Abstract
Background
Conflicting relationships have been described between anemia correction using erythropoiesis-stimulating agents (ESAs) and progression of chronic kidney disease (CKD). This study was undertaken to examine the impact of target hemoglobin on progression of kidney disease in the CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) trial.
Study design
Secondary analysis of a randomized controlled trial
Setting and participants
1432 participants with CKD and anemia
Intervention
Participants were randomized to target hemoglobin of 13.5 vs 11.3 gm/dL with the use of epoetin-alfa.
Outcomes and measurements
Cox regression was used to estimate hazard ratios for progression of CKD (a composite of doubling of creatinine, initiation of renal replacement therapy (RRT), or death). Interactions between hemoglobin target and select baseline variables (estimated glomerular filtration rate (eGFR), proteinuria, diabetes, heart failure, and smoking history) were also examined.
Results
Participants randomized to higher hemoglobin targets experienced a shorter time to progression of kidney disease in both univariate (HR, 1.25; 95% CI, 1.03–1.52; p=0.02) and multivariable models (HR, 1.22; 95% CI, 1.00–1.48; p=0.05). These differences were attributable to higher rates of RRT and death among participants in the high hemoglobin arm. Hemoglobin target did not interact with eGFR, proteinuria, diabetes, or heart failure (p>0.05 for all). In the multivariable model, hemoglobin target interacted with tobacco use (p=0.04) such that the higher target had a greater risk of CKD progression among participants that currently smoked (HR, 2.50; 95% CI, 1.23–5.09; p=0.01) which was not present among those who did not currently smoke (HR, 1.15; 95% CI 0.93–1.41; p=0.2).
Limitations
A post-hoc analysis and thus cause- effect cannot be determined.
Conclusions
These results suggest that high hemoglobin target is associated with a greater risk of progression of CKD. This risk may be augmented by concurrent smoking. Further defining the mechanism of injury may provide insight into methods to optimize outcomes in anemia management.
Since the introduction of ESAs for the treatment of anemia in chronic kidney disease (CKD), various benefits for anemia correction have been proposed. These include prevention of blood transfusion, improvement of quality of life, reduction in cardiovascular morbidity and mortality, and slowing the rate of CKD progression. It has been suggested that normalizing hemoglobin in CKD patients attenuates kidney disease progression by increasing oxygen delivery to the kidneys and thereby preventing tubular injury and the development of interstitial fibrosis. Additionally, it has been postulated that erythropoietin might counteract oxidative stress and apoptosis, and may have direct protective effects on tubular cells.1 Furthermore, a post hoc analysis of the RENAAL (Reduction in End Points in Non–Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan) study showed that a greater hemoglobin level was associated with a slower rate of progression of type 2 diabetic kidney disease.2 But, prior randomized controlled trials have identified conflicting results when analyzing the relationship between hemoglobin target and CKD progression.
A randomized controlled trial by Gouva et al. involving 88 nondiabetic predialysis CKD patients reported renoprotective effects associated with erythropoietin.3 The composite primary end point of doubling of serum creatinine, need for renal replacement therapy (RRT), or death occurred less frequently in the early erythropoietin group than in those for whom treatment was deferred until hemoglobin decreased to < 9.0 g/l (HR of 0.42 in the early vs late treatment group, p = 0.012). But a larger study of 390 subjects randomized to hemoglobin targets of 13–15 gm/dl vs. 11–12 gm/dl demonstrated no difference in the rate of decline in GFR in the higher hemoglobin arm (0.058 vs. 0.081 mL/min/month in the higher vs. lower targets, respectively; p=0.699).4
The findings of the largest trials have pointed to either a neutral or detrimental effect of correcting anemia with ESAs on CKD progression. The CHOIR trial randomized 1,432 subjects with CKD and anemia to target hemoglobin values of 13.5 gm/dL versus 11.3 gm/dL.5 The risk of initiation of dialysis was greater among those randomized to the higher target but this was not statistically significant (HR, 1.19; p=0.15) in the setting of a greater risk of death in the higher target arm. In the CREATE (Cardiovascular Risk Reduction by Early Anemia Treatment With Epoetin Beta) study, which included 603 patients with CKD and anemia randomized to a target hemoglobin value of 13.0–15.0 or 10.5–11.5 g/dL, a shorter time to dialysis initiation was seen among those randomized to the higher hemoglobin target (p=0.02).6 More recently, TREAT (Trial to Reduce Cardiovascular Events With Aranesp Therapy), a study involving 4,038 patients with type 2 diabetes, CKD, and anemia randomized to a hemoglobin goal of 13.0 gm/dL or placebo, subjects randomized to the higher arm experienced no difference in the risk of death or end-stage renal disease (ESRD) (HR, 1.06; p=0.29).7
Because of the variability in study designs and estimates of effect, as well as the limitations of examining ESRD alone as an endpoint due to the potential for informative censoring due to mortality, the present study was undertaken to examine the effect of target hemoglobin in more detail on the progression of kidney disease in the CHOIR trial. We hypothesized that higher hemoglobin targets would be associated with greater CKD progression. Furthermore, considering the varying results from different randomized trials, we hypothesized that the relationship between hemoglobin target and CKD progression would be modified by certain baseline factors, such as heart failure, diabetes, or smoking, which are known to either affect outcomes or CKD progression.8–11
METHODS
Trial design
CHOIR (ClinicalTrials.gov number NCT00211120) was an open-label randomized trial comparing the effect of treatment with epoetin-alfa to one of two hemoglobin targets (11.3 vs 13.5 g/dl). The methods, baseline characteristics, and results of CHOIR have been previously described.5
Participants
A total of 1,432 patients with CKD (defined by an estimated glomerular filtration rate (eGFR) of 15–50 ml/min/1.73 m2) and anemia (defined as hemoglobin < 11 g/dl) were enrolled and randomized into CHOIR.
Interventions
Participants were randomized 1:1 to hemoglobin of 13.5 g/dl vs 11.3 g/dl with the use of epoetin alfa administered subcutaneously.
Definition of variables
Baseline eGFR was calculated using the MDRD Study equation and serum creatinine was measured every six months thereafter using a single central laboratory.12 Diabetes mellitus (DM) was defined as either a history of DM or CKD due to type 1 or 2 DM. Smoker was defined as an individual who self-identified as currently smoking at the time of study enrollment. Participants that were not currently smoking regardless of whether they had a prior history of smoking were deemed to be non-smokers. A prior history of heart failure (HF) was defined as the composite of a history of congestive heart failure, cardiomyopathy, left ventricular dysfunction, or right ventricular dysfunction reported by the subject or in the medical record.8
Outcomes
The primary endpoint for this secondary analysis of CHOIR is progression of CKD which was defined as the composite of doubling of serum creatinine, initiation of renal replacement therapy (RRT), or death. For competing risks analysis, an alternative definition of CKD progression included a composite of doubling of creatinine or RRT while censoring for death.
