Abstract
Objectives
To assess incidence of, and risk factors for abnormal anal cytology and anal intraepithelial neoplasia (AIN) 2–3 in HIV-infected women.
Study Design
This prospective study assessed 100 HIV-infected women with anal and cervical specimens for cytology and high risk HPV testing over three semi-annual visits.
Results
Thirty-three women were diagnosed with an anal cytologic abnormality at least once. Anal cytology abnormality was associated with current CD4 count <200 cells/mm3, anal HPV infection and history of other sexually transmitted infections (STIs). Twelve subjects were diagnosed with AIN2-3: four after AIN1 diagnosis and four after ≥1 negative anal cytology. AIN2-3 trended towards an association with history of cervical cytologic abnormality and history of STI.
Conclusions
Repeated annual anal cytology screening for HIV-infected women, particularly for those with increased immunosuppression, anal and/or cervical HPV, history of other STIs, or abnormal cervical cytology, will increase the likelihood of detecting AIN2-3.
Keywords: anal cytology, anal HPV, cervical HPV, HIV
INTRODUCTION
Anal cancer rates in HIV-infected individuals have continued to increase over the past decade despite the widespread use of highly active antiretroviral therapy (HAART).1–5 Among HIV-infected women, the risk for anal cancer is approximately 14 times higher than the risk among HIV-uninfected women.6 Squamous cell carcinoma of the anus (SCCA) shares biologic similarities with cervical cancer, including detectable precancerous lesions and persistent human papillomavirus (HPV) infection. It is likely that the pathogenesis of anal cancer is similar to that of cervical cancer: that is, anal HPV infection, in conjunction with other yet to be determined factors, leads to the development of anal intraepithelial neoplasia (AIN) 2–3, a likely precursor to anal cancer.7,8 Much like cervical cytology is used to screen for cervical cancer, anal cytology can be used to screen for anal cancer. Individuals with abnormal anal cytology are referred for high resolution anoscopy (HRA) (colposcopic evaluation of the anus) with directed biopsy for histologic diagnosis.
There are limited longitudinal data describing anal HPV infection and intraepithelial neoplasia among HIV-infected women; in comparison, there have been numerous cohort studies providing extensive information on cervical HPV infection and cervical disease in HIV-infected women. The data on anal HPV infection and abnormal anal cytology in HIV-infected women have primarily focused on prevalent abnormalities.9–15 Among studies where HRA and biopsy were performed,9,10 histology results were only available in a small percentage of the study participants. Additionally, only one longitudinal study has been published describing incidence of abnormal anal cytology; and that study was conducted in the pre-HAART/early HAART era.11
We have previously published the baseline data from our prospective cohort of 100 HIV-infected women assessing the prevalence of anal cytologic abnormalities and anal HPV infection, as well as their relationship to abnormal cervical cytology and cervical HPV infections.10 At baseline, the prevalence of anal cytologic abnormalities was 17% and anal HPV was 16% in our cohort. Abnormal anal cytology was associated with cervical and anal HPV positivity, cervical cytologic abnormality, current and nadir CD4 count less than 200 cells/mm3, history of sexually transmitted infection (STI) (other than cervical/anal HPV), and alcohol use. Fourteen women (out of 19 referred) underwent HRA and AIN2-3 was detected in three (21%). We now present our longitudinal findings on the incidence and prevalence of anal cytologic abnormalities and AIN2-3, as well as the factors associated with abnormal anal cytology and AIN2-3 results.
MATERIALS AND METHODS
This prospective, observational pilot study was conducted at the Center for Infectious Diseases (CID), the primary site of HIV care at Boston Medical Center (BMC), an inner city safety net hospital in Boston, Massachusetts. English-speaking, HIV-infected women between the ages of 18 and 64 who had not had a cervical or anal cytology test, colposcopy or HRA in the six months prior to enrollment were eligible to participate. The exclusion criteria included pregnancy, use of chronic anticoagulation medication, and life expectancy of less than one year. The study participants were recruited by the nurse practitioner in CID from HIV-infected women scheduled for gynecology care (including cervical cytology). The protocol for the study was approved by the BMC Institutional Review Board.
After obtaining written informed consent, each subject provided a detailed history on routine gynecological health care and risk factors for the development of anal cytological abnormalities. The details of this questionnaire have been published previously.10 History of STI other than cervical or anal HPV was recorded based on patient self-report and included gonorrhea, Chlamydia, trichomonas, herpes, syphilis, and genital warts. Additional medical and laboratory data were collected from the electronic medical record including most recent cervical cytology results prior to enrollment, CD4 T-cell count and HIV viral load performed within the prior six months.
