Abstract
Background
Clinical evidence that ketamine, a nonselective N-methyl-D-aspartate receptor (NMDAR) antagonist, has therapeutic effects within hours in people suffering from depression suggests that modulating glutamatergic neurotransmission is a fundamental step in alleviating the debilitating symptoms of mood disorders. Acutely, ketamine increases extracellular glutamate levels, neuronal excitability and spontaneous gamma oscillations, but it is unknown whether these effects are key to ketamine's mechanism of antidepressant action.
Methods
Twenty drug-free MDD patients received a single, open-label intravenous infusion of ketamine hydrochloride (.5 mg/kg). Magnetoencephalographic recordings were made ≈3 d before and ≈6.5 h after the infusion while patients passively received tactile stimulation to the right and left index fingers, and also while they rested (eyes-closed). Antidepressant response was assessed by percent change in Montgomery-Åsberg Depression Rating Scale scores.
Results
Patients with robust improvements in depressive symptoms 230 m post-infusion (responders) exhibited increased cortical excitability within this antidepressant response window. Specifically, we found that stimulus-evoked somatosensory cortical (SS ctx) responses increase post-infusion relative to pretreatment responses in responders but not in treatment non-responders. Spontaneous SS ctx gamma-band activity during rest did not change within the same timeframe following ketamine in either responders or non-responders.
Conclusions
These findings suggest NMDAR antagonism does not lead directly to increased cortical excitability hours later, and, thus, may not be sufficient for therapeutic effects of ketamine to take hold. Rather, increased cortical excitability as depressive symptoms improve is consistent with the hypothesis that enhanced non-NMDAR-mediated glutamatergic neurotransmission via synaptic potentiation is central to ketamine's antidepressant effect.
Keywords: cortical excitability, gamma oscillation, ketamine, major depression, magnetoencephalography, NMDA antagonist
Introduction
Interest in identifying potential glutamatergic system dysfunction in mood disorders is gaining momentum (1–2). Mounting evidence, from post-mortem (3–4) and magnetic resonance spectroscopy (MRS) studies (2), points to abnormalities in glutamatergic neurotransmission in depressed individuals. Clinical research has established a range of compounds that have antidepressant properties through their direct action on the glutamatergic system. A major discovery in this context is the finding that a single subanesthetic dose of the nonselective N-methyl-D-aspartate receptor (NMDAR) antagonist ketamine can produce a sustained antidepressant effect within hours in patients with treatment-resistant major depressive disorder (MDD, [5]) and bipolar disorder (BD, [6–7]). Because conventional antidepressants that target monoaminergic systems typically take weeks to be effective, an understanding of the mechanism of ketamine's rapid antidepressant action would significantly expedite the development of fast acting and more effective drug therapies for depressive illness.
Preclinical work has provided critical insight into candidate synaptic and intracellular phenomena underlying rapid antidepressant-like effects of NMDAR blockage. For instance, ketamine's antidepressant-like effects can be neutralized by pretreatment with NBQX, an α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) antagonist (8). This finding, which has been replicated (9–11), suggests that AMPA-mediated neurotransmission is centrally involved in the rapid effects of ketamine, and, indeed, other glutamatergic-modulating compounds with antidepressant-like properties have been shown to increase AMPAR trafficking to the post-synaptic membrane (12). Ketamine administration also triggers rapid increases (i.e., at 2 h) in activity of the mammalian target of rapamycin (mTOR) pathway (11), which is followed by increased expression of synaptic proteins as well as increased density and function of spine synapses (reviewed in [13]). Notably, these effects are abolished by pretreatment with AMPAR antagonists (11). More recently, it has been demonstrated that NMDAR blockage initiates a cascade of intracellular processes that boosts translation of brain-derived neurotrophic factor (BDNF), which, in turn, promotes synaptic plasticity (9). A key role for BDNF translation is further highlighted by the finding that BDNF Val66Met knock-in mice have impaired synaptogenesis and lack an antidepressant-like response to ketamine in the forced swim test (14). Beyond a study of serum BDNF levels that did not show any change following ketamine administration or a relation to antidepressant response (15), these findings await translation to patient populations.
At the circuit level, NMDAR antagonists such as ketamine are known to increase glutamate release and neuronal excitability, acute effects attributed to dis-inhibition of pyramidal excitatory neurons due to reduced GABAergic inhibitory feedback (16–17). Thus, extracellular glutamate levels may surge and promote plastic changes such as increased AMPAR surface expression (18). Ketamine also directly induces spontaneous gamma synchrony (30–80 Hz oscillations) within cortical networks. This is a well replicated phenomenon across species, which may be driven by reduced NMDAR-mediated input specifically to fast-spiking parvalbumin-expressing GABAergic interneurons (19–23). This latter effect may be relevant to the psychotomimetic symptoms experienced during ketamine administration (24–26), and has received ample attention in schizophrenia studies (27). Whether ketamine's effects on cortical excitability and/or spontaneous cortical gamma activity last long after dissociative symptoms have dissipated is unknown, but may provide critical insight into the link between modulating glutamatergic neurotransmission and reducing depression. This question can be readily addressed noninvasively in depressed patients with whole-head magnetoencephalography (MEG) to determine whether these effects are related to rapid changes in depressive symptoms following ketamine.
