Abstract
Caveolae and caveolins, structural components of caveolae, are associated with specific ion channels in cardiac myocytes. We have previously shown that P2X purinoceptor 7 (P2X7R), a ligand gated ion channel, is increased in atrial cardiomyocytes of caveolin-1 knockout mice; however, the specific biochemical relationship of P2X7R with caveolins in the heart is not clear. The aim of this work was to study the presence of the P2X7R in atrial cardiomyocytes and its biochemical relationship to caveolin-1 and caveolin-3. Caveolin isoforms and P2X7R were predominantly localized in buoyant membrane fractions (lipid rafts/caveolae) prepared from hearts using detergent-free sucrose gradient centrifugation. Caveolin-1 knockout mice showed normal distribution of caveolin-3 and P2X7R to byoyant membranes indicating the importance of caveolin-3 to formation of caveolae. Using clear native PAGE, we showed that caveolin-1, -3 and P2X7R contribute to the same protein complex in membranes of heart atrial cardiomyocytes and in the immortal cardiomyocyte cell line HL-1. Western blot analysis revealed increased caveolin-1 and -3 protein in tissue homogenates of P2X7R knockout mice. Finally, tissue homogenates of atrial tissues from caveolin-3 knockout mice showed elevated mRNA for P2X7R in atria. The co-localization of caveolins with P2X7R in a biochemical complex and compensated upregulation of P2X7R or caveolins in the absence of any component of the complex suggests P2XR7 and caveolins may serve an important regulatory control point for disease pathology in the heart.
Keywords: P2X7R, caveolin-3, cardiomyocytes, mice, HL-1
Introduction
Caveolae are small membrane invaginations, enriched in lipids, that participate in membrane trafficking, sorting, transport and signal transduction (Parton and Simons, 2007). Caveolins are believed to play a role in the formation of the caveolae membranes, acting as scaffolding proteins organizing and concentrating caveolin-interacting proteins and lipids in caveolar microdomains (Razani and Lisanti, 2001). Currently, three different caveolin isoforms are known: caveolin-1(Cav-1), caveolin-2 (Cav-2) and caveolin-3 (Cav-3). Caveolins are proteins of 21–24 kDa, they form high molecular mass homo-oligomers. Structurally they all have N-terminal and C-terminal hydrophilic segments and a long central transmembrane domain. They are a family of integral membrane proteins which are the principal components of caveolar membranes (Razani et al., 2002). Cav-3 was identified as a muscle-specific form of the caveolin family (Galbiati et al., 2001). Cav-3 is found in both cardiac and skeletal muscle, as well as smooth muscle cells. In skeletal muscle, Cav-3 is localized to the sarcolemma, coinciding with dystrophin (Lapidos et al., 2004). It was further demonstrated that Cav-1 and Cav-3 are coexpressed in mouse and rat cardiac myocytes of the atria but not ventricles (Volonte et al., 2008).
Both Cav-1 and Cav-3 knockout mice (cav-1−/−; cav-3−/−) have been shown to develop cardiomyopathy and hypertrophy, thus indicating an important role for both caveolins in cardiac physiology. Cav-1 plays a critical role in cardiac protective signalling (Patel et al., 2007) and its scaffolding domain can inhibit matrix metalloproteinase 2 (MMP-2) activity (Cho et al., 2010). Cav-3 directly interacts with neuronal nitric oxide synthase (nNOS), the NOS isoform expressed in skeletal muscle, and binding results in the loss of NOS activity (Venema et al., 1997). Volonte et al. (Volonte et al., 2008) demonstrated that Cav-1 and Cav-3 were part of the same protein complex suggesting that both caveolin isoforms form heterooligomeric complexes in atrial cardiac myocytes.
We have previously shown in the mouse heart that P2X7R is expressed in atrial cardiomyocytes and in microvascular endothelial cells (Barth et al., 2010b). P2X7R is a 595-amino acid polypeptide with two membrane-spanning domains and intracellular N- and C-terminal domains. P2X7R is the only pore-forming P2X family member, and its activation results in the opening of a cationic channel with increased permeability to calcium and intracellular depolarization (Faria et al., 2005). Growing evidence indicates that the P2X7R is present in lipid rafts (Garcia-Marcos et al., 2006; Schwab et al., 2007; Vacca et al., 2004) and that the integrity of caveolae is important for proper ion channel function.
Some cardiac ion channels have been specifically localized to caveolae such as the ATP-sensitive KATP channel (Garg et al., 2009a), the SCN5A-encoded voltage-gated Na+ channel (hNav 1.5), the voltage-dependent K+ channel (Kv 1.5), the sodium-calcium exchanger, and the L-type Ca2+ channel (Balijepalli et al., 2006; Bossuyt et al., 2002; Lin et al., 2009). In addition, a variety of other signaling molecules and receptors have been found in caveolae of cardiac cells, including the β2 -adrenergic receptor and associated proteins of the G-protein/adenylyl cyclase/protein kinase A pathway (Barbuti et al., 2007). Therefore, caveolae can serve both to compartmentalize and to regulate ion channel function and cell signaling factors that contribute to ion channel function.
This study is the first attempt to biochemically demonstrate the presence of Cav-1, -3 and P2X7R in a protein complex within lipid rafts of atrial cardiomyocytes in vivo and in vitro.
Material and Methods
Animals
The P2X7R knockout mouse (P2rx7−/−) was generated as reported by Solle et al (Solle et al., 2001). Knockout mice were purchased from Pfizer Pharmaceuticals (B6.129P2-P2rx7tm1Gab/J).
Cav-3 knockout mice (cav3−/−) were originally obtained from Japan1 and data are from colony maintained in Dr. Hemal H. Patel’s laboratory at the University of California, San Diego. (Hagiwara et al., 2000)
Cell line and cell culture
The atrial cardiomyocyte cell line HL-1 was kindly provided by Dr. Willam C. Claycomb (Louisiana State University Health Sciences Center, New Orleans, LA, USA). Claycomb Medium was obtained from Sigma-Aldrich (St. Louis, MO, USA). The following media supplements were used: 10% (v/v) fetal bovine serum (PAN Biotech GmbH, Aidenbach, DE), 2 mM L-glutamine (Biochrom AG Seromed, Berlin, DE), 0.1 mM norepinephrine (Sigma-Aldrich). Before use, norepinephrine was made up in 30 mM ascorbic acid (Sigma-Aldrich). Trypsin/EDTA were also purchased from Biochrom AG Seromed. HL-1 cardiomyocytes (passage 48–60) were maintained in T75 cell culture flasks pre-coated with 0.00125% (v/v) fibronectin (Sigma-Aldrich) and 0.02% (v/v) gelatine (Biochrom AG Seromed). For downregulation experiments cells were plated in 12 well at a concentration of 175 × 103 cells/ml. They were grown at 37°C in a 5% CO2 atmosphere and passaged continuously. Experiments were initiated at approximately 80% confluency.
