Abstract
State-of-the-art treatment for weight management consists of a behavioral intervention to facilitate decreased energy intake and increased physical activity. These interventions are typically delivered face-to-face (FTF) by a health educator to a small group of participants. There are numerous barriers to participation in FTF clinics including availability, scheduling, the expense and time required to travel to the clinic site, and possible need for dependent care. Weight management clinics delivered by conference call have the potential to diminish or eliminate these barriers. The conference call approach may also reduce burden on providers, who could conduct clinic groups from almost any location without the expenses associated with maintaining FTF clinic space. A randomized trial will be conducted in 395 overweight/obese adults (BMI 25–39.9 kg/m2) to determine if weight loss (6 months) and weight maintenance (12 months) are equivalent between weight management interventions utilizing behavioral strategies and pre-packaged meals delivered by either a conference call or the traditional FTF approach. The primary outcome, body weight, will be assessed at baseline, 6, 12 and 18 months. Secondary outcomes including waist circumference, energy and macronutrient intake, and physical activity and will be assessed on the same schedule. In addition, a cost analysis and extensive process evaluation will be completed.
Keywords: Equivalence trial, conference call, weight loss, weight regain prevention, physical activity, pre-packaged meals
1. Introduction
The “Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults” by the National Heart Lung and Blood Institute (NHLBI) recommends decreasing energy intake and increasing physical activity to achieve a loss of 10% of baseline weight over 6 months. Changes in diet and physical activity are typically facilitated by behavioral weight loss clinics delivered face-to-face (FTF) by a health educator to a group of 12–15 individuals [1–3]. However, there are numerous barriers to participation in FTF clinics such as cost, travel to the clinic site, and availability of programs, particularly for those living in rural or low socio-economic status areas [4–6]. The logistics and cost of care for children or elderly family members can also be problematic [7]. The need to develop alternative strategies to deliver weight management programs has resulted in investigations examining the efficacy of programs delivered through the mail or email [8–10], internet [11–13], a combination of phone, internet and email [14], individual phone counseling [8, 15–19] and text messaging [20]. Weight loss and maintenance of weight loss from these approaches is unimpressive, which may be partially due to the fact that these interventions are delivered individually rather than in a group format. Several research groups [21–23] have demonstrated significantly greater weight loss for groups compared with individual behavioral counseling.
Weight management delivered by conference call rather than on an individual basis eliminates several barriers to participation while providing group support, accountability, and a sense of anonymity that some individuals prefer. In addition, the conference call format may reduce the burden on providers who could conduct clinic groups from almost any location without the expense associated with clinic space (i.e. rent, utilities, and insurance). The low provider costs may encourage additional health educators to offer weight management services and may also reduce participant costs. The results of a pilot trial conducted by our research group provide support for the concept that the phone conference approach results in clinically significant weight loss [24]. Weight loss (12 weeks) and weight maintenance (14 weeks) were assessed in 74 overweight adults (BMI 33.2 ± 3.8 kg/m2, age 25–68 years) randomly assigned to a weekly conference call (n = 25), a FTF clinic (n = 27), or a non-intervention control group (n = 22). Pre-packaged meal (PM) diets were used in both groups. While median weight loss at 12 weeks was greater in the FTF group (13.7%) than the conference call group (10.4%, p<0.05), both conference call and FTF groups lost approximately 13% of baseline weight by the conclusion of the 14-week weight maintenance phase. Both groups lost significantly more weight than controls. Thus, the conference call approach ultimately provided weight loss that was similar to that achieved in a FTF clinic and exceeded NHLBI clinical guidelines. Based on the results of this study, an 18-month randomized trial will be conducted to compare a conference call to a traditional FTF clinic for weight loss (6 months) and weight loss maintenance (12 months).
2. Materials and Methods
2.1. Overview of Study Design
To compare both weight loss and maintenance between the conference call and FTF clinics, 395 overweight/obese men and women have been randomly assigned to a weight management program (6 months weight loss/12 months of maintenance) that will be delivered by conference call (n = 201) or traditional FTF clinic (n = 194). The behaviorally-based FTF clinic with PMs will be considered the reference treatment in this equivalence trial because this method has emerged as a highly effective approach to weight management [18, 25–31]. Our research group, as well as others, have shown clinically significant weight loss over periods of 6 to 12 months [24, 32–34] that persists up to 14 months following completion of treatment [35] using this approach. The typical diet of many Americans currently involves the use of PMs in contrast to conventional, individually-prepared and cooked meals, which will increase generalizability of the findings [25]. Behavioral clinic meetings for both the conference call and FTF groups will be conducted weekly during the weight loss phase (month 0 to 6), and then gradually reduced during months 7–18. Meetings will be held twice per month during months 7–9, monthly during months 10–12, and every other month for the remainder of the 18 month trial. Outcomes will be assessed at baseline, 6, 12 and 18 months. We expect equivalent and clinically significant (≥ 10%) weight loss (months 0–6) and weight maintenance (months 7–18) with the weight at 18 months significantly below baseline in both groups. Weight was selected as the primary outcome rather than percentage of weight loss since the study was designed to test the equivalence of weight management delivery systems and randomization would assure equal distributions of weight across groups, thus the differentiation between absolute and percentage weight loss as an outcome is not critical in this design. Secondary outcomes will include waist circumference, as an indication of reduction in chronic disease risk, and measures of dietary intake and physical activity will help explain both group and individual differences in weight change. A cost analysis and extensive process evaluation will also be completed. We expect lower participant and provider costs in the phone group. Recruitment, randomization and baseline testing have been completed for the study.
