Abstract
Schizophrenia is a severe neurodevelopmental disorder with genetic and environmental etiologies. Prenatal viral/bacterial infections and inflammation play major roles in the genesis of schizophrenia. In this review, we describe a viral model of schizophrenia tested in mice whereby the offspring of mice prenatally infected with influenza at E7, E9, E16, and E18 show significant gene, protein, and brain structural abnormalities postnatally. Similarly, we describe data on rodents exposed to bacterial infection or injected with a synthetic viral mimic (PolyI:C) also demonstrating brain structural and behavioral abnormalities. Moreover, human serologic data has been indispensible in supporting the viral theory of schizophrenia. Individuals born seropositive for bacterial and viral agents are at a significantly elevated risk of developing schizophrenia. While the specific mechanisms of prenatal viral/bacterial infections and brain disorder are unclear, recent findings suggest that the maternal inflammatory response may be associated with fetal brain injury. Preventive and therapeutic treatment options are also proposed. This review presents data related to epidemiology, human serology, and experimental animal models which support the viral model of schizophrenia.
Keywords: Autism, Inflammation, Influenza, Schizophrenia, Viral infection
1. Introduction
The current review aims to highlight the impact of prenatal viral and bacterial infections and inflammation on the etiopathogenesis of schizophrenia. Genetic contributions to the genesis of schizophrenia have been widely studied, but the role of environmental influences is necessary to explain the approximately 40–55% discordance rate in monozygotic twins who do not share diagnoses of schizophrenia (reviewed in Brown, 2011).
Significant epidemiologic data accumulated over the past 30 years has established the role of environmental factors in the acquisition of neuropsychiatric disorders (Fatemi, 2005a). For instance, influenza (H1N1) infection during the first trimester of pregnancy has been associated with schizophrenia (Brown et al., 2004). Increased levels of specific immunoglobulin G (IgG) to various infectious agents, such as Toxoplasma gondii (T. gondii), have been reported in maternal sera before birth (Brown et al., 2005) and in blood from infants at birth (Mortensen et al., 2007) and have been associated with schizophrenia or schizophrenia spectrum disorder in the offspring. Additional findings have also suggested that increased susceptibility to multiple pathogens in schizophrenic patients, rather than a specific agent, may play a role in the etiology of schizophrenia (Krause et al., 2010). Krause et al. (2010) examined the antibody titres of cytomegalovirus (CMV), herpes simplex virus (HSV), Epstein–Barr virus, mycoplasma, Chlamydia, and toxoplasma in 31 schizophrenic patients and 30 healthy controls. Patients with schizophrenia were found to have a higher prevalence of antibodies in general, suggesting the possibility of an altered immune response in the central nervous systems of these individuals (Krause et al., 2010). These results are particularly salient due to immunosuppressive events that occur during pregnancy (Weinberg, 1984). Recently, Rowe et al. (2011) discovered that the expansion of Foxp3+ regulatory T cells (Tregs), a process that occurs naturally during pregnancy, is in fact an immunosuppressive event that increases prenatal susceptibility to infection. This upsurge in information regarding the association between prenatal infection and schizophrenia has initiated debate as to whether brain abnormalities result from direct infection of the fetal brain or from the maternal immune response (Aronsson et al., 2001, 2002; Fatemi et al., 2012; Gu et al., 2007; Hsiao and Patterson, 2011; Meyer, 2011, 2013–this issue; Shi et al., 2009).
2. Epidemiology
Schizophrenia is a severe psychiatric disorder affecting approximately 1% of the world’s population (APA, 1994) with an incidence of 0.2 per 1000 per year (Easton and Chen, 2006). Schizophrenia has a higher incidence in males than females, with a male:female ratio of 1.4 (McGrath, 2005), with an early mean onset at age 20 in males, compared to age 25 in females (APA, 1994; Meltzer and Fatemi, 2000). Currently, a diagnosis of schizophrenia is based on behavioral criteria that requires the patient to experience two or more of the following symptoms for a significant duration over a 1 month period: positive symptoms (hallucinations and delusions), negative symptoms (avolition, apathy, asociality), and disorganization symptoms (grossly disorganized or catatonic behavior, disorganized speech, incoherence). However only one symptom is required if the individual experiences bizarre delusions, auditory hallucinations commentating on the patient’s behavior or thoughts, or two or more voices conversing with one another (APA, 1994).
Various environmental risk factors for schizophrenia have been well documented, such as vitamin D deficiency (McGrath, 1999), being an immigrant of African descent in the United States or other industrialized countries (Dealberto, 2010; Selten et al., 2007), and birth in an urban environment (Marcelis et al., 1999). More striking, however, are the epidemiological findings associating birth season and risk of schizophrenia. An excess of approximately 5–15% of patients with schizophrenia are born during the winter months in the Northern Hemisphere (Boyd et al., 1986; Bradbury and Miller, 1985; Davies et al., 2003; Gallagher et al., 2007; Machón et al., 1983; Pallast et al., 1994; Torrey et al., 1996). One report found an interesting association between seasonality of schizophrenic births and latitude band, with significant increases in the effect of seasonal birth with increasing latitude (Davies et al., 2003). While most studies in the Northern Hemisphere demonstrate concordant results (Boyd et al., 1986; Bradbury and Miller, 1985; Davies et al., 2003; Gallagher et al., 2007; Machón et al., 1983; Pallast et al., 1994; Torrey et al., 1996), studies examining the association between winter birth and schizophrenia in the Southern Hemisphere are less robust. Several reports of the southern hemisphere have found a positive association between birth during the winter months and an increased risk of schizophrenia (Morgan et al., 2001; Syme and Illingworth, 1978). Other reports have found associations between risk of schizophrenia and birth during peak rainfall season in Northeast Brazil (de Messias et al., 2001, 2006), late spring and summer births (Berk et al., 1996), as well as an increased risk of avolition and apathy in schizophrenic patients born during the autumn and winter months in Africa (Jordaan et al., 2006). While many of these studies outline the positive associations between season of birth in the Southern Hemisphere and risk of schizophrenia, other reports were unable to replicate these findings (d’Amato et al., 1996; Morgan et al., 2001).
3. Systemic inflammation, neuroinflammation, and neuroprogression
3.1. Schizophrenia and inflammatory markers
Schizophrenia is often accompanied by systemic inflammation and cell-mediated immune (CMI) activation as shown by increased levels of cytokines, interleukin 2 receptors (IL-2Rs), interleukin 1 receptor agonist (IL-1RA) (Lin et al., 1998; Maes et al., 2000; Miller et al., 2011; Zhang et al., 2004), acute phase reactants such as IL-1β, IL-6, and transforming growth factor (TGF)-β in plasma of subjects with schizophrenia (Meyer, 2011, 2013–this issue; Miller et al., 2011). Cytokines may be divided into various categories based on their role in inflammation and their derivation. Pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, are synthesized upon activation of the innate immune system and are responsible for febrile reactions, activation of phagocytes, vascular permeability, and release of inflammatory mediators, all of which are essential to the inflammatory response (Meyer, 2011, 2013–this issue). Many anti-inflammatory cytokines, such as IL-10 and TGF-β1, act to inhibit pro-inflammatory cytokine production, and these two factors in particular have been shown to exert an anti-hypoxic effect (Curfs et al., 1997; Levin and Godukhin, 2011).
Pro-inflammatory markers of immune system activation have been widely recognized in schizophrenia, with evidence that schizophrenic patients may possess a hyper-active pro-inflammatory system (Kim et al., 2009) as supported by increased levels of IL-6, IL-1β, and TNF-α in the sera of these individuals (Kim et al., 2009; Liu et al., 2010; O’Brien et al., 2008). Polymorphisms of the IL-1 gene complex have been associated with schizophrenia (Xu and He, 2010) and, specifically, ventricular enlargement (Papiol et al., 2005). High levels of pro-inflammatory cytokines such as IL-1β, IL-6, and IL-2 have also been observed in the CSF of patients with schizophrenia, further supporting this effect (Garver et al., 2003; McAllister et al., 1995; Soderlund et al., 2009).
The role of anti-inflammatory and immunosuppressive markers in schizophrenia has also been examined. Reduced IL-4 levels have been found in schizophrenic patients during acute episodes (O’Brien et al., 2008). Interestingly, IL-4 has also been shown to induce apoptosis of microglia (Soria et al., 2011). Reductions in anti-inflammatory cytokines may indicate an inability to limit the inflammatory response in these patients. However, increases in levels of anti-inflammatory cytokines have also been found in the sera of patients with schizophrenia, notably soluble IL-1RA (sIL-1RA), IL-2R, TNF-α, IL-10, and TGF-β (reviewed in Meyer, 2011). While anti-inflammatory activity is necessary for preventing the potentially deleterious effects of over-active pro-inflammatory markers, excessive anti-inflammation may induce various physiological abnormalities and reduce an individual’s resistance to infectious agents (Meyer, 2011).
3.2. Neuroinflammation, oxidative stress, and schizophrenia
While peripheral immune activation recruits inflammatory mediators near the site of insult, this type of activation is capable of activating immune markers in the brain, such as microglia and cytokines (Patterson, 2011). Neuroinflammation as a response to bacterial and viral infections is often described as the accumulation of mobile innate and/or adaptive immune cells present in tissue that have circulated through the bloodstream (Graeber et al., 2011). These mobile cells, which may be macrophages (e.g. microglia) or neutrophils (e.g. granulocytes) possess powerful tissue-destructive effects (Rock et al., 2010).
Oxidative stress is also thought to play a role in schizophrenia (Wood et al., 2009), although the results are somewhat mixed. Several reports have shown reductions in antioxidant enzymes in schizophrenia (Dadeech et al., 2008; Raffa et al., 2009; Singh et al., 2008) while other reports have documented no change (Srivastava et al., 2001), or increased antioxidant presence in individuals with schizophrenia (Dakhale et al., 2004; Kuloglu et al., 2002; Kunz et al., 2008).
3.3. Neuroprogression in schizophrenia: structural and genetic involvement
Neuroprogression, such as neurodegeneration, apoptosis, and reduced neurogenesis/neuroprotection may be caused by oxidative stress and neuroinflammation (Andreasen et al., 2011; Berk et al., 2011; Madje, 2010; Maes et al., 2011; Niizuma et al., 2009; Sayre et al., 2008). Various neuroanatomical abnormalities in schizophrenia are indicative of tissue loss, such as ventricular enlargement and reduced hippocampal, parahippocampal gyrus, and potentially temporal lobe volume (reviewed in Csernansy, 2007). Other studies of neuroprogression in schizophrenia show evidence for apoptotic pathway involvement (Chen et al., 2009; Jarskog et al., 2004), neurodegeneration (Andreasen et al., 2011; Archer, 2010; Ashe et al., 2001) and neurotoxicity (Lahti and Reid, 2011). A few neuroprogressive genes involved in schizophrenia are outlined in Table 1. Infection via bacterial and viral agents has been shown to be relevant to the neuroprogressive pathway. Bacterial infection with Campylobacter rectus (C. rectus) has been shown to increase levels of interferon (INF)-γ (Offenbacher et al., 2005), a mediator of neuronal oxidative stress and apoptosis (Vartanian et al., 1995). Viral agents, such as Human herpesvirus 6 (HHV-6) have also been demonstrated to induce the apoptotic pathway in primary human fetal astrocytes (Gu et al., 2011). Because of the complexities involved in in vivo measurements of apoptosis and oxidative stress in relation to prenatal infection, translational models in rodents are necessary in order to gain insight into the effects of prenatal viral infection and possible therapeutic interventions on the developing brain (Meyer et al., 2009). Rodents reach adulthood quickly and have a much shorter gestational period than humans. In addition, prenatal infection with bacterial lipopolysaccharide (LPS) has been helpful for comparing the effects of bacterial and viral agents on the developing brain. While prenatal exposure to polyriboinosinic polyribocytidilic acid (PolyI:C) may mimic the viral model, it is not identical and does not affect the same genes as the influenza virus. However, this model has generated much support for other markers of immune activation that have been shown to affect fetal brain development.
Table 1.
Involvement of certain genes in schizophrenia, neuroprogression, and the viral model.
| Gene name | Involvement in schizophrenia | Involvement in neuroprogression | Involvement in viral model |
|---|---|---|---|
| Fyn proto-oncogene (Fyn) | ↑ mRNA and protein in PFC (Ohnuma et al., 2003); SNPs correlat ed with performance on the Wisconsin Card Sorting Test (Rybakowski et al., 2007) | Apoptosis (Chen et al., 2011; Saminathan et al., 2011), hypoxia (Garcia-Roman et al., 2010), inflammation (Smyth etal., 2011) | ↑ in E7 virally infected placental tissue (Fatemi et al., 2012) |
| Quaking (Qk) | ↓ mRNA in cortex and hippocampus (Haroutunian et al., 2006); ↓ mRNA in white matter (McCullumsmith et al., 2007) | Apoptosis in oligodendrocytes (Pilotte etal., 2001) | ↑ in E7 virally infected placental tissue (Fatemi et al., 2012) |
| Regulator of G-protein signaling 4 (RGS4) | Associated with depression in schizophrenia (Rethelyi et al., 2010); Polymorphisms (Lane etal., 2008) | Hypoxia (Schmidt-Kastner et al., 2006) | ↓ in E16 infected hippocampus at P0 and P14 (Fatemi et al., 2009b) |
| Semaphorin-3A (Sema3a) | ↑ in cerebellum (Eastwood et al., 2003) | Apoptosis (Guttmann-Raviv etal., 2007) | ↑ in E18 infected hippocampus at P0 (Fatemi et al., 2008a) |
| Transferrin receptor 2 (Trfr2) | ↓ mRNA in white matter (McCullumsmith et al., 2007); Polymorphisms in Transferrin gene (Qu etal., 2008) | Protection against apoptosis (Lesnikov et al., 2008) | ↑ in E18 infected cerebellum at P0 (Fatemi et al., 2008a) |
4. Animal models of viral infection
Rodent models of prenatal viral infection are important for further substantiating the viral theory of schizophrenia. Cotter et al. (1995) was the first to examine brain pathology in the offspring of pregnant mice infected with the H2N2 influenza virus during the second trimester. The offspring, which were sacrificed on postnatal day 21 (P21) did not validate the hypothesized pyramidal cell disarray (Cotter et al., 1995). Studies by Fatemi et al. (1998, 1999, 2000, 2002a,b, 2005, 2008a,b,c, 2009a,b, 2012) further investigated this topic, and discovered that prenatal viral infection with human influenza (H1N1) at various embryonic time points resulted in various brain, behavioral, and genetic abnormalities in offspring, based on time of insult. These results will be summarized and described in the next subsections (see Sections 4.1–5.2).
