Abstract
Macrophages regulate the initiation, maintenance, and resolution of chronic inflammatory responses and their function depends on their activation status. Studies in mice infected with the helminth parasite Schistosoma mansoni have been particularly helpful in defining the in vivo function of classically and alternatively activated macrophages (AAMϕs). These studies have shown that AAMϕs protect mice from acute and chronic S. mansoni infection through distinct mechanisms, which are discussed in this Viewpoint.
Keywords: Host/pathogen interactions, Immune regulation, Immunopathology, Infectious disease, Macrophages
Introduction
Macrophages play critical and plastic roles in host defense, immune regulation, and wound healing, adapting to their local environments and adopting diverse phenotypes [1–5]. This inherent plasticity and heterogeneity offers clear advantages as the immune response progresses from an acute to chronic reaction, i.e. when the host cannot eradicate persistent infection but must limit collateral tissue damage. Thus, the switch of macrophage function from killing to repair is likely critical for the host, particularly when confronting a chronic helminth infection [6]. Furthermore, the features of the activation status of macrophages are strikingly similar whether responding to Th2-eliciting parasitic, fungal, or viral infections, asthma, or other Th2-dependent diseases [3, 7]. Thus, although macrophages may cause, prevent, or repair damage by many different mechanisms, understanding how they act in one chronic disease generates findings applicable to a much broader range of disorders. Here, we briefly discuss how macrophage activation integrates the immune damage and healing responses using the example of chronic Schistosoma mansoni infection.
As a simple paradigm, microbial products plus IFN-γ ‘classically’ activate macrophages to induce microbicidal activity during Th1-dominant responses, often to the detriment of the surrounding tissue, while Th2 responses ‘alternatively’ activate macrophages using IL-4 and IL-13 [1–5]. Since IL-4/13 stimulation antagonizes the effects of IFN-γ, alternatively activated macrophages (AAMϕs, also called M2 macrophages) have been considered anti-inflammatory because they suppress Th1 immunity and limit the tissue-damaging activity of classically activated macrophages (CAMϕs, also called M1 macrophages) [1, 3, 8]. Indeed, some pathogens have been shown to subvert alternative activation to escape Th1-dependent killing by CAMϕs [1, 3, 9–11]. AAMϕs participate in many physiological and pathological processes but are particularly important in resolving the damage caused by parasites [1, 3, 6]. Among their distinctions, CAMϕs express Th1-polarizing IL-12 and inducible nitric oxide synthase (iNOS), while AAMϕs are marked by mannose receptor (MR), arginase 1 (Arg1), and IL-10 [1–5] expression. Given that their activation depends on cytokines, a primary experimental approach to analyze macrophages in vivo is to block or engineer genetic deficiencies in cytokines and their receptors. Reliance on appropriate macrophage activation is marvelously exhibited by cytokine-dependent formation of granulomas, spherical masses composed of macrophages and other inflammatory cells that confine pathogens and toxins, surround lesions, and help maintain tissue function [6].
Schistosomiasis presents an opportunity to study macrophage activation in a protective, granulomatous response against a helminth that chronically infects ~ 200 million people, creating a massive health burden and high morbidity levels that also impact AIDS, viral hepatitis, and other diseases [12, 13]. The characteristics of human and mouse macrophages only partially match [1, 3, 4], but S. mansoni infection causes similar immune responses and pathology in both hosts [13–15]. Schistosome eggs induce AAMϕ-rich granulomas and Th2-biased immunity that prevents acute mortality but ultimately causes liver fibrosis and sometimes death in chronically infected hosts [13, 14]. In some cases, adult S. mansoni may survive for decades in the venous system between the intestine and liver.
Despite repeated exposure, the immune response is typically ineffective in preventing reinfection or eliminating parasites. Each worm pair produces daily 100–300 eggs which either exit the host by crossing the intestine or are swept into the liver and become trapped in small sinusoidal vessels [6]. Eggs are metabolically active, harmful, highly antigenic, and induce intestinal and liver granulomas that wall them off from the surrounding tissues. While worms initially elicit a Th1-skewed response, eggs provoke strong, though not exclusive, Th2 immunity beginning 4–6 weeks after infection. The intensity of this ‘acute’ Th2 response peaks at week 8, then it is downregulated to a muted but persistent, ‘chronic’ stage by week 12. In the acute stage, signaling through IL-4Rα on macrophages ensures that sufficient barrier integrity is maintained despite eggs continuously moving through the wall of the intestine [16]. Although the intensity of the egg-directed immunity is negatively regulated in the chronic stage, IL-4/13-producing CD4+ T cells drive a wound-healing response causing liver inflammation and pathological scarring. Macrophages play critical but distinct roles at both the acute and chronic stages, with their activation state dictating their function.
