Abstract
As astrocytes are becoming recognized as important mediators of normal brain function, studies into their roles in neurological disease have gained significance. Across mouse models for neurodevelopmental and neurodegenerative diseases, astrocytes are considered key regulators of disease progression. In Rett syndrome and Parkinson’s disease, astrocytes can even initiate certain disease phenotypes. Numerous potential mechanisms have been offered to explain these results, but research into the functions of astrocytes in disease is just beginning. Crucially, in vivo verification of in vitro data is still necessary, as well as a deeper understanding of the complex and relatively unexplored interactions between astrocytes, oligodendrocytes, microglia, and neurons.
Introduction
Our understanding of the biological functions of glial cells (astrocytes, microglia, and oligodendrocytes) in the nervous system is undergoing a transformation. Where once they were considered accessories to the cognitively vital neurons, providing only structural and trophic support, new research is describing a paradigm in which glia are full partners with neurons in the operations of the brain. This includes roles for astrocytes in regulating basal synaptic transmission [1,2] and synaptic efficacy [2], eliciting slow inward currents [3,4], modulating cortical plasticity [5], and numerous roles during development, including synaptogenesis [6]. And as our knowledge about astrocytic function in normal physiology has expanded, exploration into their likely role in disease pathology has followed.
While microglia, oligodendrocytes, and astrocytes have been implicated in many neurological disorders, here we focus on functional studies of astrocytes in mouse models of genetic neurological diseases. Astrocytes are electrically inert cells that are derived from the same progenitors as neurons. They come predominantly in two forms, fibrous and protoplasmic, which denote their morphology and primary location in the brain (white vs. grey matter, respectively). Glial Fibrillary Acidic Protein (GFAP) is the most commonly used marker of mature astrocytes in the CNS [7], though it is also expressed transiently by radial glia progenitors [8]. Other markers include Aldh1l1 [9], Glt-1, and GLAST [10]. To date, no marker has been identified that is expressed exclusively in mature astrocytes. Moreover, no panastrocytic marker has been identified with which to determine the fraction of astrocytes that are GFAP+, although recent studies on Aldh1L1 are promising [9].
Astrocytes undergo extreme morphological and molecular changes, including upregulation of GFAP, after injury to the CNS by blunt trauma or neurodegeneration [11]. This process of astrogliosis is important to understand for clinical and therapeutic reasons, and has a long a history of study that has been reviewed extensively [12]. In contrast, newly emerging roles for astrocytes in the early stages of neurodevelopmental and neurodegenerative diseases have received less attention. A broad study of the literature suggests that astrocytes are key regulators of the progression of neuropathology after the first onset of disease. As described below, astrocytes fundamentally affect the progression of disease in Rett syndrome, Fragile X, amyotrophic lateral sclerosis, Alzheimer’s, Huntington’s, and Parkinson’s. In rare cases, astrocytes have been implicated in the initiation of some aspects of disease, including in Rett syndrome and Parkinson’s disease, which suggests as-yet unknown functions for astrocytes in normal brain function. From investigations into the roles of glia during neurological disease, we are likely to achieve a broader understanding of how the brain works, in addition to new insights into disease diagnosis and treatment.
Astrocytes in neurodevelopmental disease
Astrocytes are born later in development than neurons, but are present when the majority of synapses are formed. Recent evidence indicates that the close association of neurons and astrocytes is necessary for normal synapse development, including synaptic pruning [6,13]. This may have broad implications for neurodevelopmental diseases, as problems may arise in synaptogenesis and neuronal maturation due to astrocyte malfunction prior to the appearance of overt symptoms.
Tuberous sclerosis complex (TSC)
One of the first neurodevelopmental disorders implicating astrocyte dysfunction was tuberous sclerosis complex (TSC), which is caused by loss-of-function mutations in the genes encoding HAMARTIN (TSC1) or TUBERIN (TSC2). TSC1 or 2 mutations lead to a hallmark increase in mTOR signaling [14] and brain dysplasia, including the growth of non-malignant tumors called “tubers” (Reviewed by [15]). Resected tubers from patients and mice harboring mutations in Tsc1 or 2 are comprised mainly of enlarged, dysplastic astrocytes and neurons [16–19], suggesting at least an indirect astrocytic component to the disease. To this point, deletion of Tsc1 using a non-inducible hGFAP-cre transgene crossed to a homozygous-floxed Tsc1 mouse resulted in some neurological features reminiscent of the human disease [16]. The authors concluded that this demonstrated an astrocyte-specific growth advantage that resulted in the neuronal abnormalities. However, this interpretation is likely confounded by hGFAP-cre activity in radial glia progenitors [8].
A more tempered interpretation of the phenotype yielded by hGFAP-cre excision of the Tsc genes was suggested when the transgene was used to knockout Tsc2 [18], which also yielded TSC-like neuropathology. In this case the authors interpreted the results as demonstrating a role for Tsc2 in regulating the growth and differentiation of GFAP+ radial glia cells. This interpretation seems more congruent with studies showing that loss of Tsc2 selectively from neurons using a synapsin-I-cre transgene [20] or a calcium-calmodulin kinase II-cre transgene [21] causes severe TSC-like neuropathology. Further, removal of Tsc1 from forebrain progenitor cells using an Emx1-cre [19] indicated that Tsc1 dysfunction does not lead to increased mTOR signaling in astrocytes in vivo; however, non-canonical Tsc1/2-dependent signaling pathways relevant to TSC neuropathology have been proposed [14,22]. It still remains possible that astrocyte dysfunction contributes to TSC neuropathology, especially given the presence of enlarged astrocytes in TSC patients and mouse models, though a causal relationship between astrocyte dysfunction and TSC symptoms will have to be reconciled with the robust phenotypes yielded by neuronspecific deletion of Tsc2. One possibility is that Tsc1/2 mutation in neurons might lead to secondary dysfunction in astrocytes necessary for disease progression. For instance, loss of Tsc2 specifically from neurons causes astrogliosis [21], without necessarily causing an increase in mTOR signaling in astrocytes [20]. Further experiments, especially with conditional knockouts, are necessary to determine whether astrocyte dysfunction contributes to TSC neuropathology.