H5V cells, which were derived from murine embryonic heart endothelium, were a generous gift from Dr. Vecchi A, Padua, Italy (Garlanda et al., 1994). H5V cell line was grown in 90% DMEM and 10% FBS.
Western blot analysis
The Western blot analysis was performed as previously described (Schwab et al., 2007). The following antibodies were used: Monoclonal mouse anti-Cav-1 (clone 2297, dilution 1:500 v/v; BD Biosciences, Pharmingen, San Jose, CA, USA), monoclonal mouse anti-Cav-2 (clone 65, dilution 1:500 v/v; BD Biosciences), monoclonal mouse anti-Cav-3 (clone 26, dilution 1:1000 v/v; BD Biosciences), polyclonal rabbit anti-P2X7R (clone #APR-004, dilution 1:500 v/v; Sigma-Aldrich), polyclonal rabbit anti-protein disulfide isomerase (PDI; clone 1D3, dilution 1:1000 v/v; StressGen Biotechnologies Corp., Victoria, CA), polyclonal rabbit anti-β-coatomer protein (β-Cop; clone maD, dilution 1:500 v/v; Sigma-Aldrich), monoclonal mouse anti-flotillin-1 (clone 18, dilution 1:1000 v/v; BD Biosciences) and monoclonal mouse anti-γ-tubulin (clone GTU-88, dilution 1:1000 v/v; Sigma-Aldrich)
Detergent solubility
One subconfluent T25 tissue culture flask was washed twice in ice-cold phosphate-buffered saline (PBS) and scraped in 200 #l of MBS (25 mM Mes, 150 mM NaCl, pH 6.5) containing 1% Triton X-100 or 1% BriJ35 plus protease inhibitor cocktail (complete Mini, Roche, Prenzberg, DE). After 1 h lysis on ice, the sample was centrifuged at 17,000× g for 10 min (Allegra TM 64R, Beckman F2402H rotor; Beckman Coulter, Fullerton, CA, USA) and the supernatant was collected as soluble fraction. The pellet, equivalent to the insoluble fraction, was dissolved in 200 #l MBS containing 0.1% SDS plus protease inhibitors. The homogenate was sonicated (UP100H, Dr. Hielscher GmbH, Germany) and syringed 10 times using a 26-gauge needle. Soluble and insoluble fraction were separated by SDS-PAGE and finally subjected to Western blot analysis.
Preparation of detergent insoluble membrane fractions
The preparation of detergent insoluble membrane fractions was performed as previously described (Barth et al., 2010a).
Immunocytochemistry of tissue samples
For double immunofluorescence staining total hearts of 3-month-old wild-type cav-1−/−, or cav-3−/− mice were fixed in 4% buffered formalin for 5 h at room temperature, washed, dehydrated and embedded in paraffin. Sections of 5 #m were cut and mounted on silane-coated glas slides. The sections were dewaxed and irradiated with microwaves in 0.01 M sodium citrate buffer (pH 6.0), 2 × 5 min at 850 W. After washing in PBS, the sections were treated with 0.3% hydrogen peroxide for 30 min. Subsequently the following primary antibodies were appropriated: Monoclonal mouse anti-Cav-1 (clone 2297, dilution 1:20 v/v; BD Biosciences), monoclonal mouse anti-Cav-2 (clone 65, dilution 1:20 v/v; BD Biosciences), monoclonal mouse anti-Cav-3 (clone 26, dilution 1:40 v/v; BD Biosciences; the specificity of this antibody was additionally tested on cav-3−/− tissues, not shown), polyclonal rabbit anti-P2X7R (clone #APR-004, dilution 1:40 v/v; Sigma-Aldrich) and polyclonal rabbit anti-podocalyxin C-term peptide (dilution 1:800 v/v; Marilyn Gist Farquar, Ph.D., University of California, San Diego, CA, USA). Goat anti-mouse IgG conjugated to fluoresceine isothiocyanate (FITC; dianova, Hamburg, Germany; dilution 1:100 v/v) was used to demonstrate Cav-1, Cav-1 or Cav-3, whilst goat anti-rabbit IgG conjugated to Texas Red (dianova; dilution 1:100 v/v) was used to demonstrate P2X7R or podocalyxin immunreactivity. Finally, sections were mounted in PBS-glycerol (1:9) containing 2.5% v/v 1,4-diazabicyclo (2.2.2) octane (DABCO; Sigma, Germany) to prevent fading. For controls, the sections were solely incubated with the secondary antibody. Immunostaining of tissue sections was examined with a system microscope (Olympus BX60, Olympus optical Co., LTD, Tokyo, JP). Immunoperoxidase staining of mouse heart samples has been described earlier (Barth et al., 2010b).
High-resolution clear native-PAGE (hrCN-PAGE-3)
hrCN-PAGE-3 was performed as previously described (Weinhold et al.). The following conditions were modified: The lysis buffer contained 50 mM NaCl, 100 mM bis-tris, 5 mM 6-aminohexanoic acid and 4% digitonin. The anode buffer was made of 50 mM bis-tris/HCl (pH 7.0), whereas the cathode buffer was composed of 50 mM tricine, 15 mM bis-tris, 0.05% (w/v) Sodium Deoxycholate and 0.01% (w/v) n-Dodecyl-β-D-maltoside.
Quantitative PCR for P2X7R
RNA was isolated from the atria of wild-type and cav-3−/− mice using the fibrous tissue RNeasy kit (Qiagen, Valencia CA). Complementaty DNA (cDNA) was synthesized using the iScript cDNA synthesis kit (Biorad, Hercules CA). The qPCR was performed with Sybrgreen PCR master mix (Biorad, Hercules CA) on a CFX 96 Real Time System thermocycler (Biorad, Hercules CA). The qPCR program was an initial 3 min 95 °C followed by 40 cycles of 10s 95 °C, and 30s 67°C. Each reaction was loaded with 100ng of cDNA, and mRNA expression was normalized to GAPDH. The qPCR primers were as follows: P2X7R forward 5'ATCCACTTCCCCGGCCACAA-3', P2X7R reverse 5'-CCTCCAGTGCCGAAAACCAGG-3', GAPDH forward 5'-AACTTTGGCATTGTGGAAGG-3', GAPDH reverse 5'-ACACATTGGGGGTAGGAACA-3'. Both sets of primers are intron spanning and PCR products were assessed by real-time PCR melt curve analysis and running of products on gels but were not verified by sequencing. The PCR product sizes are as follows: P2X7R (391bp) and GAPDH (222bp).