Following the questionnaire and history, a visual examination of the lower genital tract was performed and samples were collected for cervical and anal cytology and HPV testing with Hybrid Capture 2 (HC2) (Qiagen Corporation, Gaithersburg, MD). Cervical cytology specimens were collected in the standard fashion and processed using the BD SurePath© preservative vial (Becton, Dickinson and Company, Franklin Lakes, NJ). The anal cytology specimens were collected with a small polyester swab soaked in tap water and gently inserted until resistance from the wall of the rectum was met (approximately 4.5 cm). The swab was then withdrawn with lateral pressure, using a spiral motion to sample the entire circumference of the anal canal. The swab was processed using the BD SurePath© preservative vial. After cytology testing at the BMC Pathology Laboratory was performed, the residual from the cytology specimen was run for HC2. The HC2 assay assesses the presence of 13 types of high risk associated HPV (HR-HPV) infection (HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68). HC2, however, has not been validated for detection of HR-HPV in the anus.
Follow-up was determined based on the anal and cervical results. Women with cervical cytological abnormalities: low-grade squamous intraepithelial lesion (LSIL) and high-grade squamous intraepithelial lesion (HSIL) with or without detection of HR- HPV were referred for colposcopic examination which involved microscopic visualization of cervical lesions with biopsy. Women with a cytology test showing atypical squamous cells of unknown significance (ASCUS) and a HR-HPV were also referred for colposcopic examination. Women with cervical HR-HPV and normal cytology were not assessed with colposcopy, but were recommended for repeat cytology and HR-HPV testing in six months.
Subjects with any grade of anal cytological abnormality or anal HR-HPV1 were referred for examination with HRA. HRA was performed using a lubricated plastic disposable anoscope, and applying 5% acetic acid to the anal mucosa and anoderm. Examination was then performed under colposcopic visualization of the anal canal from the dentate line to the anal verge. Identified lesions (with acetowhite changes, punctuation, abnormal vascularity and masses) were biopsied under direct visualization with a mini-Tischler biopsy forceps after application of local anesthetic. All colposcopy and HRA procedures were performed by a gynecologist (ES) with expertise in colposcopy and HRA.
Participants identified with high-grade intraepithelial neoplasia of the anus, (AIN2-3) cervix (CIN2-3), vagina (VaIN2-3), or vulva (VIN2-3) were referred for treatment as recommended clinically. Subjects with no evidence of AIN2-3 or CIN2-3 were scheduled to be followed every six months with cervical and anal evaluations for cytology and HPV for a total of three visits, with follow-up colposcopy or HRA as determined by the study protocol. The six month follow-up interval was selected based on prior published studies of anal cytologic abnormalities in HIV-infected women.11,14
It was estimated that a sample size of 100 women would be feasible for this pilot study to complete enrollment over six months. As the objective of the analysis was to compare women who were diagnosed with abnormal anal cytology at any study visit to women with normal anal cytology at every visit attended, we performed two sets of univariate and multivariate logistic regression analyses comparing women with abnormal anal cytology at any visit to: (1) women with normal anal cytology at every visit attended (which included women lost to follow-up after the first or second study visit) and (2) those who had normal anal cytology at all three study visits (not lost to follow-up). As the results were similar regardless of the number of visits, the analysis including all subjects with normal anal cytology at every visit attended regardless of number of visits is presented in order to preserve statistical power. Categorical variables were assessed using Fisher’s exact test and continuous variables were assessed with a t-test for independent samples. Multiple logistic regression analyses were performed using stepwise forward selection with a cut-off p-value of less than 0.10 for inclusion in the final model. Predictors of interest were chosen based on a cut-off p-value of less than 0.10 in univariate modeling and included current CD4 count, nadir CD4 count, current HAART use, history of STI (other than cervical/anal HPV), cigarette smoking and cervical or anal HR-HPV positive test result. Since cervical HPV and cervical cytologic abnormalities were closely related, only cervical HR-HPV was included in the multivariate model because cervical HR-HPV was more strongly associated with anal cytologic abnormalities in univariate results. We also assessed factors associated with AIN2-3 diagnosis using unadjusted odd’s ratios. As only women with abnormal anal cytology and/or anal HPV were eligible for HRA in this study, we felt the associations between HRA and abnormal anal cytology and anal HPV would be biased and thus did not include these two factors in this analysis. All analyses were performed in SAS version 9.1, SAS Institute, Inc, Cary, NC. Final results are interpreted using an α = 0.05 level of significance.
RESULTS
One hundred women were enrolled in the study between October 2006 and May 2007 and followed for completion of HRA through April 2010: 87 were followed for at least two visits and 71 took part in all three study visits. Roughly 150 women were seen for a gynecologic visit with the nurse practitioner during the period of enrollment and about 17% of those patients spoke a language other than English for a recruitment of approximately 80% of potentially eligible women. For the 87 women followed for more than one visit, the median length of follow-up, including time to final HRA if performed, was 704 days (range 73–1154 days). Thirteen (15%) of 87 participants with at least two study visits took greater than one year to return for study visit 2. Sixty-three (89%) of 71 participants with three visits took greater than one year to return for study visit 3. The study population has been described in detail elsewhere.10 In brief, 78% of subjects were black, 56% were born outside the U.S. and the median age was 40 years (Range 22–57). Eleven had a baseline CD4 count less than or equal to 200 cells/mm3, 62% had an undetectable HIV viral load and 79% were on HAART defined as three or more active HIV drugs. Only five women reported having undergone a prior anal cytology test. Twenty-two percent reported a history of receptive anal intercourse. Ten percent of women had a prior history of VIN and 27% had a history of treatment for CIN. Twenty-three percent of the women were active cigarette smokers while 15% had a history of intravenous drug use.