Rett Syndrome (RTT)
Rett syndrome is caused by sporadic loss-of-function mutations in the gene encoding methyl-CpG-binding protein 2 (MECP2). MeCP2 is a transcription factor that binds to methylated-CpGs throughout the genome [23], and was originally suggested to act as a suppressor of proximal genes by recruiting histone deacetylases and other co-repressors [24]. Other functions for MeCP2 have now been proposed including transcriptional activation [25], RNA processing [26], and long-range gene repression [27]. Genetic and behavioral studies initially suggested that RTT neuropathology was due exclusively to MeCP2 dysfunction in neurons. Most germane, loss of Mecp2 from subsets of neurons causes, to varying degrees of severity, subsets of RTT-like phenotypes consistent with the known roles of the targeted neurons [28–32]. Additionally, expression of Mecp2 in postmitotic neurons from the tau locus prevents the appearance of some RTT-like phenotypes [33]. Finally, immunohistochemical analyses from laboratories studying RTT initially did not detect MeCP2 in glia, including astrocytes [29,34,35]. However, western blot [36] and chromatin immunoprecipitation [37] analyses indicated that astrocytes express MeCP2 protein in vitro. These results were advanced by more detailed analyses demonstrating the presence of MeCP2 protein in astrocytes [38,39], as well as oligodendrocytes [38,40] and microglia [40] in vitro and in vivo. Co-culture experiments showed that MeCP2 was functional in astrocytes, because MeCP2-deficient astrocytes could not support normal dendritic morphology in wild type neurons [38,39], while wild type astrocytes supported normal dendritic architecture in MeCP2-deficient neurons [38]. In addition, MeCP2 deficiency was reported to spread to wild-type astrocytes through gap junctions in culture [39], potentially exacerbating the pathological effect of astrocytic MeCP2 loss in heterozygous females. This very interesting finding has not yet been confirmed in vivo.
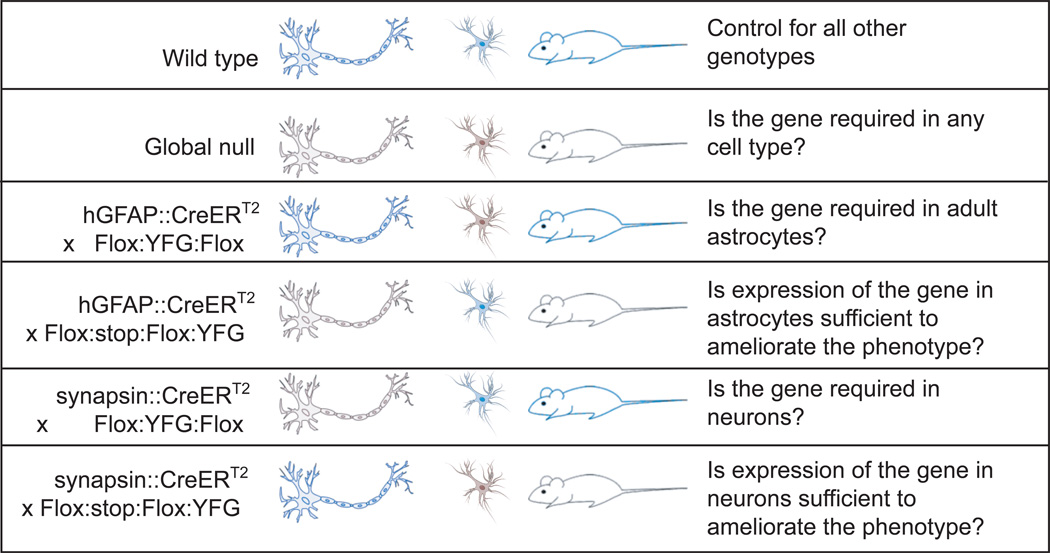
To investigate the potential role of astrocytes in RTT neuropathology in vivo, an inducible form of the hGFAP-cre transgene [41] was utilized to restore astrocytic MeCP2 in a RTT mouse model as well as remove MeCP2 selectively from astrocytes in a wild-type background [42] (Figure 1). The hGFAP-cre activity was induced in mice postnatally between three and four weeks of age to avoid recombination in GFAP+ progenitor cells. These studies showed that restoring MeCP2 in astrocytes in an otherwise null background had an unexpectedly profound influence on disease progression, because astrocyte-rescued mice showed significant improvements in several phenotypes, including increased longevity, improved locomotor abilities, and normalized respiratory patterns. Further, neuronal dendritic morphology, VGLUT1 (a synaptic transporter protein) levels, and soma sizes were restored to wild type levels in regions of efficient astrocytic MeCP2 restoration. Interestingly, loss of MeCP2 from astrocytes in an otherwise wild type mouse resulted in irregular breathing, though it did not result in lethality, and caused relatively mild impairments in motor parameters with no effect on neuronal dendritic morphology. These data were interpreted as showing that astrocytes largely control the progression of RTT neuropathology, while neurons control the initiation of most RTT-like phenotypes, excepting breathing regularity. This interpretation is also consistent with the ability of MeCP2 expression in post-mitotic neurons to prevent the appearance of several key RTT-like phenotypes [33], and is similar to the proposed role of astrocytes in genetic forms of ALS (see below).