2.2. Participant Eligibility
To improve the generalizability of the results, individuals with chronic medical conditions who received clearance from their primary care physician were allowed to participate because they represent the population of individuals typically seeking weight management and are similar to a number of weight loss trials that have established the efficacy of our reference treatment, i.e. FTF behavior clinic plus PMs [35–37]. For instance, individuals with hypertension or type 2 diabetes were not automatically excluded if their condition was controlled by medication as determined by the participant’s primary care physician. Medical conditions and medication use may be considered potential confounders; however, randomization should ensure that health status will be similar across both study groups. In addition to requiring primary care physician approval, additional inclusion and exclusion criteria (Table 1) and a comprehensive medical management plan have been developed to protect the participants in this trial. Weight loss in high risk participants (i.e. those with chronic conditions or using prescription medications) will be monitored by both the health educators and study physician. At each scheduled clinic session, all participants will be required to report changes in medications and/or adverse events to the health educators. This will take place in a private area during the FTF clinic check-in or via toll-free phone call, fax, or email to the health educator for the participants in the conference call group.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria |
|
| Exclusion Criteria |
|
2.3. Recruitment/randomization
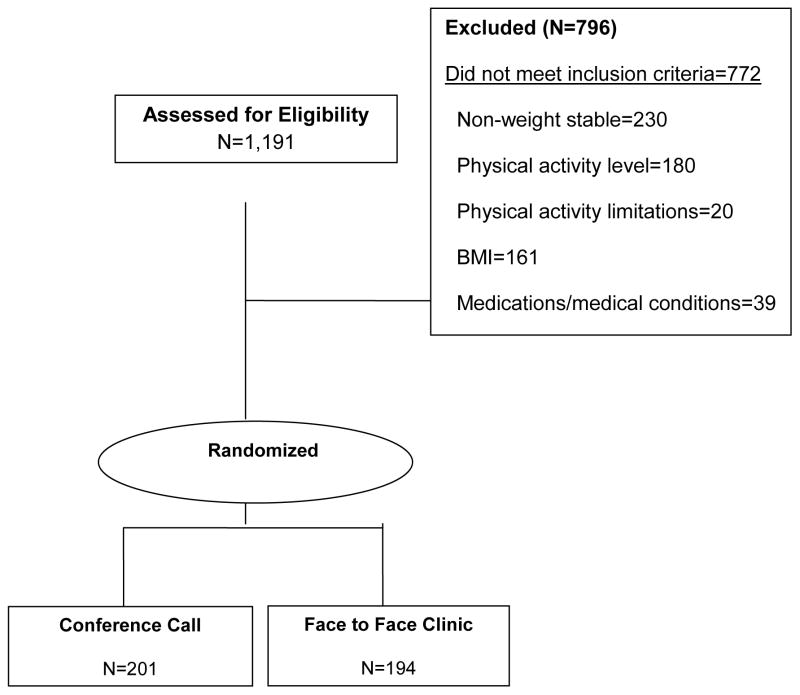
Participants were recruited using newspaper advertising, email list serves, public service messages, media contacts, word of mouth, and the waiting list for participation in our on-going University of Kansas Weight Control Research Project (WCRP). Participants were recruited over the course of ~ 3.5 years. Potential participants were asked to contact study staff via a dedicated toll-free phone line, email, or web site. Interested individuals were directed to complete a brief web-based screener on the WCRP website to provide self-reported height and weight (BMI), medication use, presence of chronic disease, smoking and drinking habits, previous attempts at weight loss, and current level of physical activity. Those satisfying the initial eligibility criteria were scheduled to attend an in-person meeting where additional screening materials (health history, physical activity questionnaire, eating behavior questionnaire, depression scale) were completed to assess final eligibility. This session also served as an orientation to the study where requirements for participation in the study were explained in detail, and participant’s questions were answered prior to obtaining written informed consent. Eligible participants were enrolled in the study by the project coordinator and randomized to the phone or clinic group. The randomization sequence was generated by an independent statistician and then sent to the project coordinator and concealed until intervention groups were assigned. Randomization was stratified by gender using random permuted blocks of size 4 for each strata with a total of 395 participants. After randomization, participants were scheduled to attend a separate 2 hr. testing appointment for collection of baseline measures by research assistants blinded to study condition (see Section 2.5). Figure 1 presents a modified Consolidated Standards of Reporting Trials (CONSORT) diagram [38] that describes the number of potential participants assessed for eligibility, the number of participants excluded or screened out and the reason, and the number randomized to the conference call or FTF clinic groups. Because the intervention was designed to compare weight management delivery systems, participants were not randomized to a true non-intervention control group. Furthermore, there is ample evidence in the weight management literature that demonstrates minimal change or increased weight for participants assigned to a non-intervention control.
Fig. 1.
The baseline characteristics of the conference call and FTF clinic groups are presented in Table 2. The study sample was over-recruited for total participants and met goals for recruitment of both minorities (e.g. all non-whites ~23%) and men (35%). There were no differences in the prevalence of comorbid conditions between the two groups.
Table 2.
Baseline demographic characteristics
| Intervention Groups | ||
|---|---|---|
| Variable | Conference Call (N=201) | FTF Clinic* (N=194) |
| Age (yrs.) | 43.2 ± 10.2 | 44.5 ± 9.9 |
| % Female | 66 | 68 |
| % Minority** | 21 | 25 |
| Weight (kg) | 100.0 ± 17.9 | 101.4 ± 18.3 |
| % Diabetes | 9 | 7 |
| % Heart problems | 5 | 5 |
| % Hypertension | 28 | 25 |
| % Hyperlipidemia | 23 | 25 |
| % Other medical problems | 19 | 17 |
Values are Mean ± SD or percentage
Face-to-face
Non-white
2.4. Intervention
This trial will compare two approaches for the delivery of a behavioral weight management intervention (FTF and conference call) for weight loss and maintenance. The weight management clinics will be based on the WCRP that has been successfully directed by the PI of the current trial (JED) for over 25 years. Based on our experience with WCRP, attrition will be minimized by frequent contact with the health educators at clinic sessions as well as mid-week assessment of participant data reports (Section 2.4.2 and 2.4.3). Health educators will place up to 3 calls to participants who fail to attend scheduled clinic sessions to encourage participation. In addition, participants will be compensated for completing scheduled outcome assessments.
2.4.1. Behavioral weight management clinics – Theoretical framework
WCRP utilizes strategies that are grounded in Social Cognitive Theory (SCT) to promote change in diet and physical activity. SCT is a triadic, dynamic model that indicates that an individual’s behavior is uniquely determined by the reciprocal interaction of personal, behavioral, and environmental factors [39] (Table 3).
Table 3.
Constructs of Social Cognitive Theory incorporated in both conference call and FTF clinic weight management interventions
| Behavioral Strategy | Operationalizing the Behavioral Strategy within the Intervention |
|---|---|
| Behavior Shaping | Participants will be taught to gradually modify their eating and physical activity behaviors according to the recommendations presented to them.. |
| Goal-Setting | Participants will be given weight loss goals of 10% of baseline body weight at month 6, and no weight regain at months 12 and 18. Health educators and lesson materials will focus on helping participants set and achieve appropriate behavioral goals (e.g., eating and physical activity) that ultimately affect weight loss and prevent weight regain Since weight maintenance is a dynamic, ever-changing process, it is expected that behavior goals will be updated and modified throughout the process in order to sustain weight loss. |
| Self-Monitoring | Participants will monitor eating (by reporting weekly consumption of fruits/vegetables and completing 3-day dietary records), physical activity (by reporting weekly exercise minutes and step counts) and weight (by weekly weigh-ins and reporting weight change to the class). Self-monitoring will also be used as a tool to identify barriers to weight management. |
| Feedback and Reinforcement | Participants will receive weekly feedback related to achievement of weight loss, physical activity, and eating behavior goals. Feedback includes reinforcement and concrete strategies for overcoming barriers. |
| Social/Peer Support | Both the in-person and phone sessions will provide opportunities for social/peer support and modeling, as much of the discussion is designed so participants can share strategies and “teach” each other. While the in-person clinic provides more informal social time, before and after class, the phone clinic also allows a few minutes of socializing prior to the start of the call. Phone participants will also be allowed to exchange email addresses and phone numbers for outside class correspondence. |
| Stimulus Control | Behavioral strategies will be provided to decrease environmental cues for less desirable behaviors and to increase the environmental cues for healthy eating and physical activity. |
| Cognitive Strategies | Cognitive strategies recognizing and countering negative thoughts with role-playing opportunities to build skills will be utilized. Examples include dealing with social relationships affected by weight, identifying/confronting self-sabotage attempts, and responding to stress with non-food techniques. |
| Relapse Prevention Strategies | Based on Marlatt and Gordon’s relapse prevention model,[75] participants will be taught to recognize precursors and consequences of lapses, and develop a plan for addressing high risk situations. |
SCT has been used in a variety of public health interventions [40] and several SCT constructs are applicable to weight management. Self-efficacy, a key construct of SCT, represents one’s confidence to execute the behaviors necessary to achieve the desired outcome. In the context of this trial, self-efficacy for a set of weight management behaviors, such as self-regulation, will be developed and practiced over time [41, 42]. Self-regulation skills emphasized in WCRP clinics include planning (e.g. how to increase vegetable consumption), self-monitoring (e.g. maintaining dietary records, recording physical activity), problem-solving, relapse prevention strategies (e.g. healthy eating while traveling, developing a plan for addressing high risk situations), and adaptive self-regulation skills specific to overcoming barriers as they arise (e.g. new job resulting in limited time for exercise). The importance of self-regulation strategies such as monitoring food intake and physical activity, weighing frequently, increasing fruit/vegetable consumption, and limiting high fat foods has been demonstrated by those who are successful at maintaining a 5–10% weight loss for at least a year [43–45].