4.1. Gene expression and human influenza viral infection
The expression of a number of important brain genes were altered in offspring infected at embryonic days 7, 9, 16, and 18 (E7, E9, E16, E18). At E7, prenatal infection was shown to induce changes in gene expression in frontal cortex and hippocampus of the exposed progeny. Many of these genes are associated with hypoxia, inflammation, and schizophrenia (Fatemi et al., 2012). Compared to other time points, these gene expression changes were minor, with significant upregulation of 21 genes and downregulation of 57 genes in the frontal cortex and hippocampus (Fatemi et al., 2012). At E9, significant upregulation of 214 genes and downregulation of 175 genes was observed in the cerebellum and neocortex of Balb/c mice (Fatemi et al., 2005; Fatemi et al., 2008b, 2008c). Many of these genes are known to regulate cell structure and function, such as cytosolic chaperone system, HSC70, Bicaudal D, aquaporin4, carbonic anhydrase 3, glycine receptor, norepinephrine transporter, and myelin basic protein (Fatemi et al., 2005). At E16, prenatal H1N1 infection was associated with a significant number of gene alterations, many of which are related to myelination in the cerebellum and hippocampus and known to be implicated in schizophrenia (Fatemi et al., 2009a,b). In total, 1463 genes were found to be upregulated and 797 genes downregulated in the cerebellum, frontal cortex, and hippocampus of the exposed offspring (Fatemi et al., 2009a,b). Examples for some of the altered genes include myelin associated glycoprotein (Mag), proteolipid protein 1 (Plp1), myelin and lymphocyte-associated protein (Mal), myelin basic protein (Mbp), myelin-associated oligodendrocytic basic protein (Mobp), myelin oligodendrocyte glycoprotein (Mog), cell adhesion molecule, neuronal 1 (Ncam1), and regulator of G protein signaling 4 (RGS4) (Fatemi et al., 2009a,b). By E18, microarray data showed 533 upregulated and 209 downregulated genes in the frontal cortex, hippocampus, and cerebellum of virally exposed offspring, many of which have been implicated in schizophrenia (Fatemi et al., 2008a). These include Sema3a, Trfr2, and Vldlr (Fatemi et al., 2008a). At this time point, several genes involved in influenza-mediated RNA processing were also upregulated in frontal cortex, hippocampus, and cerebellum and were present at P0, including non-structural protein 1(NS1) influenza binding protein and aryl hydrocarbon receptor nuclear translocator (Fatemi et al., 2008a).
4.2. Protein expression and human influenza viral infection
Protein measurements throughout various embryonic dates yielded interesting results. Neuronal nitric oxide synthase (nNOS) is an enzyme that produces nitric oxide (NO), a potentially harmful molecule implicated in fetal brain injury under hypoxia–ischemia conditions (Rao et al., 2011). In the offspring of mice infected with H1N1 on E9, nNOS was shown to increase 147% at postnatal day 35 (P35), with an eventual 29% decrease at postnatal day 56 (P56) in rostral brain areas. Interestingly, nNOS levels decreased in middle brain areas by 27% at P56 (Fatemi et al., 1998; Fatemi et al., 2000). Reelin, an extracellular secretory protein responsible for proper lamination of neurons (Quattrocchi et al., 2002), was significantly reduced in the brains of neonatal offspring of E9 virally exposed mice reflecting abnormal neuronal migration and decreased synaptic plasticity (Fatemi et al., 1999). Significant elevations in numbers of glial fibrillary acidic protein (GFAP)-positive cells were also observed in the brains of exposed offspring reflecting astrogliosis and reactive injury (Fatemi et al., 2002a). Measurement of SNAP-25 (a presynaptic brain marker) immunoreactivity varied along the septo temporal axis of hippocampus with significant overall increases in septodorsal and mid septotemporal regions and overall decreases in temporal ventral areas of the hippocampus of E9 infected offspring (Fatemi et al., 1998). SNAP-25 has roles in axonal growth and neurotransmitter release (Grabs et al., 1996; Osen-Sand et al., 1993; Oyler et al., 1989), and also been associated with death of virally infected cells (Bregano et al., 2011). At E16, protein expression of Mbp 14 kDa isoform was significantly downregulated at P14 and Mbp 18.5 and 17.2 kDa isoforms were significantly downregulated at P56 in the cerebella of exposed offspring (Fatemi et al., 2009a). Mag protein expression was also significantly downregulated at P14 and P35 in the cerebella of exposed offspring (Fatemi et al., 2009a).
4.3. Brain structural abnormalities and human influenza viral infection
At E7, increased white matter maturation as seen by increased fractional anisotropy was observed in the right middle cerebellar penduncle at P35 (Fatemi et al., 2012). At E9, reduced area measurements in the cerebral cortex and unilateral brain hemispheres were also observed in infected neonates when compared to controls (Fatemi et al., 1999). Reduced hippocampal area (18.1%) was also observed, yet did not reach statistical significance (Fatemi et al., 1999). The offspring of mice exposed on E9 and sacrificed at P0 showed significantly increased pyramidal cell density and non-significantly decreased nonpyramidal cell density (Fatemi et al., 2002b). By postnatal week 14, the exposed mice exhibited significant increases in both pyramidal and nonpyramidal cell densities (Fatemi et al., 2002b). Additionally, the effects of E9 prenatal infection were long lasting, as seen by overall increases in brain volume and decreases in ventricular area at 14 weeks (Fatemi et al., 2002b). While it is widely known that subjects with schizophrenia tend to show increased, rather than decreased, ventricular area (Chua and McKenna, 1995), slight reductions in overall brain volume have also been observed in these patients (Wright et al., 2000). At E16, exposed mice also exhibited significantly reduced cerebellar and total brain volume at P14, reduced hippocampal volume at P35, and reduced ventricular area volume at P0 (Fatemi et al., 2009a, b). Fractional anisotropy also revealed delayed maturation of white matter in the right internal capsule at P0 and accelerated maturation in the corpus callosum at P14 and right middle cerebellar penduncle at P56 (Fatemi et al., 2009a). Significant brain atrophy in E18 exposed offspring at P35 (4.3% reduction) was also observed (Fatemi et al., 2008a). Prenatal viral infection of other animal species such as the rhesus monkey, has also elicited structural abnormalities (Short et al., 2010). The offspring of rhesus monkeys infected with H3N2 1 month before term were found to have reduced gray area in much of the cortex and reduced white matter in the parietal cortex, at 1 year of age (Short et al., 2010).
4.4. Behavioral abnormalities and human influenza viral infection
Several behavioral abnormalities have also been observed in the offspring of virally exposed mice. Abnormal behavior, including decreased prepulse inhibition of the acoustic startle response (PPI) was demonstrated in offspring of E9 exposed mice (Shi et al., 2003). Other studies have replicated similar deficits of PPI in virally exposed offspring (Ibi et al., 2009; Wolff and Bilkey, 2008). These behavioral abnormalities could be reversed by chlorpromazine or clozapine (Shi et al., 2003). Neonatal Tap1−/− mice, which express lower levels of major histocompatibility complex 1, infected with the influenza A/WSN/33 virus showed reduced PPI, however infected wildtype (WT) mice did not differ from uninfected mice in adulthood (Asp et al., 2010). Other reports of mice exposed to H1N1 on E9.5 of pregnancy demonstrate increased head twitch response, a behavior typically induced by 5-HT2A agonists (Moreno et al., 2011). As these mice have been shown to express significantly elevated 5-HT2A receptor expression in the frontal cortex (Moreno et al., 2011), this supports previous findings of reduced serotonin in the cerebella of exposed offspring at P14 and P35 (Fatemi et al., 2008a; Winter et al., 2008). Additionally, Moreno et al. (2011) also reported reductions in the metabotropic glutamate receptor 2 in the frontal cortex of the exposed offspring.
4.5. Neurotransmitter abnormalities and human influenza viral infection
Abnormalities at the level of neurotransmitters have also been observed in the offspring of virally exposed mice. At E16, significant reductions in cerebellar levels of serotonin were revealed at P14, with no significant changes in dopamine expression (Winter et al., 2008). At E18, significant reductions in levels of taurine and serotonin were observed in the cerebella of virally exposed offspring, as compared to controls (Fatemi et al., 2008a). Taurine was significantly decreased at P35, serotonin was significantly decreased at P14 and P35, and 5-hydroxyindoleacetic acid was significantly decreased at P14 (Fatemi et al., 2008a). The reduction in serotonin levels during E16 and E18 is meaningful considering its role in brain development and its recent discovery in placenta (Bonnin and Levitt, 2011; Bonnin et al., 2011).
5. The role of the placenta in human influenza viral infection
The placenta plays an important role in the development and growth of the fetus (Pardi and Cetin, 2006). In mice, the placenta may be divided into three regions: the labyrinthine zone, the junctional zone, and a layer of trophoblast giant cells. The labyrinthine zone is comprised of a complex network of fetal capillaries and maternal blood spaces (MBS). The junctional zone primarily consists of spongiotrophoblast cells and glycogen cells, separating the labyrinthine zone from the maternal tissue. A layer of giant trophoblast cells lies between the junctional zone and maternal decidua. These giant trophoblast cells produce hormones and cytokines and may affect maternal physiology such that the fetal allograft is accommodated (Fig. 1). As such, abnormalities in placental gene expression and morphology can have profound effects on the developing fetus. For instance, placental insufficiency has been associated with low birth weight and an increased risk for fetal cardiovascular complications (Henriksen and Clausen, 2002). Recent reports have documented the placenta as a source of serotonin (5-HT) in the murine neonatal forebrain during early gestation (E10–E15). During later gestational periods (E16-birth), the hindbrain begins synthesizing 5-HT using maternal tryptophan in dorsal raphe neurons, which extend to the forebrain (Bonnin and Levitt, 2011; Bonnin et al., 2011). These results are particularly interesting in light of the aforementioned observed reductions of 5-HT in virally exposed mice at E16 and E18 (Fatemi et al., 2008a; Winter et al., 2008).
Fig. 1.
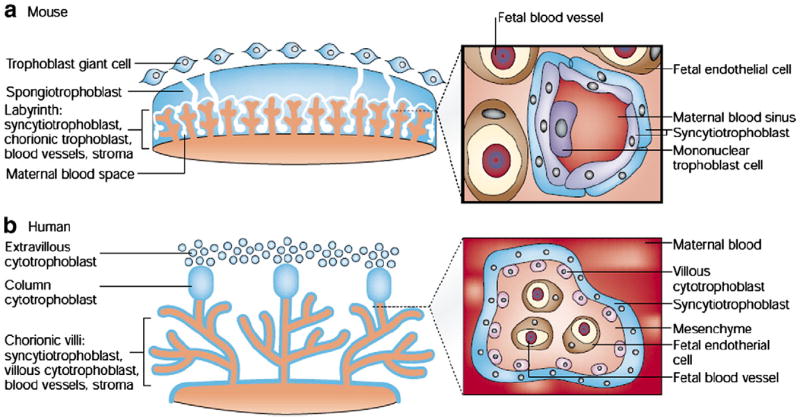
a) Structure of the mouse placenta. The inset details the fetal–maternal interface in the labyrinth. b) Structure of the human placenta. The inset image shows a cross-section through the chorionic villus; trophoblast-derived structures (blue) and mesoderm-derived tissues (orange). The inset images illustrate the number and type of cell layers between the maternal and fetal blood. doi:10.1038/35080570.
Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Genetics Volume 2, Edition 7, Rossant, J., Cross, J.C., Placental development: lessons from mouse mutants, 538–548, 2001.
5.1. Structural abnormalities of the placenta and human influenza viral infection
Dams were infected with influenza A/NWS/33 (H1N1) on E7 and their placentae were harvested on E16 (Fatemi et al., 2012). Infected placental tissues exhibited cytoarchitectual disorganization (especially in the placental labyrinth), an increased presence of immune cells (Fig. 2), and the presence of various sized thrombi (Fatemi et al., 2012). Presence of thrombi clearly shows that occlusion of vessels by thrombi could induce hypoxia via reduction in blood flow and oxygen delivery to the fetal brain. Similar reports have shown a reduction in the thickness of the labyrinthine zone upon infection with C. rectus in mouse, which may result in impaired gas exchange and flow of nutrients and waste between mother and fetus (Offenbacher et al., 2005). As discussed earlier, C. rectus infection has also been associated with an increase in INF-γ levels (Offenbacher et al., 2005), which is known to mediate neuronal stress and neuronal apoptosis (Vartanian et al., 1995).
Fig. 2.
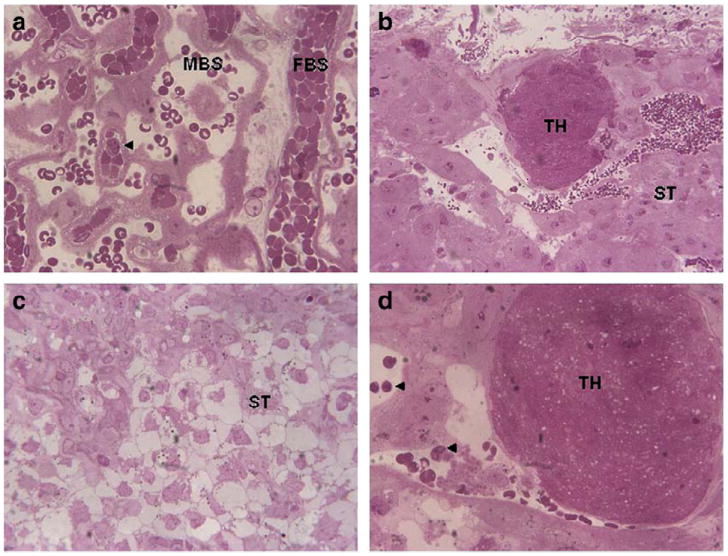
Placental abnormalities following prenatal viral infection. a. labyrinth zone of placenta (60×) from sham-infected dam showing fetal blood space (FBS) and maternal blood space. Arrow points to an inflammatory cell. b. Junctional zone of placenta (20×) from infected dam showing presence of thrombosis (TH). ST, spongiotrophoblast cells. c. Junctional zone of placenta (40×) from infected dam showing disorganization of ST cells. d. Junctional zone of placenta (60×) from infected dam showing presence of TH and inflammatory cells (arrows).