Figure 1. Genetic Strategies for Recessive Diseases.
When trying to determine the role of astrocytes in Rett syndrome, our lab used the genetic strategies outlined here. These strategies are designed to cause knockouts after the shared neural progenitor stage (Nestin+, GFAP+) by inducing excision in differentiated neurons (Synapsin::Cre is merely one example) or differentiated astrocytes (hGFAP::CreERT2 + tamoxifen). In this figure, Your Favorite Gene (YFG) takes the place of MeCP2 from Rett. Blue indicates normal expression levels and black indicates low or no expression. While useful, no Cre line is expressed in 100% of the cell type indicated, and the extent of mosaic expression will influence the interpretation of results.
How astrocytes influence the progression of RTT neuropathology remains unknown. One possible mechanism is through the secretion of trophic factors such as brain derived neurotrophic factor (BDNF) and cytokines [39], the levels of which are decreased in the absence of MeCP2 in vivo. It is also possible that improved energy metabolism contributes to the astrocyte-rescue, because the levels of the astrocyte-specific metabolite myo-inositol are decreased in RTT brains [43,44]. For respiration, multiple groups have shown that astrocytes in brainstem respiratory centers are chemosensitive and profoundly influence the firing patterns of respiratory neurons via the release of ATP in response to changes in extracellular CO2 concentrations [45]. Intriguingly, MeCP2-deficient mice have decreased CO2-sensitivity [46] and ATP levels are decreased in the brains of MeCP2-deficient mice [43]. It is possible that restoration of MeCP2 in astrocytes restores chemosensitivity of the astrocytes, therefore positively influencing respiratory patterns. Whatever the mechanism of rescue, it seems that wild type neurons do not require the same astrocytic support as MeCP2-deficient neurons, suggesting that further investigation into neuronal-glial interactions is necessary to determine precisely how astrocytes support improved neuronal function. It will also be important to investigate the involvement of other glial cell types, such as microglia [40], in the progression of RTT in vivo because microglia cause glutamate-induced neurotoxicity in vitro [40].
Fragile X Syndrome (FXS)
Fragile X syndrome is the leading cause of inherited intellectual disability in boys and is caused by CGG expansion in the 5’ non-coding region of the X-linked gene Fragile X Mental Retardation Protein 1 (FMRP1). FMRP1 is expressed primarily in the brains and gonads [47], and functions as a translational regulator of mRNAs, including transcripts important for dendritic growth and synapse formation [48–52]; however, it is currently unclear how the dysregulation of mRNA translation gives rise to FXS. Studies using FMRP1-deficient mice have clearly shown the involvement of neurons in FXS neuropathology [53]. Recent evidence suggests that astrocytes might also contribute to the neuropathology of FXS. During normal development in mice, while FMRP1 protein expression increases in neurons, it decreases in cells of the glial lineage [54], including astrocytes, such that adult GFAP+ astrocytes do not express detectable levels of the protein in vitro or in vivo [55]. This raises the possibility that a primary defect in astrocytes, due to the absence of FMRP1 activity during astrocyte differentiation, contributes to latent defects in neuronal function. In support of this idea, in co-culture FMRP1-deficient astrocytes cause decreased neuronal survival, stunted dendritic arbors, and decreased presynaptic and postsynaptic clustering of protein aggregates in wild type neurons [56]. Conversely, wild type astrocytes support normal dendritic and synaptic morphology in FMRP1-deficient neurons [56]. It remains unclear how these results might impact the FXS phenotype because it was later shown that the defects in morphology of wild type neurons caused by FMRP1-deficient astrocytes are transient [57], and the influence of astrocytes on neuronal morphology in FXS mice in vivo is not known.
A leading model for how loss of FMRP1 causes neuropathology involves the activity of the metabotropic glutamate receptor 5-protein (mGluR5) [58]. In FMRP1-deficient mice, protein synthesis is increased downstream of mGluR5 signaling, and mGluR5 antagonists can ameliorate deficits in learning and memory as well as normalize the long-term depression deficits in CA1 neurons. Further, preliminary results from clinical trials using mGluR5-signaling inhibitors suggest that mGluR5 signaling is relevant to the human disease [59]. Therefore, synaptic signaling between neurons via mGluR5 is likely a critical contributor to FXS neuropathology. Interestingly, astrocytes also express functional mGluR5, and it was recently demonstrated that astrocytes can detect single-synaptic stimulation at excitatory synapses via mGluR5 localized to the astrocytic processes that envelop synapses [1]. Upon this detection of glutamatergic signaling by mGluR5, astrocytes increase the efficiency of transmission in CA1 pyramidal cells by releasing ATP, which activates presynaptic neuronal adenosine A2A receptors. Whether, and how, mGluR5 signaling via astrocytes contributes to the excitatory synaptic defects evident in FXS mice should be explored further.
Astrocytes in neurodegenerative disease
Because astrocytes are key regulators of brain homeostasis and neuronal metabolism, degenerative diseases may be caused by lack of important astrocytic functions, such as glutamate uptake [60], or by over-reactive astrocytes that result in neuropathology [61].
Amyotrophic lateral sclerosis (ALS)
Amyotrophic lateral sclerosis is a fatal motor neuron disease for which several mouse models have been developed based on inherited dominant mutations of the gene superoxide dismutase (SOD1). In these models, mutant SOD1 expression is necessary and sufficient in motor neurons to initiate the disease, but progression depends almost entirely on its presence in both microglia and astrocytes [62]. Interestingly, recent in vitro experiments show that astrocytes expressing mutant SOD1 are toxic to even wild-type neurons [63]. These results were specific to motor neurons only, accurately reflecting the in vivo phenotype. Astrocytes derived from human patients with both familial and sporadic ALS show similar neurotoxic results [64], arguing that astrocytes may be key activators of disease, not just of the degenerative processes that follow initiation.