Statistical methods
The primary goal of this analysis was to describe the associations between hemoglobin target and progression of CKD. Baseline characteristics were described and compared between treatment groups with non-parametric Wilcoxon rank sum test. In order to identify variables associated with CKD progression for the final model, Cox proportional hazards regression using backward selection at a retention level of 0.05 was utilized. The bootstrap method (200 samples of 80% of the population) was used in combination with the backwards Cox regression analysis to select the final set of baseline variables to include in the adjusted model. A Cox proportional hazards regression with the stepwise selection process (entry level of 0.10, stay level of 0.05) was applied to every bootstrap sample. If the variable occurred in >50% of the bootstrap models, the variable was judged to be reliable and was included in the adjusted model. The treatment variable was always included in the model selection process. For continuous variables whose effects were nonlinear, piecewise-linear splines with cutpoints based on minimizing the -2 log likelihood were used. Multiple imputation method (Markov Chain Monte Carlo) was used to impute missing data so that the model development was based on all patients. Five imputed datasets were generated using SAS procedure MI. The standard errors for the parameter estimates were obtained by using SAS procedure MIANALYZE to account for uncertainty due to imputation. The variables selected in this process were used for adjustment described below.
The potential for interaction with the association between randomization group and progression of CKD was tested for baseline eGFR, baseline proteinuria, DM, HF, and current smoking. Descriptive statistics were examined based on Kaplan-Meier survival curves for subjects randomized to each treatment arm within the subgroups of interest and the unadjusted treatment effect was obtained based on the Wald test from Cox hazard models containing the main effects of treatment and the variables that interacted significantly with treatment.
The selected variables in the final model were used to obtain the adjusted treatment effect within subgroups defined by the absence or presence of current smoking based on models including the interaction of smoking with treatment. Specifically, to evaluate the treatment effect within subgroups defined by smoking, subjects without missing baseline report of smoking were used for the models. The adjusted model contains the main effects of smoking and treatment and their interaction as well as the main effects of all selected variables for adjustment. The subgroup comparisons were carried out by forming contrasts in the models to estimate the treatment effect within the subgroups of presence or absence of smoking. The multiple imputation method described above was used to deal with missing data in the selected variables for adjustment. Similar analyses were also performed for eGFR, proteinuria, DM, and HF. Analyses were also performed on datasets without imputation providing similar results (data not shown).
In order to ascertain the contribution of death vs doubling of creatinine or RRT to the primary results, two competing risks analyses were performed: (1) modeling the cause-specific hazard and (2) modeling the hazard of the cumulative incidence function. In the first approach, the adjusted relationship between hemoglobin target and hazard of doubling of creatinine/RRT while censoring for death was assessed, as well as the adjusted hazard of death while censoring for doubling of creatinine/RRT was determined. For variables with significant interactions with treatment group, these interactions were tested in these two models separately. In the second approach, the regression model for the hazard of cumulative incidence function for doubling of creatinine/RRT or death was derived separately as previously described.13, 14 All statistical analyses were performed using SAS (version 9.2, SAS Institute, www.sas.com). A p-value ≤ 0.05 was considered as statistical significant.
RESULTS
Participant flow
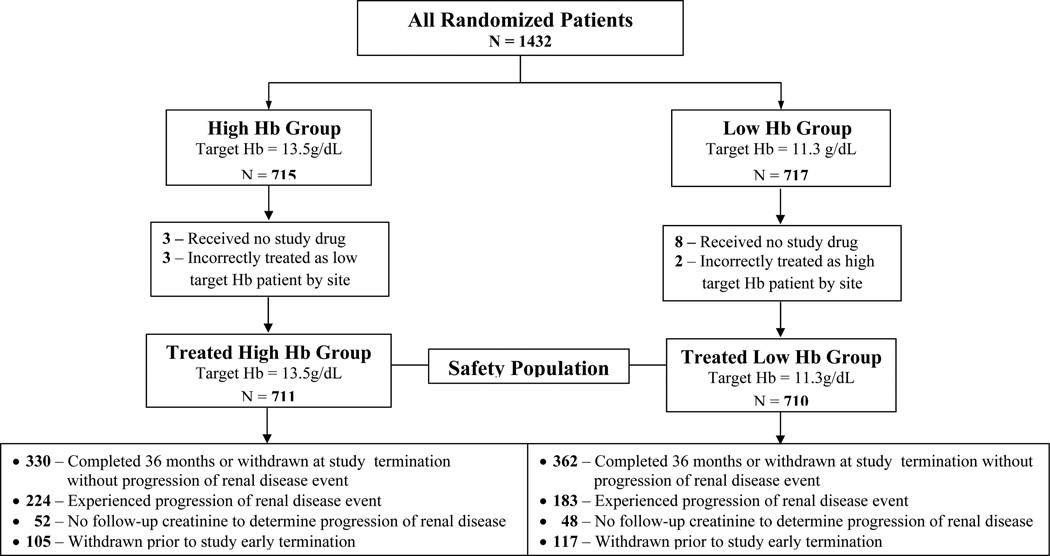
A total of 1,432 were enrolled into CHOIR and 715 were randomized to the high hemoglobin target and 717 were randomized to the low hemoglobin target. All participants who were randomized, received study drug and treated per randomization allocation were included in this analysis (1,421/1,432) (Figure 1). For the composite CKD endpoint, 100 participants did not have a repeat serum creatinine and thus 1,321 participants were analyzed.
Figure 1.
Consort Diagram Flow of Participants from CHOIR included in the Progression of Kidney Disease Analysis
Baseline characteristics
Participants randomized to each of the hemoglobin targets were similar at baseline with respect to their kidney disease (Table 1). The presence of diabetes mellitus or diagnosis of diabetes as the etiology of the kidney disease was similar between groups. Median baseline eGFR values were 25.2 and 25.4 ml/min/1.73m2 in the high and low target groups, respectively, and median baseline urine total protein–creatinine ratios were 0.6 mg/mg in both groups.
Table 1.
