Abstract
Despite growing evidence on the neural bases of emotion regulation, little is known about the mechanisms underlying individual differences in cognitive regulation of negative emotion, and few studies have used objective measures to quantify regulatory success. Using a trait-like psychophysiological measure of emotion regulation, corrugator electromyography, we obtained an objective index of the ability to cognitively reappraise negative emotion in 56 healthy men (session 1), who returned 1.3 years later to perform the same regulation task using fMRI (session 2). Results indicated that the corrugator measure of regulatory skill predicted amygdala-prefrontal functional connectivity. Individuals with greater ability to down-regulate negative emotion as indexed by corrugator at session 1 showed not only greater amygdala attenuation but also greater inverse connectivity between the amygdala and several sectors of the prefrontal cortex while down-regulating negative emotion at session 2. Our results demonstrate that individual differences in emotion regulation are stable over time and underscore the important role of amygdala-prefrontal coupling for successful regulation of negative emotion.
Keywords: emotion regulation, individual differences, corrugator electromyography, amygdala, PFC, functional connectivity
Introduction
The ability to regulate emotion according to one’s goals is a critical skill for psychological well-being and resilience. Among various forms of regulation, cognitive regulation of emotion using reappraisal as a strategy has received much scientific attention (Ochsner and Gross, 2005). Reappraisal involves reinterpreting the meaning of an emotional event; for example, creating an alternative scenario or adopting a different attitude (Gross, 2002; Ochsner et al., 2004). It is the basis of cognitive therapy (Frewen et al., 2008), has been found to be more beneficial than suppressing emotions (Ochsner et al., 2002), can be instructed or trained (Jackson et al., 2000), and varies widely across individuals (Gross and John, 2003).
Over the past decade, neuroimaging studies of reappraisal have revealed converging evidence that reappraisal engages sectors of the prefrontal cortex (PFC) and subcortical structures such as the amygdala (for a meta-analysis, see Kalisch, 2009). While the amygdala detects the significance of potentially emotion-eliciting situations and generates biobehavioral adjustments associated with that emotion (Phelps and LeDoux, 2005), the PFC provides top-down control, such as inhibiting proponent responses, maintaining affective goals, and recruiting further resources (Miller and Cohen, 2001), that steers and potentially modifies activation in subcortical circuitry including the amygdala. Supporting the PFC’s descending influence on the amygdala to achieve regulatory goals, recent studies have demonstrated a reciprocal PFC-amygdala relationship during successful reappraisal of negative emotion (Banks et al., 2007; Johnstone et al., 2007; Ochsner et al., 2002; Urry et al., 2006; Wager et al., 2008). This suggests that individuals with greater regulatory ability would be better able to engage the PFC-amygdala circuit during emotion regulation.
Most previous research on reappraisal, however, has reported group-mean findings in brain regions that are commonly activated across individuals. This approach rests on the assumption that all individuals regulate emotions in a similar way, and treats individual variation as statistical noise (Kosslyn et al., 2002). In the domain of emotion, however, variation across individuals is the rule rather than the exception (Hamann and Canli, 2004), and such individual differences in the capacity to regulate negative emotion may determine vulnerability and resilience in the face of adversity (Davidson, 2004). However, systemic investigation of the neural bases of individual differences in emotion regulation skills has been sparse, partly due to methodological issues, such as small sample sizes. Moreover, the extant neuroimaging literature has relied on self-reported negative affect as an index of regulatory success (Ochsner et al., 2002; Wager et al., 2008), or used measures reflecting non-specific arousal or effort such as eye-blink startle (Eippert et al., 2007), pupil dilation (Johnstone et al., 2007; Urry et al., 2009), and skin conductance response (Delgado et al., 2008). Arousal-dependent measures cannot differentiate between negative and positive emotions, and whether increased arousal and effort result from regulation success or failure. The use of subjective self-report measures of regulatory success can also be problematic because of demand characteristics and other biases such as inaccurate recall that plague the validity of such measures (Davidson, 1992). Furthermore, these measures were collected concurrently with the scan, which may be susceptible to state-dependent factors such as mood, fatigue, motivation, etc., and thus may not reflect stable, trait-like differences (Braver et al., 2010).
One of the most widely used and well-validated measures to objectively index negative emotion is facial electromyography (EMG) over frowning muscles (corrugator supercilii; cEMG). Activity in this muscle region reflects valence-specific negative affect (Bradley et al., 2001a), and is increased by direct intracerebral stimulation of the human amygdala (Lanteaume et al., 2007). Furthermore, cEMG activity has been shown to be systematically modulated by regulation instructions (Jackson et al., 2000; Lee et al., 2009; Ray et al., 2010), such that cEMG magnitude increases and decreases in accordance with instructions to amplify or attenuate negative emotion, respectively. In addition, these cEMG measures of emotion regulation exhibit high test-retest reliability over a four-week interval (Lee et al., 2009), suggesting that this measure may index trait-like emotion regulation ability. Regulation ability as measured by cEMG also predicts long-term adjustment in everyday life (Bonanno et al., 2004). Taken together, cEMG appears to be an objective and reliable measure to index trait-like individual differences in emotion regulatory ability.