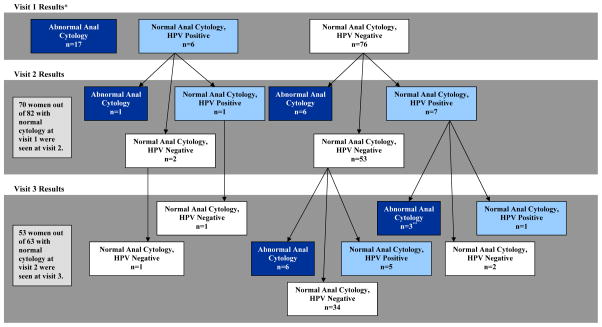
Thirty-three of 99 (33%) women were diagnosed with an anal cytologic abnormality at least once. One subject had inadequate anal cytology for analysis at the initial visit and was lost to follow-up; therefore 99 subjects are included in the analysis. The anal cytology results at each study visit, censored after initial abnormal anal cytology diagnosis are shown in Figure 1. HPV results are shown for women with concurrent normal cytology in Figure 1. Anal HPV was detected in 22 of the 33 women (67%) with abnormal anal cytology at some time point during follow-up. The highest grade of anal cytologic abnormality was ASCUS in 20 (61%) and LSIL in 13 (39%). There were no HSIL anal cytology results. Among the 70 women (out of 82) with a normal baseline anal cytology result who were followed for more than one visit, 16 (23%) had an abnormality diagnosed on a repeat cytology test: nine of whom (56%) had more than one prior negative anal cytology result. The incidence of anal cytology abnormality was 13.1 cases per 100 person-years of follow-up. Thirty-six women out of 99 had a positive anal HPV test at one or more time points and 14 (39%) of those women had no anal cytologic abnormalities during the study. One individual had an AIN2 diagnosis after positive anal HPV testing and then had abnormal anal cytology testing after HRA. Forty-eight out of 100 women had a positive cervical HPV test at any point during study follow-up (24 out of 99 at baseline) and 30 (63%) had an abnormal cervical cytology result at least once during the study (21 out of 100 at baseline).
Figure 1. Overview of Anal Cytology Results Over Time.
Note: *One baseline sample was anal cytology inadequate, HPV negative and subsequently lost to follow-up, therefore 99 subjects are included at baseline. Abnormal cytology results are noted in dark blue; normal cytology, HPV-positive are noted in light blue; and normal cytology, HPV-negative are noted in white. For simplicity, HPV result at time of abnormal anal cytology diagnosis and follow-up anal cytology and HPV results after first abnormal anal cytology diagnosis are not shown. **One individual was diagnosed with anal intraepithelial neoplasia (AIN) 1 and one was diagnosed with AIN2 between visit 2 and 3. Fifteen visit 2 and 16 visit 3 results are not shown due to prior abnormal anal cytology result.
Subject characteristics stratified by abnormal anal cytology diagnosis are shown in Table 1. In unadjusted analysis, anal cytology abnormality was associated with current or nadir CD4 count less than 200 cells/mm3, current HAART, cigarette smoking, history of treatment for CIN, cervical cytology abnormality and HPV detected in the cervix and/or anus (Table 1). History of a STI other than cervical/anal HPV and nadir CD4 count less than 200 cells/mm3 trended towards an association with anal cytologic abnormality. In multivariate modeling, anal cytologic abnormality was associated with current CD4 count less than 200 cells/mm3 (OR 12.8, 95% CI 2.0–82.0), anal HPV infection (OR 6.2 95% CI 2.2–16.9) and history of STI other than cervical/anal HPV (OR 3.6, 95% CI 1.1–11.5) (Table 1). Cervical HPV positive test result, cigarette smoking, current HAART, nadir CD4 count less than 200 cells/mm3 and history of treatment for cervical dysplasia were not significant and therefore not included in the final multivariate model.
Table 1.