AAMϕs are protective during acute infection, but Th2 immunity causes chronic pathology
Mice deficient in IL-4, IL-4/13, IL-4/10, or IL-4Rα all die during acute S. mansoni infection because they develop severe intestinal and liver pathology, Th1-biased immunity, elevated iNOS levels, oxidative damage, endotoxemia, and cachexia [13, 14, 17–22]. Mice lacking IL-4Rα in only macrophages (and neutrophils) also die in the acute stage [16]. When unresponsive to IL-4/13, macrophages in intestinal granulomas express iNOS instead of the MR, eggs move less efficiently through the intestine, and mice develop weight loss, endotoxemia, hepatocyte death, and elevated IFN-γ. Antibiotics restore survival. Therefore, during acute schistosomiasis, intestinal integrity fails in the absence of AAMϕs, leading to a lethal Th1 pathology driven in part by the increased exposure of the host to environmental bacteria (Fig. 1).
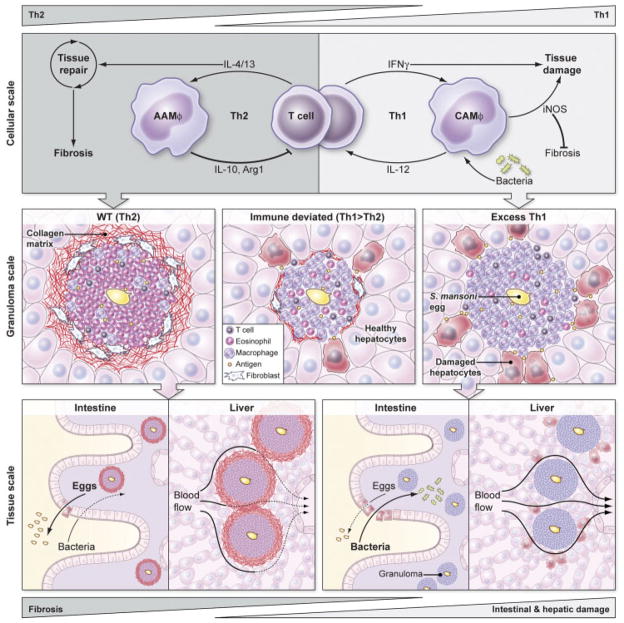
Figure 1.
A model of how macrophage activation regulates the pathogenesis of schistosomiasis at the scale of cells, granulomas, and tissues. In the WT response to S. mansoni (left), IL-4 and IL-13 from Th2 cells promote tissue repair, alternatively activate macrophages, and engage negative feedback loops by inducing AAMϕs to produce IL-10, Arg1, and other factors that limit T-cell proliferation and activation. This WT Th2 response creates eosinophil-rich granulomas around schistosome eggs surrounded by a collagen matrix deposited by IL-4/13-activated fibroblasts that protects the surrounding hepatocytes and other parenchymal cells from toxic egg-derived antigens. Normal granulomatous inflammation and AAMϕs enable schistosome eggs to cross from the mesenteric blood, through the intestine, and into the lumen while promoting wound healing, which maintains sufficient barrier integrity to shield the host from enteric bacteria. However, the accumulation of granulomas causes Th2-dependent fibrosis and collagen deposition that ultimately impedes blood flow through the liver, creating portal hypertension and dilated collateral vessels that are prone to rupture. Exaggerated Th2 immunity accelerates and exacerbates liver scarring but does not seem to significantly harm hepatocytes or disrupt liver function in the short term. If an aberrant Th1 response develops against S. mansoni eggs (right), IFN-γ classically activates macrophages that express IL-12 and iNOS, generating a positive feedback loop that can damage surrounding tissues. The excessive Th1 response generates granulomas with few eosinophils, numerous CAMϕs, and fails to activate fibroblasts to synthesize the collagen matrix which helps to contain egg antigens and protect surrounding parenchymal cells. Th1 granulomas and CAMϕs also appear to be less effective than AAMϕs at wound healing and at facilitating the transit of eggs through the wall of the intestine, leading to breaks in the epithelial barrier that cause septicemia as increased numbers of bacteria from the lumen penetrate the breach. Th1-dependent immunity induces minimal liver fibrosis and is assumed not to significantly interrupt blood flow or cause portal hypertension. Immune deviation in schistosomiasis, by vaccinating hosts with S. mansoni eggs plus IL-12, for example, can reduce both Th2- and Th1-dependent pathologies (center). A slight bias towards a Th1 response contributes to the development of more classically than alternatively activated macrophages, resulting in smaller granulomas and reduced collagen deposition via an iNOS-dependent mechanism. The reduced but still present AAMϕs and Th2 response also limits hepatocyte injury and helps preserve intestinal integrity. Thus, a mixed Th1/Th2 response or slightly biased Th1 responses appear to provide the greatest degree of protection during chronic S. mansoni infection by minimizing fibrosis while simultaneously protecting the host from intestinal and hepatic damage caused by S. mansoni eggs.