Several groups have sought to test this hypothesis in vivo. Overexpressing the G86R mutant of SOD1 selectively in astrocytes under the control of the GFAP promoter does not cause motor neuron degeneration[65]. However, transplanting glial precursor cells from G93A mutant SOD1-expressing mice into the spinal cord of wild type rats resulted in motor neuron loss near the transplants and mild behavioral and electrophysiological symptoms associated with ALS[66]. Perhaps there is some developmental compensation in the transgenic model that is not observed in vitro or in transplantations. It is also possible that different SOD1 mutations can affect astrocytes differently, though in vitro results would argue against this [64]. In agreement with the idea that astrocytes are specifically important for progression, removing mutant SOD1 from astrocytes using the GFAP:Cre transgene has no effect on the onset of disease, but slowed the rate of progression and increased longevity[67,68], similar to the results seen in RTT. Furthermore, transplantation of wild-type astrocytes into mutant SOD1-expressing mice also extended survival and attenuated motor neuron loss [69].
While clearly important for disease progression, insights into the mechanisms by which astrocytes affect motor neuron degeneration are lacking. Interactions between astrocytes and microglia are clearly important, as microgliosis is increased when astrocytes express mutant SOD1 [66,68], and reduced when astrocytes are wild type [67,69]. This activation of microglia is downstream of astrocytic signals, as minocycline, a microglial inhibitor, was able to rescue astrocyte-induced motor neuron degeneration [66]. However, NF-κB signaling does not appear to mediate this or any neurodegenerative effect of astrocytes in ALS [70]. Future investigations into the interaction between astrocytes and microglia in this and other disease models will be important to develop useful therapeutics.
Huntington’s Disease (HD)
Huntington’s disease is caused by the expansion of CAG (glutamine-encoding) repeats in the huntingtin protein that leads to selective neurodegeneration of striatal neurons. Wild type neurons co-cultured with astrocytes that overexpress a mutant form of the huntingtin protein undergo apoptosis, suggesting that this protein impairs glial support of neuronal cells [71]. This may be associated with increased excitotoxicity, as mutant astrocytes were unable to protect neurons against glutamate- or NMDA-induced toxicity in vitro.
In vivo, expression of mutant huntingtin (160Q) under the control of the GFAP promoter leads to age-dependant neurological phenotypes including weight loss, hindlimb clasping, and worsening rotarod performance [72]. While the animals die shortly after becoming symptomatic, no degeneration of neurons occurs, indicating that neuronal function is perturbed by astrocytes prior to cell death. When these mice were crossed with a line expressing mutant huntingtin primarily in neurons, they displayed more severe neurological symptoms and earlier death [72], supporting a role for astrocytes in disease severity if not neurodegeneration. Future experiments using the genetic strategies outlined in Figure 2 might be useful to explore the role of astrocytes in Huntington’s disease. Meanwhile, lentiviral overexpression of mutant huntingtin in astrocytes leads to morphological and molecular changes that are also seen in the human disease, though no reports on the effects of this perturbation on neurological function or degeneration were reported [73].
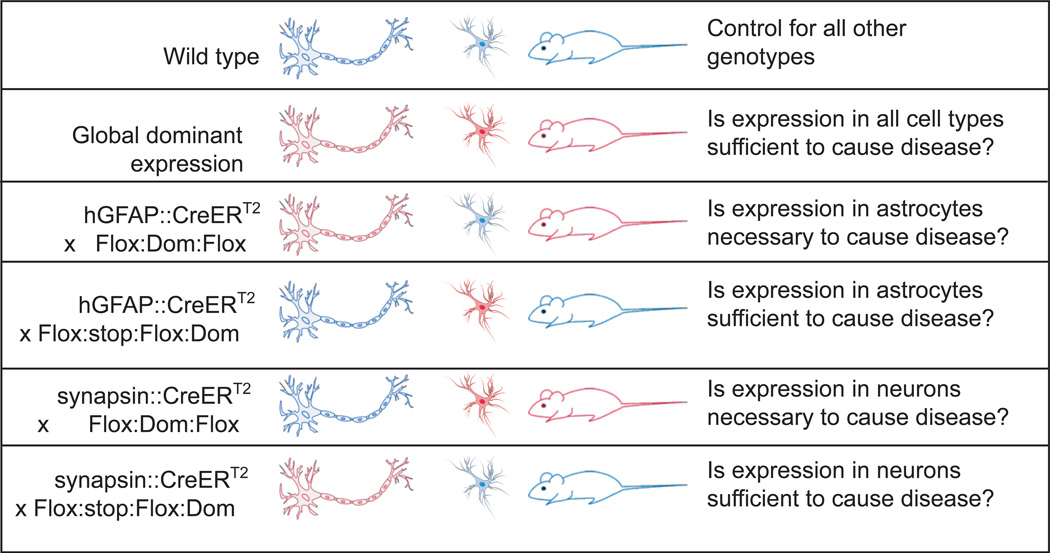
Figure 2. Genetic Strategies for Dominant Diseases.
Similar to Figure 1, these genetic strategies are designed to express the dominant gene of interest (Dom) only after the shared neural progenitor stage. Blue indicates wild type gene expression and red indicates expression or overexpression of the dominant gene. Again, mosaic expression of Cre recombinase will influence result interpretation.