In the present study, we administered a single open-label intravenous infusion of ketamine hydrochloride (0.5 mg/kg over 40 m) to drug-free patients with treatment-resistant MDD. MEG data were collected (on average) 3 d before and 6.5 h after the infusion, to compare baseline cortical activity with cortical activity within the antidepressant response window of ketamine. For pre- and post-infusion MEG sessions, we recorded neuromagnetic activity while patients received tactile stimulation of the left and right index fingers to measure stimulus-evoked somatosensory cortical (SS ctx) excitability, and also during rest (eyes-closed) to measure spontaneous SS ctx activity. The focus on SS ctx was based on evidence that synaptic plasticity (i.e., potentiation and depression) can be induced relatively easily in this cortical region (28–29). We hypothesized that enhanced cortical excitability, consistent with synaptic potentiation, rather than enhanced spontaneous cortical gamma-band activity, would be specifically linked to rapid antidepressant responses following ketamine.
Methods and Materials
Patients
All patients were studied at the National Institute of Mental Health (NIMH) in Bethesda, Maryland between January 2007 and December 2009. Twenty right-handed patients (5 women, age, mean ± SD = 46 ± 14 yr) with a DSM-IV diagnosis of MDD (30) without psychotic features met the following inclusion criteria: current major depressive episode of at least 4 w duration, current or past history of lack of response to two adequate antidepressant trials (19/20 patients met this criterion for the current episode), and a Montgomery-Asberg Depression Rating Scale (MADRS, [31]) score of ≥ 22. Diagnosis was determined by Structured Clinical Interviews for Axis I DSM-IV Disorders – Patient Version (32). Patients were hospitalized for the study duration and drug free from psychotropic medications for at least 2 w prior to MEG testing (5 w for fluoxetine). To establish treatment resistance, adequacy of past antidepressant trials was determined using the Antidepressant Treatment History Form (ATHF)-modified (33). All patients were physically healthy as determined by medical history, physical examination, electrocardiogram (ECG), chest x-ray, urinalysis and toxicology screen. The study was approved by the Combined Neuroscience Institutional Review Board of the National Institutes of Health. All subjects provided written informed consent before enrollment and were assigned a clinical research advocate from the NIMH Subject Protection Unit to monitor the consent process and research participation.
Drug administration
Patients received a single open-label infusion of 0.5 mg/kg of ketamine hydrochloride (Abbott Labs, North Chicago, IL) over 40 m via a Baxter infusion pump by an anesthesiologist. Following the infusion, patients entered a randomized, double-blind placebo-controlled 4 w clinical trial of riluzole to determine whether effects of ketamine can be modified through long term modulation of glutamatergic neurotransmission. Clinical outcome data are reported elsewhere (34). Riluzole, which has been shown to have antidepressant effects after 2 w (35), is thought to inhibit presynaptic glutamate release rather than act on postsynaptic receptors (36). Responders and non-responders to ketamine were equally likely to receive riluzole or placebo. The first oral dose of riluzole (50 mg, N=12) or placebo (N=8) was administered 5–6 h after ketamine to ensure that antidepressant effects had not begun to dissipate, and ≈1 h before the post-ketamine MEG recordings (Fig. 1). Given evidence that peak serum levels of orally-administered riluzole are achieved after 1–1.5 h in healthy volunteers (37), we did not expect riluzole to significantly influence the MEG recordings but tested this possibility. Moreover, while single doses of ≥ 250 mg can produce dizziness/vertigo, no central or peripheral side effects (e.g., ECG) at a 50 mg dose have been reported previously (37). In the present sample, side effects did not differ between the riluzole and placebo groups over the course of treatment (34).
Figure 1.
Timeline of ketamine session. Patients received a single open-label infusion of ketamine (.5 mg/kg) over 40 m (Ket). Patients' depressive, dissociative and psychotic symptoms were assessed before the infusion and at several time points afterwards (indicated by arrows). Depressive symptom change at 230 m was used to define responder and non-responder groups. Blood draws to measure plasma metabolite concentrations were taken around the same times following the infusion. Post-ketamine MEG recordings (MEG) were made ≈6.5 h after the start of infusion. An oral dose of riluzole (50 mg) or placebo was administered ≈1 h before MEG recordings.