To date, there has been no fMRI study that used cEMG to index individual differences underlying successful regulation of negative emotion. Although previous studies have found amygdala-PFC interactions important for regulation success, the findings widely diverge on which areas of the PFC critically impact regulatory success—for example, ventrolateral PFC (Wager et al., 2008), ventromedial PFC (Johnstone et al., 2007; Urry et al., 2006), dorsolateral/-medial PFC and orbitofrontal cortex (Banks et al., 2007), and anterior cingulate cortex (Ochsner et al., 2002). The direction of the relationship that these PFC regions have with the amygdala has also been inconsistent across studies. While some report an inverse amygdala-PFC relation during the down-regulation of negative emotion (Johnstone et al., 2007; Ochsner et al., 2002; Urry et al., 2006; Wager et al., 2008), others find a positive coupling associated with regulation success (Banks et al., 2007).
Thus, in the current study, we aimed to independently assess trait-like regulatory ability in a large sample using the objective measure of cEMG, and to directly examine its neural network using functional connectivity analysis during emotion regulation. To this end, we conducted two laboratory sessions of emotion regulation in which 56 participants reappraised negative emotion while recording cEMG (session 1) and BOLD fMRI (session 2; see Figure 1). We predicted that in both sessions participants would demonstrate an ability to regulate emotions according to instructions as evidenced by changes in cEMG activity and amygdala BOLD signal. We focused on the amygdala as a downstream target region of regulatory efforts for its activity has consistently been found to covary with regulatory goal (e.g., Eippert et al., 2007; Lapate et al., 2012; Ochsner et al., 2004; Urry et al., 2006; van Reekum et al., 2007). Thus, amygdala activity was used to index regulatory success in Session 2 as cEMG activity indexed regulatory success in Session 1. Next, we hypothesized that regulatory ability as indexed by cEMG in session 1 would be predictive of that as measured by the amygdala BOLD signal in session 2. Finally, given the critical role of amygdala-PFC interactions in successful down-regulation of negative emotion (Banks et al., 2007; Ochsner et al., 2002; Urry et al., 2006; Wager et al., 2008) and affective disorders (Johnstone et al., 2007; Phillips et al., 2008; Taylor and Liberzon 2007), we specifically interrogated the amygdala-PFC circuit to examine whether amygdala-PFC functional connectivity was predicted by the cEMG individual differences measure of down-regulation success.
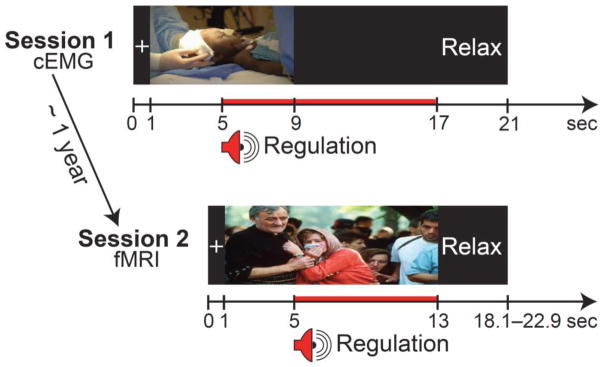
Figure 1.
Trial schematics of emotion regulation task. In session 1, following 4 seconds of picture viewing, one of three auditory regulation instructions was given (“enhance,” 00000“suppress,” “maintain”). Participants used reappraisal strategies to regulate their emotional response until they saw “Relax.” Throughout the trial, cEMG was continuously recorded. Approximately one-year later, an fMRI-variant of session 1 was conducted with a new matched set of pictures in session 2. Red bars indicate the regulation period.
Material and methods
Participants
Fifty-six male undergraduates (19.93 ± 1.81 years) were recruited from the University of Wisconsin-Madison, who were right-handed (Chapman and Chapman, 1987) and free of psychiatric/neurological disorders. Only men were included because they showed more stable emotion regulation over time (Lee et al., 2009)1, as well as to eliminate variability due to sex differences in psychophysiological (Bradley et al., 2001) and neural (McRae et al., 2008) responses in emotion regulation. All participants were paid for participation and provided informed consent for the study procedures approved by the University of Wisconsin-Madison Social & Behavioral and Health Sciences Institutional Review Boards.
Stimuli
Pictures were chosen from the International Affective Picture System (Center for the Study of Emotion and Attention [CSEA-NIMH], 1999). Two sets of 84 negative pictures (set 1: valence, 2.97 ± 0.66, arousal, 5.30 ± 0.93; set 2: valence, 2.98 ± 0.69, arousal, 5.29 ± 0.91) and 42 neutral pictures (set 1: valence, 5.02 ± 0.36, arousal, 2.75 ± 0.57; set 2: valence, 5.04 ± 0.47, arousal, 2.81 ± 0.50)2 were matched on valence and arousal ratings (Lang et al., 1999) with no repetition, and were counterbalanced across session for each participant.