Th2 immunity, on the other hand, causes fibrotic pathology during chronic schistosomiasis. IL-4 and IL-13 together determine the inflammatory phenotype of egg-induced granulomas, while IL-13 causes fibrosis [13, 14, 18–20, 23–27]. Mice deficient in IL-10, IL-12, IL-10/12, IL-13Rα2 (which acts as a decoy receptor for IL-13), and IL-10/13Rα2 all develop stronger Th2 responses yet survive past the acute stage, indicating that the intestinal integrity is maintained despite impaired Th1 immunity [25, 27]. Instead, the exaggerated Th2 cytokine response leads to the formation of larger granulomas and increased collagen deposition, and it drives excess fibrosis that scars the liver and results in portal hypertension, portosystemic venous shunts, and gastrointestinal hemorrhages, as observed in chronically infected humans exhibiting the more serious hepatosplenic form of the disease [13–15, 28].
AAMϕs exhibit immunoregulatory activity in chronic schistosomiasis
The functions of AAMϕs correlate with wound healing and fibrosis, and signals from anti-inflammatory mediators (IL-10 and glucocorticoids), damage-induced factors (IL-33, IL-25, and adenosine), and some growth factors (GM-CSF) enhance alternative activation [1, 3, 5, 29]. AAMϕs can express Arg1, an enzyme adapted from the urea cycle which converts L-arginine to L-ornithine, enabling L-ornithine decar-boxylase (ODC) to produce polyamines for cell division and L-ornithine amino-transferase (OAT) to supply proline for collagen synthesis [11, 30]. In vitro, arginine to ornithine conversion is rate limiting for proline production by macrophages; consequently stimulating IL-4Rα, inducing Arg1, and producing ornithine increases proline output [23]. In S. mansoni-infected mice, inhibiting ODC to make more ornithine available to OAT for proline synthesis increases granuloma size and deposits more collagen in the liver [23].
Immunizing mice with S. mansoni eggs plus IL-12 before infection, to deviate towards Th1 immunity, reduces chronic stage fibrosis and acts as an anti-pathology vaccine that prevents scarring without compromising host defense [31]. Immunized mice develop smaller granulomas and much less collagen deposition, although surviving the acute stage implies some IL-4/13 stimulation of macrophages [16, 21, 22]. These effects require both IFN-γ and iNOS [32]. Strikingly, Th1 deviation in iNOS-deficient mice creates granulomas that are eight times larger and leads to deposition of more collagen than in wild-type mice, forming a unique and highly dense pattern of fibrosis around the eggs [32]. These aberrant granulomas form without clear changes in cytokine or leukocyte compositions but correlate with macrophages relocating from the periphery to the center of the granuloma. To our knowledge, how nitric oxide-producing CAMϕs inhibit fibrosis remains unexplained, although the decreased availability of L-arginine in the local milieu could be a major mechanism. Since Th2 cytokine production, AAMϕ activation, and Arg1 expression correlate with fibrosis, while Th1 responses, CAMϕs, and iNOS correspond with early death but not fibrosis, AAMϕs were hypothesized to play a beneficial, repair-mediating role during acute infection while promoting fibrosis and liver pathology in chronic schistosomiasis [13, 14, 21, 23] (Fig. 1).