WCRP clinics use several behavioral strategies to assist participants in modifying their dietary and physical activity behavior, enhancing their ability to cope with stress and the temptation to engage in unhealthy behaviors, identifying and responding appropriately to internal and external cues, and implementing self-control strategies to reduce the risk of relapse. Group discussions, in-class activities, and out-of-class assignments are used to facilitate behavior change. For example, to promote the purchase of food items appropriate for weight management, a clinic lesson is devoted to interpretation of the information contained on food labels. Then, participants perform an activity where two food labels are compared and an option is selected, followed by discussion of the appropriateness of the choice. Finally, an out-of-class grocery store activity is assigned, completed, and discussed at the next clinic session.
2.4.2. FTF behavioral clinic
Sixty minute FTF behavioral clinic meetings of 11–20 participants will be scheduled in the late afternoon or early evening. Participants will be instructed to arrive 5–10 minutes prior to the start of the meeting to allow time for individual weigh-ins. Meetings will follow a standard protocol: review and discussion of self-report data including physical activity (minutes/steps via step counter) and dietary compliance (10 min); a behaviorally-based lesson on a topic related to nutrition, physical activity and lifestyle modification (30 min); and group discussion, problem solving, and assignment of activities to assist participants in developing behavioral strategies associated with successful weight management (20 min). During the weight loss phase (months 0–6), participants will provide compliance records of physical activity and diet by phone, fax, or email midway between weekly scheduled clinic meetings. This contact will provide an opportunity for the participant and health educator to interact and solve problems, if necessary. Participants will be asked to continue submitting weekly reports of physical activity and diet during weight maintenance (months 7–18), even as the frequency of meetings is reduced.
2.4.3. Phone conference call behavioral clinic
The phone conference call clinics will follow a format identical to that of the FTF clinic, with slight modifications given the logistics of no FTF contact. Since a weekly weigh-in will not occur in the phone group, these participants will provide a self-reported weight along with their weekly data report. To enter the phone conference, participants will call a toll-free number approximately 5 min prior to the scheduled meeting time and dial a unique personal identification number that will allow them to join the conference call. Participants will be expected to stay on the conference call for the duration of the clinic session. Participants will be asked to provide their undivided attention, and to keep the phone call private in the interest of confidentiality. Participants will be asked not to call in situations where safety (i.e., driving a motor vehicle) or attention (i.e., watching TV) may be compromised.
2.4.4. Standardized materials
To ensure that similar content is presented in both the FTF and conference call groups, all participants will receive identical notebooks that will provide a basic outline for the intervention. The notebooks will include detailed instructions for the weight loss and weight maintenance diets including recipes, instructions for physical activity, and scheduling of class meetings and mid-meeting contact. The notebooks will be organized by clinic session and contain handouts, worksheets, and assignments specific to each session. The notebooks will also provide general information and guidelines for participation in the program such as confidentiality, how to be recognized without speaking over others, respect for others opinions, etc.
2.4.5. Health Educators - Standardized training, skills, and quality assurance
Health educators will administer one conference call and one FTF clinic group congruently to eliminate potential between-group differences due to the health educator. The groups will be held on the same evening and the order of the classes (phone and FTF) will be randomized. All health educators delivering the weight management interventions will have backgrounds in nutrition, psychology or exercise physiology and 1–2 years of experience in weight management with the WCRP. All WCRP health educators will participate in ongoing training to improve teaching, administration, and leadership skills. This training will include weekly, 2-hour staff meetings where lessons are discussed and presentations are critiqued, monthly one-on-one training with the program coordinator who will observe ongoing classes, and attendance at professional conferences and seminars throughout the year to improve teaching skills and remain current with the latest weight management research. These procedures were successfully used in the pilot study to train health educators to deliver the phone conference call. Prior to beginning the pilot study, experienced WCRP health educators and program coordinators developed a training manual to describe how to translate FTF clinic lessons for delivery in the conference call format and recorded example conference call clinic sessions. These recordings will be used to train the health educators, who will then conduct simulated phone clinics that will be monitored and critiqued by the WCRP coordinators. These sessions will be essential for developing skills specific to the conference call delivery system such as tracking which participant is speaking, encouraging group rapport through voice communication, and keeping participants motivated and engaged throughout the session. To ensure that both the conference call and FTF clinics are delivered in an identical manner, all clinic sessions will be recorded. The recordings will be reviewed by the WCRP coordinator using a detailed check list of topics and the amount of time that was scheduled to be devoted to each topic. Health educators will be required to cover a minimum of 80% of scheduled topics during each session in both the FTF and conference call groups. If deficiencies are identified, the health educator will be notified and provided with additional training, and dismissed if he or she fails to achieve the quality of program delivery required for this trial (i.e. does not meet the 80% standard).
2.4.6. Diet - Weight loss (months 0–6)
Both the FTF and phone conference call groups will be asked to reduce energy intake to ~1,200 to 1,500 kcal/day using a combination of commercially available, shelf-stable, pre-packaged meals (PMs; Health Management Resources, Boston, MA), fruits and vegetables, and non-caloric beverages. This approach produced clinically significant weight loss in the pilot study [24] and other studies [32, 33, 46]. PMs will be provided to participants in both groups during weight loss. Participants in the FTF group will place their food orders during clinic meetings and receive their order at the next meeting. Participants in the conference call group will call, email, or fax their food orders during their midweek contact. The food will be delivered to their home via United Parcel Service typically 3–4 days following placement of the order. Participants will be asked to consume a minimum of 3 low-calorie shakes (~100 kcal each), 2 entrees (200–270 kcal each), and 5 (1-cup servings) of fruits or vegetables (no dried fruit or juices) each day. Non-caloric beverages such as diet soda, coffee, and tea will be allowed ad libitum. If participants report hunger during the diet, they will be encouraged to consume additional fruits, vegetables, or PMs. The use of PMs will provide dietary standardization across groups, allowing a more robust evaluation of the impact of clinic delivery method (FTF versus conference call) on the primary outcome of weight change. Behavioral weight management interventions using PMs achieve greater weight loss and less weight regain compared with meal plans of identical energy levels [25, 47–49]. By design, PMs reduce energy intake through portion control. PMs also reduce the time devoted to meal planning and preparation by eliminating the need to weigh and measure food [50, 51]. A large variety of PMs, both traditional entrées and low calorie beverages, are currently available. The majority of PMs can be modified to create volume [52] and variety by adding flavor extracts, fruits, and vegetables. PMs have the potential to be used indefinitely as part of a weight management strategy, and when used in combination with fruits and vegetables, carry no greater risk of adverse side effects than any other dietary approach.
2.4.7. Diet - Weight maintenance (months 7–18)
There are numerous approaches for dietary recommendations for weight maintenance following weight loss. The approach selected for this study was influenced by the Institutes of Medicine dietary recommendations for prevention of weight regain [53], over 25 years of experience with research trials and clinical weight management, and the desire to evaluate a practical and generalizable approach. Therefore, daily energy intake of estimated resting metabolic rate (RMR) * 1.4 to account for activities of daily living [54] will be recommended following weight loss. This energy intake recommendation theoretically results in a negative energy balance when including the energy expenditure of exercise. However, compensatory changes in components of energy balance such as increases in energy intake and/or decreases in components of total daily energy expenditure are anticipated, as the literature indicates most individuals regain weight subsequent to weight loss [55–60]. Thus, it is likely that this approach will maximize the potential for prevention of weight regain, and provide a reasonable compromise between scientific rigor, practicality, and generalizability. During weight maintenance, participants will receive a meal plan with suggested servings of grains, proteins, fruits, vegetables, dairy, and fats based on their energy requirements and the United States Department of Agriculture/Department of Health and Human Services Dietary Guidelines for Americans 2010 [61]. Participants will be encouraged but not required to continue to consume a minimum of 3 low-calorie shakes, 2 entrées, and 5 fruits or vegetables each day. PMs will not be provided during weight maintenance; however, participants will receive a list of suitable PMs that are commercially available (<300 kcal, <9g fat). A similar prevention of weight regain diet approach was used in our pilot study which demonstrated a weight loss of > 10% from baseline to 26 weeks [24].