Statistical analysis
Data are presented as mean ± SD. One-way analysis of variance (ANOVA) was used throughout. When significance was achieved, it was followed by post hoc Bonferroni test. Statistical analysis was performed using GraphPad Prism 5.03 (GraphPad, SanDiego, CA, USA) and significance was accepted at *p < 0.05, **p < 0.01 and ***p < 0,001. We accomplished a minimal three independent experiments.
Results
Immunohistochemistry of atrial samples of mouse hearts from wild-type and cav-1−/− animals
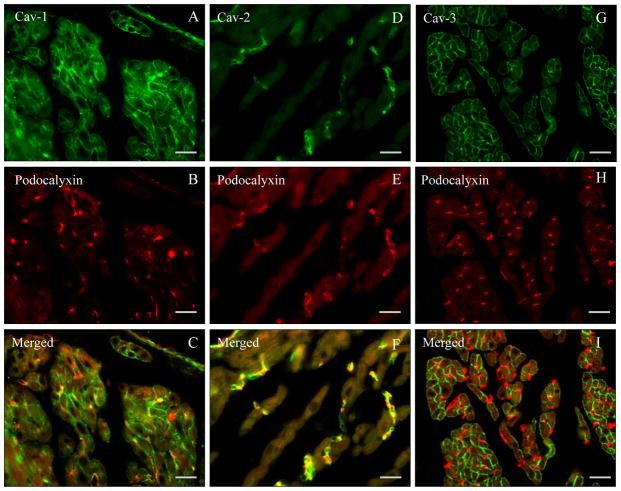
As shown in Fig. 1 using double immunofluorescence experiments with the endothelial marker podocalyxin, atrial cardiomyocytes express membrane localized Cav-1 and Cav-3 but not Cav-2. Accordingly, microvascular endothelial cells coexpress Cav-1 and podocalyxin (Fig. 1A–C) as well as Cav-2 and podocalyxin (Fig. 1D–F).
Fig. 1. Paraffin sections of mouse heart atrial tissue (wild-type).
A–I. Double label immunoflourescnce using anti caveolin-1, -2, and -3 specific antibodies (FITC-coupled secondary antibodies) and blood vessel marker anti-podocalyxin (Texas Red-coupled secondary antibody). Note the presence of Cav-1 and -3 in atrial cardiomyocytes as well as of Cav-1 and -2 in blood vessel endothelial cells. Bar = 80 μm
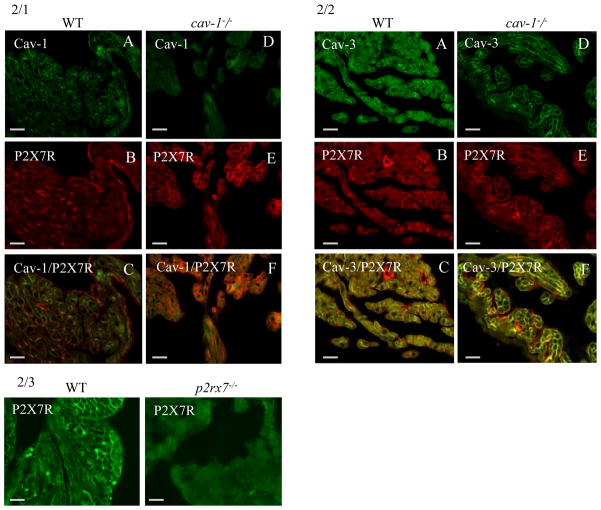
The comparison of the wild-type with the cav-1−/− animals for the expression of P2X7R (Fig. 2/1, 2/2) showed an increase of P2X7R immunoreactivity in atrial cardiomyocytes (Fig. 2/1B,E). Note the colocalization of P2X7R with Cav-1 in the microvascular endothelial cells of the wild-type sections (Fig. 2/1C). Similarly, Cav-2 colocalizes with the endothelial cells but not with the cardiomyocytes in wild-type atrial tissue (not shown). There is also a lack of Cav-2 immunoreactivity in the entire tissue of cav-1−/− animals (not shown) suggestive of earlier published observations suggesting a Golgi processing defect for Cav-2 with Cav-1 deficiency. Fig. 2/2 demonstrates that P2X7R colocalizes with Cav-3 in cardiomyocytes of both wild-type and cav-1−/− animals, whereas endothelial cells were lonely P2X7R positive.
Fig. 2. Paraffin sections of mouse heart atria (A–C: WT; D–F cav-1−/−).
2/1A–F. Double label immunofluorescence for cav-1 (FITC) and P2X7R (Texas Red). The P2X7R is present in endothelial cells and in cardiomyocytes. Note the higher immunoreactivity for P2X7R in cardiomyocytes of cav-1−/− cardiac tissue. Bar = 80 μm
2/2A–F. Double label immunofluorescence for Cav-3 (FITC) and P2X7R (Texas Red). The P2X7R is present in endothelial cells and in cardiomyocytes. Note the increased expression of Cav-3 in cardiomyocytes of cav-1−/− cardiac tissue.
2/3 Control experiment demonstrates that the immnoreactivity of the anti P2X7R antibody is abolished in atrial cardiomyocytes of the corresponding knockout tissue. Bar = 80 μm
P2X7R is localized in lipid rafts of cardiac tissues
Well-recognized biochemical characteristic of proteins localized in lipid rafts is the low buoyant density in sucrose gradient centrifugation. We tested P2X7R for these characteristics to determine whether the receptor is localized in lipid rafts of heart cell membranes.