Reprinted from Neuropharmacology, Fatemi, S.H., Folsom, T.D., Rooney, R.J., Mori, S., Kornfield, T.E., Reutiman, T.J., Kneeland, R.E., Liesch, S.B., Hua, K., Hsu, J., Patel, D.H., The viral theory of schizophrenia revisited: Abnormal placental gene expression and structural changes with lack of evidence for H1N1 viral presence in placentae of infected mice or brains of exposed offspring, 2012, with permission from Elsevier.
5.2. Genetic abnormalities of the placenta and human influenza viral infection
Significant upregulation of 77 genes and downregulation of 93 genes were observed in the placentae of pregnant dams infected at E7 (Fatemi et al., 2012). Approximately 20% of placental genes significantly affected by viral infection play a role in apoptosis, 11% in hypoxia, 11% in inflammation, 10% in the immune response, and 9.4% in psychiatric disorders (Fatemi et al., 2012). Important downregulated genes in the placentae of these animals include: glutamate receptor, ionotropic, AMPA1 (alpha 1) (Gria1), catenin (cadherin associated protein) delta 1 (Ctnn1), forkhead box O3 (foxO3), and dysferlin (Dysf) (Fatemi et al., 2012). Important upregulated genes in E7 infected placental tissues include: phosphodiesterase 10A (Pde10a), fyn proto-oncogene (Fyn), programmed cell death 2 (Pdcd2), and quaking (Qk) (Fatemi et al., 2012). Importantly, evidence for presence of the viral genes, matrix protein 1/matrix protein 2 (M1/M2), neuraminidase (NA), and nonstructural protein 1 (NS1), was not observed in the placentae of infected pregnant dams (Fatemi et al., 2012).
6. Use of PolyI:C to mimic viral infection
Similar, but not identical, neuropathological abnormalities have also been observed upon prenatal exposure with a viral mimic, PolyI:C, at critical stages of pregnancy. PolyI:C, a synthetic form of double-stranded RNA, has been demonstrated to elicit the immune response upon administration (Fortier et al., 2004; Shi et al., 2003). Activation of the maternal immune system in mice through PolyI:C administration has been demonstrated to impair fetal brain development, potentially through the pro-inflammatory cytokine, IL-6 (Smith et al., 2007). Recently, PolyI:C exposed maternal placenta was shown to express both increases in IL-6 mRNA and protein in the exposed placentae (Hsiao and Patterson, 2011). Other pro-inflammatory cytokines, such as IL-1β, TNF-α, and anti-inflammatory cytokine IL-10 induced by PolyI:C exposure have also been associated with deleterious effects on brain pathology (Meyer et al., 2005, 2006a, 2008, 2011).
6.1. PolyI:C and behavioral abnormalities
Animals treated with PolyI:C also exhibit several behavioral phenotypes similar to those seen in schizophrenia (Perry et al., 1999). The offspring of mice exposed to PolyI:C on E9 express deficits in PPI, exploratory behavior, and social interaction (Shi et al., 2003). Bitanihirwe et al. (2010) observed behavioral changes in the offspring of pregnant mice injected with PolyI:C during late gestation. These mice demonstrated social, learning, locomotor deficits, anhedonia and stereotyped behavior (Bitanihirwe et al., 2010). Another group observed sex-specific differences among the offspring of pregnant rats subjected to PolyI:C exposure, with male offspring exhibiting altered executive functioning (Zhang et al., 2012). In addition, disrupted latent inhibition (Piontkewitz et al., 2011; Zuckerman and Weiner, 2005), and rapid reversal learning (Zuckerman and Weiner, 2005) have also been observed in PolyI:C exposed animals. Disrupted latent inhibition and rapid reversal learning are both indicative of rapid behavioral switching in response to stimulus/reinforcement paradigms (Zuckerman and Weiner, 2005). Switching, also described as the tendency towards changing a learned response, has been documented in positive symptoms of schizophrenia (Yogev et al., 2003).
6.2. Time-dependent effects of PolyI:C exposure
Time-dependent effects due to prenatal PolyI:C administration and brain/behavior pathology has been observed in the exposed offspring (Fortier et al., 2007; Meyer et al., 2006a). As the maternal immune system is prone to fluctuations throughout the duration of pregnancy (Sargent, 1993), timing of prenatal infection may elicit variable deleterious consequences and may require different approaches to treatment. Prenatal PolyI:C exposure at E9 has been shown to reduce spatial exploration and impair latent inhibition, while exposure at E17 induces perseverative behavior (Meyer et al., 2006a,b).
6.3. Brain morphology and PolyI:C exposure
Brain structural abnormalities in PolyI:C exposed offspring have also been noted. Abnormal proliferation of cortical progenitor cells and impaired expression of Pak6, a regulator of gene transcription, have been found in the cerebral cortex in the offspring of mice exposed to PolyI:C injection (Soumiya et al., 2011). Additionally, altered development of the cerebellum (Shi et al., 2009), has been observed. Other groups have demonstrated concordant neuroanatomical abnormalities, such as ventriculomegaly, between offspring of rodents exposed to PolyI:C during pregnancy and patients with schizophrenia (Li et al., 2009; Piontkewitz et al., 2011). Prenatal PolyI:C exposure has also been shown to reduce the number of Reelin and Parvalbumin positive cells in the medial prefrontal cortex following exposure on E9 and E17 (Meyer et al., 2008) and in the hippocampal formation and dentate gyrus following exposure on E9 (Meyer et al., 2006a).
6.4. Neurotransmitters and PolyI:C exposure
Neurochemical abnormalities have also been reported in the offspring of animals prenatally exposed to PolyI:C injection. Increased dopamine and its major metabolites have been found in the lateral globus pallidus and prefrontal cortex of exposed offspring (Winter et al., 2009). Additionally, prenatal exposure reduced expression of serotonin and its metabolite in the hippocampus, nucleus accumbens, and lateral globus pallidus (Winter et al., 2009). While some studies have observed increased glutamate in prenatally PolyI:C exposed animals (Ibi et al., 2009), others have found glutamate to be unaffected (Winter et al., 2009).
7. Animal models infected with LPS
Other animal studies have examined the effect of prenatal administration of LPS on offspring. Investigations into the behavioral deficits and neuroanatomical abnormalities associated with prenatal LPS administration are important for determining the extent to which bacterial pathogens can alter brain development. Several studies have suggested that the offspring of animals exposed to LPS during pregnancy exhibit impaired motor activity (Kirsten et al., 2010) as well as learning and memory deficits (Hao et al., 2010). Monkeys prenatally exposed to LPS also exhibit PPI deficits, and behavioral disturbances (Willette et al., 2011).
Prenatal LPS infection has also been demonstrated to adversely affect brain development. In rhesus monkeys, LPS exposure during pregnancy at 6 weeks prior to term has been associated with increased production of white matter in offspring at 1 year of age (Willette et al., 2011). Cui et al. (2009) observed that LPS administration in pregnant rats during E15 and 16 elicited reduced dentate gyrus cell proliferation in P14 offspring while later gestational LPS administration, during E18 and 19, was associated with reduced dentate gyrus cell survival (Cui et al., 2009). Interestingly, offspring of animals exposed to LPS later in pregnancy, during E18 and 19, did not exhibit altered cell proliferation or survival in adulthood, at P60 (Cui et al., 2009). The authors of this study also observed that administration of ibuprofen was unable to block any decreases in cell survival at P14, suggesting that induction of maternal cytokines or corticosterone by LPS is likely responsible for mediating this effect (Cui et al., 2009). Rat models of prenatal bacterial infection have also led to structural and biochemical brain abnormalities in the offspring of exposed rats. Recently, Wallace et al. (2010) injected pregnant rats with Escherichia coli (E. coli) on E17. While prenatal infection with E. coli did not affect birth weight, the authors did observe “surface righting and negative geotaxis” in the offspring (Wallace et al., 2010). Interestingly, infected offspring exhibited reduced Purkinje cell counts, density, and volume, and decreased calbindin, a calcium-binding protein expressed by Purkinje cells in the cerebellum and known for its involvement in motor coordination (Barski et al., 2003).
Fortier et al. (2007) examined the effect of timing and type of prenatal infection on PPI. In this study, pregnant rats were exposed to either LPS, PolyI:C, or turpentine, which is known to induce inflammation, at E10–11, E15–16, and E18–19 (Fortier et al., 2007). While PolyI:C had no effect on PPI, PPI deficits were observed with prenatal LPS exposure during E15–E16 and E18–E19 and with turpentine injection at E15–E16. Acute LPS exposure however, had no significant effect on PPI (Fortier et al., 2007).
8. Infection via protozoa and other inflammatory agents
Infectious agents other than viral and bacterial organisms can induce inflammation during pregnancy and cause placental changes in pregnant animals and brain abnormalities in the exposed offspring. T. gondii infection in pregnant mice has been associated with increased expression of multiple chemokines, which were shown to be dependent upon IFN-γ expression (Wen et al., 2010).
Other non-organismal agents have been demonstrated to elicit the inflammatory response. Mercury has recently been shown to induce inflammatory markers such as vascular endothelial growth factor (VEGF) and IL-6 to be released by human mast cells (Kempuraj et al., 2010). In light of these findings, Kempuraj et al. (2010) have suggested that mercury may have the potential to disrupt the blood brain barrier, thereby inducing brain inflammation. In pregnant women, even low-level mercury exposure has recently been associated with reduced cerebellar size in newborns (Cace et al., 2011).While there was no significant difference in the width, the length of the cerebellum was significantly smaller in offspring of mothers whose hair mercury levels were greater than 1 μg/g (Cace et al., 2011).
Intramuscular injection of turpentine is known to induce inflammation through induction of tissue damage (Sheikh et al., 2006), promoting local release of immune factors such as TNF-α, IL-1β, and IL-6 (Aguilar-Valles et al., 2007; Luheshi et al., 1997; Tron et al., 2005; Turnbull et al., 2003). Rats prenatally exposed to turpentine at E15 exhibited reduced PPI, increased latency to reach a platform in the Morris-water maze, prolonged fear conditioning response, and enhanced locomotion in response to amphetamine exposure (Aguilar-Valles and Luheshi, 2011). Enhanced mesolimbic dopaminergic activity was also observed in these animals, and was found to be attenuated after neutralization of IL-6 levels (Aguilar-Valles et al., in press).
Inflammation-induced hypoferremia, a common physiological response to all infections whereby cytokine activation reduces serum levels of non-heme iron, has also been shown to elicit long-lasting behavioral and dopamine changes in the offspring (Aguilar-Valles et al., 2010). Thus, it becomes clear that many factors can activate inflammatory pathways and cause brain abnormalities (a description of such pathways is beyond the scope of this paper).
9. Mechanisms of viral/bacterial effects
There may be specific mechanisms differentiating the harmful effects of infectious pathogens. Foreign pathogens are known to activate the adaptive immune system, and may do so through toll-like receptors (TLRs). There are approximately 10 known members of the mammalian TLR family, each of which responds to different ligands produced by bacteria and viruses, as well as other pathogens (Akira and Hemmi, 2003). For instance, a well known ligand for mammalian TLR4 is the LPS component of Gram-negative bacteria (Poltorak et al., 1998; Qureshi et al., 1999). Recently, Watanabe et al. (2011) showed that PolyI:C causes activation of the TLR3 receptor via a cytoplasmic lipid raft protein, known as Raftlin, leading to production of interferon and inflammatory cytokines. TLR5 is found to be induced in human cells infected with H1N1 or H5N1, while TLR3 is induced in cells infected with H3N2 (Song et al., 2011). Other agents have been detected as TLR ligands, including Gram-positive bacterial peptidoglycans, double-stranded DNA (dsDNA), as well as single- and double-stranded RNA (ssRNA and dsRNA) (Ross, 2005). Interestingly, LPS treatment has been shown to increase TLR-2 expression in syncytiotrophoblast cells of the placenta (Ma et al., 2007). This finding is of particular interest as activation of TLRs cause induction of brain and behavioral abnormalities. TLR2 activation has been shown to have a deleterious effect on the developing mouse brain, as seen by reductions in white matter in the forebrain and cerebellar molecular layer and cerebral gray matter (Du et al., 2011). Tlr3 deficient mice exposed to PolyI:C injection show a lack of neural stem/ progenitor cell impairments, as compared to wildtype mice exposed to the same treatment (De Miranda et al., 2010).
9.1. Direct infection of the fetus
Several reports have postulated that the deleterious effects of prenatal viral, bacterial, or parasitic infections are due to direct infection of the fetal brain and placenta. Influenza A/WSN/33 has been detected in the central nervous system of immunodeficient mice at 17 months, post-inoculation, and with limited damage to tissues (Aronsson et al., 2001). The influenza A virus has also been discovered in fetal brain tissue 3 days after prenatal infection, and persisted for up to 90 days postnatally (Aronsson et al., 2002). By the same token, E7 H1N1 virally infected mice showed evidence of placental thrombosis, cytoarchitectural injury, and immune cell recruitment following H1N1 infection (Fatemi et al., 2012), suggesting virally induced injury to the placentae of exposed pregnant dams. However, investigation of H1N1 viral genes did not show evidence of viral RNA in placentae or brains of exposed offspring, pointing to maternal cytokine involvement in this pathogenic process.