Odds of abnormal anal cytology diagnosis versus no abnormal anal cytology (any number of visits).
| Variable | Abnormal anal cytology N=33 n (%) | No abnormal cytology N=66* n (%) | Univariate OR (95% CI) | Multivariate OR (95% CI) | |
|---|---|---|---|---|---|
| Age, mean, years ± standard deviation | 42.3 ± 8.5 | 40.0 ± 7.8 | 1.04 (0.98–1.1) | ||
| Race | Black | 26 (79) | 52 (79) | Reference | |
| White | 6 (18) | 11 (17) | 1.1 (0.4–3.3) | ||
| Hispanic | 1 (3) | 3 (5) | 0.7 (0.1–6.7) | ||
| U.S. born | 17 (52) | 26 (39) | 1.6 (0.7–3.8) | ||
| HIV diagnosed 5 or more years before enrollment | 24 (73) | 39 (59) | 1.8 (0.7–4.6) | ||
| Current CD4 count less than 200 cells/mm3 | 9 (27) | 2 (3) | 12.0 (2.4–59.6) | 12.8 (2.0–82.0) | |
| Nadir CD4 less than 200 cells/mm3 (n=97) | 17 (52) | 20 (31) | 2.3 (1.0–5.5) | NS | |
| Viral load <75 copies/mL | 20 (61) | 42 (64) | 0.9 (0.4–2.1) | ||
| Current HAART | 31 (94) | 47 (71) | 6.3 (1.4–28.8) | NS | |
| Age first coitus ≤15 years | 15 (45) | 19 (29) | 2.1 (0.9–4.9) | ||
| 6 or more lifetime partners | 16 (48) | 29 (44) | 1.2 (0.5–2.8) | ||
| History of anal sex | 9 (27) | 13 (20) | 1.5 (0.6–4.1) | ||
| History of sexually transmitted infection (other than cervical or anal HPV) | 24 (73) | 35 (53) | 2.4 (0.95–5.8) | 3.6 (1.1–11.5) | |
| Cigarette smoking | 12 (36) | 11 (17) | 2.9 (1.1–7.5) | NS | |
| Alcohol use | 11 (33) | 14 (21) | 1.9 (0.7–4.7) | ||
| IVDU | 7 (21) | 7 (11) | 2.3 (0.7–7.1) | ||
| Last cervical Pap prior to study (n=83) | NILM | 18 (72) | 52 (90) | Reference | |
| ASCUS | 1 (4.0) | 2 (3) | 1.4 (0.1–16.9) | ||
| LSIL | 5 (20) | 4 (7) | 3.6 (0.9–14.9) | ||
| HSIL | 1 (4) | 0 (0) | -- | ||
| History of treatment of cervical dysplasia | 14 (42) | 13 (20) | 3.0 (1.2–7.5) | NS | |
| Any cervical abnormality at any visit (HPV or cytology) | 25 (76) | 31 (47) | 3.5 (1.4–9.0) | † | |
| Cervical HPV+ at any visit | 24 (73) | 24 (36) | 4.7 (1.9–11.7) | NS | |
| Cervical cytological abnormality at any visit | 19 (58) | 19 (29) | 3.4 (1.4–8.0) | † | |
| Anal HPV+ any visit | 22 (67) | 14 (21) | 7.4 (2.9–18.9) | 6.2 (2.2–16.9) | |
Note: Results are presented as n (%) unless noted.
One observation had inadequate anal cytology and was excluded. OR, odds ratio; CI, confidence interval; HAART, highly active antiretroviral therapy; HPV, human papillomavirus; IVDU, intravenous drug use; NILM, no intraepithelial lesion or malignancy; ASCUS, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; NS, not significant and therefore not included in the final multivariate model.
As cervical HPV and cervical cytologic abnormalities were closely related, only cervical HPV+ was included in the multivariate model based on univariate results.
Forty-seven women were diagnosed with abnormal anal cytology or anal HPV infection at any study visit and 36 of those women (77%) underwent high resolution anoscopy. Twelve of 36 women who underwent HRA were diagnosed with AIN2-3 (33%). Cytology and histology results of those diagnosed with AIN2-3 are summarized in Table 2. In univariate analysis, women with AIN2-3 (compared to women who underwent HRA without AIN 2–3 diagnosis) trended towards an association with cervical cytologic abnormality on most recent prior cytology test (OR 10.0, 95% CI 0.97–102.9, p=0.06) and history of STI other than cervical/anal HPV (OR 7.9, 95% CI 0.9–71.1, p=0.06) (results not shown). Eleven of these 12 subjects were referred for HRA due to abnormal anal cytology results and one additional woman had AIN2-3 diagnosed after an abnormal anal HPV test with normal anal cytology. The median time from baseline cytology screening to diagnosis of AIN2-3 was 274 days (range 41–1154 days), and six of seven women diagnosed with AIN2-3 after the second cytology visit were diagnosed within one year of baseline screening. Four of the 12 subjects with AIN2-3 had at least one negative anal cytology prior to the AIN2-3 diagnosis (Table 2).
Table 2.