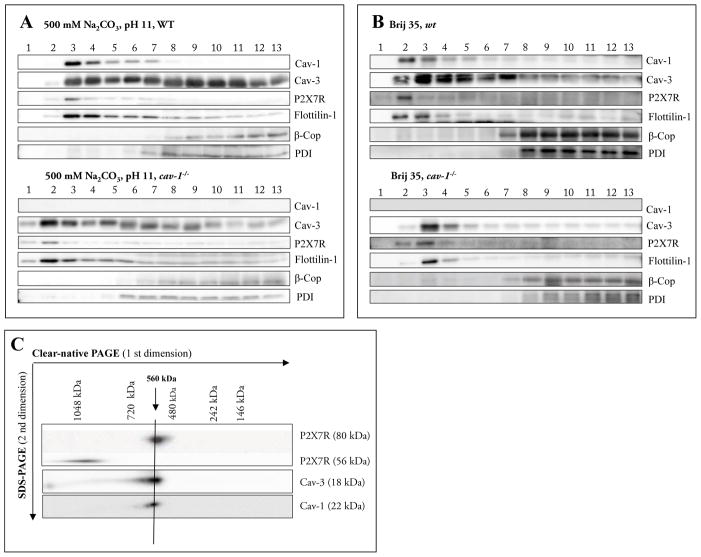
We isolated raft-like membranes using a detergent-free method (consisting of fine disruption of the membrane by sonication followed by sucrose density gradient centrifugation) (Fig. 3A/B). These fractions were analysed with Cav-1, Cav-3, flotilin 1 and the intracellular markers β-Cop and PDI. A significant proportion of P2X7R was detected in the low-density fractions together with Cav-1 (Fig. 3A), but more amounts of P2X7R were also found in the high-density fractions along with β-Cop and PDI. The detection of P2X7R in the lipid raft fractions indicated that P2X7R partially colocalized with lipid rafts. Cav-3 was detected in both fractions, the lower density fractions (3–7) and in the heavier fractions (7–13). (Head et al., 2005) and (Garg et al., 2009b) reported previously that Cav-3 is detected in both sarcolemmal and cytoplasmic fractions of cardiomyocytes. We also applied the detergent-free sucrose gradient centrifugation to determine whether Cav-3 and P2X7R are present in lipid rafts of heart cell membranes of the cav-1−/− mice. Fig. 3B shows a Western blot analysis of 13 fractions collected from top to bottom of the sucrose density gradient. The marker proteins for caveolae, caveolin-3 and flottilin-1, were found predominantly in the lower density fractions 1 to 6 (Fig. 3B). In the same fractions we detected the P2X7R. These results showed that the absence of Cav-1 had no effect on the localization of P2X7R and Cav-3 in lipid rafts.
Fig. 3.
A–C Characterization of membrane fractions prepared by sonication (3A) or by Brij35 (3B) in cell homogenates of cardiac tissue from wild-type and cav-1−/− mice.
The cardiac tissue was homogenized in a buffer containing 500 mM Na2CO3 (pH11) and raft and non-raft membranes were prepared by sonication followed by centrifugation in a discontinuous sucrose gradient. Futhermore cardiac tissue was homogenized in a buffer containing 1%Brij35 and subjected to sucrose density gradient centrifugation. Thirteen fractions were collected (fraction 1, top of the gradient; fraction 13, bottom of the gradient), and an aliquot of each fraction (20 #l) was resolved by SDS-PAGE and subjected to Western blot analysis with antibodies against Cav-1, Cav-3, P2X7R, Flottilin-1, β-Cop and PDI. As expected, Cav-1, Cav-3 and flotillin-1 were enriched in fractions 3–6 (wild-type) or 1–5 (cav- 1−/−), representing caveolae-enriched membrane fractions. Representative data from three separate experiments are shown.
C Supramolecular organization of P2X7R, Cav-1 and Cav-3.
Native membrane extracts from cardiac tissue from wild-type mice were isolated and solubilized with digitonin. Proteins were separated by hrCN-PAGE/SDS-PAGE, blotted and probed with antibodies against P2X7R, Cav-1 and Cav-3. Sizes of the molecular mass standards are indicated.
Cav-1, Cav-3 and P2X7R contribute to the same protein complex in the plasma membrane of heart atrial cardiomyocytes
To investigate the molecular organization of native P2X7 receptor complexes, digitonin lysates were prepared from cell membranes of mouse atrium cells, separated by clear native (hrCN)-PAGE, and analyzed by Western blotting with P2X7R and caveolin-specific antibodies.
In line with previous data, according to which trimeric P2X receptors have the tendency to form higher-order complexes (Boumechache et al., 2009; Dubyak, 2007; Nicke, 2008), we identified a band of P2X7R at approximately 560 kDa (Fig. 3C). For P2X7R, several interacting proteins (including Cav-1) have been identified. Therefore, the presence of Cav-1 and Cav-3 in the P2X7R complexes was investigated by probing the Western blots with Cav-1 and Cav-3 antibodies. The analysis revealed an identical separation profile as observed for P2X7R. These data suggest that the proteins may be constituents of the same 560 kDa complex. Cav-1 and Cav-3 are known to form heterooligomeric complexes in atrial cardiac myocytes (Volonte et al., 2008), which may comigrate with the P2X7R complexes. Our results indicate that the ~ 560 kDa complex harbours concomitantly P2X7R, Cav-3 and Cav-
P2rx7−/− animals show increased Cav-1 and Cav-3 expression levels
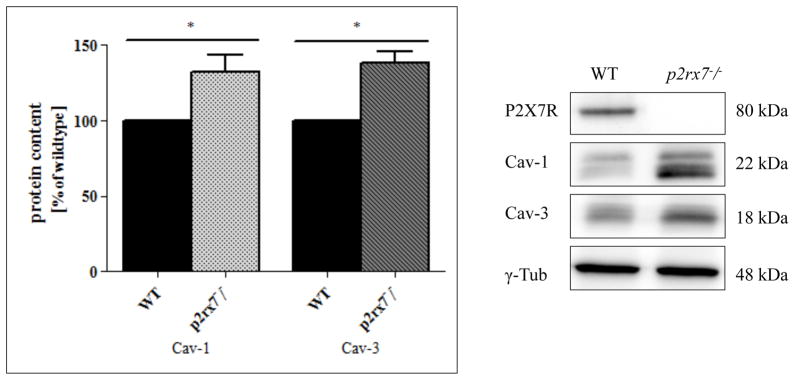
For determination of the amount of Cav-3 proteins, tissue homogenates of atrium from wild-type and P2rx7−/− mice, were prepared and subjected to Western blot analysis (Fig. 4). The Cav-1 protein content in the atrium of P2rx7−/− mice demonstrated an increase of about 30% in the atrium in comparison to the wild-type. The Cav-3 protein content in the atrium of the P2rx7−/− mice was also increased in comparison to the wild-type.
Fig. 4. Western blot analysis of mouse atrial tissues.
For determination of the amount of Cav-1, Cav-3 and P2X7R, cell homogenates of atrium from wild-type and cav-1−/− mice were prepared and subjected to western blot analysis. 50 #g of protein from each sample was loaded on the gel. Western blot analysis was performed using rabbit anti-P2X7R antibody (dilution 1:500), rabbit anti-Cav-1 antibody (dilution 1:500), mouse anti-Cav-3 antibody (dilution 1:500) and anti-γ-tubulin antibody (1:1000). γ-tubulin served as a loading control. Representative data from 5 separate experiments are shown.