Evidence for bacterial infection in the placenta has also been well documented. Han et al. (2004) found that the bacteria, Fusobacterium nucleatum (F. nucleatum), had colonized and proliferated within the mouse uterus after prenatal infection in as few as 6 h post-infection. The authors found that the infection originated in and was restricted to the deciduas basalis 24 h post-infection (Han et al., 2004). Between 48 and 72 h after infection, F. nucleatum spread to the amniotic fluid and fetus, which the authors attribute to be the likely cause of stillbirth in these animals (Han et al., 2004). Upon prenatal infection, bacterial agents such as C. rectus and Porphyromonas gingivalis (P. gingivalis) have been found in the placenta and thought to induce hypermethylation of promoter genes such as insulin-like growth factor 2 (Igf2) which is involved in placental growth (Bobetsis et al., 2007; Constancia et al., 2002). Another report examining the effect of prenatal Coxiella burnetti (C. burnetti) in goats suggests that the choriallantoic membrane trophoblast cells are the first target cells of the placenta for bacterial infection (Sanchez et al., 2006). Here, abortion was associated with proliferation of C. burnetti within the fetal trophoblast cells (Sanchez et al., 2006). Reductions in blood levels of the parasite Trypanosoma cruzi has also been associated with improved pregnancy outcome (Solana et al., 2009), providing further support for this mechanism.
9.2. Inflammatory/cytokine response
The prevailing view is that brain abnormalities occur as a result of the maternal immune response and that viral and bacterial infections may be one factor that plays a role in inflammation through activation of the adaptive and innate immune systems (Meyer, 2011, 2013–this issue). Prenatal influenza infection has been associated with secretion of IL-6 and TNF-α proteins in cultured human fetal membrane chorion cells (Uchide et al., 2006). These results are particularly important in light of recent investigations that have demonstrated an association between IL-6 exposure and fetal brain injury (Elovitz et al., 2011; Hsiao and Patterson, 2011; Smith et al., 2007).
Many reports have shown that induction of the maternal immune response in the absence of an infectious agent is sufficient to induce impaired brain development in offspring (Meyer et al., 2005, 2006b; Shi et al., 2003; Zuckerman et al., 2003). Thus ultimately, infection with a virus, synthetic viral mimic, and bacterial endotoxin all induce maternal, and perhaps fetal, cytokine production. Recently, Ellman et al. (2010) measured archived maternal serum levels of IL-8 from the mothers of 17 patients diagnosed with schizophrenia and found that prenatal IL-8 exposure during the second and third trimesters of pregnancy were associated with increased ventricular volumes. Additionally, maternal IL-8 exposure was associated with reduced volume of the right caudate, bilateral putamen, and right superior temporal gyrus (Ellman et al., 2010).
10. Correlation between human serology and animal models
Previous ecological exposure studies examining the 1957 A2 influenza epidemic found that individuals born 5 months after the peak exposure period were 88% more likely to develop schizophrenia (Mednick et al., 1988; O’Callaghan et al., 1991). However, these studies were based on associations between reported dates of peak infection and schizophrenic births. While these studies were highly valuable in reporting indirect associations, recent serologic work more reliably and directly associates the presence of maternal and fetal IgG antibodies specific to infectious pathogens and development of schizophrenia in offspring.
10.1. Prenatal and neonatal serology
Early serologic reports of maternal rubella infection demonstrated an increased risk of developing non-affective psychosis (Brown et al., 2000) and schizophrenia (Brown et al., 2001). Brown et al. (2004) observed that infection with influenza during the first trimester of pregnancy leads to a seven-fold increased risk of schizophrenia in the offspring. Other investigations have found that multiple bacterial, parasitic, and viral agents can exert similar effects. Various bacterial infections during the first trimester have been shown to increase susceptibility to developing schizophrenia by 2.53-fold in the offspring (Sorensen et al., 2009). Other reports investigating the intracellular protozoan T. gondii have replicated these results, associating maternal infection with a three-fold increased risk of developing schizophrenia in the offspring (Brown et al., 2005). A more recent report has shown a strong association between seroposivity in maternal sera and presence of maternal T. gondii antibodies in newborn children (Pedersen et al., 2011). Because newborn children will only begin producing T. gondii antibodies around 3 months of age (Wilson and McAuley, 1999), this demonstrates the interesting possibility of maternal T. gondii antibodies crossing the placenta and interacting with the fetus. To further investigate whether prenatal infection is associated with brain abnormalities, a recent study investigated the prenatal brain using magnetic resonance imaging in eight fetuses exposed to T. gondii (Malinger et al., 2011, in press). Two of these fetuses were diagnosed with congenital toxoplasmosis during the second trimester, while the remaining six were diagnosed during the third trimester of pregnancy (Malinger et al., 2011, in press). Interestingly, seven of these fetuses exhibited multiple echo-dense nodular foci in the parenchyma, periventricular zone, or caudothalamic zone (Malinger et al., 2011, in press), while one fetus exhibited severe brain damage due to ventriculomegaly (Malinger et al., 2011, in press), and brain disorganization resulting from infection.
10.2. Postnatal human serology
Postnatally, subjects with schizophrenia infected with HSV-1 have been shown to have decreased posterior cingulate gyrus gray matter and a decreased performance on the Wisconsin Card Sorting Test compared to subjects with schizophrenia who were HSV-1 negative (Prasad et al., 2011). Several other postnatal reports have found associations between the presence of antibodies reactive against infectious agents and risk of schizophrenia. In adult life, individuals with a high T. gondii IgG level are shown to be 1.73 times more likely to develop schizophrenia or associated spectrum disorders than those with lower IgG levels (Pedersen et al., 2011). Dickerson et al. (2006) found that individuals with deficit schizophrenia were more likely to have been infected with CMV compared to those with non-deficit schizophrenia. Exposure to HSV-1 and CMV in schizophrenic subjects is also linked with impaired cognitive function (Shirts et al., 2008; Yolken et al., 2011), and elevations of HSV-2 in children have been associated with later development of schizophrenia (Buka et al., 2001).
11. Preventive measures and therapeutic interventions
Since prenatal infections play such an important role in increasing the incidence of schizophrenia, it would be of special interest to consider preventive measures to mitigate the teratogenic effects of intrauterine infections. A primary prophylactic treatment is important for affecting change in the mother, prior to pregnancy, in order to prevent infection. For instance, use of influenza vaccines before a planned pregnancy could be a potentially beneficial means for preventing the teratogenic effects of viral infection (Cono et al., 2006). The current mandate by the CDC to vaccinate pregnant mothers does not take into account the potential activation of maternal cytokine production. During pregnancy there is a concern that influenza vaccination may incite the generation of antibodies against influenza-related epitopes, inducing the maternal inflammatory response (Nahmias et al., 2006; Patterson, 2007). Additionally, use of oseltamivir to treat viral infection during pregnancy may not be fully safe as its administration in pregnant mice may affect fetal brain gene expression during postnatal development (vide infra) (Fatemi et al., unpublished observations). A recent study by Kanduc (2010) examines the peptide sequence homology of the influenza A H5N1 virus with human proteins. Indeed, many proteins that exhibit sequence homologies with H5N1 are involved in brain cell proliferation, development, and differentiation. Kanduc (2010) observed overlapping gene sequence homologies between the H5N1 virus and genes known to be abnormally regulated in schizophrenia. Interestingly, Kanduc (2010) demonstrated that both Neurexin 1 alpha and 1 beta, Reelin, and disrupted in schizophrenia 1 protein, contain sequence homologies with H5N1; these proteins are also known to be implicated in the etiology of schizophrenia (Fatemi et al., 2005; Fatemi, 2005b; Voineskos et al., 2011). This peptide-virus overlap may have a profound effect on the immune system’s ability to identify foreign antigens as self antigens. High sequence homology between human proteins and infectious agents may also induce cross reactivity, eliciting an autoimmune response.
11.1. Prenatal treatment with oseltamivir
We have performed a pilot study to test the teratogenicity of oseltamivir by infecting or sham-infecting pregnant mice with H1N1 at E16 and concurrently treating them with either oseltamivir (20 mg/kg/day, oral gavage (o.g.) N = 20) or saline (200 μL, o.g.; N = 20). Pilot data demonstrate that oseltamivir had no major effects on pregnancy (all dams delivered pups), litter size/survival, weight of dams, body weights of pups at birth (P0; birth) and brain weights at birth (Table 2) (Unpublished observations, Fatemi et al.).
Table 2.
Effect of oseltamivir on litter size, weight of dams, weight of pups, and brain weight at birth following E16 infection with H1N1.
| −E16C | −E16V | + E16C | + E16V | |
|---|---|---|---|---|
| Wt. of dams at sacrifice (g) | 27.42 ± 0.98 | 25.85 ± 1.21 | 26.0 ± 1.15 | 26.0 ± 1.82 |
| Litter size | 7.21 ± 1.49 | 6.28 ± 1.49 | 6.85 ± 1.95 | 5.85 ± 2.11 |
| Wt. of pups P0 (g) | 1.40 ± 0.07 (n = 11) | 1.45 ± 0.13 (n = 11) | 1.36 ± 0.12 (n = 11) | 1.44 ± 0.14 (n = 7) |
| Brain wt. at P0 (g) | 0.086 ± 0.009 (n = 11) | 0.11 ± 0.27* (n = 11) | 0.085 ± 0.009 (n = 11) | 0.081 ± 0.011 (n = 7) |
(−) in absence of oseltamivir; (+) in presence of oseltamivir (dose = 20 mg/kg/day), oral gavage (o.g.); C = control, V = virally exposed;
= p<0.05.
Gene expression results in hippocampus at P0 showed that in the offspring of infected mice with no oseltamivir, there were 95 downregulated genes and 145 upregulated genes with a fold change of at least 2.0 and p<0.05 when compared with sham-infected controls with no oseltamivir. These results are similar to what we have obtained previously for E16 infection at P0 (117 downregulated and 115 upregulated with a fold change of 2.0 and p<0.05). Significantly, there were no gene expression changes meeting our criteria (fold change of 2.0 and p<0.05) when comparing oseltamivir-treated infected versus oseltamivir-treated controls (Unpublished observations, Fatemi et al.). Therefore, treatment with oseltamivir dramatically reduced virally induced changes in gene expression. Moreover, when comparing control animals treated with oseltamivir and control animals with no drug treatment, only six genes were upregulated and zero downregulated by oseltamivir, while infected animals treated with oseltamivir versus infected animals without drug showed upregulation of 12 genes and downregulation of 22 genes at P0 (Unpublished observations, Fatemi et al.). Interestingly, 10 of the 12 upregulated genes were downregulated in the infected animals that did not receive oseltamivir. Similarly, 18 of the 22 genes downregulated in infected animals additionally treated with oseltamivir were upregulated in infected animals that did not receive oseltamivir (Unpublished observations, Fatemi et al.). These results suggest that oseltamivir treatment alone can affect the direction of change in expression of some hippocampal genes. These results are significant because they are expected to provide interventions that may be used to prevent the “winter birth effect” in humans and thus, decrease the chances of individuals developing schizophrenia. Moreover, oseltamivir acts as a neuraminidase inhibitor, preventing viral replication, without any obvious effects on cytokine production. Its prevention of gene expression changes in the brains of virally exposed offspring suggest that maternal cytokine activation may not be totally responsible for gene expression changes seen in this model. Despite these early positive results, the effects of oseltamivir on gene regulation in other postnatal stages of brain development are unknown and require further investigation.
11.2. Early antipsychotic treatment
Another therapeutic/preventive step is the use of low dose antipsychotics in children susceptible to developing schizophrenia. For example, once prodromal signs of schizophrenia have already occurred, treatment with a low-dose antipsychotic may be helpful. Clozapine treatment has been shown to improve memory deficits in the offspring of mice infected with PolyI:C on day 17 of pregnancy without affecting adult hippocampal neurogenesis (Meyer et al., 2010). Low-dose treatment with other antipsychotics has shown normalization of extracellular glutamate levels (Roenker et al., 2011), which are significantly increased upon prenatal infection with PolyI:C (Roenker et al., 2011). Additionally, PolyI:C exposed rats treated with risperidone and clozapine during adolescence did not develop the brain structural abnormalities found in untreated PolyI:C exposed rats (Piontkewitz et al., 2009, 2010, 2011, 2012). PolyI:C exposed rats treated with clozapine and/or risperidone on post-natal days 34–47 did not the exhibit the same ventricular enlargement and hippocampal reduction as compared to the PolyI:C saline treated rats (Piontkewitz et al., 2009, 2010, 2011, 2012). While use of certain antipsychotics as an early treatment option may be helpful, there is debate over the ethics of such early treatment. Several recent reports have highlighted the controversy over administration of antipsychotics in children, which include risk of weight gain and metabolic effects (Maayan and Correll, 2011; Pringsheim et al., 2011), induction of diabetes mellitus (Andrade et al., 2011), and neurologic side effects (Pringsheim et al., 2011).
11.3. Antipsychotic action and the immune system
Recently, the immune system has been put forth as a potential target for the action of certain antipsychotics (Drzyzga et al., 2006). Various cytokines have been associated as state and trait markers of schizophrenia (Miller et al., 2011). During acute exacerbations, IL-1β, IL-6, and TGF-β were shown to be increased in patients with schizophrenia, yet normalized upon treatment with antipsychotics, thus acting as state markers of disease (Miller et al., 2011). In contrast, other cytokines and their receptors, e.g. IL-12, IFN-γ , TNF-α, and soluble IL-2 receptor (sIL-2R) acted as trait markers of schizophrenia because they remained elevated both in acute exacerbations and after antipsychotic treatment (Miller et al., 2011). Individuals with treatment resistant forms of schizophrenia have been shown to express higher IL-1RA, IL-6 and sIL-2R levels, as compared to controls (Maes et al., 2000; Miller et al., 2011). While Maes et al. (2000) and Zhang et al. (2004) were unable to observe reductions in IL-6 levels after prolonged treatment with clozapine, risperidone, or haloperidol, other reports suggest that use of neuroleptics and atypical antipsychotics may reduce serum levels of IL-6R and increase IL-2R (Maes et al., 1994; Muller et al., 1997). Increased IL-17 levels have been shown to be associated with chlorpromazine, clozapine, d-clozapine, haloperidol, and quetiapine treatment, at nearly all concentrations, in healthy female volunteers without history of psychiatric disorder (Himmerich et al., 2011). While clozapine, which has the potential to induce fever during the first stages of administration, has been associated with increased sIL-2r levels (Pollmacher et al., 1996), haloperidol and risperidol have been demonstrated to reduce plasma IL-2 (Kim et al., 2000; Zhang et al., 2004). This is particularly relevant, as significant elevations in sIL-2R alpha and IL-1RA have been observed in the sera of schizophrenic patients, as compared to controls (Suvisaari et al., 2011). Furthermore, anti-inflammatory therapy has gained support for the treatment of negative and cognitive symptoms of schizophrenia (reviewed in Meyer, 2011).