Anal Cytology and HPV Results for Subjects Diagnosed with Anal Intraepithelial Neoplasia (AIN) 2–3
| VISIT 1 | VISIT 2 | VISIT 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Anal Cytology Result | Anal HPV | HRA Result | Days to HRA Result† | Anal Cytology Result | Anal HPV | HRA Result | Days to HRA Result† | Anal Cytology Result | Anal HPV | HRA Result | Days to HRA Result† |
| 1 | LSIL | − | AIN2 | 90 | ||||||||
| 2 | LSIL | + | AIN3 | 41 | ||||||||
| 3 | LSIL | + | AIN3 | 125 | ||||||||
| 4 | NILM | − | N/A | N/A | NILM | + | AIN2 | 363 | ||||
| 5 | ASCUS | + | AIN1 | 52 | ASCUS | + | AIN2 | 283 | ||||
| 6 | ASCUS | + | Refused | N/A | LSIL | + | AIN2 | 615 | ||||
| 7 | LSIL | − | AIN1 | 30 | LSIL | − | AIN2 | 219 | ||||
| 8 | LSIL | − | AIN1 | 30 | ASCUS | + | AIN2 | 240 | ||||
| 9 | ASCUS | + | Refused | N/A | ASCUS | + | AIN3 | 346 | ||||
| 10 | NILM | − | N/A | N/A | LSIL | − | AIN3 | 265 | ||||
| 11 | ASCUS | − | AIN1 | 125 | NILM | − | N/A | N/A | ASCUS | − | AIN3 | 895 |
| 12 | NILM | + | Not Referred* | N/A | ASCUS | + | NILM | 419 | LSIL | + | AIN3 | 1154 |
Note: HPV, human papillomavirus; HRA, high resolution anoscopy; NILM, no intraepithelial lesion or malignancy; ASCUS, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; AIN, anal intraepithelial neoplasia; N/A, not applicable.
Days from baseline cytology result to HRA.
Visit 1 occurred prior to decision to refer subjects with isolated HPV+ for HRA.
COMMENT
This study shows that abnormal anal cytology is a common finding in a diverse population of HIV-infected women and repeated anal cytologic testing increases the likelihood of an abnormal anal cytology diagnosis, resultant referral for HRA, and thus, AIN2-3 diagnosis. Incident anal cytologic abnormalities were detected in 23% of women with a normal baseline anal cytology result. The high rate of incident anal cytologic abnormalities (13.1 per 100 person-years of follow-up) is comparable to the rate found in Durante et al (22 per 100 person-years, 95% C.I. 14–33), from the early HAART era.11
Similar to cervical cytology, the majority of AIN2-3 is diagnosed after a minimally abnormal anal cytology; however, the likelihood of finding AIN2-3 given minimally abnormal cytology is much higher in the anus than in the cervix.16–18 Of 32 participants with a minimally abnormal cytology prior to HRA, 11 (34%) had biopsy proven AIN2-3. Four women with AIN1 on HRA for minimally abnormal anal cytology were diagnosed with AIN2-3 on a second HRA done for a repeat minimally abnormal anal cytology: AIN2-3 diagnosis occurred within one year of initial HRA in three of those four cases. Possible explanations for this difference include rapid disease progression, missed lesion on HRA or discrepancy in pathologic interpretation. While any of these reasons are possible, we had an experienced anoscopist perform every HRA and an experienced pathologist read all pathology results. One limitation of this study is that we did not have an additional blinded pathologist review the results for confirmation. As one-third of the AIN2-3 cases were found after an AIN1 diagnosis and one-third had at least one normal cytology result prior, it appears that there is benefit for repeated screening in at least a subset of individuals. These results will need to be replicated in larger studies.
Several factors were associated with abnormal anal cytology in unadjusted and multivariate analyses. Cervical HR-HPV diagnosis was associated with anal HR-HPV positivity; and trended towards an association with history of STI other than cervical/anal HPV. While it appears that history of STI other than cervical/anal HPV is associated with abnormal anal cytology, the sample size was too small to look at other STIs separately. It would be important to tease out in future studies whether specific STIs are driving this association or whether history of other STI may simply be a marker for history of cervical and/or anal HPV infection. Low CD4 count has been associated with AIN or anal cytology abnormalities in HIV-infected women previously.9,11,13,14 Although only 11 subjects had a current CD4 count less than 200 cells/mm3, low CD4 count was the strongest predictor of anal cytology abnormality in this population, which highlights the importance of immune reconstitution.
Our plan was for repeated anal cytology and HPV screening every six months over three visits. While a large majority did return for at least one follow-up visit, many subjects had delays in the planned follow-up. While 86% of women with AIN2-3 diagnosed after a second anal cytology result were diagnosed within one year of baseline cytology, we cannot tell from this study whether faster time to AIN2-3 diagnosis made a difference in outcome. In addition, 20% of women in this study diagnosed with abnormal anal cytology during follow-up had ≥one prior normal anal cytology result. While the optimal frequency of anal cancer screening in HIV-infected women remains unclear, it appears that women with risk factors including low CD4 count, and history of anal or cervical HPV or other STIs should undergo repeated cytology screenings regardless of initial normal anal cytology results.