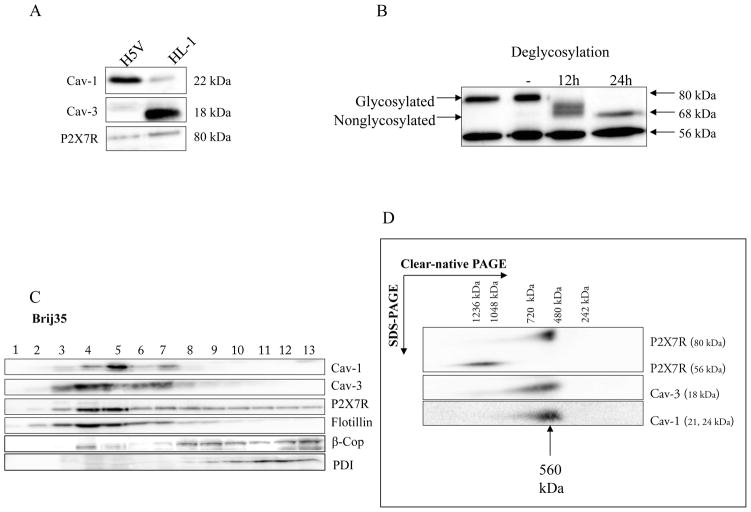
Presence of the P2X7 receptor and its modification in the atrial cardiomyocyte cell line HL-1
First we tested the expression of Cav-1, Cav-3 and P2X7R in the immortal atrial cardiomyocyte cell line HL-1 in comparison to the heart microvascular endothelial cell line H5V using Western blot analysis (Fig. 5A). Cav-1, but not Cav-3 was found in H5V endothelial cell line as expected, whereas Cav-3 was the dominant caveolin isotype in the cardiomyocyte cell line HL-1. P2X7R was found in both cell lines.
Fig. 5. Biochemical analysis of immortal cell lines.
A Analysis of Cav-1, Cav-3 and P2X7R expression in H5V and HL-1 cells.
Cav-1, Cav-3 and P2X7R expression was measured in cell lysates of H5V and HL-1 cells by SDS-PAGE and Western blot analysis. 50 #g of protein from each sample was loaded on the gel. Western blot analysis was performed using rabbit anti-P2X7R, anti-Cav-1, anti-Cav-3 and anti-γ-tubulin antibody. γ-tubulin served as a loading control. Representative data from three separate experiments are shown.
B P2X7 receptors are glycosylated in HL-1 cells.
Membranes were incubated in the precence of N-glycosidase F for 12 h and 24 h and compared with nontreated membranes (-). Full deglycosylation of the 80 kDa band was indicated by a reduction in molecular mass of approximately 12 kDa to 68 kDa. The mass of the 56 kDa band was unaffected by deglycosylation. Representative data from three separate experiments are shown.
C Characterization of membrane fractions prepared by Brij35 from HL-1 cells.
HL-1 cells were homogenized in a buffer containing 1% Brij35 and subjected to sucrose density gradient centrifugation. Thirteen fractions were collected and an aliquot of each fraction (20 #l) was resolved by SDS-PAGE and subjected to Western blot analysis with antibodies against Cav-1, Cav-3, P2X7R, PDI, β-Cop and flotillin-1. As expected, Cav-1, Cav-3 and flotillin-1 were enriched in fractions 3–7, representing caveolae-enriched membrane fractions. Representative data from three separate experiments are shown.
D Supramolecular organization of P2X7R, Cav-1 and Cav-3 in HL-1 cells.
Native membrane extracts from HL1- cells were isolated and solubilized with digitonin. Proteins were separated by hrCN-PAGE/SDS-PAGE, blotted and probed with antibodies against P2X7R, Cav-1 and Cav-3. Sizes of the molecular mass standards are indicated.
Further, we detected two major bands of 80 kDa and 56 kDa by Western blot analysis using anti-P2X7R antibodies directed against the extreme C-terminus (aa 576–595) in HL-1 cell membrane extracts (Fig. 5B). The 80 kDa band was consistent with the pattern previously described for mouse P2X7R (Li et al., 2005; Young et al., 2007) and N-glycosidase F treatment resulted in a decrease in the molecular mass of the 80 kDa band to approximately 68 kDa, consistent with the calculated molecular mass of non-glycosylated P2X7R (Fig. 5B). The 56 kDa band was unchanged by N-glycosidase F treatment, and its molecular mass was too low to represent non-glycosylated full-length P2X7R, raising the possibility that it might be a non-specific band. However, immunoreactivity to both the 80 kDa and 56 kDa bands was abolished following pre-incubation with the antibody control peptide (not shown), implying that the 56 kDa band shared at least a portion of the epitope recognised by the C-terminal P2X7R antibody.
Isolation of lipid rafts and localization of the P2X7R in vitro
A main characteristic of lipid rafts is low buoyant density in sucrose gradient centrifugation. We isolated raft-like membranes from HL-1 cells using Brij35 treatment at 4ºC (Fig. 5C). When rafts were prepared using Triton Brij35 treatment followed by sucrose density gradient centrifugation, only a small portion of P2X7R protein was detected in the caveolin-1- and flotillin-1-containing low-density raft fractions (Fig. 5C, lanes 4–7), and was faintly detected in the non-raft fractions (Fig. 5C, lanes 8–13). P2X7R protein was also detected in the high-density fractions from the Brij35 isolation method, along with markers for Golgi apparatus and endoplasmic reticulum (β-COP and PDI). The detection of P2X7R in the lipid raft fractions of HL-1 cells indicated that P2X7R partially colocalizes with lipid rafts.
Detection of native P2X receptor complex in the plasma membrane of HL-1 cells
To investigate the molecular organization of native P2X7 receptor complexes, digitonin lysates were prepared from cell membranes of mouse HL-1 cells, separated by high resolution clear native (hrCN)-PAGE, and analyzed by Western blotting with P2X-specific antibodies. In line with previous data, according to which trimeric P2X receptors have the tendency to form higher-order complexes (Nicke, 2008), we identified a band of the P2X7R at approximately 560 kDa (Fig. 5D). The presence of Cav-1 and Cav-3 in the P2X7R complexes was investigated by probing the Western blots with Cav-1 and Cav-3 antibodies. The analysis revealed an identical separation profile as observed for P2X7R. These data suggest that Cav-1 and Cav-3 may be constituents of the same 560 kDa complexes (data not shown). Comigration of the two P2XR receptors and Cav-1 in native gels was confirmed by a second dimension BN-/SDS PAGE (Fig 5D). Our results indicate that the ~ 560 kDa complex harbours concomitantly P2X7R, Cav-1 and Cav-3 and this data is consistent with that presented from atrial tissue.