11.4. Alternative treatments
Other potential avenues for treatment include anti-inflammatory medications such as cyclooxygenase-2 (COX-2) inhibitors (Akhondzadeh et al., 2007; Muller, 2010; Muller et al., 2002) and acetylsalicylic acid (aspirin) (Laan et al., 2010). COX-2 is an important and selective mediator of inflammation in the periphery as well as the brain. Ablation of COX-2 expressing neurons in the forebrain has been demonstrated to elicit neuroprotective effects and reduce inflammation after seizure (Serrano et al., 2011). COX-2 inhibitors, such as celecoxib, added as an adjunctive therapy to antipsychotic treatment, have been demonstrated to greatly improve the total score on the Positive and Negative Syndrome Scale (PANSS) in patients with schizophrenia after 5 weeks (Muller et al., 2002) or 8 weeks (Akhondzadeh et al., 2007) of treatment. While celecoxib has been associated with cardiovascular complications (Solomon et al., 2005, 2006), other more cardioprotective and non-selective COX inhibitors, such as aspirin, may also exert potentially beneficial effects (Hayden et al., 2002). Aspirin is a nonsteroidal anti-inflammatory drug shown to non-selectively inhibit both COX-1 and COX-2 enzymes (Laan et al., 2010). Furthermore, when co-administered with antipsychotic treatment, aspirin has been shown to reduce symptoms of schizophrenia over a period of 3 months, as measured by PANSS, and was shown to be more effective in patients with high anti-inflammatory cytokine levels (Laan et al., 2010). While the authors of this study agree that the reduction in PANSS scores after celecoxib treatment is more profound than after treatment with aspirin, more investigation into the long-term effects of aspirin is needed.
Antibiotics, such as minocycline, have been reported to reduce psychotic symptoms in patients with schizophrenia (Miyaoka et al., 2007). Minocycline has also been shown to positively affect cognitive function and negative symptoms (Levkovitz et al., 2010). Minocycline has been postulated to have an anti-inflammatory effect (Chu et al., 2007) and can interfere with activation of microglia (Hashimoto, 2008). Medicinal plants containing bioactive compounds such as polyphenols, flavonoids, saponins, glucosides, and alkaloids have also been shown to disturb viral propagation (reviewed in Wang et al., 2006). More specifically, flavonoids have been demonstrated to exert an anti-influenza effect (Parker-Athill et al., 2009; Fatemi, 2009; Liu et al., 2008; Nagai et al., 1995).
12. Conclusions
There is now considerable experimental and clinical evidence to show the significant risks of prenatal infection/inflammation as triggers for development of schizophrenia. Future studies to prevent and/or mitigate these insults, are needed to reduce the risks of developing certain types of psychotic disorders.
Acknowledgments
We would like to thank Tim Folsom for the critical review of this manuscript. The work of S.H. Fatemi was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grants 5R01HD046589-04, 3R01HD046589-04S1, and Young & the Phyllis and Perry Schwartz Established Investigator Awards from NARSAD.
Abbreviations
- IgG
immunoglobulin
- PolyI:C
polyriboinosinic polyribocytidilic acid
- IL
interleukin
- sIL-R
soluble IL-receptor
- TNF
tumor necrosis factor
- TGF
transforming growth factor
- LPS
lipopolysaccharide
- TLR
toll-like receptor
- CMV
cytomegalovirus
- HSV
herpes simplex virus
References
- Aguilar-Valles A, Luheshi GN. Alterations in cognitive function and behavioral response to amphetamine induced by prenatal inflammation are dependent on the stage of pregnancy. Psychoneuroendocrinology. 2011;36(5):634–48. doi: 10.1016/j.psyneuen.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Aguilar-Valles A, Poole S, Mistry Y, Williams S, Luheshi GN. Attenuated fever in rats during late pregnancy is linked to suppressed interleukin-6 production after localized inflammation with turpentine. J Physiol. 2007;583:391–403. doi: 10.1113/jphysiol.2007.132829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Valles A, Flores C, Luheshi GN. Prenatal inflammation-induced hypoferremia alters dopamine function in the adult offspring in rat: relevance for schizophrenia. PLoS One. 2010;5(6):e10967. doi: 10.1371/journal.pone.0010967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Valles A, Jung S, Poole S, Clores C, Luheshi GN. Leptin and interleukin-6 alter the function of mesolimbic dopamine neurons in a rodent model of prenatal inflammation. Psychoneuroendocrinology in press; [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S, Tabatabaee M, Amini H, Ahmadi Abhari SA, Abbasi SH, Benham B. Cel-ecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo controlled trial. Schizophr Res. 2007;90(1–3):179–85. doi: 10.1016/j.schres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Washington D.C: APA; 1994. [Google Scholar]
- Andrade SE, Lo JC, Roblin D, Fouayzi H, Connor DF, Penfold RB, et al. Antipsychotic medication use among children and risk for diabetes mellitus. Pediatrics. 2011;128(6):1135–41. doi: 10.1542/peds.2011-0855. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain changes in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry. 2011;70(1):672–9. doi: 10.1016/j.biopsych.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer T. Neurodegeneration in schizophrenia. Expert Rev Neurother. 2010;10(7):1131–41. doi: 10.1586/ern.09.152. [DOI] [PubMed] [Google Scholar]
- Aronsson F, Karlsson H, Ljunggren HG, Kristensson K. Persistence of the influenza A/WSN/33 virus RNA at midbrain levels of immunodefective mice. J Neurovirol. 2001;7:117–24. doi: 10.1080/13550280152058771. [DOI] [PubMed] [Google Scholar]
- Aronsson F, Lannebo C, Paucar M, Brask J, Kristensson K, Karlsson H. Persistence of viral RNA in the brain of offspring to mice infected with influenza A/WSN/33 during pregnancy. J Neurovirol. 2002;8:353–7. doi: 10.1080/13550280290100480. [DOI] [PubMed] [Google Scholar]
- Ashe PC, Berry MD, Boulton AA. Schizophrenia, a neurodegenerative disorder with neurodevelopmental antecedents. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:691–707. doi: 10.1016/s0278-5846(01)00159-2. [DOI] [PubMed] [Google Scholar]
- Asp L, Holtze M, Powell SB, Karlsson H, Erhardt S. Neonatal infection with neurotropic influenza A virus induces the kynurenine pathway in early life and disrupts sensorimotor gating in adult Tap1-/- mice. Int J Neuropsychopharmacol. 2010;13(4):475–85. doi: 10.1017/S1461145709990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski J, Hartmann J, Rose C, Hoebeek F, Morl K, Noll-Hussong D, et al. Calbindin in cerebellar Purkinje cells is a critical determinant of the precision of motor coordination. J Neurosci. 2003;23:3469–77. doi: 10.1523/JNEUROSCI.23-08-03469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Terre-Blanche MJ, Maude C, Lucas MD, Mendelsohn M, O’Neill-Kerr AJ. Season of birth and schizophrenia: southern hemisphere data. Aust N Z J Psychiatry. 1996;30(2):220–2. doi: 10.3109/00048679609076097. [DOI] [PubMed] [Google Scholar]
- Berk M, Kapczinkski F, Andreazza AC, Dean OM, Giorlando F, Maes M, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35(3):804–17. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BKY, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology. 2010;35:2462–78. doi: 10.1038/npp.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobetsis YA, Barros SP, Lin DM, Weidman JR, Dolinoy DC, Jirtle RL, et al. Bacterial infection promotes DNA hypermethylation. J Dent Res. 2007;86(2):169–74. doi: 10.1177/154405910708600212. [DOI] [PubMed] [Google Scholar]
- Bonnin A, Levitt P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience. 2011;197:1–7. doi: 10.1016/j.neuroscience.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Goedin N, Chen K, Wilson ML, King J, Shih JC, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472(7343):347–50. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JH, Pulver AE, Stewart W. Season of birth: schizophrenia and bipolar disorder. Schizophr Bull. 1986;12(2):173–86. doi: 10.1093/schbul/12.2.173. [DOI] [PubMed] [Google Scholar]
- Bradbury TN, Miller GA. Season of birth in schizophrenia: a review of evidence, methodology, and etiology. Psychol Bull. 1985;98(3):569–94. [PubMed] [Google Scholar]
- Bregano LC, Agostinho SD, Roncatti FL, Pires MC, Riva HG, Luvizotto MC, et al. Immunohistochemical detection of metalloproteinase-9 (MMP-9), antioxidant like 1 protein (AOP-1) and synaptosomal-associated protein (SNAP-25) in the cerebella of dogs naturally infected with spontaneous canine distemper. Folia Histochem Cytobiol. 2011;49(1):41–8. doi: 10.5603/fhc.2011.0007. [DOI] [PubMed] [Google Scholar]
- Brown AS. The environment and susceptibility to schizophrenia. Prog Neurobiol. 2011;93:23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Cohen P, Greenwald MA, Susser E. Nonaffective psychosis after prenatal exposure to rubella. Am J Psychiatry. 2000;157:438–43. doi: 10.1176/appi.ajp.157.3.438. [DOI] [PubMed] [Google Scholar]
- Brown AS, Cohen P, Harkavy-Friedman J, Babulas V, Malaspina D, Gorman JM, et al. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol Psychiatry. 2001;49(6):473–86. doi: 10.1016/s0006-3223(01)01068-x. [DOI] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravestein S, Schaefer CA, Wyatt RJ, Bresnahan M, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61(8):774–80. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown AS, Schaefer CA, Quesenberry CP, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162:767–73. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry. 2001;58:1032–7. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- Cace IB, Milardovic A, Prpic I, Krajina R, Petrovic O, Vukelic P, et al. Relationship between the prenatal exposure to low-level mercury and the size of a newborn’s cerebellum. Med Hypotheses. 2011;76(4):514–6. doi: 10.1016/j.mehy.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Chen X, Sun C, Chen Q, O’Neill FA, Walsh D, Fanous AH, et al. Apoptotic engulfment pathway and schizophrenia. PLoS One. 2009;4(9):e6875. doi: 10.1371/journal.pone.0006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Cai L, Zhu J, Chen M, Chen J, Li ZH, et al. Fyn requires HnRNPA2B1 and Sam68 to synergistically regulate apoptosis in pancreatic cancer. Carcinogenesis. 2011;32(10):1419–26. doi: 10.1093/carcin/bgr088. [DOI] [PubMed] [Google Scholar]
- Chu LS, Fang SH, Zhou Y, Yu GL, Wang ML, Zhang WP, et al. Minocycline inhibits 5-lipoxygenase activation and brain inflammation after focal cerebral ischemia in rats. Acta Pharmacol Sin. 2007;28(6):763–72. doi: 10.1111/j.1745-7254.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- Chua SE, McKenna PJ. Schizophrenia—a brain disease? A critical review of structural and functional cerebral abnormality in the disorder. Br J Psychiatry. 1995;166(5):563–82. doi: 10.1192/bjp.166.5.563. [DOI] [PubMed] [Google Scholar]
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections and bioterrorism emergencies. Emerg Infect Dis. 2006;12(11):1631–7. doi: 10.3201/eid1211.060618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, et al. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417:945–8. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- Cotter D, Takei N, Sham P, Quinn P, Larkin C, Oxford J, et al. Does prenatal exposure to influenza induce pyramidal cell disarray in the dorsal hippocampus? Schizophr Res. 1995;16(3):233–41. doi: 10.1016/0920-9964(94)e0082-i. [DOI] [PubMed] [Google Scholar]
- Csernansy JG. Neurodegeneration in schizophrenia: evidence from in vivo neuroimaging studies. ScientificWorldJournal. 2007;7:135–43. doi: 10.1100/tsw.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui K, Ashdown H, Luheshi GN, Boksa P. Effects of prenatal immune activation on hippocampal neurogenesis in the rat. Schizophr Res. 2009;113(2–3):288–97. doi: 10.1016/j.schres.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Curfs JH, Meis JF, Hoogkamp-Korstanje JA. A primer on cytokines: sources, receptors, effects, and inducers. Clin Microbiol Rev. 1997;10(4):742–80. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Amato T, Guillaud-Bataille JM, Rochet T, Jay M, Mercier C, Terra JL, et al. No season-of-birth effect in schizophrenic patients from a tropical island in the Southern Hemisphere. Psychiatry Res. 1996;60(2–3):205–10. doi: 10.1016/0165-1781(96)02794-1. [DOI] [PubMed] [Google Scholar]
- Dadeech G, Mishra S, Gautam S, Sharma P. Evaluation of antioxidant deficit in schizophrenia. Indian J Psychiatry. 2008;50:16–20. doi: 10.4103/0019-5545.39753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakhale G, Khanzode S, Khanzode A, Saoji A, Khobragade L, Turankar A. Oxidative damage and schizophrenia: the potential benefit by atypical antipsychotics. Neuropsychobiology. 2004;49:205–9. doi: 10.1159/000077368. [DOI] [PubMed] [Google Scholar]
- Davies G, Welham J, Chant D, Torrey EF, McGrath J. A systematic review and meta-analysis of Northern Hemisphere season of birth studies in schizophrenia. Schizophr Bull. 2003;29(3):587–93. doi: 10.1093/oxfordjournals.schbul.a007030. [DOI] [PubMed] [Google Scholar]
- de Messias EL, Cordeiro NF, Sampaio JJ, Bartko JJ, Kirkpatrick B. Schizophrenia and season of birth in a tropical region: relationship to rainfall. Schizophr Res. 2001;48(2–3):227–34. doi: 10.1016/s0920-9964(00)00058-x. [DOI] [PubMed] [Google Scholar]
- de Messias E, Mourao C, Maia J, Campos JP, Ribeiro K, Ribeiro L, et al. Season of birth and schizophrenia in Northeast Brazil: relationship to rainfall. J Nerv Ment Dis. 2006;194(11):870–3. doi: 10.1097/01.nmd.0000243762.63694.e6. [DOI] [PubMed] [Google Scholar]