Clinical measures
Patients were rated 60 m prior to ketamine infusion and at multiple time points post-infusion (40 m, 80 m, 110 m, 230 m). Rating scales included the MADRS (31), which was the primary outcome measure. Values for items on this scale that do not change over brief time periods (e.g., sleep, appetite) were carried forward from the baseline ratings. Responders were defined as patients exhibiting ≥ 50% reduction in MADRS scores at 230 m relative to baseline scores. Secondary outcome measures were the Brief Psychiatric Rating Scale positive symptoms subscale (BPRS-pos, [38]) and the Clinician Administered Dissociative States Scale (CADSS, [39]), which measure psychotic and dissociative symptoms, respectively.
Plasma measurements
Ketamine and norketamine plasma levels were obtained along with clinical ratings following the infusion. Analyses were performed by Gas Chromatograph-Mass Spectrometer (Agilent Technologies, Santa Clara, CA) at NMS Labs (Willow Grove, PA; for detailed methods, see reference [6]).
MEG and MRI acquisition
Baseline MEG recordings were made 1–6 d before the infusion, and post-ketamine MEG recordings were made 6–7 h after the infusion. Patients completed two runs (250 s/run) while receiving tactile stimulation of the left and right index fingers (500 stimuli, 25 ms duration, 2 Hz average rate). Tactile stimulation was controlled by a pneumatic stimulating device that uses a brief burst of air (30 psi) to displace a plastic membrane resting against the skin of the distal phalange. Patients also completed two runs (250 s/run) in which they rested with their eyes closed.
For each run, neuromagnetic activity was recorded by a CTF-OMEGA 275-channel whole-head magnetometer (VSM MedTech, Ltd., Canada) in a magnetically-shielded room (Vacuumschmelze, Germany), using synthetic 3rd order balancing for active noise cancellation. Data were acquired at 1200 Hz with a bandwidth of 0–300 Hz. Anatomical T1-weighted MRIs were obtained from each patient using a 1.5 T or 3 T GE whole-body scanner (Milwaukee, WI) in a separate session.
Time-frequency analysis
All MEG analyses were done by an experimenter (B.R.C.) who was blind to patients' clinical ratings and session information. Time-frequency analyses were conducted on the sensor data to examine stimulus-evoked response characteristics. Stimulus epochs (−100 to 300 ms, locked to stimulus onset) were time-domain averaged before applying a Stockwell transform (40) to the data. Plots of evoked power were normalized by pre-stimulus power, and averaged across sensors overlying the hemisphere contralateral to stimulation. Source analyses were tailored to the response characteristics shown in these plots.
Source analysis: stimulus-evoked responses
A minimum-variance adaptive beamformer algorithm (41) was used to determine sources of the stimulus-evoked gamma-band responses (for similar methods, see references [42–43]). For each dataset (2 runs × 2 sessions), a single covariance matrix was calculated from 500 unaveraged epochs, from −100 to 300 ms relative to stimulus onset, with a 30–50 Hz bandpass filter. A multi-sphere source space model derived from patients' MRIs was used for source power estimation. Beamformer weights were calculated with a normalized vector formulation that determines optimal source orientation in three-dimensional space for computing the biomagnetic forward solution at each voxel (44). Virtual sensor time series were projected by vector multiplication of the raw data by the beamformer weights, and subsequently averaged in the time domain to attenuate noise and extract the stimulus-locked evoked response at each voxel (implemented by SAMerf[45]). From the time course of evoked gamma power observed in the time-frequency plots, we contrasted power in the 30–60 ms post-stimulus window with power in a 30-ms pre-stimulus window.
Using AFNI (46), individual-subject source volumes, which represent distributions of baseline-normalized stimulus-evoked power, were co-registered to their anatomical MRIs and spatially warped to a Talairach template for group-level analyses. For visualization, group-averaged maps for baseline and post-ketamine sessions were overlayed on a standardized MRI and statistically thresholded based on one-sample t tests at a false discovery rate (47) of .001. Peak responses were identified in left and right sensorimotor cortices for contralateral stimulation after averaging group-maps across baseline and post-ketamine sessions. Spherical masks of 5-mm radii were centered at each source to extract mean power estimates. The same source identified in each hemisphere for contralateral stimulation was used for extracting individual mean power estimates for ipsilateral stimulation.
Source analysis: resting-state activity
A similar minimum-variance adaptive beamformer algorithm was employed to determine changes in cortical resting-state (eye-closed) activity before and after ketamine (for similar methods, see reference [48]). For each dataset (2 runs × 2 sessions), a single covariance matrix was calculated over 4 m of rest, with a 30–50 Hz bandpass filter. Source power at each voxel was estimated over the single 4 m epoch and normalized by a constant noise estimate, which is derived from the same covariance matrix, to correct for the depth bias in beamformer power estimates (pseudo-Z deviate [41]). The same spherical masks defined by the previous analyses of stimulus-evoked responses were used to extract mean gamma-band power from left and right SS ctx before and after ketamine.