Clinical and demographic features of participants by randomization arm
| High Hb [13.5 g/dL] target (n=711) |
Low Hb [11.3 g/dL] target (n=710) |
P-Value | |
|---|---|---|---|
| Age (y) | 66.0 ± 14.3 | 66.3 ± 13.5 | 0.7 |
| Female gender | 401 (56.4%) | 382 (53.8%) | 0.3 |
| Race White Black American Indian/Alaskan Native Asian or Pacific Islander Other |
443 (62.5) 201 (28.3) 1 (0.1) 24 (3.4) 40 (5.6) |
434 (61.1) 208 (29.3) 3 (0.4) 23 (3.2) 42 (5.9) |
0.9 |
| Hispanic Ethnicity | 89/708 (12.6%) | 95/709 (13.4%) | 0.6 |
| Current Smoker (vs. Never/Past Smoker) | 40/708 (5.6%) | 50/710 (7.0%) | 0.3 |
| Comorbid Conditions Atrial fibrillation/flutter Diabetes mellitus Coronary artery disease 1 Cerebrovascular disease 2 Heart failure 3 Hypertension History of solid organ malignancy Thromboembolic disease 4 Peripheral vascular disease 5 Cardiovascular disease composite 6 |
63/668 (9.4) 435/707 (61.5) 232/669 (34.7) 88/670 (13.1) 191/668 (28.6) 641/669 (95.8) 90/664 (13.6) 37/669 (5.5) 115/668 (17.2) 319/669 (47.7) |
58/667 (8.7) 452/709 (63.8) 214/668 (32.0) 98/665 (14.7) 181/667 (27.1) 623/669 (93.1) 93/665 (14.0) 48/665 (7.2) 115/665 (17.3) 319/665 (47.9) |
0.6 0.4 0.3 0.4 0.6 0.03 0.8 0.2 0.9 0.9 |
| Baseline Laboratory Values eGFR (mL/min/1.73m^2) Albumin (mg/dl) UPCR (mg/mg) Cholesterol (mg/dl) Ferritin (mg/dl) Transferrin saturation (%) Hb (g/dl) |
25.2. [19.6–32.7] 3.8 [3.4–4.0] 0.6 [0.2–2.2] 178 [149–212] 119 [59.8–223] 23.1 [17.6–30.2] 10.2 [9.6–10.7] |
25.4 [19.8–33.0] 3.8 [3.5–4.1] 0.6 [0.2–1.8] 175 [151–211] 123 [62.9–236] 23.2 [18.1–30.2] 10.2 [9.6–10.7] |
0.6 0.2 0.2 0.8 0.2 0.8 0.5 |
| Inflammation or malnutrition* | 282/709 (39.8) | 258/707 (36.5) | 0.2 |
Values for continuous variables given as mean ± SD or median [25th–75th percentile]; values for categorical variables given as number (percentage) or n/N (%).
eGFR, estimated glomerular filtration rate; UPCR, urine total protein–creatinine ratio; Hb, hemoglobin.
Conversion factor for cholesterol in mg/dL to mmol/L, ×0.02586.
Coronary artery disease = prior myocardial infarction, angina, coronary artery bypass graft or percutaneous intervention
Cerebrovascular disease = prior cerebrovascular accident or transient ischemic attack
Heart failure = prior congestive heart failure, cardiomyopathy, left or right ventricular dysfunction
Prior thromboembolic disease = prior pulmonary embolism, arterial thrombosis, deep venous thromboembolism, or hypercoagulable state
Peripheral vascular disease = prior PVD or lower extremity amputation
Cardiovascular disease composite = CAD, CVA/TIA, or PVD
albumin <3.6 mg/dL or ferritin >600 mg/dL
Effect of hemoglobin target on CKD progression
The median time to the first event was 351.5 days for those in the high hemoglobin arm vs. 323.0 days for participants in the low hemoglobin arm. However, a greater proportion of participants in the higher hemoglobin target group experienced the composite endpoint of CKD progression (34.0% vs. 27.6%, respectively). The unadjusted rates of the components of the primary endpoint and the event rates per 1000 patient years for are listed in Table 2.
Table 2.
Unadjusted numbers and rates reaching the primary endpoint and its components
| High Hb [13.5 g/dL] target (n=711) | Low Hb [11.3 g/dL] target (n=710) | |
|---|---|---|
| Doubling of Serum Creatinine, RRT,1 or Death No. reaching endpoint* event rate (per 1000 patient-years) First Outcome in the Composite of Kidney Disease Progression Either Doubling Serum Creatinine or RRT No. reaching endpoint** event rate (per 1000 patient-years) 2 Doubling of Serum Creatinine No. reaching endpoint** event rate (per 1000 patient-years) 2 RRT No. reaching endpoint** event rate (per 1000 patient-years) 2 Doubling of Serum Creatinine and RRT** Death No. reaching endpoint** event rate (per 1000 patient-years) 2 |
224/659 (34.0%) 240.981 n=659 172 (26.1%) 186.00 37 (5.6%) 47.94 130 (19.7%) 160.42 5 (0.8%) 52 (7.9%) 54.14 |
183/662 (27.6%) 193.121 n=662 148 (22.4%) 156.73 37 (5.6%) 46.95 107 (16.2%) 137.73 4 (0.6%) 35 (5.3%) 36.75 |
Hb, hemoglobin
RRT = Renal replacement therapy
only the first event within an individual is counted.
Given as n/N (%).
Given as no. (%).
While doubling of serum creatinine occurred at a similar rate between groups (5.6%), there was a greater rate of initiation of RRT and higher mortality among participants in the high vs low hemoglobin target arm (19.7% vs 16.2% for RRT and 7.9% vs 5.3% for death among high vs low hemoglobin, respectively).
Cox hazards models of CKD progression
A higher hemoglobin target was associated with a shorter time to progression of kidney disease in the univariate model (HR, 1.25; 95% CI, 1.03–1.52; p=0.02) (Table 3, model a). In the fully adjusted model, hemoglobin target remained significantly associated with a shorter time to CKD progression (HR, 1.22; 95% CI, 1.00–1.48; p=0.05) (Table 3, model b).
Table 3.
Cox regression on time to progression of kidney disease
| Model1 | Variable(s) | HR (95% CI) | P-Value |
|---|---|---|---|
| a | Hb target of 13.5 g/dL (vs. 11.3 g/dL) {unadjusted} | 1.25 (1.03–1.52) | 0.02 |
| b | Hb target of 13.5 g/dL (vs. 11.3 g/dL) {adjusted1,2} | 1.22 (1.00–1.48) | 0.05 |
| c | Hb target of 13.5 g/dL (vs. 11.3 g/dL) Smoking status (Current Smoker vs. Never/Past Smoker) Interaction of High-Hb target Group and smoking status Contrasts Current Smoking Patients with Hb target of 13.5 g/dL (vs. 11.3 g/dl) Never/Past Smoking Patients with Hb target of 13.5 g/dL (vs. 11.3 g/dl) |
1.15 (0.93–1.41) 0.98 (0.57–1.68) 2.18 (1.04–4.58) 2.50 (1.23–5.09) 1.15 (0.93–1.41) |
0.2 0.9 0.04 0.01 0.2 |
| d | Hb target of 13.5 g/dL (vs. 11.3 g/dL) DM Interaction of High-Hb target Group and DM Contrasts DM Patients with Hb target of 13.5 g/dL (vs. 11.3 g/dl) Non-DM Patients with Hb target of 13.5 g/dL (vs. 11.3 g/dl) |
1.29 (0.90–1.85) 1.03 (0.73–1.44) 0.91 (0.59–1.40) 1.17 (0.92–1.49) 1.29 (0.90–1.85) |
0.2 0.9 0.7 0.2 0.2 |
| e | Hb target of 13.5 g/dL (vs. 11.3 g/dL) Heart failure Interaction of High-Hb target Group and heart failure Contrasts Heart failure Patients with Hb target of 13.5 g/dL (vs. 11.3 g/dl) Non-heart failure Patients with Hb target of 13.5 g/dL (vs. 11.3 g/dl) |
1.31 (1.02–1.67) 1.43 (1.05–1.94) 0.82 (0.54–1.23) 1.07 (0.77–1.48) 1.31 (1.02–1.67) |
0.03 0.02 0.3 0.7 0.03 |
| f | Hb target of 13.5 g/dL (vs. 11.3 g/dL) Baseline eGFR Linear Splines* eGFR < 26 mL/min/1.73m^2 eGFR >= 26 mL/min/1.73m^2 Interaction of Hb 13.5 g/dL Group and Baseline eGFR Linear Splines* eGFR < 26 mL/min/1.73m^2 eGFR >=26 ml/min/1.73m^2 |
0.88 (0.27–2.91) 0.88 (0.84–0.91) 0.97 (0.93–1.01) 1.01 (0.95–1.07) 1.04 (0.98–1.09) |
0.8 <0.001 0.1 0.7 0.2 |
| g | Hb target of 13.5 g/dL (vs. 11.3 g/dL) Baseline UPCR Linear Splines** UPCR < 1 mg/mg UPCR >= 1 mg/mg Interaction of High-Hb target Group and Baseline UPCR Linear Splines** UPCR < 1 mg/mg UPCR >= 1 mg/mg |
1.75 (1.08–2.84) 2.81 (1.70–4.62) 1.13 (1.06–1.21) 0.57 (0.30–1.09) 1.03 (0.95–1.11) |
0.02 <0.001 <0.001 0.09 0.5 |
Kidney disease progression defined as doubling of creatinine, progression to RRT, or death.