- De Miranda J, Yaddanapudi K, Hornig M, Villar G, Serge R, Lipkin WI. Induction of toll-like receptor 3-mediated immunity during gestation inhibits cortical neurogenesis and causes behavioral disturbances. MBio. 2010;1(4):e00176–10. doi: 10.1128/mBio.00176-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dealberto MJ. Ethnic origin and increased risk for schizophrenia in immigrants to countries of recent and longstanding immigration. Acta Psychiatr Scand. 2010;121(5):325–39. doi: 10.1111/j.1600-0447.2009.01535.x. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Kirkpatrick B, Boronow J, Stallings C, Origoni A, Yolken R. Deficit schizophrenia: association with serum antibodies to cytomegalovirus. Schizophr Bull. 2006;32(2):396–400. doi: 10.1093/schbul/sbi054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzyzga L, Obuchowicz E, Marcinowska A, Herman ZS. Cytokines in schizophrenia and the effects of antipsychotic drugs. Brain Behav Immun. 2006;20:532–45. doi: 10.1016/j.bbi.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Du X, Fleiss B, Li H, D’angelo B, Sun Y, Zhu C, et al. Systemic stimulation of TLR2 impairs neonatal mouse brain development. PLoS One. 2011;6(5):e19583. doi: 10.1371/journal.pone.0019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton WW, Chen CY. Epidemiology. In: Lieberman JA, Stroup TS, Perkins DO, editors. The American psychiatric publishing textbook of schizophrenia. Washington DC: American Psychiatric Publishing Inc; 2006. pp. 17–38. [Google Scholar]
- Eastwood SL, Law AJ, Everall IP, Harrison PJ. The axonal chemorepellant semaphoring 3A is increased in the cerebellum in schizophrenia and may contribute to its synaptic pathology. Mol Psychiatry. 2003;8(2):148–55. doi: 10.1038/sj.mp.4001233. [DOI] [PubMed] [Google Scholar]
- Ellman LM, Deicken RF, Vinogradov S, Kremen WS, Poole JH, Kern DM, et al. Structural brain alterations in schizophrenia following fetal exposure to the inflammatory cytokine interleukin-8. Schizophr Res. 2010;121(1–3):46–54. doi: 10.1016/j.schres.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovitz MA, Brown AG, Breen K, Anton L, Maubert M, Burd I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci. 2011;29(6):663–71. doi: 10.1016/j.ijdevneu.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH. Prenatal viral infection, brain development, and schizophrenia. In: Fatemi SH, editor. Neuropsychiatric disorders and infection. London: Taylor & Francis; 2005a. pp. 107–30. [Google Scholar]
- Fatemi SH. Reelin glycoprotein in autism and schizophrenia. Int Rev Neurobiol. 2005b;71:179–87. doi: 10.1016/s0074-7742(05)71008-4. [DOI] [PubMed] [Google Scholar]
- Fatemi SH. Multiple pathways in prevention of immune-mediated brain disorders: implications for the prevention of autism. J Neuroimmunol. 2009;217(1–2):8–9. doi: 10.1016/j.jneuroim.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Sidwell R, Kist D, Akhter P, Meltzer HY, Bailey K, et al. Differential expression of synaptosome-associated protein 25 kDa [SNAP-25] in hippocampi of neonatal mice following exposure to human influenza virus in utero. Brain Res. 1998;800(1):1–9. doi: 10.1016/s0006-8993(98)00450-8. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, et al. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry. 1999;4(2):145–54. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Cuadra AE, El-Fakahany EE, Sidwell RW, Thuras P. Prenatal viral infection causes alterations in nNOS expression in developing mouse brains. Neuroreport. 2000;11(7):1493–6. [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Sidwell RW, Kist DA, Stary JM, Earle JA, et al. Human influenza viral infection in utero alters glial fibrillary acidic protein immunoreactivity in the developing brains of neonatal mice. Mol Psychiatry. 2002a;7(6):633–40. doi: 10.1038/sj.mp.4001046. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, et al. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002b;22(1):25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Pearce DA, Brooks AI, Sidwell RW. Prenatal viral infection in mouse causes differential expression of genes in brains of mouse progeny: a potential animal model for schizophrenia and autism. Synapse. 2005;57:91–9. doi: 10.1002/syn.20162. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Huang H, Oishi K, Mori S, et al. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophr Res. 2008a;99(1–3):56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Sidwell RW. Viral regulation of aquaporin 4, connexin 43, microcephalin and nucleolin. Schizophr Res. 2008b;98(1–3):163–77. doi: 10.1016/j.schres.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Sidwell RW. The role of cerebellar genes in pathology of autism and schizophrenia. Cerebellum. 2008c;7(3):279–94. doi: 10.1007/s12311-008-0017-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Abu-Odeh D, Mori S, Huang H, et al. Abnormal expression of myelination genes and alterations in white matter fractional anisotropy following prenatal viral influenza infection at E16 in mice. Schizophr Res. 2009a;112(1–3):46–53. doi: 10.1016/j.schres.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Huang H, Oishi I, Mori S. Prenatal viral infection of mice at E16 causes changes in gene expression in hippocampi of the offspring. Eur Neuropsychopharmacol. 2009b;19(9):648–53. doi: 10.1016/j.euroneuro.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Rooney RJ, Mori S, Kornfield TE, Reutiman TJ, Kneeland RE, Liesch SB, Hua K, Hsu J, Patel DH. The viral theory of schizophrenia revisted: Abnormal placental gene expression and structural changes with lack of evidence for H1N1 viral presence in placentae of infected mice or brains of exposed offspring. Neuropharmacology. 2012;62(3):1290–8. doi: 10.1016/j.neuropharm.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2004;287:R759–66. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- Fortier M, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–7. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Gallagher BJ, III, Jones BJ, McFalls JA, Jr, Pisa AM. Schizophrenic subtype, seasonality of birth and social class: a preliminary analysis. Eur Psychiatry. 2007;22(2):123–8. doi: 10.1016/j.eurpsy.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Garcia-Roman J, Ibarra-Sanchez A, Lamas M, Gonzalez Espinosa C. VEGF secretion during hypoxia depends on free radicals-induced Fyn kinase activity in mast cells. Biochem Biophys Res Commun. 2010;401(2):262–7. doi: 10.1016/j.bbrc.2010.09.047. [DOI] [PubMed] [Google Scholar]
- Garver DL, Tamas RL, Holcomb JA. Elevated interleukin-6 in the cerebrospinal fluid of a previously delineated schizophrenia subtype. Neuropsychopharmacology. 2003;28(8):1515–20. doi: 10.1038/sj.npp.1300217. [DOI] [PubMed] [Google Scholar]
- Grabs D, Bergmann M, Post A, Gratzl M. Rab3 proteins and SNAP-25, essential components of the exocytosis machinery in conventional synapses, are absent from ribbons synapses of the mouse retina. Eur J Neurol. 1996;8:162–8. doi: 10.1111/j.1460-9568.1996.tb01177.x. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Li W, Rodriguez ML. Role of microglia in CNS inflammation. FEBS Lett. 2011;585(23):3798–805. doi: 10.1016/j.febslet.2011.08.033. [DOI] [PubMed] [Google Scholar]
- Gu J, Xie Z, Gao Z, Liu J, Korteweg C, Ye J, et al. H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet. 2007;370(9593):1137–45. doi: 10.1016/S0140-6736(07)61515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Zhang GF, Li LY, Zhou F, Feng DJ, Ding CL, et al. Human herpesvirus 6A induces apoptosis of primary human fetal astrocytes via both caspase-dependent and – independent pathways. Virology. 2011;8(1):530. doi: 10.1186/1743-422X-8-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, Guimaraes-Sternberg C, Kessler O, Neufeld G. Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem. 2007;282(36):26294–305. doi: 10.1074/jbc.M609711200. [DOI] [PubMed] [Google Scholar]
- Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. 2004;72(4):2272–9. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao LY, Hao XQ, Li SH, Li XH. Prenatal exposure to lipopolysaccharide results in cognitive deficits in age-increasing offspring rats. Neuroscience. 2010;166(3):763–70. doi: 10.1016/j.neuroscience.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Katsel P, Dracheva S, Davis KL. The human homolog of the QKI gene affected in the severe dysmyelination “quaking” mouse phenotype: downregulated in multiple brain regions in schizophrenia. Am J Psychiatry. 2006;163(10):1834–7. doi: 10.1176/ajp.2006.163.10.1834. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Microglial activation in schizophrenia and minocycline treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(7):1758–9. doi: 10.1016/j.pnpbp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the US Preventative Services Task Force. Ann Intern Med. 2002;136(2):161–72. doi: 10.7326/0003-4819-136-2-200201150-00016. [DOI] [PubMed] [Google Scholar]
- Henriksen T, Clausen T. The fetal origins hypothesis: placental insuficiency and inheritance versus maternal malnutrition in well-nourished populations. Acta Obstet Gynecol Scand. 2002;81:112–4. doi: 10.1034/j.1600-0412.2002.810204.x. [DOI] [PubMed] [Google Scholar]
- Himmerich H, Schonherr J, Fulda S, Sheldrick AJ, Bauer K, Sack U. Impact of antipsychotics on cytokine production in-vitro. J Psychiatr Res. 2011;45(10):1358–65. doi: 10.1016/j.jpsychires.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25:604–15. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi D, Nagai T, Kitahara Y, Mizoguchi H, Koike H, Shiraki A, et al. Neonatal polyI:C treatment in mice results in schizophrenia-like behavioral and neurochemical abnormalities in adulthood. Neurosci Res. 2009;64(3):297–305. doi: 10.1016/j.neures.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Selinger ES, Lieberman JA, Gilmore JH. Apoptotic proteins in the temporal cortex in schizophrenia: high Bax/Bcl-2 ratio without caspase-3 activation. Am J Psychiatry. 2004;161(1):109–15. doi: 10.1176/appi.ajp.161.1.109. [DOI] [PubMed] [Google Scholar]
- Jordaan E, Niehaus DJ, Koen L, Seller C, Mbanga I, Emsley RA. Season of birth, age and negative symptoms in Xhosa schizophrenia sample from the Southern Hemisphere. Aust N Z J Psychiatry. 2006;40(8):698–703. doi: 10.1080/j.1440-1614.2006.01870.x. [DOI] [PubMed] [Google Scholar]
- Kanduc D. Describing the hexapeptide identity platform between the influenza A H5N1 and Homo sapiens proteomes. Biologics. 2010;4:245–61. doi: 10.2147/btt.s12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempuraj D, Asadi S, Zhang B, Manola A, Hogan J, Peterson E, et al. Mercury induces inflammatory mediator release from human mast cells. J Neuroinflammation. 2010;7:20. doi: 10.1186/1742-2094-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Kim L, Lee MS. Relationships between interleukins, neurotransmitters, and psychopathology in drug-free male schizophrenics. Schizophr Res. 2000;44:165–75. doi: 10.1016/s0920-9964(99)00171-1. [DOI] [PubMed] [Google Scholar]
- Kim YK, Myint AM, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Cytokine changes and tryptophan metabolites in medication-naïve and medication-free schizophrenic patients. Neuropsychobiology. 2009;59(2):123–9. doi: 10.1159/000213565. [DOI] [PubMed] [Google Scholar]
- Kirsten TB, Taricano M, Florio JC, Palermo-Neto J, Bernardi MM. Prenatal lipopolysaccharide reduces motor activity after an immune challenge in adult male offspring. Behav Brain Res. 2010;211(1):77–82. doi: 10.1016/j.bbr.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Krause D, Matz J, Weidinger E, Wagner J, Wildenauer A, Obermeier M, et al. The association of infectious agents and schizophrenia. World J Biol Psychiatry. 2010;11(5):739–43. doi: 10.3109/15622971003653246. [DOI] [PubMed] [Google Scholar]
- Kuloglu M, Ustundag B, Atmaca M, Canatan H, Tezcan AE, Cinkilinc N. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct. 2002;20:171–5. doi: 10.1002/cbf.940. [DOI] [PubMed] [Google Scholar]
- Kunz M, Gama CS, Andreazza AC, Salvador M, Cereser KM, Gomes FA, et al. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1677–81. doi: 10.1016/j.pnpbp.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H. Adjuvant asprin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71(5):520–7. doi: 10.4088/JCP.09m05117yel. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Reid MA. Is there evidence for neurotoxicity in the prodromal and early stages of schizophrenia? Neuropsychopharmacology. 2011;36:1779–80. doi: 10.1038/npp.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane HY, Liu YC, Huang CL, Chang YC, Wu PL, Huang CH, et al. RGS4 polymorphisms predict clinical manifestations and responses to risperidone treatment in patients with schizophrenia. J Clin Psychopharmacol. 2008;28(1):64–8. doi: 10.1097/jcp.0b013e3181603f5a. [DOI] [PubMed] [Google Scholar]
- Lesnikov V, Gorden N, Fausto N, Spaulding E, Campbell J, Shulman H, et al. Transferrin fails to provide protection against Fas-induced hepatic injury in mice with deletion of functional transferring-receptor type 2. Apoptosis. 2008;13(8):1005–12. doi: 10.1007/s10495-008-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin SG, Godukhin OV. Anti-inflammatory cytokines, TGF- β1 and IL-10, exert anti-hypoxic action and abolish posthypoxic hyperexcitability in hippocampal slice neurons: comparative aspects. Exp Neurol. 2011;232(2):329–32. doi: 10.1016/j.expneurol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Mendlovich S, Riwkes S, Braw Y, Levkovitch-Verbin H, Gal G, et al. A double-blind randomized study of minocycline treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2010;71(2):138–49. doi: 10.4088/JCP.08m04666yel. [DOI] [PubMed] [Google Scholar]
- Li Q, Cheung C, Wei R, Hui ES, Feldon J, Meyer U, et al. Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: evidence from MRI in a mouse model. PLoS One. 2009;4(7):e6354. doi: 10.1371/journal.pone.0006354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Kenis G, Bignotti S, Tura GJ, De Jong R, Bosmans E, et al. The inflammatory response system in treatment-resistant schizophrenia: increased serum interleukin-6. Schi-zophr Res. 1998;32:9–15. doi: 10.1016/s0920-9964(98)00034-6. [DOI] [PubMed] [Google Scholar]