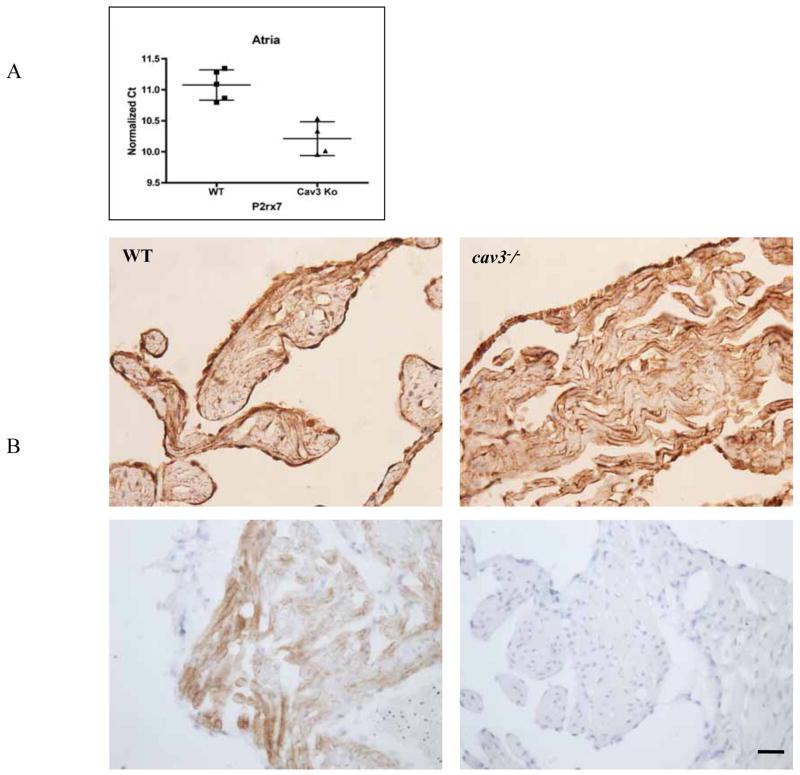
Cav-3−/− animals show elevated levels of P2X7R mRNA
We observed a greater that 2-fold increase in P2X7R mRNA expression in atrial tissue of cav3−/− compared to wild-type mice. The normalized cycle threshold values are normalized to GAPDH with lower values indicative of higher expression (Fig. 6A). Immunohistochemical investigations, however, showed no remarkable differences in the immunoreactivity for Cav-1 but a weak increase of P2X7R immunoreactivity in the atrial cardiomyocytes (Fig. 6B).
Fig. 6. Protein and mRNA measurements in cav-3−/− mice.
A P2X7R quantitative mRNA expression in atrial tissue was assessed. Cycle threshold (Ct) values were normalized to GAPDH expression. Lower Ct value equates to higher mRNA expression.
B Immunoperoxidase demonstration of P2X7R (upper panel) and Cav-3 (lower panel; immunohistochemical control experiment for the specificity of the anti-Cav-3 antibody) in atrial tissues of mouse heart (left: wild-type; right: cav-3−/− ).
Discussion
It was recently shown by Volonte et al. (Volonte et al., 2008) that Cav-1 and Cav-3 were part of the same protein complex in atrial cardiac myocytes. In the present investigation we demonstrated by hrCN-PAGE and coimmunoprecipitation experiments an additional presence of P2X7R in a complex with Cav-1 and Cav-3 in atrial cardiomyocytes in vivo and in vitro.
Whereas Cav-3 is the major caveolin family member expressed in cardiac myocytes of the mouse and rat heart ventricles, in some mouse strains Cav-1 also seems to play a role in ventricular cardiomyocytes (for discussion of this matter see also (Cho et al., 2007; Volonte et al., 2008), atrial cardiomyocytes strongly express both Cav-1 and Cav-3 in substantial amounts (Volonte et al., 2008).
In previous studies using lung epithelial cells, we found that a part of P2X7R is organized in the same complex with Cav-1 in lipid rafts (Barth et al., 2008). Here we found that the P2X7R is localized in caveolin-enriched membrane fractions of immortal cardiomyocyte cell line HL-1 and that it was associated and/or colocalized with Cav-3. The functional implication of this interaction is not yet clear. It is possible that the subcellular localization of the P2X7R to caveolin-enriched microdomains on the plasma membrane of cardiac myocytes is essential for the activation or regulation of the receptor.
Information about the distribution and role of purinergic receptors in the cardiomyocytes are rare (for a recent review see (Musa et al., 2009)). Activation of P2X receptors, ATP-gated cationic channels, modulates intracellular Ca2+ transients and contractility in cardiomyocytes (Shen et al., 2007; Shen et al., 2006). Extracellular ATP is released from ischemic and hypoxic myocytes. P2Y receptors, which are coupled to G proteins, have been shown to mobilize intracellular Ca2+ stores or decrease intracellular AMP levels with all physiological consequences for the heart (Musa et al., 2009).
Cardiac Cav-3 has been shown to be involved in spatial regulation and localization of various ion channels such as CaV3.2 T-type Ca2+ channels (Markandeya et al., 2011), cardiac K(ATP) channel (Garg et al., 2009a; Sun and Hu, 2010), hyperpolarization-activated cyclic nucleotide-gated channel (HCN) 4 (Ye et al., 2008), and also associates with sodium channels (Lin et al., 2009). Some of them are also ATP-dependent (Alvarez et al., 1990; Matsuura and Ehara, 1997). Cav-3 is further required for organizing the signalling complexes which trigger protein kinase A-dependent phosphorylation of proteins that regulate intracellular calcium in ventricular myocyte (Nichols et al., 2010).
Cav-3 knockout mice develop a progressive cardiomyopathic phenotype with dissociation of alpha sarcoglycan, a member of the dystrophin-glycoprotein complex, from lipid rafts and hyperactivation of the p42/44 MAPK cascade (Woodman et al., 2002). When we checked cardiac tissues from cav-3−/− animals for the cellular and biochemical distribution of Cav-1 and P2X7R in their atrial cardiomyocytes, increased amounts of P2X7R mRNA were found. Interestingly, P2rx7−/− animals showed elevated levels of Cav-1 and Cav-3. To underline evidence for a functional Cav-1/Cav-3/P2X7R complex, as shown in vitro by high resolution clear native (hrCN)-PAGE, Cav-1−/− cardiomyocytes had elevated levels of P2X7R and Cav-3. It is plausible to suggest that loss of any one component may drive the compensated upregulation of other components of the complex. Such as interconnected relationship suggests that the complex may serve an important homeostatic or regulator function in the heart that needs compensation.
Inflammation is a key component in cardiovascular disease. Controlling inflammatory events and their subsequent processes holds the potential for novel therapeutic treatment options. Cytokines are the propagators of inflammation. (Kahlenberg et al., 2005) have shown that stimulation of the P2X7R is able to induce the acceleration of caspase-1 activation. We found also an increased expression of caspase 1 and IL1-β in the atrium of cardiac myocytes Barth, unpublished observations). Caspase-1 is a member of the proinflammatory family of caspases (Bauernfeind et al., 2010) was discovered through its proteolytic activation of pro-IL-1β and -18 into their active form. It was shown by Merkle et al. (Merkle et al., 2007) that caspase-1 acts as a potent proapoptotic caspase in isolated cardiomyocytes. A recent study suggest that caveolins may be binding partners for intracellular localized activated caspases (Sharma et al., 2011). The link between caveolins and P2X7R in regulating inflammation in the heart is not clear but may pose an important avenue for future investigations.