Statistical analyses
Mixed-effects ANOVAs were conducted on extracted data in SPSS 18 (α = .05). For stimulus-evoked responses, a 2 (Group: responders vs. non-responders) × 2 (Hemisphere: left vs. right) × 2 (Stimulation: contralateral vs. ipsilateral) × 2 (Time: pre-ketamine vs. post-ketamine) mixed-effects ANOVA was conducted. For resting activity, a 2 (Group) × 2 (Hemisphere) × 2 (Time) mixed-effects ANOVA was conducted. We also ran these analyses by replacing the Group variable with MADRS % change scores as a continuous between-subjects variable to ensure that any null effects related to treatment response were not due to loss of power after dichotomization. The same statistical outcomes were obtained and thus are not reported. Both models were also expanded by the additional grouping factor of Riluzole (riluzole vs. placebo) to determine whether administration of this drug affected the results.
For responders and non-responders separately, Pearson correlations were calculated to test for relationships between mean stimulus-evoked SS ctx responses and plasma concentrations of ketamine and norketamine taken at four time points. The aim of these correlation analyses was to provide evidence for a direct link between ketamine administration and post-ketamine cortical responses given the absence of a placebo control. Due to positive skew in plasma concentration levels, a log10 transformation was applied to these data. Statistical significance (two-tailed) was determined using a Bonferroni correction for multiple correlation tests (α = .05/16 = .0031).
Results
Symptomatic change
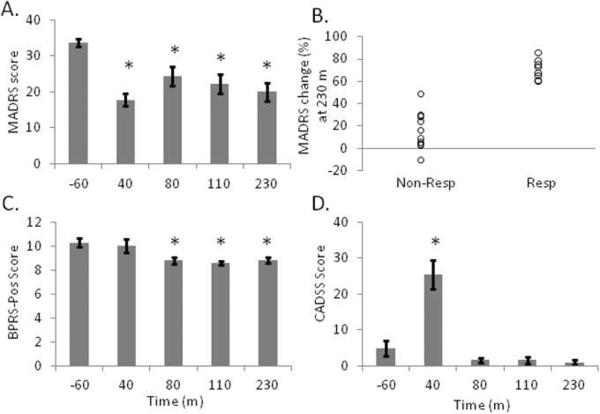
Paired t tests (Bonferroni-corrected) were conducted to assess symptom change at each time point following ketamine relative to baseline scores taken 60 m prior to the infusion. One patient was missing a MADRS score at 40 m, a BPRS-pos score at 40 m and 80 m, and a CADSS score at 80 m. A second patient was missing a CADSS score at 40 m. MADRS scores were significantly reduced at all time points (all t's > 4.7, p's < .001, Fig 2A). Nine patients reached an response criterion of ≥ 50% symptom improvement (“responders”) at 230 m; 11 patients showed little to no improvement at this time point (“non-responders”, Fig. 2B). Antidepressant response was not associated with participant gender: of the 9 responders, 2 were female patients and 7 were male patients, and of the 11 non-responders, 3 were female patients and 8 were male patients, χ21 = .07, p = .80. Responders and non-responders were also equally randomly-assigned to receive riluzole (6 of 9 responders received riluzole and 6 of 11 non-responders received riluzole), χ21 = .30, p = .58. BPRS-pos scores were significantly reduced at 80 m (t18 = 4.63, p < .001), 120 m (t19 = 4.59, p < .001) and 230 m (t19 = 3.88, p = .001), but not at 40 m (t18 = 0.73, p =.47, Fig. 2C). CADSS scores were significantly increased at 40 m (t18 = −5.95, p < .001, Fig. 2D), but not at later time points (all t's < 2.64, p's > .015).
Figure 2.