2.4.8. Physical activity
Both the FTF and conference call clinic groups will be asked to perform 300 min/week of moderate to vigorous physical activity during the 18-month trial. This will include both structured exercise and lifestyle physical activity. Participants will start at 45 min/week during week 1 and progress in the number of days/week and min/session to reach the 300 min/week goal at week 11 (Table 4). Weekly minutes of physical activity will be recorded by health educators at each clinic session and during the mid-meeting contact. In addition, all participants will receive a step counter during their baseline laboratory visit as a motivational tool to provide immediate and concrete feedback regarding their level of physical activity. Both reported minutes of physical activity and step counter data will be used to stimulate group discussion and will be included in a process analysis discussed in section 2.6.
Table 4.
Exercise Progression
| Week | Days per Week | Minutes per Day | Total for Week |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 1 | 3 | 15 | 45 |
| 2 | 3 | 20 | 60 |
| 3 | 4 | 20 | 80 |
| 4 | 4 | 25 | 100 |
| 5 | 4 | 30 | 120 |
| 6 | 5 | 25 | 125 |
| 7 | 5 | 30 | 150 |
| 8 | 5 | 40 | 200 |
| 9 | 5 | 45 | 225 |
| 10 | 5 | 50 | 250 |
| 11+ | 5 | 60 | 300 |
2.5. Outcome assessments
Outcome assessments including anthropometrics (weight, height, and waist circumference), physical activity (accelerometry), and energy and macronutrient intake (food records) for both intervention groups will be obtained at baseline, 6, 12 and 18 months by trained research assistants blinded to study condition. Given the nature of the intervention, it will not be possible to blind either participants or those administering the interventions to group assignment. However, the participants will not be aware of the study hypotheses. Data collection will be scheduled on separate days for participants in the FTF and conference call groups to avoid any potential contamination among participants.
2.5.1. Anthropometrics (Weight, height, and waist circumference)
Anthropometric assessments will be obtained between the hours of 6:00 and 10:00 AM prior to breakfast and after attempting to void. Participants will be weighed wearing shorts and a t-shirt using a digital scale accurate to ± 0.1 kg (Befour Inc. Model #PS6600, Saukville, WI). Height will be measured using a stadiometer (Model PE-WM-60–84, Perspective Enterprises, Portage MI) and body mass index (kg/m2) will be calculated. Waist circumference, a measure that will be used as a surrogate for abdominal adiposity, will be measured using the procedures of Lohman et al. [62]. Three measurements will be obtained and the average of the 2 closest measurements will be used for analysis.
2.5.2. Physical activity
Participants will wear an ActiGraph GT1X portable accelerometer (ActiGraph LLC, Pensacola, FL) on a belt over the non-dominant hip for 7 consecutive days at each assessment time point. Matthews et al. [63] have shown that a 7-day monitoring period provides measures of both physical activity and inactivity with a reliability of 90%. Accelerometer data will be collected in 1-min epochs with a minimum of 12 hours constituting a valid monitored day. Day 1 of the 7-day monitoring period will begin on the day following baseline data collection. At the completion of the 7-day period, participants will return the ActiGraph accelerometer using a padded postage-paid envelope provided by the investigators. At subsequent data collection points, ActiGraph accelerometers will be mailed to participants and returned in postage paid envelopes. The main outcome variable will be the average ActiGraph counts/min over the 7-day period. In addition, the average number of min/day over 7-day spent at various activity levels will be assessed using the cut-points used in the National Health and Nutrition Examination Survey as described by Troiano et al. [64]. A SAS data reduction program will be used to complete the analyses described.
2.5.3. Energy and macronutrient intake
Participants in both the FTF and conference call groups will be asked to complete 3-Day Food Record Booklets to record all foods and beverages consumed on 2 weekdays and 1 weekend day prior to anthropometric outcome assessments (at baseline, 6, 12, 18 months). To facilitate record-keeping, all participants will be mailed a pamphlet prepared by the research staff entitled, “How to record your food record” along with their dietary record form. Research staff will also contact participants by phone as a reminder to complete the diet record and to answer any questions they may have. Prior to obtaining anthropometric assessments, the Food Record Booklets will be reviewed by research staff (registered dietitians) who will seek to clarify any questionable dietary record entries. Data from the 3-Day Food Record Booklets will be entered into the most recent version of Nutrient Data System for Research (NDS-R) for analysis of total energy and macronutrient intake.
2.6. Process Analysis
Qualitative and quantitative process evaluation data will be collected for both the FTF and conference call groups to determine the extent to which the interventions were delivered as intended, the degree to which participants and health educators met the goals and objectives of the intervention, participant satisfaction with the intervention, and the type and amount of resources necessary for intervention implementation.
2.6.1. Process Data - Participants
To compare the level of participation across the intervention groups, health educators will record the following information over the course of the 18-month intervention collected from conference call or FTF clinics: 1) the number of PMs consumed each week; 2) the number of fruits and vegetables consumed each week; 3) self-reported physical activity (minutes and steps) each week; 4) attendance at scheduled meetings; and 5) the number of mid-meeting check-ins completed.
In conjunction with end-study collection of anthropometric data (month 18), brief (~5–10 min) structured interviews with be conducted by a co-investigator (CG) with all participants to assess: 1) overall satisfaction with the general features of the intervention; 2) attributes of the intervention important to the participant (i.e., convenience/ease of participation, timeliness, flexibility with scheduling, group rapport, cost); and 3) the extent to which the intervention met the participant’s expectations; i.e., perceived quality of the intervention, suggestions for improvements, etc. Formal focus group discussions will also be conducted with participants who fail to complete the 18 month intervention. Separate focus groups will be conducted for the FTF and conference call clinic groups. A focus group moderator’s guide [65, 66] will be developed and used to obtain information about to the participants’ perceptions of the weight management intervention, potential benefits and barriers to participation, and reasons for not completing the intervention. In addition to note-taking by the moderator and assistant moderator during the focus group session, all discussions will be recorded for later transcription. Four focus group sessions are proposed (6 to 10 individuals per group) but in the event of a diverse range of responses to a topic, more groups will be used to attain saturation [67]. Non-completers who are unavailable for focus group sessions will be asked to complete a brief semi-structured interview by phone or email. Finally, to determine the degree to which external or competing factors may have influenced the outcomes of the interventions and thus threaten internal validity [68], participants will be asked if they have participated in any new programs while enrolled in this study which had a goal of altering energy or macronutrient intake or increasing physical activity.
2.7. Cost analysis
A potential advantage to delivery of weight management by conference call may be lower fixed and variable costs for both participants and providers. Fixed costs are independent of the number of individuals participating while variable costs are directly associated with the number of participants enrolled [69]. Costs depend on the resources used and the cost of those resources, and valid cost analyses are based on a clear understanding of the actual process being evaluated [70, 71]. To assure a complete accounting of the resources used in the FTF and conference call groups, a process flow chart was created and validated by consensus of the investigators [72]. Information about resource use will be obtained by conducting structured interviews with providers (i.e. investigators/health educators) and surveys of a random sample of participants (20%) equally distributed across the FTF and conference call groups. This resource use information will be completed at months 6, 12, and 18. As described in the following sections, both provider and participant resource use will be evaluated at each time point.