Parkinson’s Disease (PD)
Parkinson’s disease is characterized by the presence of α-synuclein protein inclusions in neurons throughout the nervous system and progressive neurodegeneration of dopaminergic neurons in the substantia nigra (SN), which leads to severe motor dysfunction. Several mouse models for this disease exist that phenocopy the disease to varying degrees, including MPTP- and rotenone-induced neurodegeneration, overexpression of mutant forms of α-synuclein, or knockouts of Parkinson’s-linked genes Parkin, PINK1, or DJ-1, [74]. Media conditioned from Parkin-null astrocytes leads to increased apoptosis in neuronal cultures [75]. In addition, DJ-1 knockout astrocytes lack the ability to protect neurons from rotenone-induced neurodegeneration [76], providing a non-neuronal link between these models of PD.
Exciting new results have shown that selective expression of mutant α-synuclein protein in astrocytes in vivo results in SN neurodegeneration, behavioral dysfunction and shortened lifespan [77]. In fact, 100% of the animals died after three months, a much faster rate than when mutant α-synuclein protein is expressed under control of the Huα or PrP promoters, which express in fewer astrocytes but more neurons [78,79]. This suggests that astrocytic dysfunction might be crucial for disease initiation, not just for downstream neurodegenerative effects. This might also explain how grafted neurons end up showing α-synuclein pathology in long term transplants [80,81]. To further support this, no neuronal-specific knockouts of Parkinson’s-related genes have led to neurodegeneration in the SN [74]. Conditional astrocyte-specific knockouts in these genes would help answer whether astrocytes are the primary cell type for disease initiation (Figure 1).
Alzheimer’s Disease (AD)
Several mouse models of Alzheimer’s disease are generated by overexpression of various truncations of the amyloid-β (Aβ) protein, which form Aβ plaques in the brain that, along with cognitive deficits and tau neurofibrillary tangles, are diagnostic for AD [82]. Aβ pre-treatment of astrocytes leads to decreased neuronal viability in vitro, and co-culture of astrocytes accelerates and exacerbates neuronal death caused by Aβ treatment [83,84]. These effects and inflammatory signals are reduced by treatment with minocycline, suggesting that microglia are upstream of astrocyte-induced cell death observed in this paradigm [84]. These results need to be verified in vivo (Figure 2).
A possible mechanism is suggested by the finding that Aβ-induced microglia signal an increase in the levels of the hemichannel protein connexin43 (Cx43) in astrocytes, which leads to increased toxic glutamate and ATP release [85,86]. Blocking hemichannel release in vivo leads to improved memory without affecting Aβ plaque deposition [87], arguing that these downstream glial events are important for the cognitive decline observed in this disease. Genetically ablating hemichannel function in microglia and astrocytes in AD models should further demonstrate their importance to disease pathology. Other proposed mechanisms for the Aβ-induced neurotoxicity of astrocytes include the activation of neutral sphingomyelinase [88], overexpression of S100B [89], calcium dysregulation [90], and metabolic dysfunction [83]. Furthermore, transplantation of wild-type astrocytes into the brains of Aβ-expressing mice resulted in Aβ clearance [91], supporting the notion that astrocytes should be targets of therapeutic investigations in the future.
Conclusions
An increasing body of evidence is accumulating that astrocytes are key regulators of neurological disease, in both developmental and degenerative contexts. Numerous mechanisms have been proposed for these effects, including glutamate dysregulation, ATP release, metabolic deficiency, phagocytosis, and inflammatory signaling. This highlights just how many important functions astrocytes, in addition to microglia and oligodendrocytes, have in normal CNS physiology, and indicates that much more remains to be learned about their roles in normal and diseased states. Only once the complicated network of actions and reactions between these cell types is untangled can we begin to address the true underlying causes of neurological disorders, and have more hope of developing effective treatments.
Highlights.
Astrocytes have fundamental roles in neurological disease progression.
In RTT and PD, astrocytes can independently cause disease phenotypes.
Much remains to be studied regarding the roles of astrocytes in other diseases.
More rigorous use of genetic tools should aid in these studies.
Acknowledgements
The authors would like to thank Dr. Nurit Ballas and Dr. Paul Barnes for comments on the manuscript and grants from the NIH and Rett Syndrome Research Trust to GM. GM is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the last two years, have been highlighted as:
* of special interest
** of outstanding interest
- 1. Panatier A, Vallee J, Haber M, Murai KK, Lacaille JC, Robitaille R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell. 146:785–798. doi: 10.1016/j.cell.2011.07.022. ** Wild type astrocytes sense excitatory synaptic activity by sensing pre-synaptic release of glutamate via mGluR5 receptors. In turn, astrocytes promote pre-synaptic release of glutamate by releasing ATP to bind to pre-synaptic adenosine A2A receptors. These studies might implicate gliotransmission in the mGluR5-related synaptic defects in FXS.