CI, confidence interval; HR, hazard ratio; Hb, hemoglobin; DM, diabetes mellitus; UPCR, urine protein-creatinine ratio; eGFR, estimated glomerular filtration rate;
Candidate variables for the model included baseline laboratory measurements (eGFR, albumin, total cholesterol, ferritin, transferrin saturation, Hb, UPCR, and the composite indicating the presence of inflammation or malnutrition defined as albumin < 3.6 or ferritin > 600) and demographic and clinical measurements (age, gender, race, ethnicity, coronary artery disease, heart failure, DM, cerebrovascular disease, thromboembolic disease, hypertension, peripheral vascular disease, any malignancy, cigarette smoking and prior atrial fibrillation).
All models (except a) are adjusted for the variables specified plus the following: smoking status, baseline eGFR linear splines (< 26 mL/min/1.73m^2 and >=26 mL/min/1.73m^2), baseline albumin linear splines (< 3.5 g/dL and >= 3.5 g/dL), baseline cholesterol linear splines (< 240 mg/dL and >= 240 mg/dL), baseline UPCR linear splines (< 1 mg/mg and >= 1 mg/mg), age linear splines (< 75 years and >= 75 years), female gender, prior heart composite (CHF, cardiomyopathy, LVD, or RVD) and prior atrial fibrillation/flutter. Due to one participant missing smoking status, 1,320/1,321 were included in models b–g.
HR per 1-mL/min/1.73m^2 decrease
HR per 1-mg/mg increase
Subgroups associated with CKD progression
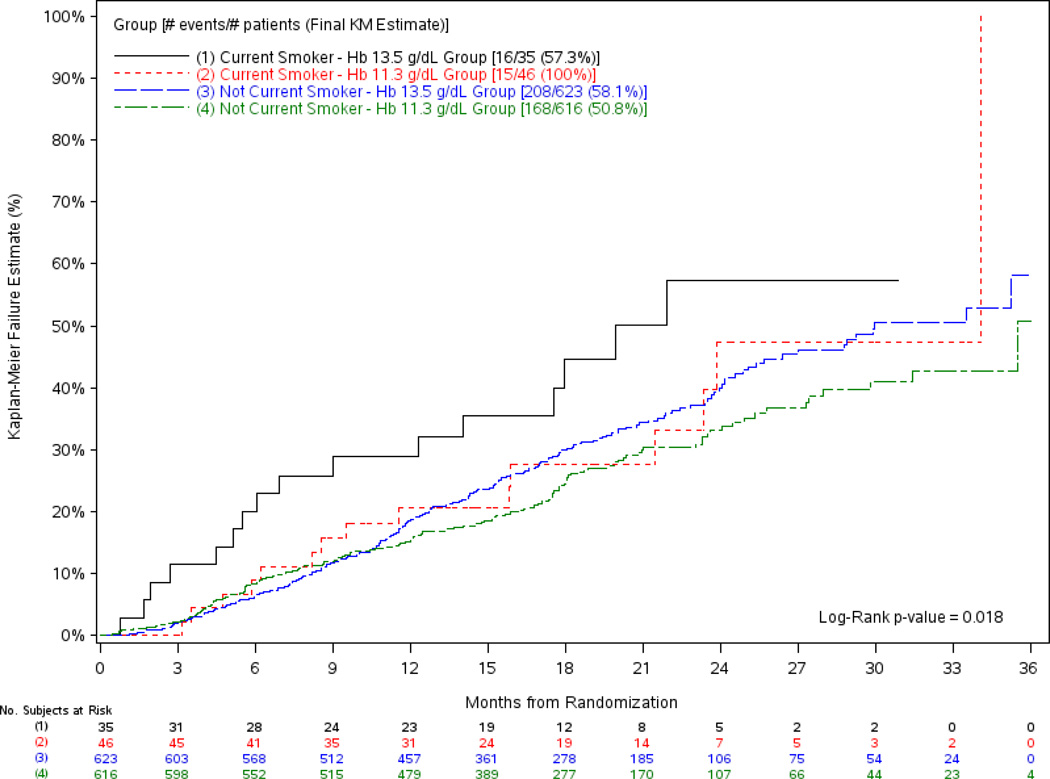
In the multivariable model, hemoglobin target interacted with tobacco use (p=0.04) (Table 3, model c) such that the higher hemoglobin target had a greater associated risk of progression of kidney disease among current smoking participants only (HR, 2.50; 95% CI, 1.23–5.10; p=0.01). No risk associated with hemoglobin target was identified among those who currently did not use tobacco (HR, 1.15; 95% CI, 0.93–1.41; p=0.2). The difference in risk was also apparent in univariate analyses (Figure 2) (p=0.02). Hemoglobin target did not interact with DM (Table 3, model d), the presence of heart failure (Table 3, model e), baseline eGFR (Table 3, model f), or with baseline protein-creatinine ratio (Table 3, model g).
Figure 2.
Kaplan-Meier Plot of the Time to the First Progression of Kidney Disease Outcome (Doubling of Creatinine, RRT or Death) by Treatment Group and Smoking Status
To further explore the association between smoking status and higher CKD progression among those with higher hemoglobin targets, we evaluated baseline characteristics, ESA dose, and achieved hemoglobin among current smokers vs nonsmokers/prior smokers within treatment arm (Table 4). Baseline characteristics were similar between treatment groups among smokers and nonsmokers/prior smokers. ESA dose was significantly higher among participants in the high vs low hemoglobin arm overall, but there was no stepwise difference in ESA dose between current smokers and nonsmokers/prior smokers within the high hemoglobin arm. However, ability to achieve target hemoglobin within the initial 4-month titration phase and throughout the study was lowest among those randomized to the high hemoglobin arm and who were also current smokers.