In conclusion, we show that Cav-1, Cav-3, and P2X7R forms a complex in atrial membranes and in HL-1 cardiac myocytes cultures. The Cav-1/Cav-3/P2X7R complex appears to be enriched predominantly in buoyant caveolar fractions in vivo and in vitro. The Cav-1/Cav-3/P2X7R complex also appears to be transcriptionally regulated dependent on the protein expression of the various complex partners suggesting a novel unrecognized control point for potential regulation of atrial cardiac myocyte function. Further biochemical and functional elucidation of the Cav-1/Cav-3/P2X7R complex has likely implications for cardiovascular pathology.
Acknowledgments
This work was supported by grants from National Institutes of Health HL091071 (HHP).
References
- Alvarez JL, Mongo K, Scamps F, Vassort G. Effects of purinergic stimulation on the Ca current in single frog cardiac cells. Pflugers Arch. 1990;416:189–195. doi: 10.1007/BF00370241. [DOI] [PubMed] [Google Scholar]
- Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103:7500–7505. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbuti A, Terragni B, Brioschi C, DiFrancesco D. Localization of f-channels to caveolae mediates specific beta2-adrenergic receptor modulation of rate in sinoatrial myocytes. J Mol Cell Cardiol. 2007;42:71–78. doi: 10.1016/j.yjmcc.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Barth K, Blasche R, Kasper M. T1alpha/podoplanin shows raft-associated distribution in mouse lung alveolar epithelial E10 cells. Cell Physiol Biochem. 2010a;25:103–112. doi: 10.1159/000272065. [DOI] [PubMed] [Google Scholar]
- Barth K, Pfleger C, Linge A, Sim JA, Surprenant A, Steinbronn N, Strasser RH, Kasper M. Increased P2X7R expression in atrial cardiomyocytes of caveolin-1 deficient mice. Histochem Cell Biol. 2010b;134:31–38. doi: 10.1007/s00418-010-0716-8. [DOI] [PubMed] [Google Scholar]
- Barth K, Weinhold K, Guenther A, Linge A, Gereke M, Kasper M. Characterization of the molecular interaction between caveolin-1 and the P2X receptors 4 and 7 in E10 mouse lung alveolar epithelial cells. Int J Biochem Cell Biol. 2008;40:2230–2239. doi: 10.1016/j.biocel.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Bauernfeind F, Ablasser A, Bartok E, Kim S, Schmid-Burgk J, Cavlar T, Hornung V. Inflammasomes: current understanding and open questions. Cell Mol Life Sci. 2010;68:765–783. doi: 10.1007/s00018-010-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossuyt J, Taylor BE, James-Kracke M, Hale CC. The cardiac sodium-calcium exchanger associates with caveolin-3. Ann N Y Acad Sci. 2002;976:197–204. doi: 10.1111/j.1749-6632.2002.tb04741.x. [DOI] [PubMed] [Google Scholar]
- Boumechache M, Masin M, Edwardson JM, Gorecki DC, Murrell-Lagnado R. Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells. J Biol Chem. 2009;284:13446–13454. doi: 10.1074/jbc.M901255200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WJ, Chow AK, Schulz R, Daniel EE. Matrix metalloproteinase-2, caveolins, focal adhesion kinase and c-Kit in cells of the mouse myocardium. J Cell Mol Med. 2007;11:1069–1086. doi: 10.1111/j.1582-4934.2007.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WJ, Chow AK, Schulz R, Daniel EE. Caveolin-1 exists and may function in cardiomyocytes. Can J Physiol Pharmacol. 2010;88:73–76. doi: 10.1139/Y09-114. [DOI] [PubMed] [Google Scholar]
- Dubyak GR. Go it alone no more--P2X7 joins the society of heteromeric ATP-gated receptor channels. Mol Pharmacol. 2007;72:1402–1405. doi: 10.1124/mol.107.042077. [DOI] [PubMed] [Google Scholar]
- Faria RX, Defarias FP, Alves LA. Are second messengers crucial for opening the pore associated with P2X7 receptor? Am J Physiol Cell Physiol. 2005;288:C260–271. doi: 10.1152/ajpcell.00215.2004. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Razani B, Lisanti MP. Caveolae and caveolin-3 in muscular dystrophy. Trends Mol Med. 2001;7:435–441. doi: 10.1016/s1471-4914(01)02105-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Marcos M, Pochet S, Tandel S, Fontanils U, Astigarraga E, Fernandez-Gonzalez JA, Kumps A, Marino A, Dehaye JP. Characterization and comparison of raft-like membranes isolated by two different methods from rat submandibular gland cells. Biochim Biophys Acta. 2006;1758:796–806. doi: 10.1016/j.bbamem.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Garg V, Jiao J, Hu K. Regulation of ATP-sensitive K+ channels by caveolin-enriched microdomains in cardiac myocytes. Cardiovasc Res. 2009a;82:51–58. doi: 10.1093/cvr/cvp039. [DOI] [PubMed] [Google Scholar]
- Garg V, Sun W, Hu K. Caveolin-3 negatively regulates recombinant cardiac K(ATP) channels. Biochem Biophys Res Commun. 2009b;385:472–477. doi: 10.1016/j.bbrc.2009.05.100. [DOI] [PubMed] [Google Scholar]
- Garlanda C, Parravicini C, Sironi M, De Rossi M, Wainstok de Calmanovici R, Carozzi F, Bussolino F, Colotta F, Mantovani A, Vecchi A. Progressive growth in immunodeficient mice and host cell recruitment by mouse endothelial cells transformed by polyoma middle-sized T antigen: implications for the pathogenesis of opportunistic vascular tumors. Proc Natl Acad Sci U S A. 1994;91:7291–7295. doi: 10.1073/pnas.91.15.7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara Y, Sasaoka T, Araishi K, Imamura M, Yorifuji H, Nonaka I, Ozawa E, Kikuchi T. Caveolin-3 deficiency causes muscle degeneration in mice. Hum Mol Genet. 2000;9:3047–3054. doi: 10.1093/hmg/9.20.3047. [DOI] [PubMed] [Google Scholar]
- Head BP, Patel HH, Roth DM, Lai NC, Niesman IR, Farquhar MG, Insel PA. G-protein-coupled receptor signaling components localize in both sarcolemmal and intracellular caveolin-3-associated microdomains in adult cardiac myocytes. J Biol Chem. 2005;280:31036–31044. doi: 10.1074/jbc.M502540200. [DOI] [PubMed] [Google Scholar]
- Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol. 2005;175:7611–7622. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- Lapidos KA, Kakkar R, McNally EM. The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ Res. 2004;94:1023–1031. doi: 10.1161/01.RES.0000126574.61061.25. [DOI] [PubMed] [Google Scholar]