Depressive symptoms improve after ketamine. A, Depressive symptoms measured by the Montgomery-Asberg Depressive Rating Scale (MADRS) decrease 40 m following the infusion and remain low through 230 m. B, Patients were divided into responders (Resp, N = 9) and non-responders (Non-Resp, N = 11) based on percent change in MADRS scores. C, Psychotic symptoms measured by the Brief Psychiatric Rating Scale-positive symptoms subscale (BPRS-pos) decrease after 80 m and remain low through 230 m. D, Dissociative symptoms measured by the Clinician Administered Dissociative States Scale (CADSS) spike at 40 m but return to baseline by 80 m post-infusion. *significantly different than baseline (−60 m, Bonferroni-corrected)
Stimulus-evoked SS ctx responses
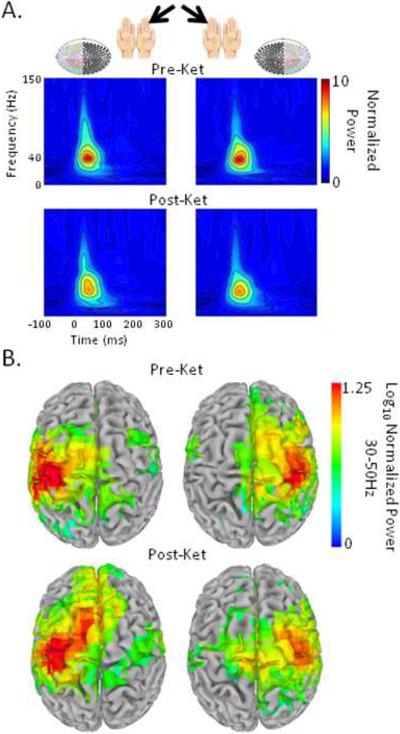
Time-frequency analyses revealed that stimulation elicited an evoked response ≈45 ms post-stimulus with a spectral peak in the gamma band over contralateral hemisphere (Fig. 3A). Group analyses revealed peak evoked power in left and right central sulci (Brodmann Area 3/4) following contralateral and ipsilateral stimulation (Fig. 3B), confirming that early stimulus-evoked gamma-band responses (GBRs) observed before and after ketamine reflect bottom-up sensory processing in left and right primary SS ctx. Spherical ROIs for extracting individual stimulus-evoked responses were centered at group-averaged peak locations after averaging pre- and post-ketamine data (for left SS ctx: −37, 17, 47 mm in Talairach space; for right SS ctx: 43, 17, 52 mm).
Figure 3.
Tactile stimulation of the left and right finger elicits an early stimulus-evoked response that is localized to left and right primary SS ctx. A, Grand-averaged (N = 20) Stockwell time-frequency plots before (Pre-Ket) and after ketamine administration (Post-Ket) show robust stimulus-evoked power to the tactile stimulus (relative to a pre-stimulus baseline) in sensors overlying the contralateral hemisphere (colored circles). B, Source analyses of evoked power (relative to pre-stimulus power) revealed peaks in left and right central sulci (BA 3/4). Whole brain maps of evoked power are statistically thresholded based on one-sample t tests (false discovery rate = .001) and overlayed on a standardized brain template.
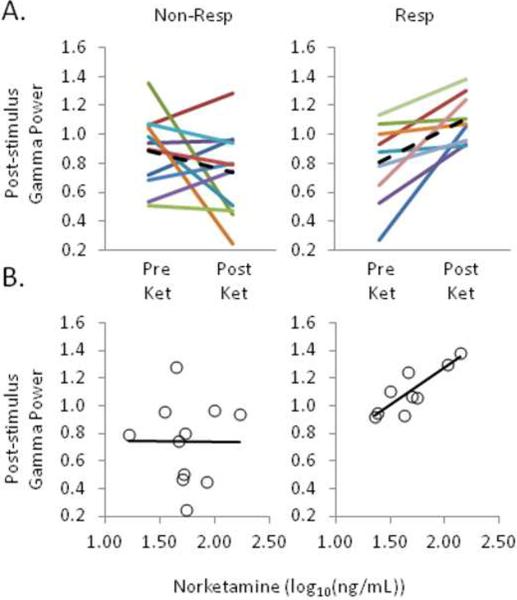
A 4-way mixed-effects ANOVA revealed that responders showed significantly increased SS ctx GBRs after ketamine relative to baseline (FTime(1,8) = 12.19, p = .008); non-responders showed no change (FTime(1,10) = 1.51, p = .25; FResponse-by-Time(1,18) = 8.38, p = .01, Fig. 4A). This interaction effect was not lateralized to one hemisphere (FHemisphere-by-Response-by-Time(1,18) = 2.81, p = .11), nor driven differentially by contralateral or ipsilateral stimulation (FStimulation-by-Response-by-Time(1,18) < 1). No acute effect of riluzole on stimulus-evoked SS ctx GBRs was detected (FRiluzole-by-Time(1,16) < 1). Moreover, the significant response-by-time interaction effect was not modulated by riluzole (FRiluzole-by-Response-by-Time(1,16) < 1, Figure S1 in the Supplement). Effect sizes for the Response-by-Time interaction for patients receiving placebo (N = 8, partial η2 = .42) vs. riluzole (N = 12, partial η2 = .24) further suggest that riluzole did not significantly influence the results.
Figure 4.
Rapid improvement in depressive symptoms following ketamine is linked to increased SS ctx excitability. A, Stimulus-evoked SS ctx gamma-band responses from pre- to post-ketamine infusion, collapsed across left and right hemispheres and contralateral and ipsilateral stimulation, were selectively increased in responders (N = 9, right) but not non-responders (N= 11, left). Solid lines represent individual patients, and the dotted lines represent the group means. B, Post-ketamine SS ctx responses were positively correlated with plasma norketamine levels (40 m relative to the start of infusion) in responders (right) but not non-responders (left).