- 2.Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirttimaki TM, Hall SD, Parri HR. Sustained neuronal activity generated by glial plasticity. J Neurosci. 31:7637–7647. doi: 10.1523/JNEUROSCI.5783-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- 5.Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, Hirase H. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci. 31:18155–18165. doi: 10.1523/JNEUROSCI.5289-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung WS, Barres BA. The role of glial cells in synapse elimination. Curr Opin Neurobiol. doi: 10.1016/j.conb.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43:429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- 8.Casper KB, McCarthy KD. GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol Cell Neurosci. 2006;31:676–684. doi: 10.1016/j.mcn.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 11.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 12.Kang W, Hebert JM. Signaling pathways in reactive astrocytes, a genetic perspective. Mol Neurobiol. 43:147–154. doi: 10.1007/s12035-011-8163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker AJ, Koch SM, Reed J, Barres BA, Ullian EM. Developmental control of synaptic receptivity. J Neurosci. 2008;28:8150–8160. doi: 10.1523/JNEUROSCI.1744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neuman NA, Henske EP. Non-canonical functions of the tuberous sclerosis complex-Rheb signalling axis. EMBO Mol Med. 3:189–200. doi: 10.1002/emmm.201100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomasoni R, Mondino A. The tuberous sclerosis complex: balancing proliferation and survival. Biochem Soc Trans. 39:466–471. doi: 10.1042/BST0390466. [DOI] [PubMed] [Google Scholar]
- 16.Uhlmann EJ, Apicelli AJ, Baldwin RL, Burke SP, Bajenaru ML, Onda H, Kwiatkowski D, Gutmann DH. Heterozygosity for the tuberous sclerosis complex (TSC) gene products results in increased astrocyte numbers and decreased p27-Kip1 expression in TSC2+/− cells. Oncogene. 2002;21:4050–4059. doi: 10.1038/sj.onc.1205435. [DOI] [PubMed] [Google Scholar]
- 17.Sosunov AA, Wu X, Weiner HL, Mikell CB, Goodman RR, Crino PD, McKhann GM., 2nd Tuberous sclerosis: a primary pathology of astrocytes? Epilepsia. 2008;49(Suppl 2):53–62. doi: 10.1111/j.1528-1167.2008.01493.x. [DOI] [PubMed] [Google Scholar]
- 18.Way SW, McKenna J, 3rd, Mietzsch U, Reith RM, Wu HC, Gambello MJ. Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse. Hum Mol Genet. 2009;18:1252–1265. doi: 10.1093/hmg/ddp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Magri L, Cambiaghi M, Cominelli M, Alfaro-Cervello C, Cursi M, Pala M, Bulfone A, Garcia-Verdugo JM, Leocani L, Minicucci F, et al. Sustained activation of mTOR pathway in embryonic neural stem cells leads to development of tuberous sclerosis complex-associated lesions. Cell Stem Cell. 9:447–462. doi: 10.1016/j.stem.2011.09.008. * This group showed that loss of TSC1 selectively from forebrain neural progenitor cells is sufficient to recapitulate most aspects of TSC neuropathology. Though tubers developed consisting of enlarged neurons and astrocytes, markers of mTOR signaling were increased in neurons, but not astrocytes.
- 20. Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. * Loss of TSC1 selectively from neurons in an otherwise wild type mouse causes a severe TSC1-like neuropathology.
- 21. Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. * Loss of TSC2 selectively from excitatory forebrain neurons is sufficient to cause severe TSC1-like neuropathology.
- 22.Banerjee S, Crouse NR, Emnett RJ, Gianino SM, Gutmann DH. Neurofibromatosis-1 regulates mTOR-mediated astrocyte growth and glioma formation in a TSC/Rheb-independent manner. Proc Natl Acad Sci U S A. 108:15996–16001. doi: 10.1073/pnas.1019012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skene PJ, Illingworth RS, Webb S, Kerr AR, James KD, Turner DJ, Andrews R, Bird AP. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol Cell. 37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guy J, Cheval H, Selfridge J, Bird A. The role of MeCP2 in the brain. Annu Rev Cell Dev Biol. 27:631–652. doi: 10.1146/annurev-cellbio-092910-154121. [DOI] [PubMed] [Google Scholar]
- 25.Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young JI, Hong EP, Castle JC, Crespo-Barreto J, Bowman AB, Rose MF, Kang D, Richman R, Johnson JM, Berget S, et al. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc Natl Acad Sci U S A. 2005;102:17551–17558. doi: 10.1073/pnas.0507856102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasui DH, Peddada S, Bieda MC, Vallero RO, Hogart A, Nagarajan RP, Thatcher KN, Farnham PJ, Lasalle JM. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. Proc Natl Acad Sci U S A. 2007;104:19416–19421. doi: 10.1073/pnas.0707442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 30.Gemelli T, Berton O, Nelson ED, Perrotti LI, Jaenisch R, Monteggia LM. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biol Psychiatry. 2006;59:468–476. doi: 10.1016/j.biopsych.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 31.Fyffe SL, Neul JL, Samaco RC, Chao HT, Ben-Shachar S, Moretti P, McGill BE, Goulding EH, Sullivan E, Tecott LH, et al. Deletion of Mecp2 in Sim1-expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress. Neuron. 2008;59:947–958. doi: 10.1016/j.neuron.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samaco RC, Mandel-Brehm C, Chao HT, Ward CS, Fyffe-Maricich SL, Ren J, Hyland K, Thaller C, Maricich SM, Humphreys P, et al. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc Natl Acad Sci U S A. 2009;106:21966–21971. doi: 10.1073/pnas.0912257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luikenhuis S, Giacometti E, Beard CF, Jaenisch R. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc Natl Acad Sci U S A. 2004;101:6033–6038. doi: 10.1073/pnas.0401626101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shahbazian MD, Antalffy B, Armstrong DL, Zoghbi HY. Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum Mol Genet. 2002;11:115–124. doi: 10.1093/hmg/11.2.115. [DOI] [PubMed] [Google Scholar]
- 35.Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci. 2004;27:306–321. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Rusconi L, Salvatoni L, Giudici L, Bertani I, Kilstrup-Nielsen C, Broccoli V, Landsberger N. CDKL5 expression is modulated during neuronal development and its subcellular distribution is tightly regulated by the C-terminal tail. J Biol Chem. 2008;283:30101–30111. doi: 10.1074/jbc.M804613200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuwabara T, Hsieh J, Nakashima K, Taira K, Gage FH. A small modulatory dsRNA specifies the fate of adult neural stem cells. Cell. 2004;116:779–793. doi: 10.1016/s0092-8674(04)00248-x. [DOI] [PubMed] [Google Scholar]
- 38. Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. * MeCP2-deficient astrocytes cause dendritic atrophy in wild type neurons, and wild type astrocytes support dendritic architecture in MeCP2-deficient neurons in vitro. The molecule(s) relevant for these dendritic morphological changes are astrocyte-secreted molecules.