Table 4.
Baseline Characteristics, ESA dose, and Hb Response by smoking status and Treatment Group1
| Current Smokers | NonSmokers/Past Smokers | |||
|---|---|---|---|---|
| High Hb [13.5 g/dL] target (n=40) | Low Hb [11.3 g/dL] target (n=50) | High Hb [13.5 g/dL] target (n=668) | Low Hb [11.3 g/dL] target (n=660) | |
| Age | 61.7 ± 14.4 | 62.2 ± 11.7 | 66.2 ± 14.3 | 66.6 ± 13.6 |
| Comorbidity Atrial fibrillation/flutter Diabetes mellitus Coronary artery disease Cerebrovascular disease Heart failure |
2/38 (5.3%) 26/39 (66.7%) 13/38 (34.2%) 9/38 (23.7%) 11/38 (28.9%) |
4/49 (8.2%) 27/50 (54.0%) 10/49 (20.4%) 10/49 (20.4%) 11/49 (22.4%) |
61/628 (9.7%) 407/666 (61.1%) 218/629 (34.7%) 78/630 (12.4%) 179/628 (28.5%) |
54/618 (8.7%) 425/659 (64.5%) 204/619 (33.0%) 88/616 (14.3%) 170/618 (27.5%) |
| ESA dose (U/wk)2 | 10,408.2 [7060.5–13149.5] | 5,493.0 [3412.1–7866.0] | 10,840.7 [7724.8–14204.2] | 4,498.5 [2942.6–7379.2] |
| Achieved Hb target by month 4 | 19/39 (48.7%) | 47/50 (94.0%) | 390/662 (58.9%) | 606/652 (92.9%) |
| Achieved Hb target by the CKD endpoint 3 | 27/35 (77.1%) | 44/46 (95.7%) | 530/623 (85.1%) | 598/614 (97.4%) |
Values for continuous variables given as mean ± SD or median [25th–75th percentile]; values for categorical variables given as n/N (%).
ESA, erythropoiesis-stimulating agent; CKD, chronic kidney disease; Hb, hemoglobin
Three of the 1,421 participants were missing smoking status and were not included in this table
ESA dose was defined as the sum of EPO doses from baseline through the day of kidney disease progression endpoint divided by the number of weeks of follow-up
Defined as the maximum Hb after day 1 through the day of the kidney disease progression endpoint >=13.1 g/dL if the target Hb group = 13.5 or >=11.1 g/dL if the target Hb group = 11.3
Competing Risks Analysis
To evaluate the extent to which the higher mortality rate in the higher hemoglobin target arm contributed to the associations described here, models based on cause-specific hazards as well as models based on hazard of cumulative incidence functions were explored for the outcome of doubling of creatinine or RRT and the outcome of death (Table 5).
Table 5.
Competing risk analyses on time to doubling of creatinine or RRT and on time to death
| Model | Variable(s)1 | Based on cause-specific hazard |
Based on hazard of cumulative incidence function |
||
|---|---|---|---|---|---|
| HR (95% CI) | P–Value | HR (95% CI) | P-Value | ||
| Regression Models of the Time to the Doubling of Creatinine or RRT (whichever occurred first) | |||||
| a | Hb target of 13.5 g/dL (vs. 11.3 g/dL) {unadjusted} | 1.19 (0.95–1.48) | 0.1 | 1.17 (0.94, 1.46) | 0.2 |
| b | Hb target of 13.5 g/dL (vs. 11.3 g/dL) {adjusted1} | 1.12 (0.90–1.41) | 0.3 | 1.09 (0.87, 1.36) | 0.5 |
| c | Hb target of 13.5 g/dL (vs. 11.3 g/dL) Current Smoker (vs. Never/Past Smoker) Interaction of High-Hb target Group and Current Smoker Contrasts Current Smoker Patients with Hb target of 13.5 g/dL (vs. 11.3 g/dl) Never/Past Smoker Patients with Hb target of 13.5 g/dL (vs. 11.3 g/dl) |
1.03 (0.82–1.30) 0.77 (0.41–1.44) 3.22 (1.38–7.50) 3.32 (1.48–7.49) 1.03 (0.82–1.30) |
0.8 0.4 0.007 0.004 0.8 |
0.99 (0.79, 1.25) 0.73 (0.39, 1.37) 3.36 (1.42, 7.97) 3.34 (1.47, 7.62) 0.99 (0.79, 1.25) |
0.9 0.3 0.006 <0.001 0.9 |
| Regression Models of the Time to Death | |||||
| a | Hb target of 13.5 g/dL (vs. 11.3 g/dL) {unadjusted} | 1.52 (0.99–2.33) | 0.06 | 1.49 (0.97, 2.30) | 0.07 |
| b | Hb target of 13.5 g/dL (vs. 11.3 g/dL) {adjusted1} | 1.49 (0.97–2.30) | 0.07 | 1.48 (0.96, 2.29) | 0.08 |
| c | Hb target of 13.5 g/dL (vs. 11.3 g/dL) Current Smoker (vs. Never/Past Smoker) Interaction of High-Hb target Group and Current Smoker Contrasts Current Smoker Patients with Hb target of 13.5 g/dL (vs. 11.3 g/dl) Never/Past Smoker Patients with Hb target of 13.5 g/dL (vs. 11.3 g/dl) |
1.55 (0.99–2.44) 2.46 (0.85–7.10) 0.60 (0.12–2.91) 0.93 (0.20–4.25) 1.55 (0.99–2.44) |
0.06 0.1 0.5 0.9 0.06 |
1.57 (0.99, 2.47) 2.49 (0.87, 7.08) 0.50 (0.11, 2.26) 0.78 (0.18, 3.31) 1.57 (0.99, 2.47) |
0.05 0.09 0.4 0.3 0.05 |
CI, confidence interval; HR, hazard ratio; Hb, hemoglobin; RRT, renal replacement therapy
All models (EXCEPT a) adjusted for the variables specified plus the following: baseline eGFR linear splines (< 26 mL/min/1.73m^2 and >=26 mL/min/1.73m^2), baseline albumin linear splines (< 3.5 g/dL and >= 3.5 g/dL), baseline cholesterol linear splines (< 240 mg/dL and >= 240 mg/dL), baseline urine protein-creatinine ratio linear splines (< 1 mg/mg and >= 1 mg/mg), age linear splines (< 75 years and >= 75 years), female gender, prior CHF composite (CHF, cardiomyopathy, LVD, or RVD), prior atrial fibrillation/flutter and current smoker. Due to one participant missing smoking status, 1,320/1,321 were included in models b–c.