A series of Pearson correlations revealed that norketamine levels at 40 m post-infusion positivelycorrelated with post-ketamine SS ctx responses in responders (r7 = .85, p = .003, Fig. 4B); no such correlation was found in non-responders (r9 = −.01, p = .977). Plasma ketamine and norketamine at later time points were not significantly correlated with post-ketamine SS ctx responses in either group (all p's > .11).
Spontaneous SS ctx gamma activity
A 3-way mixed-effects ANOVA revealed greater spontaneous gamma-band power, averaged over two runs, in the left (dominant) SS ctx compared to the right SS ctx (FHemisphere(1,18) < 10.27, p = .005), but no evidence of an increase after ketamine relative to baseline (FTime(1,18) < 1), or a relation to antidepressant response (FResponse-by-Time(1,18) < 1). Spontaneous gamma-band activity in SS ctx during rest was also not affected by riluzole (FRiluzole-by-Time(1,16) < 1).
Discussion
Here we investigated cortical changes associated with ketamine administration in the context of treatment response in treatment-resistant MDD patients. Stimulus-evoked responses and spontaneous somatosensory cortical (SS ctx) activity were measured with MEG before and after a single infusion of ketamine to extend our previous work that focused exclusively on pretreatment brain-based predictorsof treatment response (43, 49–50). For those patients exhibiting a rapid and robust reduction indepressive symptoms (responders), we found a uniform increase in stimulus-evoked SS ctx responses following the infusion (Fig. 4A). Among these responders, we also found a positive correlation between increased cortical excitability and plasma norketamine levels (Fig. 4B), a major active metabolite of ketamine with a relatively long half life (≈5 h) compared to ketamine (≈2 h, [50]). Patients exhibitinglittle or no improvement in depressive symptoms within hours after the infusion (non-responders), on average, showed no change in stimulus-evoked responses and no correlation with plasma metabolite levels. These results show that enhanced cortical excitability differentiates responders from non-responders to ketamine, as opposed to spontaneous cortical gamma activity that did not change in either responders or non-responders, and thus provides the first cortical marker of ketamine's rapid antidepressant action.
Notably, our findings fill a critical gap in our understanding of the mechanism underlying ketamine's effects. NMDAR blockage is known to acutely increase spontaneous gamma activity (19–25), and computational modeling supports the hypothesis that these abnormal oscillations result directly fromreducing NMDAR-mediated input to fast-spiking GABAergic interneurons (26). We, however, did not observe increases in spontaneous SS ctx gamma activity hours after the infusion in either responders or non-responders. This could reflect the dose used here (.5mg/kg), which is substantially lower than doses used in most preclinical work, as well as the timing of our measurements. To our knowledge, evidence that NMDAR antagonists increase spontaneous gamma is limited to the period just after drug intake when psychotomimetic symptoms are most prominent. Thus, ketamine-related increases in spontaneous cortical gamma may be more relevant to the acute, dissociative effects than the antidepressant effects of NMDAR antagonism. Resting-state measurements obtained during or directly after ketamine infusion could evaluate this possibility. Nevertheless, because changes in spontaneous gamma in our patients were not apparent hours after the infusion and did not correlate with changes in their depressive symptoms, the immediate effects of NMDAR antagonism may not be sufficient to elicit rapid clinical improvements. Additional processes must account for the sustained profile of increased cortical excitability in responders.
We should also note that increased cortical excitability 6–7 h after ketamine administration is not likely due to lingering differences in extracellular glutamate levels between responders and non-responders. In animals, glutamate levels return to baseline within 2 h after variable doses of ketamine (17). Likewise, recent MRS studies in humans have reported evidence of a surge of glutamate during ketamine administration (52–53), but not following its administration or, more importantly, a link between glutamate level and antidepressant response (54–55). We can speculate that ketamine-induced dis-inhibition and increased glutamatergic activity are too transient to figure directly into rapid alleviation of depressive symptoms. Nevertheless, these processes could trigger critical plastic changes at excitatory synapses that mediate the relatively long-lasting increases in cortical excitability in patients exhibiting symptomatic improvement. An upregulation of AMPAR surface expression is a strong candidate mechanism underlying this effect as emerging preclinical work indicates (8–11). Given a critical role for AMPAR-mediated neurotransmission in recruitment of fast-spiking GABAergic interneurons (56), greater sensory-driven perturbation of gamma activity in responders (i.e., increased stimulus-evoked gamma-band responses) following ketamine might reflect enhanced AMPAR-mediated glutamatergic drive of interneuronal networks.
