, Colombo A. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation* 1. Exp. Neurol. 1999;156:71–83. doi: 10.1006/exnr.1998.6998. [DOI] [PubMed] [Google Scholar]
- [55].Keirstead H. Stem cell transplantation into the central nervous system and the control of differentiation. J. Neurosci. Res. 2001;63:233–236. doi: 10.1002/1097-4547(20010201)63:3<233::AID-JNR1016>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- [56].Yancopoulos G, Davis S, Gale N, Rudge J, Wiegand S, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- [57].Hobson M, Green C, Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J. Anatomy. 2001;197:591–605. doi: 10.1046/j.1469-7580.2000.19740591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gratchev A, Kzhyshkowska J, Köthe K, Muller-Molinet I, Kannookadan S, Utikal J, Goerdt S. M [φ] 1 and M [φ] 2 can be repolarized by Th2 or Th1 cytokines, respectively, and respond to exogenous danger signals. Immunobiology. 2006;211:473–486. doi: 10.1016/j.imbio.2006.05.017. [DOI] [PubMed] [Google Scholar]
- [59].Stout R, Jiang C, Matta B, Tietzel I, Watkins S, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- [60].Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- [61].Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat. Med. 2006;12:518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- [62].Herz J, Zipp F, Siffrin V. Neurodegeneration in autoimmune CNS inflammation. Exp. Neuro. 2009 doi: 10.1016/j.expneurol.2009.11.019. [DOI] [PubMed] [Google Scholar]
- [63].Noël W, Raes G, Hassanzadeh G, Ghassabeh, De Baetselier P, Beschin A. Alternatively activated macrophages during parasite infections. Trends Parasitol. 2004;20:126–133. doi: 10.1016/j.pt.2004.01.004. [DOI] [PubMed] [Google Scholar]
- [64].Schwartz M, Butovsky O, Brück W, Hanisch U. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- [65].Shaked I, Tchoresh D, Gersner R, Meiri G, Mordechai S, Xiao X, Hart R, Schwartz M. Protective autoimmunity: Interferon- enables microglia to remove glutamate without evoking inflammatory mediators. J. Neurochem. 2005;92:997–1009. doi: 10.1111/j.1471-4159.2004.02954.x. [DOI] [PubMed] [Google Scholar]
- [66].Brück W, Brück Y, Friede R. TNF-[alpha] suppresses CR3-mediated myelin removal by macrophages. J. Neuroimmunol. 1992;38:9–17. doi: 10.1016/0165-5728(92)90085-y. [DOI] [PubMed] [Google Scholar]
- [67].Saha R, Liu X, Pahan K. Up-regulation of BDNF in astrocytes by TNF- : a case for the neuroprotective role of cytokine. J. Neuroimmune Pharmacol. 2006;1:212–222. doi: 10.1007/s11481-006-9020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mason J, Suzuki K, Chaplin D, Matsushima G. Interleukin-1 {beta} Promotes Repair of the CNS. J. Neurosci. 2001;21:7046. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].O'Kusky J, Ye P, D'Ercole A. Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development. J. Neurosci. 2000;20:8435–42. doi: 10.1523/JNEUROSCI.20-22-08435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hsieh J, Aimone J, Kaspar B, Kuwabara T, Nakashima K, Gage F. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J. Cell Biol. 2004;164:111–122. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lichtenwalner R, Forbes M, Bennett S, Lynch C, Sonntag W, Riddle D. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- [72].Napoli I, Neumann H. Protective effects of microglia in multiple sclerosis. Exp. Neurol. 2009 doi: 10.1016/j.expneurol.2009.04.024. [DOI] [PubMed] [Google Scholar]
- [73].Mukhatyar V, Karumbaiah L, Yeh J, Bellamkonda R. Tissue engineering strategies designed to realize the endogenous regenerative potential of peripheral nerves. Adv. Mater. 2009;21:4670–4679. [Google Scholar]
- [74].McNeeley K, Karathanasis E, Annapragada A, Bellamkonda R. Masking and triggered unmasking of targeting ligands on nanocarriers to improve drug delivery to brain tumors. Biomaterials. 2009;30:3986–3995. doi: 10.1016/j.biomaterials.2009.04.012. [DOI] [PubMed] [Google Scholar]
- [75].Kim Y, Caldwell J, Bellamkonda R. Nanoparticle-mediated local delivery of methylprednisolone after spinal cord injury. Biomaterials. 2009;30:2582–2590. doi: 10.1016/j.biomaterials.2008.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kim Y, Haftel V, Kumar S, Bellamkonda R. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials. 2008;29:3117–3127. doi: 10.1016/j.biomaterials.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Dodla M, Bellamkonda R. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials. 2008;29:33–46. doi: 10.1016/j.biomaterials.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Jain A, Kim Y, McKeon R, Bellamkonda R. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials. 2006;27:497–504. doi: 10.1016/j.biomaterials.2005.07.008. [DOI] [PubMed] [Google Scholar]
- [79].Clements I, Kim Y, English A, Lu X, Chung A, Bellamkonda R. Thin-film enhanced nerve guidance channels for peripheral nerve repair. Biomaterials. 2009;30:3834–3846. doi: 10.1016/j.biomaterials.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]