Compliance for our screening visits was good: 87% of subjects in our study were followed for two visits and 71% were followed for three visits—however over 20% of women who were eligible for HRA based on anal cytologic or HPV criteria refused follow-up HRA. Poor compliance with HRA is not unique to our cohort as only 50% of the subjects in the WIHS cohort underwent the recommended HRA.9 It appears from our anecdotal experience that HIV-positive women are more likely to delay or avoid HRA compared to men with HIV and generally express more discomfort with undergoing anoscopy. The HRA visits did not take place in the HIV clinic at the time of this study and the separate HRA location may have been an additional barrier for women who were already uncomfortable about having the procedure. HIV-infected women from this population cited fear of pain or sexual assault flashbacks, embarrassment, and lack of social support as reasons for avoidance of HRA in a recent study (unpublished data). Our patient population is generally covered by Medicaid insurance, therefore cost for the visit was not likely to be a barrier, however additional barriers such as transportation, child care or missing work could all play a role. A better understanding of the reasons for non-adherence with recommended HRA evaluation will be necessary if anal cancer screening is to be adopted as standard of care in HIV-infected women.
While the prevalence of anal cytologic abnormalities at baseline was only 17%, overall, 33% of our subjects had at least one abnormal anal cytology over up to three years of cytologic follow-up. This is comparable to a prevalence of abnormal anal cytology of 31–38% in other recently reported cohorts of HIV-infected women,9,12,19 and 67–81 % in HIV-infected men who have sex with men.18,20,21 The reasons for the lower rates of abnormal anal cytology in our cohort at baseline are unclear. The patient population at BMC is unusual in that the majority of the study population was born outside of the U.S., acquired HIV through heterosexual contact, and 37% were diagnosed with HIV within the past five years. Our patient population differed from that of the Women’s Interagency HIV Study (WIHS)9 and Study to Understand the Natural History of HIV and AIDS in the Era of Effective Therapy (SUN)12,19 cohorts in several respects: reported history of anal intercourse (WIHS 47%9 vs. SUN 40%19 vs. BMC 22%10), current cigarette smoking (WIHS 56% vs. SUN 52% vs. BMC 23%), and history of injection drug use (WIHS 34% vs. SUN 3% (within the past six months) vs. BMC 15%). These factors are suggestive of differences in the composition of the patient populations.
In this study of HIV-infected women, anal HPV was detected with HC2 utilizing the residual liquid cervical cytology SurePath specimen. A much lower proportion of participants had detectable prevalent anal HR-HPV infection in our cohort compared with others (16% in BMC versus 44% in WIHS and 84% in SUN). The prevalent cervical HR-HPV infection rates were comparable between the WIHS (19%)9 and BMC (24%)10 cohorts; although much higher in the SUN Study (70%).12 In comparison, rates of anal HPV in HIV-infected men range from 60–95%.18,21–25 Both the WIHS and SUN studies collected the anal HPV specimens in 1cc standard transport media (STM) and used PCR testing whereas in our study, the specimens were the residual from the 10cc liquid cytology specimen (SurePath) (as is routine for detection of high-risk HPV from cervical specimens in the clinical setting). A recent study of anal cancer screening of HIV-positive and negative men utilizing HRA, anal cytology (with PreservCyt, Hologic Inc, Marlborough, MA) and HC2 testing from both STM and liquid residual from cytology found that HC2 had a sensitivity of 91% in detecting AIN2-3 with no difference whether STM versus liquid residual from cytology was used.26 It is unclear if our lower rates of anal HPV detection reflect differences in liquid cytology medium (SurePath vs. ThinPrep), differences in method of HPV detection (HC2 vs. PCR) or a truly lower prevalence of anal HPV in our patient population. In addition, multiple subjects with normal cytology throughout follow-up were transiently HPV positive (Figure 1). It is unclear if these represent false negative results or if these women were transiently shedding or clearing their infections.
Despite our small sample size, prior abnormal cervical cytology result and history of non-cervical/anal HPV STI were the strongest predictors of AIN2-3 diagnosis in our population. While abnormal cervical cytology tests and STIs are common in HIV-positive women, either diagnosis should likely prompt increased surveillance for AIN2-3. Abnormal anal cytology result appears to be more common in HIV-infected women with AIN2-3 in this population compared to anal HPV positivity; the proportion with anal HPV diagnosis was lower than anticipated and the number of subjects who underwent HRA was too small to say this definitively. While we continue to perform anal HC2 HPV testing on anal cytology samples at our institution, the additive value of HC2 HPV testing in HIV-infected women is less clear from our findings. A large study on HIV-infected women including both HC2 and PCR testing with HRA for all subjects will be necessary to better understand the value of anal HPV testing with HC2 prior to making stronger recommendations for or against its use.