2.7.1. Provider costs
The cost of space rental to conduct the interventions will be estimated by multiplying the local market value for space cost/ft2 by the amount of meeting space required for the FTF clinic and office space to complete the phone conference calls. Monthly billing statements will provide a measure of utility costs including toll-free access. We anticipate that fixed costs (rent/utilities) will be lower for the conference call group due to the elimination of the need for both meeting space and storage of food products (PMs). Variable provider costs including provider time, travel, and long distance services will be tracked. The costs of provider time (i.e. wages/benefits) will be estimated as the number of hours worked obtained from provider maintained logs, multiplied by the provider’s wage. The costs associated with provider travel (logs – travel time x wage), long distance services (billing statements), and the cost of PMs/shipping during the weight loss phase will be calculated.
2.7.2. Participant costs
The cost of participant time associated with travel to and attendance at clinic meetings will be estimated from self-report of time spent in these activities multiplied by the median wage in the region. Transportation costs will be estimated from self-reported spending (i.e. bus, taxi) or driving mileage multiplied by the federal mileage reimbursement rate. The survey will query about other costs, including child care, supplies, PMs/shipping during the maintenance phase, or any other participation-related expenses. Lower participant costs are expected in the conference call groups primarily from reductions in travel costs.
2.8. Statistical Power and Analysis
2.8.1. Statistical power
The primary goal of this trial is to evaluate the equivalence of weight loss achieved at 6 months by a FTF behavioral intervention compared to the same intervention delivered by conference call. Non- statistically significant differences between treatment groups cannot be interpreted to mean that the response to treatment in both groups is equivalent, as failing to reject the null hypothesis (i.e. no between-group difference) does not prove the null true. Therefore, an equivalency analysis will be conducted for this trial. For this analysis, equivalence will be defined as a difference in weight loss between groups of < 4 kg. There are no clear guidelines for defining equivalence of weight loss. The proposed 4 kg represents a difference of approximately 1–2 BMI units for men or women of average height (men = 178cm, women = 167cm), a difference that is associated with risk of chronic disease [73]. Mu (FTF) represents the mean weight loss in the FTF clinic group, Mu(CC) represents the mean weight loss in the conference call group. The statistical hypotheses of interest are:
whereas Ho is the null hypothesis and Ha is the alternative hypothesis. Thus, if HO is rejected based on the t-statistic, it would be concluded the two interventions are equivalent in the average amount of weight loss at 6 months. In our pilot study, the average baseline weight was 95 kg with a standard deviation of 16 kg. Based on our pilot results, we expect both the phone and clinic groups to lose an average of ~11 to 13 kg over 6 months with a standard deviation of 7 kg. The median weight loss in the pilot study was slightly greater for the phone group than the clinic group; however, to be conservative, we estimated that in a larger-scale study, the clinic group may lose 1.0 kg more on average than the phone group. Under these assumptions, 116 participants in each group results in 90% power to demonstrate equivalence with an overall type I error rate of 5%. Power calculations were done using nQuery Advisor® [75, 76].
2.8.2. Analysis plan- Primary outcomes
The primary goal of this trial is to evaluate the equivalence of weight loss (≤ 4 kg) achieved at 6 months by a FTF behavioral intervention compared to the same intervention delivered by conference call [77]. Our primary analysis will be a two-sample t-test for equivalence, for which the equivalent limit difference must be specified and two one-sided tests for equivalence conducted, under intention-to-treat using multiple imputation to impute missing data. Secondarily, a per-protocol analysis will also be performed. Per-protocol will be defined as attending ≥ 75% of all scheduled meetings and weight data at all assessments (baseline, 6, 12, 18 months). The equivalence (≤ 4 kg) of weight regain for participants with weight data at 6 and 18 months will be evaluated in a similar manner, for those subjects who have data at both time points (6 and 18 months). The significance of within-group weight change from baseline to 18 months will be evaluated using a paired t-test.
We expect weight to decline from baseline to 6 months and then rise somewhat when measured at 18 months. Therefore, in an exploratory manner we will longitudinally model weight change across the entire 18 month period. Given the potential rebound in weight loss, both time and time squared will be included in the linear mixed model to handle this potential nonlinear result. We will also look at other baseline factors, such as gender, age, BMI and waist circumference as well as process measures including PA, fruit and vegetable consumption, and compliance to the interventions to assess their impact on weight loss over time. The linear mixed model approach allows us to have both single time point and time varying covariates in the model, assuming an autoregressive correlation structure over time. Even though baseline BMI is a covariate in the above model, all analyses performed on weight will also be performed on BMI.
2.8.3. Analysis plan – Process evaluation
Process evaluation procedures will be included to provide detailed information regarding the implementation both the FTF and conference call interventions. Data regarding the extent and quality of the implementation of the interventions will protect against “Type III error” in which an intervention is not fully implemented as planned and does not provide a true test of the experimental hypotheses [78]. Descriptive statistics will be used to provide a general description of the context in which the programs are being conducted. For example, participant characteristics may contribute to or detract from intervention effects. The type and amount of resources necessary for program implementation will also be assessed. Self-reported data from the weekly reports (number of PMs, fruit and vegetable intake, physical activity) will be examined to monitor adherence to the diet and physical activity program components in the conference call and FTF groups. In addition, the transcribed recordings and notes from the focus group discussions will be used to identify common themes [66]. Linear mixed models as proposed for weight loss will be utilized to determine if any difference occur in the process measures between the two groups longitudinally.
2.8.4. Analysis plan - Cost
A two-sample t-test will be used to compare the average costs for both participants and providers between the FTF and conference call groups. To increase the generalizability of fixed cost estimates, data on rental space costs will be obtained from Kansas City Chamber of Commerce, Grubb & Ellis commercial real estate, and various governmental agencies.
3. Discussion
This paper describes the rationale, design, and baseline characteristics for a randomized trial to compare weight loss (6 months) and weight regain (12 months) between behavioral weight loss interventions delivered by conference call and the FTF approach. The conference call approach was selected rather than other technology-based alternatives (email, web-accessed programs [9, 10, 79], or internet [11, 12] because the telephone is a relatively inexpensive and ubiquitous form of communication that is currently available in 98.1% of US households [80], compared to only 70% of US households (58% among blacks, 46 % household income < $25,000) with computer internet access [81]. Also, the phone has the potential to provide weight management services to a large segment of the population as phone service is available across the spectrum of SES and geographic location (urban and rural). In addition, to date internet-delivered interventions for weight management have shown only modest success [9, 82, 83].
This is illustrated by the results of Harvey-Berino [11] from a 6-month comparison of weight loss from behavioral interventions delivered by internet, in-person, or a combination (internet + in-person). Significant differences were noted in the proportion of participants who achieved a 7% weight loss in the internet (37.3%) and in-person (56.3%) groups, with no significant difference between the combination group (44.4%) and any other group.
Traditional FTF weight management clinics present a number of barriers that limit both participation and compliance [84–86]. In many locations, particularly in low SES and rural areas, weight management clinics are non-existent. Clinic fees are often high, and the time and/or expense required to travel to a clinic site, find parking, etc. may discourage participation. Travel to clinic meetings may be especially problematic for older individuals and those with physical disabilities. The logistics and expense of arranging child care are potential barriers to participation in both FTF and conference call clinics, particularly single parents. However, child care issues likely present a greater barrier to participant in FTF clinics as they require participants to leave their home.