- 39. Maezawa I, Swanberg S, Harvey D, LaSalle JM, Jin LW. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J Neurosci. 2009;29:5051–5061. doi: 10.1523/JNEUROSCI.0324-09.2009. * MeCP2-deficient astrocytes cause dendritic atrophy in wild type neurons via a gap-junction dependent mechanism.
- 40.Maezawa I, Jin LW. Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J Neurosci. 30:5346–5356. doi: 10.1523/JNEUROSCI.5966-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirrlinger PG, Scheller A, Braun C, Hirrlinger J, Kirchhoff F. Temporal control of gene recombination in astrocytes by transgenic expression of the tamoxifen-inducible DNA recombinase variant CreERT2. Glia. 2006;54:11–20. doi: 10.1002/glia.20342. [DOI] [PubMed] [Google Scholar]
- 42. Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N, et al. A role for glia in the progression of Rett's syndrome. Nature. 475:497–500. doi: 10.1038/nature10214. ** Restoration of MeCP2 selectively in astrocytes of an MeCP2-deficient mouse significantly attenuates the progression of key RTT-like symptoms, and reverses respiratory abnormalities and dendritic atrophy. However, MeCP2-deficient astrocytes were unable to initiate most RTT-like phenotypes. These results were interpreted as showing astrocytes affect disease progression of RTT.
- 43.Saywell V, Viola A, Confort-Gouny S, Le Fur Y, Villard L, Cozzone PJ. Brain magnetic resonance study of Mecp2 deletion effects on anatomy and metabolism. Biochem Biophys Res Commun. 2006;340:776–783. doi: 10.1016/j.bbrc.2005.12.080. [DOI] [PubMed] [Google Scholar]
- 44.Viola A, Saywell V, Villard L, Cozzone PJ, Lutz NW. Metabolic fingerprints of altered brain growth, osmoregulation and neurotransmission in a Rett syndrome model. PLoS One. 2007;2:e157. doi: 10.1371/journal.pone.0000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erlichman JS, Leiter JC, Gourine AV. ATP, glia and central respiratory control. Respir Physiol Neurobiol. 173:305–311. doi: 10.1016/j.resp.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Su J, Cui N, Gai H, Wu Z, Jiang C. The disruption of central CO2 chemosensitivity in a mouse model of Rett syndrome. Am J Physiol Cell Physiol. 301:C729–C738. doi: 10.1152/ajpcell.00334.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinds HL, Ashley CT, Sutcliffe JS, Nelson DL, Warren ST, Housman DE, Schalling M. Tissue specific expression of FMR-1 provides evidence for a functional role in fragile X syndrome. Nat Genet. 1993;3:36–43. doi: 10.1038/ng0193-36. [DOI] [PubMed] [Google Scholar]
- 48.Brown V, Jin P, Ceman S, Darnell JC, O'Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 49.Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci U S A. 2001;98:7101–7106. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee A, Li W, Xu K, Bogert BA, Su K, Gao FB. Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development. 2003;130:5543–5552. doi: 10.1242/dev.00792. [DOI] [PubMed] [Google Scholar]
- 51.Miyashiro K, Eberwine J. Fragile X syndrome: (What's) lost in translation? Proc Natl Acad Sci U S A. 2004;101:17329–17330. doi: 10.1073/pnas.0408034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao L, Park SK, Xu T, Vanderklish P, Yates JR., 3rd Quantitative proteomic analysis of primary neurons reveals diverse changes in synaptic protein content in fmr1 knockout mice. Proc Natl Acad Sci U S A. 2008;105:15281–15286. doi: 10.1073/pnas.0804678105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H, Ku L, Osterhout DJ, Li W, Ahmadian A, Liang Z, Feng Y. Developmentally-programmed FMRP expression in oligodendrocytes: a potential role of FMRP in regulating translation in oligodendroglia progenitors. Hum Mol Genet. 2004;13:79–89. doi: 10.1093/hmg/ddh009. [DOI] [PubMed] [Google Scholar]
- 55.Pacey LK, Doering LC. Developmental expression of FMRP in the astrocyte lineage: implications for fragile X syndrome. Glia. 2007;55:1601–1609. doi: 10.1002/glia.20573. [DOI] [PubMed] [Google Scholar]
- 56. Jacobs S, Doering LC. Astrocytes prevent abnormal neuronal development in the fragile x mouse. J Neurosci. 30:4508–4514. doi: 10.1523/JNEUROSCI.5027-09.2010. * FMRP1-deficient astrocytes induce defects in dendritic morphology and the organization of synaptic markers of wild type neurons, and conversely, wild type astrocytes prevented these alterations from occurring in FMRP1- deficient neurons in vitro.
- 57. Jacobs S, Nathwani M, Doering LC. Fragile X astrocytes induce developmental delays in dendrite maturation and synaptic protein expression. BMC Neurosci. 11:132. doi: 10.1186/1471-2202-11-132. * The altered neuronal-dendritic morphology and disorganized synaptic markers caused by FMRP1-deficient astrocytes in vitro is transient, suggesting that the affects of FMRP1-deficient astrocytes on neuronal morphology and synapses are developmental.