- Liu A, Liu B, Qin H, MingYuen Lee S, Wang Y, Du G. Anti-influenza virus activities of flavinoids from the medicinal plant Elsholtzia rugulosa. Planta Med. 2008;74:847–51. doi: 10.1055/s-2008-1074558. [DOI] [PubMed] [Google Scholar]
- Liu L, Jia F, Yuan G, Chen Z, Yao J, Li H, Fang C. Tyrosine hydroxylase, interleukin-1beta and tumor necrosis factor-alpha are over expressed in peripheral blood mononuclear cells from schizophrenia patients as determined by semi-quantitative analysis. Psychiatry Res. 2010;176(1):1–7. doi: 10.1016/j.psychres.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Luheshi GN, Stefferl A, Turnbull AV, Dascombe MJ, Brouwer S, Hopkins SJ, et al. Febrile response to tissue inflammation involves both peripheral and brain IL-1 and TNF-alpha in the rat. Am J Physiol. 1997;272:R862–8. doi: 10.1152/ajpregu.1997.272.3.R862. [DOI] [PubMed] [Google Scholar]
- Ma Y, Krikun G, Abrahams VM, Mor G, Guller S. Cell type-specific expression and function of toll-like receptors 2 and 4 in human placenta: implications for fetal infection. Placenta. 2007;28:1024–31. doi: 10.1016/j.placenta.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan L, Correll CU. Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol. 2011;21(6):517–35. doi: 10.1089/cap.2011.0015. [DOI] [PubMed] [Google Scholar]
- Machón RA, Mednick SA, Schulsinger F. The interaction of seasonality, place of birth, genetic risk and subsequent schizophrenia in a high risk sample. Br J Psychiatry. 1983;143:383–8. doi: 10.1192/bjp.143.4.383. [DOI] [PubMed] [Google Scholar]
- Madje JA. Neuroinflammation resulting from covert brain invasion by common viruses—a potential role in local and global neurodegeneration. Med Hypotheses. 2010;75(2):204–13. doi: 10.1016/j.mehy.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Bosmans E. Immune-inflammatory markers in schizophrenia: comparison to normal controls and effects of clozapine. Acta Psychiatr Scand. 1994;89(5):346–51. doi: 10.1111/j.1600-0447.1994.tb01527.x. [DOI] [PubMed] [Google Scholar]
- Maes M, Bocchio Chiavetto L, Bignotti S, Battisa Tura G, Pioli R, Boin F, et al. Effects of atypical antipsychotics on the inflammatory response system in schizophrenic patients resistant to treatment with typical neuroleptics. Eur Neuropsychopharmacol. 2000;10(2):119–24. doi: 10.1016/s0924-977x(99)00062-0. [DOI] [PubMed] [Google Scholar]
- Maes M, Leonard B, Fernandez A, Kubera M, Nowak G, Veerhuis R, et al. (Neuro)inflammation and neuroprogression as new pathways and drug targets in depression: from antioxidants to kinase inhibitors. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):659–63. doi: 10.1016/j.pnpbp.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Malinger G, Werner H, Rodriguez Leonel JC, Rebolledo M, Buque M, Mizyrycki S, Lerman-Sagie T, Herrera M. Prenatal brain imaging in congenital toxoplasmosis. Prenat Diagn in press; [DOI] [PubMed] [Google Scholar]
- Marcelis M, Takei N, van Os J. Urbanization and risk for schizophrenia: does the effect operate before or around the time of illness onset. Psychol Med. 1999;29(5):1197–203. doi: 10.1017/s0033291799008983. [DOI] [PubMed] [Google Scholar]
- McAllister CG, van Kammen DP, Rehn TJ, Miller AL, Gurklis J, Kelley M, et al. Increases in CSF levels of interleukin-2 in schizophrenia: effects of recurrence of psychosis and medication status. Am J Psychiatry. 1995;152(9):1291–7. doi: 10.1176/ajp.152.9.1291. [DOI] [PubMed] [Google Scholar]
- McCullumsmith RE, Gupta D, Beneyto M, Kreger E, Haroutunian V, Davis KL, et al. Expression of transcripts for myelination-related genes in the anterior cingulated cortex in schizophrenia. Schizophr Res. 2007;90(1–3):15–27. doi: 10.1016/j.schres.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. Hypothesis: is low prenatal vitamin D a risk-modifying factor for schizophrenia? Schizophr Res. 1999;40(3):173–7. doi: 10.1016/s0920-9964(99)00052-3. [DOI] [PubMed] [Google Scholar]
- McGrath JJ. Myths and plain truths about schizophrenia epidemiology—the NAPE lecture 2004. Acta Psychiatry Scand. 2005;111(1):4–11. doi: 10.1111/j.1600-0447.2004.00467.x. [DOI] [PubMed] [Google Scholar]
- Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45(2):189–92. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Fatemi SH. Schizophrenia and other psychotic disorders. In: Ebert MH, Loosen PT, Nurcombe B, editors. Current diagnosis and treatment in psychiatry. Norwalk, CT: Appleton and Lange; 2000. pp. 260–77. [Google Scholar]
- Meyer U. Anti-inflammatory signaling in schizophrenia. Brain Behav Immun. 2011;25:1507–18. doi: 10.1016/j.bbi.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Meyer U. Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:20–34. doi: 10.1016/j.pnpbp.2011.11.003. this issue. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29(6):913–47. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006a;26(18):4752–62. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal–foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immunol. 2006b;20(4):378–88. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry. 2008;13:208–21. doi: 10.1038/sj.mp.4002042. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009;33(7):1061–79. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Meyer U, Knuesel I, Nyffeler M, Feldon J. Chronic clozapine treatment improves prenatal infection-induced working memory deficits without influencing adult hippocampal neurogenesis. Psychopharmacology (Berl) 2010;208(4):531–43. doi: 10.1007/s00213-009-1754-6. [DOI] [PubMed] [Google Scholar]
- Meyer U, Schwarz MJ, Muller N. Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther. 2011;132(1):96–110. doi: 10.1016/j.pharmthera.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:633–71. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaoka T, Yasukawa R, Yasuda H, Hayashida M, Inagaki T, Horiguchi J. Possible anti-psychotic effects of minocycline in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):304–7. doi: 10.1016/j.pnpbp.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Moreno JL, Kurita M, Holloway T, Lopez J, Cadagan R, Martinez-Sobrido L, et al. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HT2A and mGlu2 receptors in the adult offspring. J Neurosci. 2011;31(5):1863–72. doi: 10.1523/JNEUROSCI.4230-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan VA, Jablensky AV, Castle DJ. Season of birth in schizophrenia and affective psychoses in Western Australia 1916-61. Acta Psychiatr Scand. 2001;104(2):138–47. doi: 10.1034/j.1600-0447.2001.00188.x. [DOI] [PubMed] [Google Scholar]
- Mortensen PB, Norgaard-Pedersen B, Waltoft BL, Sorensen TL, Hougaard D, Torrey EF, et al. Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biol Psychiatry. 2007;61(5):688–93. doi: 10.1016/j.biopsych.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Muller N. COX-2 inhibitors as antidepressants and antipsychotics: clinical evidence. Curr Opin Investig Drugs. 2010;11(1):31–42. [PubMed] [Google Scholar]
- Muller N, Empl M, Riedel M, Schwarz M, Ackenheil M. Neuroleptic treatment increases soluble IL-2 receptors and decreases soluble IL-6 receptors in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1997;247(6):308–13. doi: 10.1007/BF02922260. [DOI] [PubMed] [Google Scholar]
- Muller N, Riedel M, Scheppach C, Brandstatter B, Sokullu S, Krampe K, et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159(6):1029–34. doi: 10.1176/appi.ajp.159.6.1029. [DOI] [PubMed] [Google Scholar]
- Nagai T, Moriguchi R, Suzuki Y, Tomimori T, Yamada H. Mode of action of the anti-influenza virus activity of plant flavinoid, 5,7,4′trihydroxy-8-methoxyflavone, from the roots of Scutellaria baicalensis. Antiviral Res. 1995;26:11–25. doi: 10.1016/0166-3542(94)00062-d. [DOI] [PubMed] [Google Scholar]
- Nahmias AJ, Nahmias SB, Danielsson D. The possible role of transplacentally-acquired antibodies to infectious agents, with molecular mimicry to nervous system sialic acid epitopes, as causes of neuromental disorders: prevention and vaccine implications. Clin Dev Immunol. 2006;3(2–4):167–83. doi: 10.1080/17402520600801745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niizuma K, Endo H, Chan PH. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J Neurochem. 2009;109(Suppl 1):133–8. doi: 10.1111/j.1471-4159.2009.05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien SM, Scully P, Dinan TG. Increased tumor necrosis factor-alpha concentrations with interleukin-4 concentrations in exacerbations of schizophrenia. Psychiatry Res. 2008;160(3):256–62. doi: 10.1016/j.psychres.2007.11.014. [DOI] [PubMed] [Google Scholar]
- O’Callaghan E, Sham P, Takei N, Glover G, Murray RM. Schizophrenia after prenatal exposure to 1957 A2 influenza epidemic. Lancet. 1991;337(8752):1248–50. doi: 10.1016/0140-6736(91)92919-s. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Riche EL, Barros SP, Bobetsis YA, Lin D, Beck JD. Effects of maternal Campylobacter rectus infection on murine placenta, fetal and neonatal survival, and brain development. J Periodontol. 2005;76:2133–43. doi: 10.1902/jop.2005.76.11-S.2133. [DOI] [PubMed] [Google Scholar]
- Ohnuma T, Kato H, Arai H, McKenna PJ, Emson PC. Expression of Fyn, a non-receptor tyrosine kinase in prefrontal cortex from patients with schizophrenia and its correlation with clinical onset. Brain Res Mol Brain Res. 2003;112(1–2):90–4. doi: 10.1016/s0169-328x(03)00051-2. [DOI] [PubMed] [Google Scholar]
- Osen-Sand A, Catsicas M, Staple JK, Jones KA, Ayala G, Knowles J, et al. Inhibition of axonal growth by SNAP-25 antisense oligonucleotides in vitro and in vivo. Nature. 1993;364:445–8. doi: 10.1038/364445a0. [DOI] [PubMed] [Google Scholar]
- Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom F, et al. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989;109:3039–52. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallast EG, Jongbloet PH, Straatman HM, Zielhuis GA. Excess seasonality of births among patients with schizophrenia and seasonal ovopathy. Schizophr Bull. 1994;20(2):269–76. doi: 10.1093/schbul/20.2.269. [DOI] [PubMed] [Google Scholar]
- Papiol S, Molina V, Desco M, Rosa A, Reig S, Gispert JD, et al. Ventricular enlargement in schizophrenia is associated with a genetic polymorphism at the interleukin-1 receptor agonist gene. Neuroimage. 2005;27:1002–6. doi: 10.1016/j.neuroimage.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Pardi G, Cetin I. Human fetal growth and organ development: 50 years of discoveries. Am J Obstet Gynecol. 2006;194:1088–99. doi: 10.1016/j.ajog.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Parker-Athill E, Luo D, Bailey A, Giunta B, Tian J, Shytle RD, et al. Flavonoids, a prenatal prophylaxis via targeting JAK2/STAT3 signaling to oppose IL-6/MIA associated autism. J Neuroimmunol. 2009;217(1–2):20–7. doi: 10.1016/j.jneuroim.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH. Maternal effects on schizophrenia risk. Science. 2007;318(5850):576–7. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Brain–immune connections, stress, and depression. In: Patterson PH, editor. Brain–immune connections in autism, schizophrenia, and depression. Boston: MIT Press; 2011. pp. 10–28. [Google Scholar]
- Pedersen MG, Stevens H, Pedersen CB, Norgaard-Pedersen B, Mortensen PB. Toxoplasma infection and later development of schizophrenia in mothers. Am J Psychiatry. 2011;168:814–21. doi: 10.1176/appi.ajp.2011.10091351. [DOI] [PubMed] [Google Scholar]
- Perry W, Geyer MA, Braff DL. Sensorimotor gating and thought disturbance measured in close temporal proximity in schizophrenic patients. Arch Gen Psychiatry. 1999;56(3):277–81. doi: 10.1001/archpsyc.56.3.277. [DOI] [PubMed] [Google Scholar]
- Pilotte J, Larocque D, Richard S. Nuclear translocation controlled by alternatively spliced isoforms inactivates the QUAKING apoptotic inducer. Genes Dev. 2001;15(7):845–58. doi: 10.1101/gad.860301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Tracing the development of psychosis and its prevention: what can be learned from animal models. Neuropharmacology. 2012;62(3):1273–89. doi: 10.1016/j.neuropharm.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Assaf Y, Weiner I. Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biol Psychiatry. 2009;66(11):1038–46. doi: 10.1016/j.biopsych.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Risperidone administered during asymptomatic period of adolescence prevents the emergence of brain structural pathology and behavioral abnormalities in an animal model of schizophrenia. Schizophr Bull. 2010;37(6):1257–69. doi: 10.1093/schbul/sbq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Abnormal trajectories of neurodevelopment and behavior following in utero insult in the rat. Biol Psychiatry. 2011;70(9):842–51. doi: 10.1016/j.biopsych.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Pollmacher T, Hinze-Selch D, Mullington J. Effects of clozapine on plasma cytokine and soluble cytokine receptor levels. J Clin Psychopharmacol. 1996;16:403–9. doi: 10.1097/00004714-199610000-00011. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, van Huffel C, Ku X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in the Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Eack SM, Goradia D, Pancholi KM, Keshavan MS, Yolken RH, et al. Progressive gray matter loss and changes in cognitive functioning associated with exposure to herpes simplex virus 1 in schizophrenia: a longitudinal study. Am J Psychiatry. 2011;168:822–30. doi: 10.1176/appi.ajp.2011.10101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsheim T, Lam D, Ching H, Patten S. Metabolic and neurological complications of second-generation anti-psychotic use in children: a systematic review and meta-analysis of randomized controlled trials. Drug Saf. 2011;34(8):651–68. doi: 10.2165/11592020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Qu M, Yue W, Tang F, Wang L, Han Y, Zhang D. Polymorphisms of transferrin gene are associated with schizophrenia in Chinese Han population. J Psychiatr Res. 2008;42(11):877–83. doi: 10.1016/j.jpsychires.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Quattrocchi CC, Wannenes F, Persico AM, Ciafre SA, D’Arcangelo G, Farace MG, et al. Reelin is a serine protease of the extracellular matrix. J Biol Chem. 2002;277:303–9. doi: 10.1074/jbc.M106996200. [DOI] [PubMed] [Google Scholar]
- Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, et al. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–25. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa M, Mechri A, Othman LB, Fendri C, Gaha L, Kerkeni A. Decreased glutathione levels and antioxidant activities in untreated and treated schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1178–83. doi: 10.1016/j.pnpbp.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Rao S, Lin Z, Drobyshevsky A, Chen L, Ji X, Ji H, et al. Involvement of neuronal nitric oxide synthase in ongoing fetal brain injury following near-term rabbit hypoxia–ischemia. Dev Neurosci. 2011;33(3–4):288–98. doi: 10.1159/000327241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethelyi JM, Bakker SC, Polgar P, Czobor P, Strengman E, Pasztor PI, et al. Association study of NRG1, DTNBP1, RGS4, G72/G30, and PIP5K2A with schizophrenia and symptom severity in a Hungarian sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(3):792–801. doi: 10.1002/ajmg.b.31049. [DOI] [PubMed] [Google Scholar]
- Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–42. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenker NL, Gudelsky G, Ahlbrand R, Bronson SL, Kern JR, Waterman H, et al. Effect of paliperidone and risperidone on extracellular glutamate in the prefrontal cortex of rats exposed to prenatal immune activation or MK-801. Neurosci Lett. 2011;500(3):167–71. doi: 10.1016/j.neulet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SR. Viral pathogenesis and toll-like receptors. In: Palese P, editor. Modulation of host gene expression and innate immunity by viruses. The Netherlands: Springer; 2005. [Google Scholar]
- Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, Way SS. Foxp3+ regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe. 2011;10(1):54–64. doi: 10.1016/j.chom.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Skibinska M, Hauser J. Polymorphisms of the Fyn kinase gene and a performance on the Wisconsin Card Sorting Test in schizophrenia. Psychiatr Genet. 2007;17(3):201–4. doi: 10.1097/YPG.0b013e3280991219. [DOI] [PubMed] [Google Scholar]
- Saminathan H, Asaithambi A, Anantharam V, Kanthasamy AG, Kanthasamy A. Environmental neurotoxic pesticide dieldrin activates a non receptor tyrosine kinase to promote pkcδ-mediated dopaminergic apoptosis in a dopaminergic neuronal cell model. Neurotoxicology. 2011;32(5):567–77. doi: 10.1016/j.neuro.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J, Souriau A, Buendia AJ, Arricau-Bouvery N, Martinez CM, Salinas J, et al. Experimental Coxiella burnetii infection in pregnant goats: a histopathological and immunohistochemical study. J Comp Pathol. 2006;135(2–3):108–15. doi: 10.1016/j.jcpa.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Sargent IL. Maternal and fetal immune responses during pregnancy. Exp Clin Immunogenet. 1993;10(2):85–102. [PubMed] [Google Scholar]
- Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem Res Toxicol. 2008;21(1):172–88. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, van Os J, Steinbusch HWM, Schmitz C. Gene regulation by hypoxia and the neurodevelopmental origin of schizophrenia. Schizophr Res. 2006;84(2–3):253–71. doi: 10.1016/j.schres.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Selten JP, Cantor-Graae E, Kahn RS. Migration and schizophrenia. Curr Opin Psychiatry. 2007;20(2):111–5. doi: 10.1097/YCO.0b013e328017f68e. [DOI] [PubMed] [Google Scholar]
- Serrano GE, Lelutiu N, Rojas A, Cochi S, Shaw R, Makinson CD, et al. Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J Neurosci. 2011;31(42):14850–60. doi: 10.1523/JNEUROSCI.3922-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh N, Tron K, Dudas J, Ramadori G. Cytokine-induced neutrophils chemoattractant-1 is released by the noninjured liver in a rat acute-phase model. Lab Invest. 2006;86:800–14. doi: 10.1038/labinvest.3700435. [DOI] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23(1):116–23. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirts BH, Prasad KM, Pogue-Geile MF, Dickerson F, Yolken RH, Nimgaonkar VL. Antibodies to cytomegalovirus and Herpes Simplex Virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106(2–3):268–74. doi: 10.1016/j.schres.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short SJ, Lubach GR, Karasin AI, Olsen CW, Styner M, Knickmeyer RC, et al. Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiatry. 2010;67(10):965–73. doi: 10.1016/j.biopsych.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh OP, Chakraborty I, Dasgupta A, Datta S. A comparative study of oxidative stress and interrelationship of important antioxidants in haloperidol and olanxapine patients suffering from schizophrenia. Indian J Psychiatry. 2008;50:171–6. doi: 10.4103/0019-5545.43627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27(40):10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D, Phan V, Wang A, McKay DM. Interferon-γ-induced increases in intestinal epithelial macromolecular permeability requires the Src kinase Fyn. Lab Invest. 2011;91(5):764–77. doi: 10.1038/labinvest.2010.208. [DOI] [PubMed] [Google Scholar]
- Soderlund J, Schroder J, Nordin C, Samuelsson M, Walther-Jallow L, Karlsson H, et al. Activation of brain interleukin-1beta in schizophrenia. Mol Psychiatry. 2009;14:1069–71. doi: 10.1038/mp.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solana ME, Alba Soto CD, Fernandez MC, Poncini CV, Postan M, Gonzalez Cappa SM. Reduction of parasite levels in blood improves pregnancy outcome during experimental Trypanosoma cruzi infection. Parasitology. 2009;136:627–39. doi: 10.1017/S0031182009005770. [DOI] [PubMed] [Google Scholar]
- Solomon SD, McMurray JJV, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352(11):1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Pfeffer MA, McMurray JJV, Fowler R, Finn P, Levin B, et al. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation. 2006;114(10):1028–35. doi: 10.1161/CIRCULATIONAHA.106.636746. [DOI] [PubMed] [Google Scholar]
- Song BM, Kang YM, Kim HS, Seo SH. Induction of inflammatory cytokines and toll-like receptors in human normal respiratory epithelial cells infected with seasonal H1N1, 2009 pandemic H1N1, seasonal H3N2, and highly pathogenic H5N1 influenza virus. Viral Immunol. 2011;24(3):179–87. doi: 10.1089/vim.2010.0125. [DOI] [PubMed] [Google Scholar]
- Sorensen HJ, Mortensen EL, Reinisch JM, Mednick SA. Association between prenatal exposure to bacterial infection and risk of schizophrenia. Schizophr Bull. 2009;35(3):631–7. doi: 10.1093/schbul/sbn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria JA, Arroyo DS, Gaviglio EA, Rodriguez-Galan MC, Wang JM, et al. Interleukin 4 induces the apoptosis of mouse microglial cells by a caspase-dependent mechanism. Neurobiol Dis. 2011;43(3):616–24. doi: 10.1016/j.nbd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumiya H, Fukumitsu H, Furukawa S. Prenatal immune challenge compromises the normal course of neurogenesis during development of the mouse cerebral cortex. J Neurosci Res. 2011;89(10):1575–85. doi: 10.1002/jnr.22704. [DOI] [PubMed] [Google Scholar]
- Srivastava N, Barthwal MK, Dalal PK, Agarwal AK, Nag D, Srimal RC, et al. Nitrite content and antioxidant enzyme levels in the blood of schizophrenia patients. Psychopharmacology (Berl) 2001;158:140–5. doi: 10.1007/s002130100860. [DOI] [PubMed] [Google Scholar]
- Suvisaari J, Saarni SE, Haukka J, Perala J, Saarni SI, Viertio S, et al. Inflammation in psychotic disorders: a population-based study. Psychiatry Res. 2011;189(2):305–11. doi: 10.1016/j.psychres.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Syme GJ, Illingworth DJ. Sex differences in birth patterns of schizophrenics. J Clin Psychol. 1978;34(3):633–5. doi: 10.1002/1097-4679(197807)34:3<633::aid-jclp2270340310>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Rawlings RR, Ennis JM, Merrill DD, Flores DS. Birth seasonality in bipolar disorder, schizophrenia, schizoaffective disorder, and stillbirths. Schizophr Res. 1996;21(3):141–9. doi: 10.1016/0920-9964(96)00022-9. [DOI] [PubMed] [Google Scholar]
- Tron K, Novosyadlyy R, Dudas J, Samoylenko A, Kietzmann T, Ramadori G. Upregulation of heme oxygenase-1 gene by turpentine oil-induced inflammation: involvement of interleukin-6. Lab Invest. 2005;85(376):387. doi: 10.1038/labinvest.3700228. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Prehar S, Kennedy AR, Little RA, Hopkins SJ. Interleukin-6 is an afferent signal to the hypothalamo-pituitary-adrenal axis during local inflammation in mice. Endocrinology. 2003;144:1894–906. doi: 10.1210/en.2002-220964. [DOI] [PubMed] [Google Scholar]
- Uchide N, Suzuki A, Ohyama K, Bessho T, Toyoda H. Secretion of bioactive interleukin-6 and tumor necrosis factor-α proteins from primary cultured human fetal membrane chorion cells infected with influenza virus. Placenta. 2006;27:678–90. doi: 10.1016/j.placenta.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Vartanian T, Li Y, Zhao M, Steffansson K. Interferon-γ-induced oligodendrocyte cell death: implications for the pathogenesis of multiple sclerosis. Mol Med. 1995;1:732–43. [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Lett TA, Lerch JP, Tiwari AK, Ameis SH, Rajji TK, et al. Neurexin-1 and frontal lobe white matter: an overlapping intermediate phenotype for schizophrenia and autism spectrum disorders. PLoS One. 2011;6(6):e20982. doi: 10.1371/journal.pone.0020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace K, Veerisetty S, May W, Miquel-Hidalgo JJ, Bennett W. Prenatal infection decreases calbindin, decreases Purkinje cell volume and density and produces long-term motor deficits in Sprague–Dawley rats. Dev Neurosci. 2010;32(4):302–12. doi: 10.1159/000319506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jia W, Zhao A, Wang X. Anti-influenza agents from plants and traditional Chinese medicine. Phytother Res. 2006;20:335–41. doi: 10.1002/ptr.1892. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Tatematsu M, Saeki K, Shibata S, Shime H, Yoshimura A, et al. Raftlin is involved in the nucleocapture complex to induce poly(I:C)-mediated TLR3 activation. J Biol Chem. 2011;286(12):10702–11. doi: 10.1074/jbc.M110.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ED. Pregnancy-associated depression of cell-mediated immunity. Rev Infect Dis. 1984;6(6):814–31. doi: 10.1093/clinids/6.6.814. [DOI] [PubMed] [Google Scholar]
- Wen X, Kudo T, Payne L, Wang X, Rodgers L, Suzuki Y. Predominant interferon-γ-mediated expression of CXCL9, CXCL10, and CCL5 proteins in the brain during chronic infection with Toxoplasma gondii in BALB/c mice resistant to development of toxoplasmic encephalitis. J Interferon Cytokine Res. 2010;30(9):653–60. doi: 10.1089/jir.2009.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette AA, Lubach GR, Knickmeyer RC, Short SJ, Styner M, Gilmore JH, et al. Brain enlargement and increased behavioral and cytokine reactivity in infant monkeys following acute prenatal endotoxemia. Behav Brain Res. 2011;219(1):108–15. doi: 10.1016/j.bbr.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, McAuley JB. Toxoplasma. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of clinical micro-biology. Washington, DC: American Society for Microbiology; 1999. pp. 1374–82. [Google Scholar]
- Winter C, Reutiman TJ, Folsom TD, Sohr R, Wolf RJ, Juckel G, et al. Dopamine and serotonin levels following prenatal viral infection in mouse—implications for psychiatric disorders such as schizophrenia and autism. Eur Neuropsychopharmacol. 2008;18(10):712–6. doi: 10.1016/j.euroneuro.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C, Djodari-Irani A, Sohr R, Morgenstern R, Feldon J, Juckel G, et al. Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int J Neuropsychopharmacol. 2009;12(4):513–24. doi: 10.1017/S1461145708009206. [DOI] [PubMed] [Google Scholar]
- Wolff AR, Bilkey DK. Immune activation during mid-gestation disrupts sensorimotor gating in rat offspring. Behav Brain Res. 2008;190(1):156–9. doi: 10.1016/j.bbr.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Yucel M, Pantelis C, Berk M. Neurobiology of schizophrenia spectrum disorders: the role of oxidative stress. Ann Acad Med Singapore. 2009;38(5):396–406. [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Xu M, He L. Convergent evidence shows a positive association of interleukin-1 gene complex locus with susceptibility to schizophrenia in the Caucasian population. Schizophr Res. 2010;120:131–42. doi: 10.1016/j.schres.2010.02.1031. [DOI] [PubMed] [Google Scholar]
- Yogev H, Hadar U, Gutman Y, Sirota P. Perseveration and over-switching in schizophrenia. Schizophr Res. 2003;61(2–3):315–21. doi: 10.1016/s0920-9964(02)00323-7. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF, Lieberman JA, Yang S, Dickerson FB. Serological evidence of exposure to Herpes Simplex Virus type 1 is associated with cognitive deficits in the CATIE schizophrenia sample. Schizophr Res. 2011;128(1–3):61–5. doi: 10.1016/j.schres.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Cau LY, Zhang PY, Wu GY, Shen YC. Changes in serum interleukin-2, -6, and — 8 levels before and during treatment with risperidone and haloperidol: relationship to outcome in schizophrenia. J Clin Psychiatry. 2004;65:940–7. doi: 10.4088/jcp.v65n0710. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cazakoff BN, Thai CA, Howland JG. Prenatal exposure to a viral mimetic alters behavioural flexibility in male, but not female, rats. Neuropharmacology. 2012;62(3):1299–307. doi: 10.1016/j.neuropharm.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39(3):311–23. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–89. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]