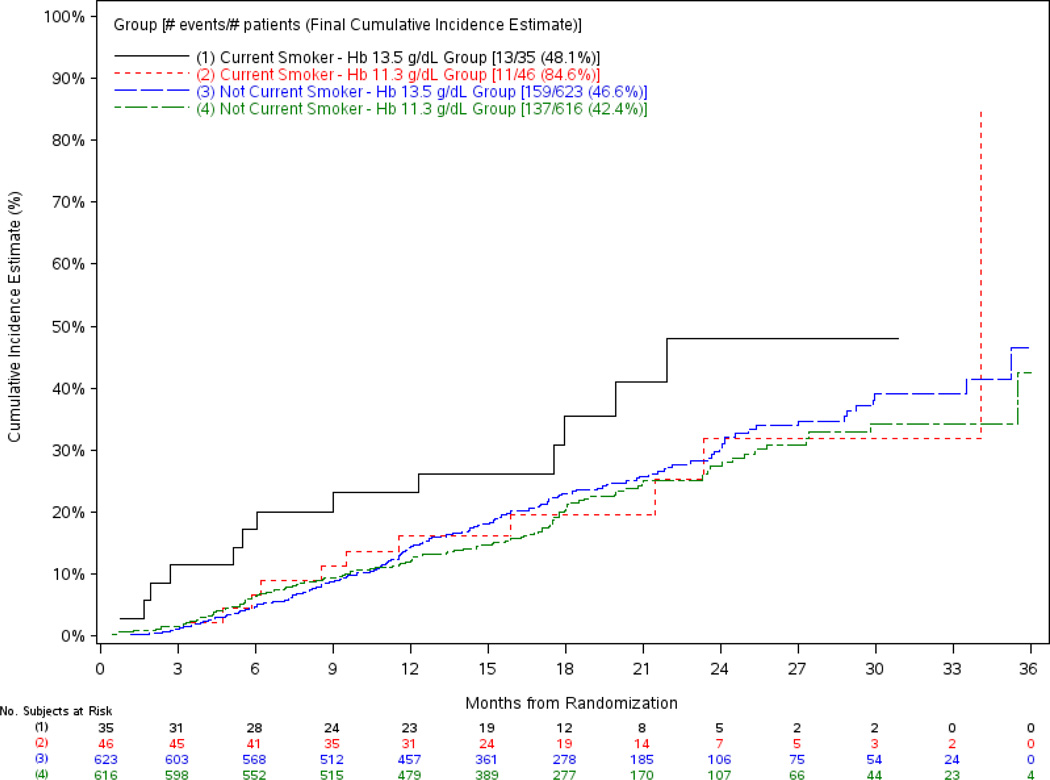
The regression models on time to doubling of creatinine or RRT showed that the associations between treatment arm and the composite outcome of doubling of creatinine or RRT were similar in magnitude to those seen when death was included in the endpoint but did not reach conventional levels of statistical significance (Table 5, models a and b). Like the original model, there was a significant interaction between smoking status and treatment arm. The unadjusted cumulative incidence of doubling of creatinine or RRT among smokers and nonsmokers within treatment arm is shown in Figure 3. The adjusted association between high hemoglobin target and a greater risk of doubling of creatinine or RRT was significant among smokers (with larger hazard ratio than the original model) but again not among those who did not currently smoke (Table 5, model c).
Figure 3.
Cumulative Incidence Plot of the Time to Doubling of Creatinine or RRT by Treatment Group and Smoking Status
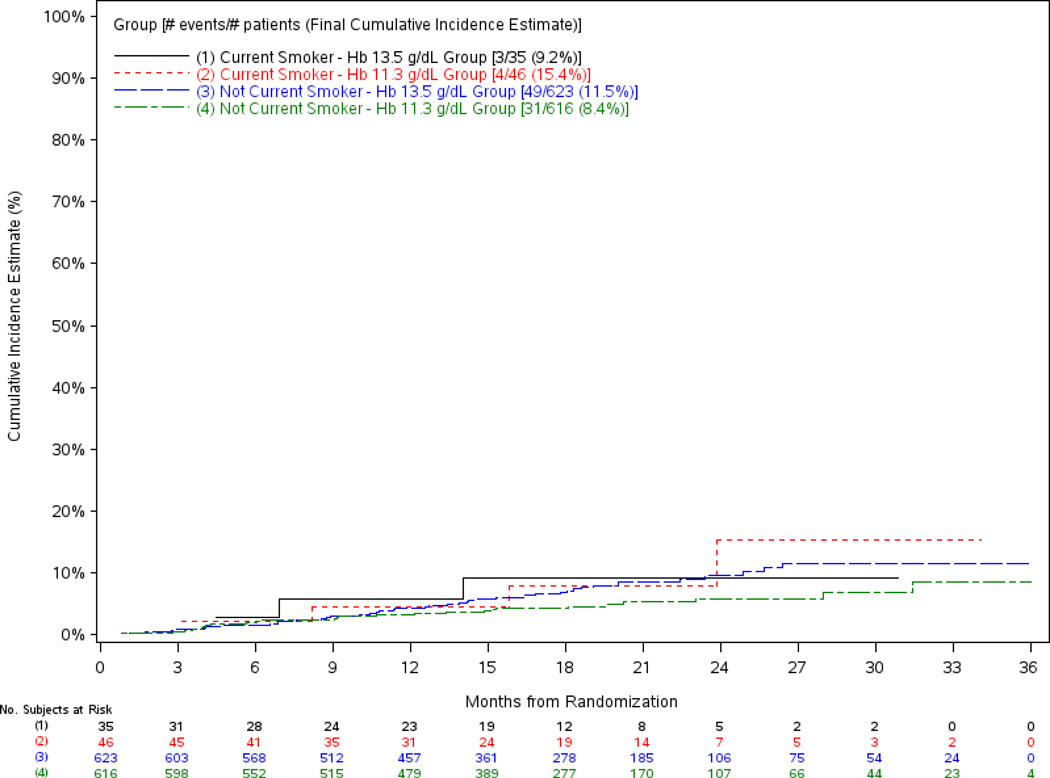
The regression models on time to death showed the association between treatment arm and the outcome of death yielded slightly higher hazard estimates than the original models (composite endpoint of doubling of creatinine or RRT or death), but also did not reach conventional levels of statistical significance (Table 5, models a and b). However, there was no interaction between smoking status and hazard of death and the unadjusted cumulative incidence of death among smokers and nonsmokers within treatment arm is shown in Figure 4. The adjusted association between hemoglobin target and death was not significant for smokers, but close to significance for nonsmokers (Table 5, model c).
Figure 4.
Cumulative Incidence Plot of the Time to Death by Treatment Group and Smoking Status
DISCUSSION
This secondary analysis of the CHOIR trial demonstrates that targeting a higher hemoglobin may be an independent predictor progression of kidney disease among patients with CKD treated for anemia. While higher hemoglobin target was associated with a shorter time to progression in the group overall, the rate appeared to be greatest among participants in the trial who were currently smoking.