- 58.Dolen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008;586:1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krueger DD, Bear MF. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med. 62:411–429. doi: 10.1146/annurev-med-061109-134644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenberg PA, Aizenman E. Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neurosci Lett. 1989;103:162–168. doi: 10.1016/0304-3940(89)90569-7. [DOI] [PubMed] [Google Scholar]
- 61.Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 13:584–591. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, Song S, Likhite S, Murtha MJ, Foust KD, et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 29:824–828. doi: 10.1038/nbt.1957. * The authors derived astrocytes from neural progenitor cells retrieved from dead patients with both familial and sporadic ALS and show that they all lead to motor neuron-specifc defects, including atrophy of neurites and cell death. Significantly, they rescued these effects by knocking down endogenous SOD1.
- 65.Gong YH, Parsadanian AS, Andreeva A, Snider WD, Elliott JL. Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J Neurosci. 2000;20:660–665. doi: 10.1523/JNEUROSCI.20-02-00660.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Papadeas ST, Kraig SE, O'Banion C, Lepore AC, Maragakis NJ. Astrocytes carrying the superoxide dismutase 1 (SOD1G93A) mutation induce wild-type motor neuron degeneration in vivo. Proc Natl Acad Sci U S A. 108:17803–17808. doi: 10.1073/pnas.1103141108. * Transplanted glial precursor cells from mutant SOD1 mice led to motor neuron loss, loss of forelimb grip, and microgliosis.
- 67.Wang L, Gutmann DH, Roos RP. Astrocyte loss of mutant SOD1 delays ALS disease onset and progression in G85R transgenic mice. Hum Mol Genet. 20:286–293. doi: 10.1093/hmg/ddq463. [DOI] [PubMed] [Google Scholar]
- 68.Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, Rothstein JD, Maragakis NJ. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crosio C, Valle C, Casciati A, Iaccarino C, Carri MT. Astroglial inhibition of NF-kappaB does not ameliorate disease onset and progression in a mouse model for amyotrophic lateral sclerosis (ALS) PLoS One. 6:e17187. doi: 10.1371/journal.pone.0017187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shin JY, Fang ZH, Yu ZX, Wang CE, Li SH, Li XJ. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J Cell Biol. 2005;171:1001–1012. doi: 10.1083/jcb.200508072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bradford J, Shin JY, Roberts M, Wang CE, Li XJ, Li S. Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Proc Natl Acad Sci U S A. 2009;106:22480–22485. doi: 10.1073/pnas.0911503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Faideau M, Kim J, Cormier K, Gilmore R, Welch M, Auregan G, Dufour N, Guillermier M, Brouillet E, Hantraye P, et al. In vivo expression of polyglutamine-expanded huntingtin by mouse striatal astrocytes impairs glutamate transport: a correlation with Huntington's disease subjects. Hum Mol Genet. 19:3053–3067. doi: 10.1093/hmg/ddq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson's disease. Neuron. 66:646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Solano RM, Casarejos MJ, Menendez-Cuervo J, Rodriguez-Navarro JA, Garcia de Yebenes J, Mena MA. Glial dysfunction in parkin null mice: effects of aging. J Neurosci. 2008;28:598–611. doi: 10.1523/JNEUROSCI.4609-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mullett SJ, Hinkle DA. DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol Dis. 2009;33:28–36. doi: 10.1016/j.nbd.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gu XL, Long CX, Sun L, Xie C, Lin X, Cai H. Astrocytic expression of Parkinson's disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol Brain. 3:12. doi: 10.1186/1756-6606-3-12. ** Using a conditional driver, the authors demonstrate that expression of mutant alpha-synuclein specifically in astrocytes results in neuronal loss in the subtantia nigra, loss of weight, and eventual paralysis
- 78.Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL. Human alpha-synuclein-harboring familial Parkinson's disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 80.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 81.Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 82.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 83.Allaman I, Gavillet M, Belanger M, Laroche T, Viertl D, Lashuel HA, Magistretti PJ. Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: impact on neuronal viability. J Neurosci. 30:3326–3338. doi: 10.1523/JNEUROSCI.5098-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garwood CJ, Pooler AM, Atherton J, Hanger DP, Noble W. Astrocytes are important mediators of Abeta-induced neurotoxicity and tau phosphorylation in primary culture. Cell Death Dis. 2:e167. doi: 10.1038/cddis.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Orellana JA, Shoji KF, Abudara V, Ezan P, Amigou E, Saez PJ, Jiang JX, Naus CC, Saez JC, Giaume C. Amyloid beta-induced death in neurons involves glial and neuronal hemichannels. J Neurosci. 31:4962–4977. doi: 10.1523/JNEUROSCI.6417-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C, Saez JC. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem. 118:826–840. doi: 10.1111/j.1471-4159.2011.07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takeuchi H, Mizoguchi H, Doi Y, Jin S, Noda M, Liang J, Li H, Zhou Y, Mori R, Yasuoka S, et al. Blockade of gap junction hemichannel suppresses disease progression in mouse models of amyotrophic lateral sclerosis and Alzheimer's disease. PLoS One. 6:e21108. doi: 10.1371/journal.pone.0021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jana A, Pahan K. Fibrillar amyloid-beta-activated human astroglia kill primary human neurons via neutral sphingomyelinase: implications for Alzheimer's disease. J Neurosci. 30:12676–12689. doi: 10.1523/JNEUROSCI.1243-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mori T, Koyama N, Arendash GW, Horikoshi-Sakuraba Y, Tan J, Town T. Overexpression of human S100B exacerbates cerebral amyloidosis and gliosis in the Tg2576 mouse model of Alzheimer's disease. Glia. 58:300–314. doi: 10.1002/glia.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pihlaja R, Koistinaho J, Kauppinen R, Sandholm J, Tanila H, Koistinaho M. Multiple cellular and molecular mechanisms are involved in human Abeta clearance by transplanted adult astrocytes. Glia. 59:1643–1657. doi: 10.1002/glia.21212. [DOI] [PubMed] [Google Scholar]