Unexpectedly, selectively deleting Arg1 in macrophages exacerbates Th2-mediated pathology during chronic schistosomiasis, turning the infection lethal [33]. These mice fail to down-modulate granulomatous inflammation, a regulation step which normally marks the acute-to-chronic stage transition [13, 14]. Arg1 deficiency does not interfere with alternative activation, inhibiting iNOS does not ameliorate pathology, and death is not associated with the development of endotoxemia or hepatocyte death. Instead, Arg1 expression by macrophages restricts T-cell proliferation in vitro and in vivo, which can be explained if macrophages locally deplete the available extracellular stores of L-arginine. Competition for L-arginine might also limit the functions of macrophages versus fibroblasts, or CAMϕs versus AAMϕs [7, 11, 34]. Thus, IL-4Rα and Arg1 deficiency cause fundamentally different defects in macrophage function during schistosomiasis [16, 33].
Arg1 may represent one element in a pattern of feedback inhibition, where IL-4Rα-stimulated AAMϕs produce factors limiting the T-cell responses that triggered their expression [3, 5, 34]. Like Arg1, Relmα is induced in macrophages by IL-4, repressed by IFN-γ, and limits Th2 cytokine responses to S. mansoni eggs in the liver and lung [35, 36]. Relmα-deficient mice develop higher IgE titers, larger granulomas, and more collagen deposition, corresponding with greater IL-4 and IL-5 production by T cells, than wild-type mice. However, Relmα expression is not limited to macrophages; hence, the role of Relmα derived from epithelial cells and eosinophils still needs to be determined.
Macrophage activation also induces ligands for PD-1, a T-cell inhibitory receptor. In vitro, IFN-γ and LPS upregulate PD-L1 but not PD-L2, while IL-4 promotes PD-L2 expression, suggesting CAMϕs and AAMϕs may limit T-cell activation using different genes of the same pathway [37]. Splenic macrophages from mice infected using only male S. mansoni, to study the immune response to worms but not eggs, inhibit T-cell proliferation in culture but via PD-L1 rather than PD-L2 [38]. However, T cells acquire intrinsic hyporesponsiveness during chronic schistosomiasis linked to the anergy-associated E3-ubitquitin ligase GRAIL [39]. This hyporesponsiveness remains evident in vitro without including APCs and neither correlates with PD-1 expression nor (in data not shown) abates with PD-1 blockade. These results might be reconciled if PD-1-mediated inhibition is localized, for example, if AAMϕs deter T-cell proliferation in granulomas but the liver-infiltrating population is renewed by T cells from elsewhere.
IL-10 plays an anti-pathological role during many infections, including schistosomiasis, and the distinction between IL-12 versus IL-10 secretion underlies the concept of macrophage polarization [1, 2, 5, 13, 14]. Egg-induced Th2 differentiation depends on IL-10, but IL-10 reduces both Th1 and Th2-mediated damage [18]. In adoptive transfer experiments, CD4+ T-cell-derived IL-10 plays a more important role than macrophage-derived IL-10, but nonetheless protects in cooperation with IL-10 from RAG-independent cells, presumably macrophages [40]. IL-10 also changes the sensitivity of macrophage activation by boosting IL-4Rα levels and preventing IL-4Rα down-regulation by LPS [29]. In addition, IL-10 sustains MR expression in a subset of IL-4Rα-deficient granuloma macrophages, suggesting IL-10 contributes to the AAMϕ phenotype independently of IL-4/13 [41]. Whether by cause or effect, macrophage-mediated protection during schistosomiasis relies heavily on IL-10.