- Li J, Liu D, Ke HZ, Duncan RL, Turner CH. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem. 2005;280:42952–42959. doi: 10.1074/jbc.M506415200. [DOI] [PubMed] [Google Scholar]
- Lin E, Hung VH, Kashihara H, Dan P, Tibbits GF. Distribution patterns of the Na+-Ca2+ exchanger and caveolin-3 in developing rabbit cardiomyocytes. Cell Calcium. 2009;45:369–383. doi: 10.1016/j.ceca.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Markandeya YS, Fahey JM, Pluteanu F, Cribbs LL, Balijepalli RC. Caveolin-3 regulates protein kinase A modulation of the Ca(V)3.2 (alpha1H) T-type Ca2+ channels. J Biol Chem. 2011;286:2433–2444. doi: 10.1074/jbc.M110.182550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura H, Ehara T. Selective enhancement of the slow component of delayed rectifier K+ current in guinea-pig atrial cells by external ATP. J Physiol. 1997;503 (Pt 1):45–54. doi: 10.1111/j.1469-7793.1997.045bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle S, Frantz S, Schon MP, Bauersachs J, Buitrago M, Frost RJ, Schmitteckert EM, Lohse MJ, Engelhardt S. A role for caspase-1 in heart failure. Circ Res. 2007;100:645–653. doi: 10.1161/01.RES.0000260203.55077.61. [DOI] [PubMed] [Google Scholar]
- Musa H, Tellez JO, Chandler NJ, Greener ID, Maczewski M, Mackiewicz U, Beresewicz A, Molenaar P, Boyett MR, Dobrzynski H. P2 purinergic receptor mRNA in rat and human sinoatrial node and other heart regions. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:541–549. doi: 10.1007/s00210-009-0403-2. [DOI] [PubMed] [Google Scholar]
- Nichols CB, Rossow CF, Navedo MF, Westenbroek RE, Catterall WA, Santana LF, McKnight GS. Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ Res. 2010;107:747–756. doi: 10.1161/CIRCRESAHA.109.216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicke A. Homotrimeric complexes are the dominant assembly state of native P2X7 subunits. Biochem Biophys Res Commun. 2008;377:803–808. doi: 10.1016/j.bbrc.2008.10.042. [DOI] [PubMed] [Google Scholar]
- Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, Huang D, Moreno AL, Patel PM, Insel PA, Roth DM. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. Faseb J. 2007;21:1565–1574. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- Razani B, Lisanti MP. Caveolins and caveolae: molecular and functional relationships. Exp Cell Res. 2001;271:36–44. doi: 10.1006/excr.2001.5372. [DOI] [PubMed] [Google Scholar]
- Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- Schwab W, Harada H, Goetz W, Nowicki M, Witt M, Kasper M, Barth K. Immunocytochemical and biochemical detection of EMMPRIN in the rat tooth germ: differentiation-dependent co-expression with MMPs and co-localization with caveolin-1 in membrane rafts of dental epithelial cells. Histochem Cell Biol. 2007;128:195–203. doi: 10.1007/s00418-007-0313-7. [DOI] [PubMed] [Google Scholar]
- Sharma V, Sharma A, Saran V, Bernatchez PN, Allard MF, McNeill JH. beta-receptor antagonist treatment prevents activation of cell death signaling in the diabetic heart independent of its metabolic actions. Eur J Pharmacol. 2011;657:117–125. doi: 10.1016/j.ejphar.2011.01.044. [DOI] [PubMed] [Google Scholar]
- Shen JB, Cronin C, Sonin D, Joshi BV, Gongora Nieto M, Harrison D, Jacobson KA, Liang BT. P2X purinergic receptor-mediated ionic current in cardiac myocytes of calsequestrin model of cardiomyopathy: implications for the treatment of heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H1077–1084. doi: 10.1152/ajpheart.00515.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen JB, Pappano AJ, Liang BT. Extracellular ATP-stimulated current in wild-type and P2X4 receptor transgenic mouse ventricular myocytes: implications for a cardiac physiologic role of P2X4 receptors. Faseb J. 2006;20:277–284. doi: 10.1096/fj.05-4749com. [DOI] [PubMed] [Google Scholar]
- Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- Sun W, Hu K. Role for SUR2A in coupling cardiac K(ATP) channels to caveolin-3. Cell Physiol Biochem. 2010;25:409–418. doi: 10.1159/000303045. [DOI] [PubMed] [Google Scholar]
- Vacca F, Amadio S, Sancesario G, Bernardi G, Volonte C. P2X3 receptor localizes into lipid rafts in neuronal cells. J Neurosci Res. 2004;76:653–661. doi: 10.1002/jnr.20069. [DOI] [PubMed] [Google Scholar]
- Venema VJ, Ju H, Zou R, Venema RC. Interaction of neuronal nitric-oxide synthase with caveolin-3 in skeletal muscle. Identification of a novel caveolin scaffolding/inhibitory domain. J Biol Chem. 1997;272:28187–28190. doi: 10.1074/jbc.272.45.28187. [DOI] [PubMed] [Google Scholar]
- Volonte D, McTiernan CF, Drab M, Kasper M, Galbiati F. Caveolin-1 and caveolin-3 form heterooligomeric complexes in atrial cardiac myocytes that are required for doxorubicin-induced apoptosis. Am J Physiol Heart Circ Physiol. 2008;294:H392–401. doi: 10.1152/ajpheart.01039.2007. [DOI] [PubMed] [Google Scholar]
- Weinhold K, Krause-Buchholz U, Rodel G, Kasper M, Barth K. Interaction and interrelation of P2X7 and P2X4 receptor complexes in mouse lung epithelial cells. Cell Mol Life Sci. 2010;67:2631–2642. doi: 10.1007/s00018-010-0355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J, Tang B, Jelicks LA, Kitsis RN, Christ GJ, Factor SM, Tanowitz HB, Lisanti MP. Caveolin-3 knockout mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002;277:38988–38997. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- Ye B, Balijepalli RC, Foell JD, Kroboth S, Ye Q, Luo YH, Shi NQ. Caveolin-3 associates with and affects the function of hyperpolarization-activated cyclic nucleotide-gated channel 4. Biochemistry. 2008;47:12312–12318. [PMC free article] [PubMed] [Google Scholar]
- Young MT, Pelegrin P, Surprenant A. Amino acid residues in the P2X7 receptor that mediate differential sensitivity to ATP and BzATP. Mol Pharmacol. 2007;71:92–100. doi: 10.1124/mol.106.030163. [DOI] [PubMed] [Google Scholar]