This relationship is similar to the CREATE trial.6 Looking at time to initiation of RRT alone, the patients randomized to the higher hemoglobin target treatment experienced a statistically greater risk (p=0.03). In TREAT, however, the risk of initiation of RRT or death was similar between the groups receiving active treatment with darbepoetin targeting a hemoglobin of 13 gm/dL and those receiving placebo (HR, 1.06; 95%, CI 0.95–1.19; P = 0.3).7 Differences in study design (i.e. comparison to a placebo arm vs. two different hemoglobin targets and inclusion of only participants with type 2 DM) as well as differences in outcome assessed (i.e. RRT alone, RRT and death, and doubling of creatinine with RRT and death) need to be considered in comparing the conclusions of these two trials with those drawn from smaller trials where patients randomized to the higher arm had better renal outcomes.3 A meta-analysis of recently summarized the treatment effect of different hemoglobin treatment strategies on progression to ESRD.15 While the meta-analysis demonstrated that larger trials consistently pointed to a trend for an increased risk associated with a higher target, when pooled, no difference between arms could be detected (HR, 1.08; 95% CI, 0.97–1.20). This meta-analysis should be interpreted with the results presented here in the context that time to doubling of creatinine as well as the potential for informative censoring related to mortality were accounted for in the current analysis.
Smoking has been demonstrated in epidemiology studies as a consistent predictor of decline in kidney function. In a study of 23,534 people in Maryland, cigarette smoking was significantly associated with the prevalent risk of CKD in both men and women (HRs of 2.9 [95% CI, 1.7–5.0] and 2.4 [95% CI, 1.5–4.0] in women and men, respectively).9 Similarly, in a large community-based sample of an elderly Italian population showing no evidence of reduced kidney function, smokers were more likely to experience a loss of kidney function (OR, 2.3; 95% CI, 1.0–5.3; p=0.05).10 Supporting the mechanism for this association is a study that measured renal parameters in 30 subjects who were smokers and 24 nonsmoking subjects.11 In this study, smokers were shown to have a significant reduction in renal plasma flow as reflected by MAG3 clearance as well as an increase in plasma endothelin-1 concentration (both p<0.001) as compared with nonsmokers. Further, smoking has been demonstrated to induce an increase in mean arterial pressure, change in filtration fraction, and an increase in urinary albumin-creatinine ratio in patients with IgA nephropathy.16 The relationship between smoking and hemoglobin target however is largely unexplored.
The vascular effects of erythropoietin have been well defined, and many studies demonstrate that erythropoeitin can be protective in experimental settings of acute kidney injury.17, 18 In the setting of nitric oxide (NO) inhibition, erythropoeitin counteracts the decrease in renal perfusion, suggesting that erythropoeitin increases local NO bioavailability.19 While this increase in NO bioavailability is seen in intact vessels, the administration of erythropoeitin, however, causes vasoconstriction in injured arteries.20, 21 Additionally, in eNOS-deficient mice, erythropoeitin significantly increases systolic blood pressure and enhances medial thickening of injured carotid arteries. Smoking has been demonstrated to increase eNOS expression but decrease its activity in patients with normal kidney function. Given that erythropoeitin significantly enhances the medial thickening of injured carotid arteries in eNOS-deficient mice (18), it is possible that a decrement in eNOS activity among smokers treated with erythropoeitin could result in a similar situation in which the erythropoeitin had direct detrimental effects on “injured” blood vessels in the kidney because of a smoking-induced functional decrease in eNOS activity.
But, the mechanism for the identified increased risk of CKD progression with higher hemoglobin targets among current smokers remains unknown. We explored whether smoking status may purely be a surrogate marker for another known risk factor known to increase risk of CKD progression but found no difference in factors such as diabetes or congestive heart failure to explain our identified differences.8 Further, there was no difference in ESA dose between current smokers and nonsmokers/prior smokers treated to high hemoglobin targets. However, it is notable that smokers in the high hemoglobin arm were less likely to achieve the target hemoglobin, which is a marker of a high-risk patient population.22
Similar to the association reported here is the association between thromboembolic events and smoking with the use of oral contraceptives (OCP). Both smoking and OCPs independently increase the risk of several events such as myocardial infarction, venous thrombosis, and subarachnoid hemorrhage, resulting in significant risk when both are used concurrently.23, 24 While some reports suggest that the use of both agents augments their additive risk implying a synergy of effect 24, others state that the risk is merely additive and the two factors do interact. The package insert for many of the oral contraceptives however carries the warning against concurrent smoking.25
The major limitations of this analysis include the consideration of the validity of post hoc subgroup analyses as well as the limited power that exists within a cohort categorized by a variable. The limitations of subgroup analyses particularly with respect to chance findings have been widely discussed 26–28 but should not be understated. These limitations are pertinent to the interpretation of the subgroup analyses presented here. Chance findings may give a significant result among subgroups even though no treatment effect was seen in the cohort overall. Additionally, our inability to characterize type, duration, and quantity of tobacco utilized will likely contribute to a misclassification of subjects potentially biasing these results towards the null. In addition, residual confounding may exist and thus these findings should be considered hypothesis generating. Finally, the competing mortality risk in the higher target arm should be recognized. While the greater mortality risk in the higher arm contributed to the significance of the finding in the composite analysis in which death was included, the point estimate for risk of increased creatinine and progression to dialysis was similar though decreased in power when death was removed from the composite. Further, in this setting where death was excluded from the composite the risk of higher target was still significant in the subgroup that smoked.
In summary, the results of this analysis indicate that the correction of anemia to a higher target may be associated with a greater decline in kidney function, but that this risk may be restricted to the subgroup with the additional risk factor of smoking. These data shed light as to a potential reason why studies to date examining the association between anemia correction and loss of kidney function have not been consistent. Owing to different populations of patients with differing likelihoods of behaviors such as smoking which may be a critical part of the risk cascade, it is not unexpected that the conclusions across studies may be different. While our findings support the current clinical practice of educating CKD patients against smoking, further validation of our findings is required.
Acknowledgements
Support: The original CHOIR trial was supported by Ortho Biotech Clinical Affairs and Johnson & Johnson Pharmaceutical Research and Development, both subsidiaries of Johnson & Johnson. This analysis was supported by a grant from the National Institutes of Health- NIDDK (R01 DK080094-01A1). Dr. Inrig is supported by NIH grants 1KL2 RR024127 and K23 HL092297.