Weight management delivered by conference call has the potential to remove a number of these barriers while preserving the personal connection between the health educator and the participants, thus allowing for aspects of counseling such as empathy, sharing of personal information, and modeling that have been shown to be helpful in facilitating behavior change [87]. The conference call approach (as opposed to individually delivered phone interventions) also allows participants to be part of a group and interact with other participants in real time, a feature that can increase accountability and rapport while preserving a sense of anonymity that some individuals may prefer. Interestingly, the combination of anonymity and group membership was consistently identified as a desirable feature of the conference call approach in our pilot investigation [24]. The importance of group counseling is supported by data suggesting that group counseling may be more effective than individual counseling for lifestyle modification. For example, Renjilian et al. [22] have demonstrated that participants randomized to group treatment achieved significantly greater weight loss than those randomized to individual counseling, regardless of their expressed pre-intervention group preference. Thus, the group format appears advantageous over individual counseling and is inherently more efficient in the context of individual phone interventions which would require multiple phone calls.
The conference call approach may also diminish the burden on individuals such as health educators and dietitians who provide weight management services. Providers could conduct clinics from any location without incurring the time and expense associated with maintaining a clinic meeting space and time and expense of travel to a clinic site. Although products could be direct shipped to FTF clinics, products are traditionally processed and stored and later distributed at clinic meetings. With the conference call approach, weight management products are typically shipped directly from the vendor to the participant, eliminating the usual cost and logistics associated with processing and storage. The low provider costs associated with the use of conference calls may encourage additional individuals to provide weight management services, which may decrease the cost of these services for participants over the long term. However, not all individuals will prefer conference calls and instead may want FTF contact with a health educator. Gregarious individuals may prefer the FTF contact with their peers and the ability to build personal relationships to a greater extent than likely with the conference call approach. Conference calls rely on technology and although phone calls are generally reliable, poor connections or lack of connections may occur occasionally.
To our knowledge, there have been no investigations on the effectiveness of a weight management intervention (weight loss/prevention of weight regain) delivered by conference call. One strength of the study is the use of PMs to standardize how weight is lost and reduce the bias for comparison of the delivery systems, which is our main objective. PMs are widely available and used in research and by individuals for weight management or simply for convenience [51]. In fact, the use of PMs likely resembles the way many Americans now eat in contrast to conventional, individually-prepared and cooked meals, which will increase generalizability of the findings. Additional strengths of the study include the length of follow up, the sample size, and cost analysis. Limitations include the inability to blind health educators to treatment condition, and potential differences in motivation to lose weight between participants who agree to participate in a research study and the general public.
If the conference call is shown to provide weight loss and maintenance equivalent to the traditional FTF approach, it may provide a viable weight management option for individuals who are unable or unwilling to participate in a traditional FTF weight management program. The phone conference option may be particularly attractive for individuals with physical disabilities, the elderly, those without transportation, or individuals in rural areas or other locations where access to traditional weight management programs is limited. The phone conference approach could be easily incorporated into medical practice, clinical practice, or commercial weight management programs.
Acknowledgments
Funding: National Institute of Diabetes, Digestive and Kidney Disease R01 - DK76063
The authors would like to thank Health Management Resources for their contribution to the project.
Abbreviations
- FTF
face-to-face
- SES
socio-economic status
- BMI
body mass index
- NHLBI
National Heart Lung and Blood Institute
- WCRP
Weight Control Research Project
- SCT
Social Cognitive Theory
- IOM
Institute of Medicine
- PM
pre-packaged meal
- RMR
resting metabolic rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kate Lambourne, Email: katel@ku.edu.
Richard A. Washburn, Email: rwashburn@ku.edu.
Cheryl Gibson, Email: cgibson@kumc.edu.
Debra K. Sullivan, Email: dsulliva@kumc.edu.
Jeannine Goetz, Email: jgoetz@kumc.edu.
Robert Lee, Email: rlee2@kumc.edu.
Bryan K. Smith, Email: bryasmi@siue.edu.
Matthew S. Mayo, Email: mmayo@kumc.edu.
References
- 1.Wing R. Behavioral approaches to the treatment of obesity. 2. New York: Marcel Dekker, Inc; 2004. [Google Scholar]
- 2.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12 (Suppl):151S–62S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 3.Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Arnold ME, Steinberg CM. Effects of sibutramine plus orlistat in obese women following 1 year of treatment by sibutramine alone: a placebo-controlled trial. Obesity Research. 2000;8:431–7. doi: 10.1038/oby.2000.53. [DOI] [PubMed] [Google Scholar]
- 4.Setse R, Grogan R, Cooper LA, Strobino D, Powe NR, Nicholson W. Weight loss programs for urban-based, postpartum African-American women: perceived barriers and preferred components. Matern Child Health J. 2008;12:119–27. doi: 10.1007/s10995-007-0211-6. [DOI] [PubMed] [Google Scholar]
- 5.Phillips CD, McLeroy KR. Health in rural America: remembering the importance of place. Am J Public Health. 2004;94:1661–3. doi: 10.2105/ajph.94.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeffery RW. Socioeconomic status, ethnicity and obesity in women. Ann Epidemiol. 1996;6:263–5. doi: 10.1016/s1047-2797(96)00070-1. [DOI] [PubMed] [Google Scholar]
- 7.Mayer-Davis EJ, D’Antonio AM, Smith SM, Kirkner G, Levin Martin S, Parra-Medina D, et al. Pounds off with empowerment (POWER): a clinical trial of weight management strategies for black and white adults with diabetes who live in medically underserved rural communities. Am J Public Health. 2004;94:1736–42. doi: 10.2105/ajph.94.10.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeffery RW, Sherwood NE, Brelje K, Pronk NP, Boyle R, Boucher JL, et al. Mail and phone interventions for weight loss in a managed-care setting: Weigh-To-Be one-year outcomes. Int J Obes Relat Metab Disord. 2003;27:1584–92. doi: 10.1038/sj.ijo.0802473. [DOI] [PubMed] [Google Scholar]
- 9.Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e-mail counseling, computer-automated tailored counseling, and no counseling in an Internet weight loss program. Arch Intern Med. 2006;166:1620–5. doi: 10.1001/archinte.166.15.1620. [DOI] [PubMed] [Google Scholar]