Prior to the adoption of universal screening for anal dysplasia in HIV-infected women, there are several questions that need to be answered. Whether anal cytology is an optimal screening tool for anal cancer and AIN2-3 is not known. Studies to evaluate sensitivity of anal cytology have been conducted in MSM; however, as the disease prevalence is likely much higher in HIV-infected MSM compared with women it is not clear that cytology would be the best screening tool for HIV-infected women. We also do not know the true prevalence of AIN2-3 in HIV-infected women as there have been no studies where all HIV-infected women underwent HRA regardless of cytology results. Lastly, the natural history of anal HPV infections and neoplasia and the efficacy of treatment of AIN2-3 to prevent the development of invasive anal cancer remain unknown.
As HIV-infected women live longer, they continue to be at risk for HPV associated diseases including intraepithelial neoplasia and cancer of the cervix, vulva and anus despite treatment with anti-retroviral therapy. Anal cytology may be a better screening test for AIN2-3 compared with anal HPV testing with HC2 in this population; however repeated anal cytology screening appears necessary for at least a subset of HIV-positive women including those with advanced immunosuppression, history of abnormal cervical cytology, anal and/or cervical HPV or other STIs.
Acknowledgments
Funding: This work was supported by departmental funds and through National Institute of Health grants [5 T32 AI52074-05 and 5 K12 HD043444-07] (A.S.B.).
Footnotes
Presentation Information:
- Infectious Disease Society of America (IDSA) Annual Meeting, San Diego, CA; October 2007.
- 24th International Papillomavirus Conference and Clinical Workshop, Beijing, China; October 2007.
- ASCCP (American Society for Colposcopy and Cervical Pathology) Biennial Meeting, Orlando, FL; March 2008.
- American College of Obstetricians and Gynecologists Annual Meeting, New Orleans, LA; May 2008.
- 48th Annual ICAAC/46th IDSA Annual Meeting, Washington, DC., October, 2008,
- 25th International Papillomavirus Conference and Clinical Workshop, Malmö, Sweden, May, 2009.
- 26th International Papillomavirus Conference and Clinical Workshop, Montreal, Canada, July 2010.
Disclosure: E.A.S. is funded on an NIH grant (R01 CA163103-01) for which Qiagen has agreed to provide Hybrid Capture 2 supplies for HPV testing.
Because the utility of screening for anal intraepithelial neoplasia based on anal HPV alone in HIV-infected women is unknown, the protocol was amended during the study to refer participants with anal HR-HPV detected by HC2 for HRA regardless of the anal cytology result. Only four women with anal HPV diagnosed at the baseline visit were not referred for HRA prior to this change in protocol.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crum-Cianflone N, Hullsiek K, Marconi V. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23:41–50. doi: 10.1097/QAD.0b013e328317cc2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guiguet M, Boue F, Cadranel J, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10:1152–1159. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 3.D’Souza G, Wiley D, Li X. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2008;48:491–499. doi: 10.1097/QAI.0b013e31817aebfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bower M, Powles T, Newsom-Davis T, Thirlwell C, Stebbing J, Mandalia S. HIV-associated anal cancer: Has highly active retroviral therapy reduced the incidence or improved the outcome? J Acquir Immune Defic Syndr. 2004;37:1563–1565. doi: 10.1097/00126334-200412150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Diamond C, Taylor TH, Aboumrad T, Bringman D, Anton-Culver H. Increased incidence of squamous cell anal cancer among men with AIDS in the era of highly active antiretroviral therapy. Sex Transm Dis. 2005;32:314–320. doi: 10.1097/01.olq.0000162366.60245.02. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101:1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: Updating the natural history of HPV and anogenital cancer. Vaccine. 2006 Aug 31;24(Suppl 3):S3/42–51. doi: 10.1016/j.vaccine.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Chin-Hong PV, Palefsky JM. Human papillomavirus anogenital disease in HIV-infected individuals. Dermatol Ther. 2005 Jan-Feb;18:67–76. doi: 10.1111/j.1529-8019.2005.05009.x. [DOI] [PubMed] [Google Scholar]