To add another layer of complexity, questions of whether and how macrophage immunoregulatory mechanisms affect regulatory CD4+ T lymphocytes (Tregs) are only now beginning to be explored [42, 43]. Suppression by both Foxp3+ Tregs and IL-10 derived from Foxp3− T cells modulate immunity and reduce pathology during schistosomiasis [40, 42, 44]. Effector and regulatory T cells may be similarly receptive to cues from AAMϕs, such as arginine depletion, PD-1 ligation, and Relmα stimulation, which limit T-cell responses within granulomas. Alternatively, Tregs may be less susceptible to such stimuli, enabling AAMϕs and Tregs to cooperatively suppress Th2 responses. A matching pattern of chemokine and chemokine receptors also suggests that AAMϕs recruit Tregs and Th2 cells to the same location, which might facilitate suppression [5]. At least in some circumstances, interactions with AAMϕs favor differentiation into Tregs and, reciprocally, Treg-derived IL-10 could promote macrophage polarization [5, 45].
It is important to consider the discrepancies reported between similar schistosomiasis studies. Differences in parasite dose, mouse strain, experimental design, and, presumably, environmental bacteria may contribute to different outcomes [13–15]. Examples include mortality rates in IL-4-deficient mice [18–20], Arg1 reducing chronic liver versus acute intestinal pathology [33, 45], the efficacy of blocking oxidative damage versus IL-12 in preventing Th1-mediated disease [21, 33, 45], and the protective effect of eggs plus IL-12 versus the pathological consequences of eggs plus CFA immunization in some strains of mice [31, 46]. However, these differences should not confuse the more important point that most data are concordant and support a consensus that AAMϕs limit immune-mediated damage.
Future directions
These schistosomiasis studies demonstrate that it is possible to separate host defense from immune-mediated fibrosis through the functions of AAMϕs. Furthermore, immune deviation experiments reveal an alternative, iNOS-dependent mechanism of reducing granuloma formation and liver fibrosis without compromising host survival [31]. The arginine metabolic pathway links these findings: both Arg1 and iNOS reduce scarring, both enzymes consume arginine, and T cells require this amino acid to proliferate while fibroblasts use it to synthesize collagen [11, 30, 34]. Therefore, competition between activated macrophages, T cells, and fibroblasts for scarce nutritional resources likely influences the outcome of tissue inflammation and remodeling.
One highly desirable outcome is to resolve inflammation, reverse fibrosis, and restore normal tissue architecture and function. Since macrophages are capable of remodeling the extracellular matrix, both directly by producing matrix-digesting enzymes and their inhibitors and indirectly by regulating T cells and fibroblasts, therapies designed to manipulate macrophage activation appear well suited to these goals [5, 7, 34]. Praziquantel and other drugs cure schistosome infections quickly, effectively, and cheaply in patients and mice, and, although the dead worms further provoke the immune response, varying degrees of collagen resorption and liver regeneration follow treatment [15, 28, 47]. However, this resolution-reversal stage is little studied compared with investigations of acute or chronic schistosomiasis even though recovering from fibrosis is clinically important to this infection and potentially relevant to a wide range of diseases. The data to date are mostly based on pathology and collagen degradation with more limited cellular or molecular analysis and, to our knowledge, no study has yet directly targeted macrophages during the resolution phase of schistosomiasis.
And yet, even these briefly presented examples show the challenge of interpreting correlative evidence and demonstrating the causal and critical mechanisms of different macrophage subsets. Communication between different types of cells in multiple organs over a series of disease stages governs the pathology caused by schistosomiasis, and the activation status of macrophages is likely dynamic throughout infection [7, 13, 14, 34]. Furthermore, the processes by which macrophages can deactivate fibroblasts – phagocytosing dead cells and microbes, remodeling the extracellular matrix, and ceasing to produce pro-fibrotic factors such as TGF-β, PDGF, and IL-1β – are problematic to measure in vivo. Macrophages might transiently adopt a pro-inflammatory, pro-fibrotic phenotype in a newly forming granuloma that switches, when the egg dies, to resolve the inflammation and remove the collagen deposits. If so, this switch would be obscured in a cross-sectional analysis of the entire macrophage population. Similarly, macrophages’ functions may determine their location in granulomas, or vice-versa, but addressing this topic demands painstaking experiments that, although intriguing, still fall short of a causative explanation [32, 41, 48].