Importantly, although our findings are consistent with enhanced AMPAR-mediated glutamatergic neurotransmission via synaptic potentiation, we did not specifically test the effects of an AMPAR antagonist (or agonist), and thus cannot exclude other mechanisms. Moreover, despite positive preclinical findings (reviewed in [1]), there is currently no clinical proof of concept data for AMPAR potentiators in depressed patients. Nonetheless, we can raise the question of whether NMDAR antagonism is a necessary starting point for modifying cortical circuitry in a way that proves to be clinically beneficial. If not, the possibility of directly triggering synaptic potentiation, rather than indirectly by NMDAR antagonists, remains a promising target for the development of potent, rapid-acting antidepressants. Alternatively, although enhanced non-NMDAR glutamatergic transmission may be an important endpoint, NMDAR antagonism may be a critical antecedent to all downstream cellular phenomena, such as local BDNF translation (9), which ultimately support the reduction of depressive symptoms.
Despite clear results, the administration of riluzole before the post-ketamine MEG recordings introduces potential complications in interpreting effects of ketamine on cortical responses. We found no evidence to indicate that riluzole contributed to post-ketamine changes in stimulus-driven responses (Figure S1 in the Supplement); indeed, effect sizes for each group (riluzole vs. placebo) were comparable. Thus, we remain confident that the timing of riluzole administration was relatively unproblematic with regards to our findings. Moreover, the open-label administration of ketamine might leave some doubt regarding the specificity of the effects. In previous clinical trials (5–6), the rate of placebo responding at 230 m was very low (1/16 and 1/18). As we used the same patient inclusion criteria for treatment resistance and symptom severity for our sample, nonspecific effects seem unlikely. The fact that, in responders, norketamine levels correlated positively with post-ketamine stimulus-evoked SS ctx responses provides additional support for a specific link between increased cortical excitability and ketamine administration. Placebo-controlled investigations with larger sample sizes are needed to confirm this link.
To conclude, our findings identify the first cortical marker of ketamine's rapid antidepressant effects, and they are consistent with the hypothesis that enhanced non-NMDAR-mediated glutamatergic neurotransmission through synaptic potentiation is central to ketamine's mechanism of action. Further work should examine whether increased cortical excitability persists for the duration of antidepressant response following a single dose of ketamine (e.g., up to 1 week [5]), and whether it attenuates upon relapse. It would also be important to determine whether similar effects of increased cortical excitability can be obtained with other NMDAR antagonists that have antidepressant action, particularly those with milder dissociative effects than ketamine (e.g., NR2B subunit-selective NMDAR antagonist, [57]). More generally, whether increased cortical excitability is a state-dependent correlate of depressive mood improvement, and thus broadly associated with clinical response to conventional antidepressants and other therapeutic interventions, or specific to glutamatergic-modulating drugs should also be considered in future studies. Finally, extending the current results to cortical regions that have been implicated in the pathophysiology of MDD and show activities that are predictive of treatment response to ketamine (e.g., rostral anterior cingulate cortex [43, 49]) may be critical to linking local cortical circuit abnormalities to specific phenotypic characteristics of mood disorders.
Supplementary Material
Acknowledgments
This research was supported by the intramural research program of the National Institute of Mental Health, and National Alliance for Research on Schizophrenia and Depression awards to C.A.Z. and G.S.
This work was conducted at the Intramural Research Program at the National Institute of Mental Health. C.A.Z. is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression. C.A.Z. has assigned his rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government. G.S. is now a full time employee of Johnson & Johnson Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures All other authors declare no biomedical financial interests or potential conflicts of interest.