- 10.van Wier MF, Ariens GA, Dekkers JC, Hendriksen IJ, Smid T, van Mechelen W. Phone and e-mail counselling are effective for weight management in an overweight working population: a randomized controlled trial. BMC Public Health. 2009;9:6. doi: 10.1186/1471-2458-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey-Berino J, West D, Krukowski R, Prewitt E, VanBiervliet A, Ashikaga T, et al. Internet delivered behavioral obesity treatment. Prev Med. 2010;51:123–8. doi: 10.1016/j.ypmed.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manzoni GM, Pagnini F, Corti S, Molinari E, Castelnuovo G. Internet-based behavioral interventions for obesity: an updated systematic review. Clin Pract Epidemiol Ment Health. 2011;7:19–28. doi: 10.2174/1745017901107010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krukowski RA, Tilford JM, Harvey-Berino J, West DS. Comparing behavioral weight loss modalities: incremental cost-effectiveness of an internet-based versus an in-person condition. Obesity (Silver Spring) 2011;19:1629–35. doi: 10.1038/oby.2010.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365:1959–68. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellerstedt WL, Jeffery RW. The effects of a telephone-based intervention on weight loss. Am J Health Promot. 1997;11:177–82. doi: 10.4278/0890-1171-11.3.177. [DOI] [PubMed] [Google Scholar]
- 16.Digenio AG, Mancuso JP, Gerber RA, Dvorak RV. Comparison of methods for delivering a lifestyle modification program for obese patients: a randomized trial. Ann Intern Med. 2009;150:255–62. doi: 10.7326/0003-4819-150-4-200902170-00006. [DOI] [PubMed] [Google Scholar]
- 17.Neve M, Morgan PJ, Collins CE. Weight change in a commercial web-based weight loss program and its association with website use: cohort study. J Med Internet Res. 2011;13:e83. doi: 10.2196/jmir.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rock CL, Flatt SW, Sherwood NE, Karanja N, Pakiz B, Thomson C. Effect of a free prepared meal and incentivized weight loss program on weight loss and weight loss maintenance in obese and overweight women. JAMA. 2010;304:1803–10. doi: 10.1001/jama.2010.1503. [DOI] [PubMed] [Google Scholar]
- 19.Sherwood NE, Crain AL, Martinson BC, Hayes MG, Anderson JD, Clausen JM, et al. Keep it off: a phone-based intervention for long-term weight-loss maintenance. Contemp Clin Trials. 2011;32:551–60. doi: 10.1016/j.cct.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patrick K, Raab F, Adams MA, Dillon L, Zabinski M, Rock CL, et al. A text message-based intervention for weight loss: randomized controlled trial. J Med Internet Res. 2009;11:e1. doi: 10.2196/jmir.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Befort CA, Donnelly JE, Sullivan DK, Ellerbeck EF, Perri MG. Group versus individual phone-based obesity treatment for rural women. Eating behaviors. 2010;11:11–7. doi: 10.1016/j.eatbeh.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renjilian D, Perri M, Nezu S, McKelvey W, Shermer R, Anton S. Individual versus group therapy for obesity: effects of matching participants to their treatment peferences. J Consult Clin Psychol. 2001;69:717–21. [PubMed] [Google Scholar]
- 23.Cresci B, Tesi F, La Ferlita T, Ricca V, Ravaldi C, Rotella CM, et al. Group versus individual cognitive-behavioral treatment for obesity: results after 36 months. Eat Weight Disord. 2007;12:147–53. doi: 10.1007/BF03327591. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly JE, Smith BK, Dunn L, Mayo MS, Jacobsen DJ, Stewart EE, et al. Comparison of a phone vs clinic approach to achieve 10% weight loss. Int J Obes. 2007;31:1270–6. doi: 10.1038/sj.ijo.0803568. [DOI] [PubMed] [Google Scholar]
- 25.Heymsfield SB. Meal replacements and energy balance. Physiol Behav. 2010;100:90–4. doi: 10.1016/j.physbeh.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Heymsfield SB, van Mierlo CAJ, van der Knaap HCM, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord. 2003;27:537–49. doi: 10.1038/sj.ijo.0802258. [DOI] [PubMed] [Google Scholar]
- 27.Wadden TA, West DS, Neiberg RH, Wing RR, Ryan DH, Johnson KC, et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009;17:713–22. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:2226–38. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 29.Annunziato RA, Timko CA, Crerand CE, Didie ER, Bellace DL, Phelan S, et al. A randomized trial examining differential meal replacement adherence in a weight loss maintenance program after one-year follow-up. Eating behaviors. 2009;10:176–83. doi: 10.1016/j.eatbeh.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Franz MJ. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. Journal of the American Dietetic Association. 2007;107:1755–67. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 31.National Heart L, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults; The evidence report. National Institutes of Health; 1998. [PubMed] [Google Scholar]
- 32.LeCheminant JD, Gibson CA, Sullivan DK, Hall S, Washburn R, Vernon MC, et al. Comparison of a low carbohydrate and low fat diet for weight maintenance in overweight or obese adults enrolled in a clinical weight management program. Nutr J. 2007;6:36. doi: 10.1186/1475-2891-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zemel MB, Donnelly JE, Smith BK, Sullivan DK, Richards J, Morgan-Hanusa D, et al. Effects of dairy intake on weight maintenance. Nutr Metab. 2008;5:28. doi: 10.1186/1743-7075-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashley JM, St Jeor ST, Schrage JP, Perumean-Chaney SE, Gilbertson MC, McCall NL, et al. Weight control in the physician’s office. Arch Intern Med. 2001;161:1599–604. doi: 10.1001/archinte.161.13.1599. [DOI] [PubMed] [Google Scholar]
- 35.Befort CA, Stewart EE, Smith BK, Gibson CA, Sullivan DK, Donnelly JE. Weight maintenance, behaviors and barriers among previous participants of a university-based weight control program. Int J Obes (Lond) 2008;32:519–26. doi: 10.1038/sj.ijo.0803769. [DOI] [PubMed] [Google Scholar]
- 36.Berkel LA, Poston WS, Reeves RS, Foreyt JP. Behavioral interventions for obesity. J Am Diet Assoc. 2005;105:S35–43. doi: 10.1016/j.jada.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 37.Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (Lond) 2005;29:1153–67. doi: 10.1038/sj.ijo.0802982. [DOI] [PubMed] [Google Scholar]
- 38.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Journal of Clinical Epidemiology. 2010;63:e1–e37. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, New Jersey: Prentice-Hall; 1986. [Google Scholar]
- 40.Dunn AL, Anderson RE, Jakicic JM. Lifestyle physical activity interventions. History, short- and long-term effects, and recommendations. Am J Prev Med. 1998;15:398–412. doi: 10.1016/s0749-3797(98)00084-1. [DOI] [PubMed] [Google Scholar]
- 41.Bandura A. Social Cognitive Theory: an agentic perspective. Annu Rev Psychol. 2001:1–25. doi: 10.1146/annurev.psych.52.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Bandura A. Self-Efficacy: The Exercise of Control. New York: WH Freemand & Co; 1997. [Google Scholar]
- 43.Astrup A. The role of dietary fat in the prevention and treatment of obesity. Efficacy and safety of low-fat diets. Int J Obes Relat Metab Disord. 2001;25 (Suppl 1):S46–50. doi: 10.1038/sj.ijo.0801698. [DOI] [PubMed] [Google Scholar]
- 44.McGuire M, Wing R, Hill J. The prevalence of weight loss maintenance among American adults. Int J Obes. 1999;23:1314–9. doi: 10.1038/sj.ijo.0801075. [DOI] [PubMed] [Google Scholar]
- 45.McGuire M, Wing R, Klem M, Hill J. Behavioral strategies of individuals who have maintained long-term weight losses. Obes Res. 1999;7:334–41. doi: 10.1002/j.1550-8528.1999.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 46.Bailey BW, Jacobsen DJ, Donnelly JE. Weight loss and maintenance outcomes using moderate and severe caloric restriction in an outpatient setting. Dis Manag. 2008;1:176–80. doi: 10.1089/dis.2007.0002. [DOI] [PubMed] [Google Scholar]