Another pertinent question is whether resident and recruited macrophages play distinct and limited roles or whether activating either cell type enables similar functions [34, 49, 50]. Schistosome eggs cause leukocyte emigration into the liver on a large scale, creating the plausible assumption that the resident Kupffer cell macrophages have scant influence because they would be greatly outnumbered by macrophages derived from monocytes that migrate from the blood and differentiate [14, 48]. This assumption should be reexamined, because other parasite infections drive the proliferation of resident macrophages in many different tissues, and treating with IL-4 is sufficient to induce Kupffer cell proliferation [50]. The resolution of peritoneal inflammation caused by zymosan injection likewise includes a stage when resident macrophages enter cell cycle and renew their population [49]. These discoveries support a hypothesis that recruited macrophages play an anti-microbial but destructive role later supplanted by proliferating resident macrophages, which repair damage, such as by deconstructing granulomas [50]. Where experimentally possible, a more precise choice of which activated macrophages to analyze might transform what seems a heterogeneous population responding to the same mix of cytokines and other stimuli into discrete families of cells following a series of activation steps [4, 34].
Several broad questions emerge from these observations. First, what distinguishes a ‘regulatory’ from a ‘wound-healing’ phenotype [1, 3], especially if macrophages in both activation states are present in the same local environment at the same time? Also, although the gene expression profiles of CAMϕs and AAMϕs are distinct, can cells in separate activation states employ different genes to accomplish the same function? Furthermore, these activation states have been primarily defined and manipulated by cytokine stimulation, but what other stimuli are important? Finally, it will be interesting and important to compare the roles of different macrophage activation states between schistosomiasis and other chronic diseases, such as asthma, idiopathic pulmonary fibrosis, systemic sclerosis, and viral cirrhosis, as well as search for parallels with myeloid-derived suppressor cells and the immune response to tumors. All these questions warrant expanding the characterization and analysis of macrophages isolated from interesting in vivo contexts. If conserved anti-pathological mechanisms can be identified by comparing macrophage functions in very different diseases, these similarities might reveal novel therapeutic strategies [7, 34].
Summary
Macrophages have the capability to promote, restrict, or resolve inflammation and fibrosis, and their role depends on their activation status [1, 3, 7]. AAMϕs are required to survive the acute phase of S. mansoni infection because, if these cells cannot respond to IL-4 or IL-13, parasite eggs crossing the intestine reduce barrier integrity and cause endotoxemia and an excessive and lethal Th1 and CAMϕ response against enteric bacteria [13, 14, 16] (Fig. 1). AAMϕs are also critical to reduce morbidity and mortality during the chronic stage of infection. Although IL-4/13 production, alternative macrophage activation, and Arg1 all correlate with liver fibrosis during schistosomiasis, Arg1-expressing AAMϕs act in a negative feedback loop on Th2 cells, and directly or indirectly on fibroblasts, to reduce tissue scarring and pathology [33] (Fig. 1). Macrophage-derived Relmα, PD-1 ligands, and IL-10 display similar regulatory roles, feeding back to restrict the Th2 immune response that induced their expression [35, 36, 38, 40]. These studies nicely illustrate how AAMϕs protect mice from acute and chronic S. mansoni infection through distinct mechanisms.
Acknowledgments
The authors thank Margaret Mentink-Kane, Thirumalai Ramalingam, Robert Thompson, Kevin Vannella, Lee Borthwick, and Allen Cheever for helpful discussions, and Ethan Tyler for transforming scratch paper sketches into appealing graphics. This work was supported by the intramural research program of the NIAID/NIH.