Financial Disclosure: Dr Inrig has received investigator initiated research support from Genzyme. Dr. Barnhart reports receiving consulting fees and grant support from Ortho Biotech Clinical Affairs. Dr. Reddan reports receiving consulting fees from Ortho Biotech Clinical Affairs and Shire Pharmaceuticals; lecture fees from Amgen, Novartis, Pfizer, AstraZeneca, and General Electric; and grant support from Ortho Biotech Clinical Affairs, Amgen, and Novartis. Dr. Patel has received Research/Grant support from Abbott Laboratories and Merck & Co. Dr. Califf reports receiving research support from Novartis and Schering Plough; receiving lecture support from the HEART.ORG, Novartis, and Kowa Research; serving as a consultant for Annenberg, Heart.org, Kowa Research Institute, NITROX LLC, Novartis Pharmaceutical, and Schering Plough; and holding equity interest in NITROX LLC. Dr. Singh reports receiving consulting fees from Johnson and Johnson and Watson and lecture fees from Johnson and Johnson and Watson; and receiving grant support from Ortho Biotech Clinical Affairs, Johnson & Johnson, Amgen, and Watson. Dr. Szczech reports receiving consulting fees from Ortho Biotech Clinical Affairs, Nabi Pharmaceuticals, Gilead, Fresenius Medical Care, Kureha, Affymax, and Acologix; lecture fees from Nabi Biopharmaceuticals, Fresenius Medical Care, GlaxoSmithKline, Gilead, Genzyme, Abbott, Amgen, and Ortho Biotech; and grant support from GlaxoSmithKline, Pfizer, and Genzyme.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ms Sapp declares that she has no relevant financial interests.
REFERENCES
- 1.Sharples EJ, Patel N, Brown P, et al. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004 Aug;15(8):2115–2124. doi: 10.1097/01.ASN.0000135059.67385.5D. [DOI] [PubMed] [Google Scholar]
- 2.Keane WF, Zhang Z, Lyle PA, et al. Risk scores for predicting outcomes in patients with type 2 diabetes and nephropathy: the RENAAL study. Clin J Am Soc Nephrol. 2006 Jul;1(4):761–767. doi: 10.2215/CJN.01381005. [DOI] [PubMed] [Google Scholar]
- 3.Gouva C, Nikolopoulos P, Ioannidis JP, Siamopoulos KC. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int. 2004 Aug;66(2):753–760. doi: 10.1111/j.1523-1755.2004.00797.x. [DOI] [PubMed] [Google Scholar]
- 4.Rossert J, Levin A, Roger SD, et al. Effect of early correction of anemia on the progression of CKD. Am J Kidney Dis. 2006 May;47(5):738–750. doi: 10.1053/j.ajkd.2006.02.170. [DOI] [PubMed] [Google Scholar]
- 5.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006 Nov 16;355(20):2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 6.Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006 Nov 16;355(20):2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009 Nov 19;361(21):2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 8.Szczech LA, Barnhart HX, Sapp S, et al. A secondary analysis of the CHOIR trial shows that comorbid conditions differentially affect outcomes during anemia treatment. Kidney Int. 2010 Feb;77(3):239–246. doi: 10.1038/ki.2009.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol. 2003 Nov;14(11):2934–2941. doi: 10.1097/01.asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 10.Baggio B, Budakovic A, Perissinotto E, et al. Atherosclerotic risk factors and renal function in the elderly: the role of hyperfibrinogenaemia and smoking. Results from the Italian Longitudinal Study on Ageing (ILSA) Nephrol Dial Transplant. 2005 Jan;20(1):114–123. doi: 10.1093/ndt/gfh553. [DOI] [PubMed] [Google Scholar]
- 11.Gambaro G, Verlato F, Budakovic A, et al. Renal impairment in chronic cigarette smokers. J Am Soc Nephrol. 1998 Apr;9(4):562–567. doi: 10.1681/ASN.V94562. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. Jan;101(1):87–93. doi: 10.1016/j.cmpb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 15.Palmer SC, Navaneethan SD, Craig JC, et al. Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med. 2010 Jul 6;153(1):23–33. doi: 10.7326/0003-4819-153-1-201007060-00252. [DOI] [PubMed] [Google Scholar]
- 16.Ritz E, Benck U, Franek E, Keller C, Seyfarth M, Clorius J. Effects of smoking on renal hemodynamics in healthy volunteers and in patients with glomerular disease. J Am Soc Nephrol. 1998 Oct;9(10):1798–1804. doi: 10.1681/ASN.V9101798. [DOI] [PubMed] [Google Scholar]
- 17.Johnson DW, Pat B, Vesey DA, Guan Z, Endre Z, Gobe GC. Delayed administration of darbepoetin or erythropoietin protects against ischemic acute renal injury and failure. Kidney Int. 2006 May;69(10):1806–1813. doi: 10.1038/sj.ki.5000356. [DOI] [PubMed] [Google Scholar]
- 18.Abdelrahman M, Sharples EJ, McDonald MC, et al. Erythropoietin attenuates the tissue injury associated with hemorrhagic shock and myocardial ischemia. Shock. 2004 Jul;22(1):63–69. doi: 10.1097/01.shk.00001276869.21260.9d. [DOI] [PubMed] [Google Scholar]
- 19.Sartelet H, Fabre M, Castaing M, et al. Expression of erythropoietin and its receptor in neuroblastomas. Cancer. 2007 Sep 1;110(5):1096–1106. doi: 10.1002/cncr.22879. [DOI] [PubMed] [Google Scholar]
- 20.d'Uscio LV, Smith LA, Santhanam AV, Richardson D, Nath KA, Katusic ZS. Essential role of endothelial nitric oxide synthase in vascular effects of erythropoietin. Hypertension. 2007 May;49(5):1142–1148. doi: 10.1161/HYPERTENSIONAHA.106.085704. [DOI] [PubMed] [Google Scholar]
- 21.Barua RS, Ambrose JA, Eales-Reynolds LJ, DeVoe MC, Zervas JG, Saha DC. Dysfunctional endothelial nitric oxide biosynthesis in healthy smokers with impaired endothelium-dependent vasodilatation. Circulation. 2001 Oct 16;104(16):1905–1910. doi: 10.1161/hc4101.097525. [DOI] [PubMed] [Google Scholar]
- 22.Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008 Sep;74(6):791–798. doi: 10.1038/ki.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petitti DB, Wingerd J, Pellegrin F, Ramcharan S. Oral contraceptives, smoking, and other factors in relation to risk of venous thromboembolic disease. Am J Epidemiol. 1978 Dec;108(6):480–485. doi: 10.1093/oxfordjournals.aje.a112646. [DOI] [PubMed] [Google Scholar]
- 24.Petitti DB, Wingerd J. Use of oral contraceptives, cigarette smoking, and risk of subarachnoid haemorrhage. Lancet. 1978 Jul 29;2(8083):234–235. doi: 10.1016/s0140-6736(78)91745-2. [DOI] [PubMed] [Google Scholar]
- 25. [accessed on August 4, 2010];Yaz prescribing information. 2010 Available at http://www.yaz-us.com/.
- 26.Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000 Mar 25;355(9209):1064–1069. doi: 10.1016/S0140-6736(00)02039-0. [DOI] [PubMed] [Google Scholar]
- 27.Pocock SJ, Hughes MD, Lee RJ. Statistical problems in the reporting of clinical trials. A survey of three medical journals. N Engl J Med. 1987 Aug 13;317(7):426–432. doi: 10.1056/NEJM198708133170706. [DOI] [PubMed] [Google Scholar]
- 28.Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. Jama. 1991 Jul 3;266(1):93–98. [PubMed] [Google Scholar]