- 47.Heymsfield SB, van Mierlo CA, van der Knaap HC, Heo M, Frier HI. Weight management using a meal replacement strategy: Meta and pooling analysis from six studies. Int J Obes Relat Metab Disord. 2003;27:537–49. doi: 10.1038/sj.ijo.0802258. [DOI] [PubMed] [Google Scholar]
- 48.Davis LM, Coleman C, Kiel J, Rampolla J, Hutchisen T, Ford L, et al. Efficacy of a meal replacement diet plan compared to a food-based diet plan after a period of weight loss and weight maintenance: a randomized controlled trial. Nutr J. 2010;9:11. doi: 10.1186/1475-2891-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheskin LJ, Mitchell AM, Jhaveri AD, Mitola AH, Davis LM, Lewis RA, et al. Efficacy of meal replacements versus a standard food-based diet for weight loss in type 2 diabetes: a controlled clinical trial. The Diabetes educator. 2008;34:118–27. doi: 10.1177/0145721707312463. [DOI] [PubMed] [Google Scholar]
- 50.Wing RR, Jeffery RW, Burton LR, Thorson C, Nissinoff KS, Baxter JE. Food provision vs structured meal plans in the behavioral treatment of obesity. Int J Obes Relat Metab Disord. 1996;20:56–62. [PubMed] [Google Scholar]
- 51.Keogh JB, Clifton PM. The role of meal replacements in obesity treatment. Obes Rev. 2005;6:229–34. doi: 10.1111/j.1467-789X.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 52.Rolls B. The Volumetrics Eating Plan. New York, New York: HarperCollins Publishers Inc; 2005. [Google Scholar]
- 53.Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102:1621–30. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 54.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–7. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 55.Barte JC, Ter Bogt NC, Bogers RP, Teixeira PJ, Blissmer B, Mori TA, et al. Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obes Rev. 2010;1:899–906. doi: 10.1111/j.1467-789X.2010.00740.x. [DOI] [PubMed] [Google Scholar]
- 56.Borg P, Kukkonen-Harjula L, Fogelholm M, Pasanen M. Effects of walking or resistance training on weight loss maintenance in obese, middle-aged men: a randomized trial. Int J Obes. 2002;26:676–83. doi: 10.1038/sj.ijo.0801962. [DOI] [PubMed] [Google Scholar]
- 57.Foreyt JP, Goodrick GK. Evidence for success of behavior modification in weight loss and control. Ann Intern Med. 1993;119:698–701. doi: 10.7326/0003-4819-119-7_part_2-199310011-00014. [DOI] [PubMed] [Google Scholar]
- 58.Phelan S, Wing RR, Loria CM, YK, Lewis CE. Prevalance and predictors of weight-loss maintenance in a biracial cohort: Results from the Coronary Artery Risk Development in Young Adults Study. Am J Prev Med. 2010;39:546–54. doi: 10.1016/j.amepre.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toubro S, Astrup A. Randomised comparison of diets for maintaining obese subjects’ weight after major weight loss: ad lib, low fat, high carbohydrate diet v fixed energy intake. BMJ. 1997;314:29–34. doi: 10.1136/bmj.314.7073.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiss EC, Galuska DA, Kettel Khan L, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med. 2007;33:34–40. doi: 10.1016/j.amepre.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 61.U.S. Department of Agriculture, U.S. Department of Health and Human Services. [Accessed April 20, 2011.];Dietary Guidelines for Americans. 2010 http://www.cnpp.usda.gov/dietaryguidelines.htm.
- 62.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, Ill: Human Kinetics Books; 1988. [Google Scholar]
- 63.Matthews CE, Ainsworth BE, Thompson RW, Bassett DRJ. Sources of variance in daily physical activity levels as measured by an accelerometer. Med Sci Sports Exerc. 2002;34:1376–81. doi: 10.1097/00005768-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 64.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 65.Krueger RA. Focus groups: a practical guide for applied research. Thousand Oaks: Sage; 1988. [Google Scholar]
- 66.Krueger RA. Focus groups: a practical guide for applied research. Thousand Oaks, CA: Sage Publications; 1988. [Google Scholar]
- 67.Glaser BG, Strauss AL. Discovery of grounded theory: strategies for qualitative research. Chicago: Aldine; 1967. [Google Scholar]
- 68.Cook TD, Campbell DT. Quasi-experimentation: Design and analysis issues for field settings. Boston: Houghton Mifflin; 1979. [Google Scholar]
- 69.McLean RA. Financial Management in Healthcare Organizations. Albany, NY: Delmar Publishers; 1997. [Google Scholar]
- 70.McLaughlin CP, Kaluzny AD. Continuous Quality Improvement in Health Care: Theory, Implementation, and Applications. Gaithersburg, MD: Aspen; 1999. [Google Scholar]
- 71.Lee RH, Bott MJ, Forbes S, Redford L, Swagerty DL, Taunton RL. Process-based costing. J Nurs Care Qual. 2003;18:259–66. doi: 10.1097/00001786-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 72.Dranove D. Measuring Costs. In: Sloan FA, editor. Valuing Health Care: Costs, Benefits, and Effectiveness of Pharmaceuticals and Other Medical Technologies. New York, NY: Cambridge University Press; 1996. pp. 61–80. [Google Scholar]
- 73.Bray GA. Overweight is risking fate. Definition, classification, prevalence, and risks. Ann N Y Acad Sci. 1987;499:14–28. doi: 10.1111/j.1749-6632.1987.tb36194.x. [DOI] [PubMed] [Google Scholar]
- 74.U.S.Department of Health and Human Services F. Guidance for Industry Food-Effect Bioavailability and Fed Bioequivalence Studies. Washington, D.C: 2002. [Google Scholar]
- 75.Dixon WJ, Massey FJ. Introduction to statistical analysis. 4. Boston: McGraw-Hill; 1983. [Google Scholar]
- 76.O’Brien RG, Muller KE. Applied analysis of variance in behavioral science. New York: Marcel Decker; 1993. [Google Scholar]
- 77.Wellek S. Testing Statistical Hypotheses of Equivalence. Boco Raton FL: Chapman Hall; 2003. [Google Scholar]
- 78.Basch CE, Sliepcevich EM, Gold RS, Duncan DF, Kolbe LJ. Avoiding type III errors in health education program evaluations: a case study. Health Ed Q. 1985;12:315–31. doi: 10.1177/109019818501200311. [DOI] [PubMed] [Google Scholar]
- 79.van Wier MF, Dekkers JC, Hendriksen IJM, Heymans MW, Ariëns GAM, Pronk NP, et al. Effectiveness of phone and e-mail lifestyle counseling for long term weight control among overweight employees. Journal of Occupational and Environmental Medicine. 2011;53:680–6. doi: 10.1097/JOM.0b013e31821f2bbb. [DOI] [PubMed] [Google Scholar]
- 80.Wireless substitution: Early release of estimates from the National Health Interview Survey, January–June 2010. Washington, DC: Department of Health and Human Services, National Center for Health Statistics; 2010. [Google Scholar]
- 81.The 2012 Statistical Abstract: The National Data Book. Table 1155: Household internet usage in and outside of the home by selected characteristics: 2010. U.S. Census Bureau; 2011. [Google Scholar]
- 82.Tate DF, Jackvony EH, Wing RR. Effects of internet behavioral counseling on weight loss in adults at risk for type 2 diabetes - A randomized trial. JAMA. 2003;289:1833–6. doi: 10.1001/jama.289.14.1833. [DOI] [PubMed] [Google Scholar]
- 83.Tate DF, Wing RR, Winett RA. Using Internet technology to deliver a behavioral weight loss program. JAMA. 2001;285:1172–7. doi: 10.1001/jama.285.9.1172. [DOI] [PubMed] [Google Scholar]
- 84.Foster GD, Wadden TA, Phelan S, Sarwer DB, Sanderson RS. Obese patients’ perceptions of treatment outcomes and the factors that influence them. Arch Intern Med. 2001;161:2133–9. doi: 10.1001/archinte.161.17.2133. [DOI] [PubMed] [Google Scholar]
- 85.Perri MG, Nezu PAM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem-solving therapy in the long-term management of obesity. J Consult Clin Psychol. 2001;69:722–6. [PubMed] [Google Scholar]
- 86.Foreyt J, Poston W. The challenge of diet, exercise and lifestyle modification in the management of the obese diabetic patient. Int J Obes Relat Metab Disord. 1999;23:S5–S11. doi: 10.1038/sj.ijo.0800955. [DOI] [PubMed] [Google Scholar]
- 87.Kipnis D. The technological perspective. Psychol Sci. 1991;2:62–29. [Google Scholar]