- 9.Hessol NA, Holly EA, Efird JT, Minkoff H, Schowalter K, Darragh TM. Anal intraepithelial neoplasia in a multisite study of HIV-infected and high-risk HIV-uninfected women. AIDS. 2009;23:59–70. doi: 10.1097/QAD.0b013e32831cc101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tandon R, Baranoski AS, Huang F, et al. Abnormal anal cytology in HIV-infected women. Am J Obstet Gynecol. 2010;203:21.e21–26. doi: 10.1016/j.ajog.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durante AJ, Williams AB, Da Costa M, Darragh TM, Khoshnood K, Palefsky JM. Incidence of anal cytological abnormalities in a cohort of human immunodeficiency virus-infected women. Cancer Epidemiol Biomarkers Prev. 2003;12:638–642. [PubMed] [Google Scholar]
- 12.Kojic EM, Cu-Uvin S, Conley L, et al. Human papillomavirus infection and cytologic abnormalities of the anus and cervix among HIV-infected women in the study to understand the natural history of HIV/AIDS in the era of effective therapy (the SUN study) Sex Transm Dis. 2011;38:253–259. doi: 10.1097/OLQ.0b013e3181f70253. [DOI] [PubMed] [Google Scholar]
- 13.Palefsky JM, Holly EA, Ralston ML, Da Costa M, Greenblatt RM. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. J Infect Dis. 2001;183:383–391. doi: 10.1086/318071. [DOI] [PubMed] [Google Scholar]
- 14.Holly EA, Ralston ML, Darragh TM, Greenblatt RM, Jay N, Palefsky JM. Prevalence and risk factors for anal squamous intraepithelial lesions in women. J Natl Cancer Inst. 2001;93:843–849. doi: 10.1093/jnci/93.11.843. [DOI] [PubMed] [Google Scholar]
- 15.Gingelmaier A, Weissenbacher T, Kost B, Kaestner R, Sovric M, Mylonas I. Anal cytology as a screening tool for early detection of anal dysplasia in HIV-infected women. Anticancer Research. 2010;30:19–24. [PubMed] [Google Scholar]
- 16.Berry JM, Palefsky JM, Jay N, Cheng SC, Darragh TM, Chin-Hong PV. Performance characteristics of anal cytology and human papillomavirus testing in patients with high-resolution anoscopy-guided biopsy of high-grade anal intraepithelial neoplasia. Dis Colon Rectum. 2009;52:239–247. doi: 10.1007/DCR.0b013e31819793d9. [DOI] [PubMed] [Google Scholar]
- 17.Nathan M, Singh N, Garrett N, Hickey N, Prevost T, Sheaff M. Performance of anal cytology in a clinical setting when measured against histology and high-resolution anoscopy findings. AIDS. 2010;24:373–379. doi: 10.1097/QAD.0b013e328333ab8e. [DOI] [PubMed] [Google Scholar]
- 18.Salit IE, Lytwyn A, Raboud J, et al. The role of cytology (Pap tests) and human papillomavirus testing in anal cancer screening. AIDS. 2010;24:1307–1313. doi: 10.1097/QAD.0b013e328339e592. [DOI] [PubMed] [Google Scholar]
- 19.Conley L, Bush T, Darragh TM, Palefsky JM, Unger ER, Patel P. Factors associated with prevalent abnormal anal cytology in a large cohort of HIV-infected adults in the United States. J Infect Dis. 2010;202:1567–1576. doi: 10.1086/656775. [DOI] [PubMed] [Google Scholar]
- 20.Cranston RD, Hart SD, Gornbein JA, Hirschowitz SL, Cortina G, Moe AA. The prevalence, and predictive value, of abnormal anal cytology to diagnose anal dysplasia in a population of HIV-positive men who have sex with men. Int J STD AIDS. 2007;18:77–80. doi: 10.1258/095646207779949772. [DOI] [PubMed] [Google Scholar]
- 21.Palefsky JM, Holly EA, Efirdc JT, et al. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS. 2005;19:1407–1414. doi: 10.1097/01.aids.0000181012.62385.4a. [DOI] [PubMed] [Google Scholar]
- 22.Goldstone SE, Kawalek AZ, Goldstone RN, Goldstone AB. Hybrid Capture II detection of oncogenic human papillomavirus: A useful tool when evaluating men who have sex with men with atypical squamous cells of undetermined significance on anal cytology. Dis Colon Rectum. 2008;51:1130–1136. doi: 10.1007/s10350-008-9306-4. [DOI] [PubMed] [Google Scholar]
- 23.Palefsky JM, Holly EA, Ralston ML, Jay N. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men. J Infect Dis. 1998;177:361–367. doi: 10.1086/514194. [DOI] [PubMed] [Google Scholar]
- 24.Gohy LG, Isabella, Rouleau D, Ghattas G, et al. Genotyping of human papillomavirus DNA in anal biopsies and anal swabs collected from HIV-seropositive men with anal dysplasia. J Acquir Immune Defic Syndr. 2008;49:32–39. doi: 10.1097/QAI.0b013e318183a905. [DOI] [PubMed] [Google Scholar]
- 25.Wilkin TJ, Palmer S, Brudney KF, Chiasson MA, Wright TC. Anal intraepithelial neoplasia in heterosexual and homesexual HIV-positive men with access to antiretroviral therapy. J Infect Dis. 2004;190:1685–1691. doi: 10.1086/424599. [DOI] [PubMed] [Google Scholar]
- 26.Goldstone SE, Lowe B, Rothmann T, Nazarenko I. Evaluation of the Hybrid Capture 2 assay for detecting anal high-grade dysplasia. Int J Cancer. 2012 Jan 10; doi: 10.1002/ijc.27431. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]