Abbreviations
- AAMϕ
alternatively activated macrophage
- Arg1
arginase 1
- CAMϕ
classically activated macrophage
- iNOS
inducible nitric oxide synthase
- Treg
regulatory CD4+ T lymphocyte
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
See accompanying Viewpoint: http://dx.doi.org/10.1002/eji.201141743
The complete Macrophage Viewpoint series is available at: http://onlinelibrary.wiley.com/doi/10.1002/eji.v41.9/issuetoc
References
- 1.Mosser DM, Edwards JP. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez FO, et al. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 3.Martinez FO, et al. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 4.Murray PJ, Wynn TA. J Leukoc Biol. 2011;89:557–563. doi: 10.1189/jlb.0710409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas SK, Mantovani A. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 6.Allen JE, Wynn TA. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wynn TA, Barron L. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbas AK, et al. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 9.Das P, et al. PLoS Pathog. 2010;6:e1000899. doi: 10.1371/journal.ppat.1000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Kasmi KC, et al. Nat Immunol. 2008;9:1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munder M. Br J Pharmacol. 2009;158:638–651. doi: 10.1111/j.1476-5381.2009.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotez PJ, et al. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearce EJ, MacDonald AS. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 14.Wynn TA, et al. Immunol Rev. 2004;201:156–167. doi: 10.1111/j.0105-2896.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- 15.Cheever AW, et al. Mem Inst Oswaldo Cruz. 2002;97:917–940. doi: 10.1590/s0074-02762002000700002. [DOI] [PubMed] [Google Scholar]
- 16.Herbert DR, et al. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 17.Jankovic D, et al. J Immunol. 1999;163:337–342. [PubMed] [Google Scholar]
- 18.Hoffmann KF, et al. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 19.Fallon PG, et al. J Immunol. 2000;164:2585–2591. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- 20.Brunet LR, et al. J Immunol. 1997;159:777–785. [PubMed] [Google Scholar]
- 21.La Flamme AC, et al. J Immunol. 2001;166:1903–1911. doi: 10.4049/jimmunol.166.3.1903. [DOI] [PubMed] [Google Scholar]
- 22.Herbert DR, et al. J Immunol. 2008;180:4948–4955. doi: 10.4049/jimmunol.180.7.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hesse M, et al. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 24.Ramalingam TR, et al. Nat Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mentink-Kane MM, et al. Proc Natl Acad Sci USA. 2004;101:586–590. doi: 10.1073/pnas.0305064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiaramonte MG, et al. J Clin Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiaramonte MG, et al. J Exp Med. 2003;197:687–701. doi: 10.1084/jem.20020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrade ZA. Parasite Immunol. 2009;31:656–663. doi: 10.1111/j.1365-3024.2009.01157.x. [DOI] [PubMed] [Google Scholar]
- 29.Lang R, et al. J Immunol. 2002;169:2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 30.Bronte V, Zanovello P. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 31.Wynn TA, et al. Nature. 1995;376:594–596. doi: 10.1038/376594a0. [DOI] [PubMed] [Google Scholar]
- 32.Hesse M, et al. Am J Pathol. 2000;157:945–955. doi: 10.1016/S0002-9440(10)64607-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pesce JT, et al. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barron L, Wynn TA. Am J Physiol Gastrointest Liver Physiol. 2011;300:G723–G728. doi: 10.1152/ajpgi.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pesce JT, et al. PLoS Pathog. 2009;5:e1000393. doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nair MG, et al. J Exp Med. 2009;206:937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loke P, Allison JP. Proc Natl Acad Sci USA. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith P, et al. J Immunol. 2004;173:1240–1248. doi: 10.4049/jimmunol.173.2.1240. [DOI] [PubMed] [Google Scholar]
- 39.Taylor JJ, et al. J Clin Invest. 2009;119:1019–1028. doi: 10.1172/JCI36534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hesse M, et al. J Immunol. 2004;172:3157–3166. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 41.Dewals BG, et al. PLoS Negl Trop Dis. 2010;4:e689. doi: 10.1371/journal.pntd.0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson MS, et al. Immunol Cell Biol. 2007;85:148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Layland LE, et al. J Immunol. 2010;184:713–724. doi: 10.4049/jimmunol.0901435. [DOI] [PubMed] [Google Scholar]
- 44.Dewals B, et al. Eur J Immunol. 2010;40:2837–2847. doi: 10.1002/eji.200940075. [DOI] [PubMed] [Google Scholar]
- 45.Herbert DR, et al. J Immunol. 2010;184:6438–6446. doi: 10.4049/jimmunol.0902009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rutitzky LI, Stadecker MJ. Eur J Immunol. 2011;41:2677–2687. doi: 10.1002/eji.201041327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang YJ, et al. PLoS One. 2011;6:e20247. doi: 10.1371/journal.pone.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burke ML, et al. PLoS Negl Trop Dis. 2010;4:e598. doi: 10.1371/journal.pntd.0000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davies LC, et al. Eur J Immunol. 2011;41:2155–2164. doi: 10.1002/eji.201141817. [DOI] [PubMed] [Google Scholar]
- 50.Jenkins SJ, et al. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]