Rapid Antidepressant Effects of Ketamine in Major Depression; ClinicalTrials.gov; NCT00088699
References
- 1.Sanacora G, Zarate CA, Jr, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yüksel C, Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, et al. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry. 2011;16:634–646. doi: 10.1038/mp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci USA. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 6.DiazGranados N, Ibrahim L, Brutsche N, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine's antidepressant efficacy in bipolar depression: A randomized controlled add-on trial. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2011.12.010. Online: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: Role of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P-F. NMDA receptor blockage at rest triggers rapid behavioural antidepressant response. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 11.Li N, Lee B, Liu R-J, Dwyer JM, Iwata M, Li X-Y, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, et al. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: Relationship to clinical effects in mood disorders. Neuropsychopharmacol. 2007;32:793–802. doi: 10.1038/sj.npp.1301178. [DOI] [PubMed] [Google Scholar]
- 13.Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35(1):47–56. doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu R-J, Lee FS, Li X-Y, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotropic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.09.030. Online: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machado-Viera R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, et al. Brain-derived neurotrophic factor and initial antidepressant response to an N-Methyl-D-Aspartate antagonist. J Clin Psychiatry. 2009;70:1662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;11:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- 19.Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.31. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazarawicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci. 2009;22:1452–1464. doi: 10.1162/jocn.2009.21305. [DOI] [PubMed] [Google Scholar]
- 21.Maksimow A, Särkelä M, Långsjö JW, Salmi E, Kaisti KK, Yli-Hankala A, et al. Increase in high frequency EEG activity explains the poor performance of EEG spectral entropy monitor during S-ketamine anesthesia. Clin Neurophysiol. 2006;117:1660–1668. doi: 10.1016/j.clinph.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anver H, Ward PD, Magony A, Vreugdenhil M. NMDA receptor hypofunction phase couples independent γ-oscillations in the rat visual cortex. Neuropychopharmacol. 2011;36:519–528. doi: 10.1038/npp.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong LE, Summerfelt A, Buchanan RW, O'Donnell P, Thaker GK, Weiler MA, et al. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacol. 2010;35:632–640. doi: 10.1038/npp.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant γ oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Spencer KM. The functional consequences of cortical circuit abnormalities on gamma oscillations in schizophrenia: insights from computational modeling. Front Hum Neurosci. 2009 doi: 10.3389/neuro.09.033.2009. doi: 10.3389/neuro.09.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 28.Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res Bull. 2006;69:86–84. doi: 10.1016/j.brainresbull.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- 30.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: 1994. [Google Scholar]
- 31.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 32.First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Patient edition Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- 33.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62:10–17. [PubMed] [Google Scholar]
- 34.Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs. add-on riluzole: Results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacol. 2012 doi: 10.1038/npp.2011.338. Online: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zarate CA, Jr, Payne JL, Quiroz J, Sporn J, Denicoff KD, Luckenbaugh D, et al. An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry. 2004;161:171–174. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]
- 36.Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci. 2000;12:3567–3574. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- 37.Le Liboux, Lefebvre P, Le Roux Y, Truffinet P, Aubeneau M, Kirkesseli S, et al. Single- and multiple-dose pharmacokinetics of riluzole in white subjects. J Clin Pharmacol. 1997;37:820–827. doi: 10.1002/j.1552-4604.1997.tb05630.x. [DOI] [PubMed] [Google Scholar]
- 38.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:790–812. [Google Scholar]
- 39.Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS) J Trauma Stress. 1998;11:25–36. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 40.Stockwell RG, Mansinha L, Lowe RP. Localization of the complex spectrum: The S transform. IEEEE Trans Signal Process. 1996;44:998–1000. [Google Scholar]
- 41.Vrba J, Robinson SE. Signal processing in magnetoencephalography. Methods. 2001;25:249–271. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- 42.Cornwell BR, Carver FW, Coppola R, Johnson L, Alvarez R, Grillon C. Evoked amygdala responses to negative faces revealed by adaptive MEG beamformers. Brain Res. 2008;1244:103–112. doi: 10.1016/j.brainres.2008.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sekihara K, Nagarajan SS, Poeppel D, Marantz A, Miyashita Y. Reconstructing spatio-temporal activities of neural sources using an MEG vector beamformer technique. IEEE Trans Biomed Eng. 2001;48:760–771. doi: 10.1109/10.930901. [DOI] [PubMed] [Google Scholar]
- 45.Robinson SE. Localization of event-related activity by SAM(erf) Neurol Clin Neurophysiol. 2004;30:109. [PubMed] [Google Scholar]
- 46.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 47.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using false discovery rates. Neuroimage. 2002;15:772–786. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 48.Rutter L, Carver FW, Holroyd T, Nadar SR, Mitchell-Francis J, Apud J, et al. Magnetoencephalographic gamma power reduction in patients with schizophrenia during resting condition. Hum Brain Mapp. 2009;30:3254–3264. doi: 10.1002/hbm.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, et al. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacol. 2010;34(6):819–821. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salvadore G, van der Veen JW, Zhang Y, Marenco S, Machado-Vieira R, Baumann J, et al. An investigation of amino-acid neurotransmitters as potential predictors of clinical improvement to ketamine in depression. Int J Neuropsychopharmacol. 2011 doi: 10.1017/S1461145711001593. Online: 10.1017/S1461145711001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, et al. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacol. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 52.Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry. 2005;162:394–396. doi: 10.1176/appi.ajp.162.2.394. [DOI] [PubMed] [Google Scholar]
- 53.Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed JH, et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012 doi: 10.1038/mp.2011.171. doi: 10.1038/mp.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor MJ, Tiangga ER, Ni Mhuircheartaigh R, Cowen PJ. Lack of effect of ketamine on cortical glutamate and glutamine in healthy volunteers: a proton magnetic resonance spectroscopy study. J Psychopharmacol. 2011 doi: 10.1177/0269881111405359. doi: 10.1177/0269881111405359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [1H]-MRS. Psychiatry Res: Neuroimage. 2011;191:122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, LeBeau FEN, Bannerman DM, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associative behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 57.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.