Procedure
Participants underwent two sessions of emotion regulation in response to standardized affective pictures, one in which cEMG was measured (session 1) and one wherein BOLD responses were collected (session 2). In the first session, EMG sensors were placed on the corrugator supercilii muscle (Tassinary et al., 1989), and 6 negative and 4 neutral pictures were presented to familiarize participants with the protocol. During the experiment, 126 pictures (1-s fixation; 8-s/picture; 12-s intertrial interval) were presented in 6 blocks. Four seconds after picture onset, one of three auditory regulation instructions was presented: “enhance” (increase intensity of emotional response), “suppress” (decrease intensity of emotional response), or “maintain” (sustain initial intensity of emotional response) (see below for more detail). Participants were instructed to continue regulating their emotional response for 12 s until the word “Relax” appeared on the screen (Figure 1). Negative pictures were paired with each of the 3 regulation cues, whereas neutral pictures were paired only with the maintain instruction. Pictures were quasi-randomly presented with the constraint that no more than 3 trials of the same valence or instruction occurred consecutively. Following an average interval of 15.2 months (range: 11–19 months) participants returned to complete the fMRI-variant emotion regulation task. Prior to the experiment, participants completed a simulation scan to become familiar with the scanning environment and to practice emotion regulation. Using a non-repeating matched picture set, 126 pictures (1-s fixation; 12-s/picture; 5.1–9.9-s intertrial interval) were presented in 4 scan runs. After 4 seconds of uninstructed picture viewing, participants received one of three regulation instructions: “enhance,” “suppress” or “maintain.”3 Participants were instructed to continue regulating for 8 s until they saw “Relax” (Figure 1). In addition, pupil dilation was concurrently measured as an index of cognitive demand to ascertain the paradigm validity (Siegle et al., 2008).
Participants used cognitive reappraisal strategies to increase or decrease negative emotion, such as imagining a different outcome of the situation depicted in the picture or varying their level of personal involvement in the scene. For example, in order to reduce negative emotion to a picture of a child in surgery, participants might imagine that the outcome of the surgery turned out to be successful. In order to amplify negative emotion to a picture depicting mourning at a funeral, participants could imagine themselves in place of the individual in the scene. Participants were allowed to choose reappraisal strategies that they deemed most effective and similar to what they might use in their everyday lives, but were instructed to avoid non-cognitive strategies such as breathing, gaze aversion, or outward facial expression.
Data collection and analysis
Session 1: cEMG
Raw signal was continuously collected using two Ag/AgCl electrodes placed above the eyebrow, counterbalanced for laterality across subjects. EMG signals were amplified (10 k) and filtered (1–400 Hz) (SA Instrumentation Co., Encinitas, CA), corrected for artifacts, segmented into 500-ms Hamming-windowed chunks (50% overlap), and calculated for baseline-corrected (2-s) spectral power density (log10 μV2 for the 45–200-Hz EMG band). We chose spectral power density estimate over raw signal because it provides a cleaner measure by excluding noise from lower frequency bands (e.g., eye movements; Van Boxtel, 2001). A paired t-test was conducted for pre-instruction period (0–4-s post-picture onset) to test the effect of valence (negative vs. neutral pictures), and a repeated measures ANOVA was conducted for post-instruction period (4–6-s post-picture onset) to test the effects of regulation (negative pictures: enhance, maintain, suppress).
Session 2: FMRI
MR images were collected on a 3T scanner (General Electric Medical Systems, Waukesha, WI) with a whole-head transmit-receive quadrature coil. Anatomical images were acquired using a T1-weighted inversion recovery fast gradient echo [124 × 1 mm axial slices; 256 × 256 matrix; 240 mm field of view (FOV)]. Functional images were acquired using a T2*-weighted gradient-echo echo planar imaging pulse sequence (30 × 4 mm sagittal slices, 1 mm interslice gap; 64 × 64 matrix; 240 mm FOV; 2-s repetition time; 30-ms echo time; 90° flip). The functional MRI data were processed and analyzed using AFNI (Analysis of Functional NeuroImages; Cox, 1996). Images were slice-time and motion corrected. Single-subject GLM included separate regressors for each regulation condition (negative pictures: enhance, maintain, suppress, and neutral pictures: maintain) to estimate hemodynamic response functions (HRFs; modeled by a set of tent basis functions), six motion estimate covariates (Johnstone et al., 2006), and a second-order polynomial to model the baseline and slow signal drift. The estimated HRFs were converted to percent signal change values, and an area-under-the-curve (AUC) metric was calculated by averaging across time points corresponding to the peak response during the regulation period (7–6-s post-picture onset) minus those prior to the instruction (0–4-s post-picture onset). We attempted to control for variance occurring prior to the instruction cue, such as the marked variable time to onset in the amygdala following an emotional stimulus (e.g., Larson et al., 2006), so as to obtain a more accurate estimate of the “regulation effect” which was our main interest. The AUC estimates were manually normalized to Talairach space for better alignment of limbic structures, particularly the amygdala (Nacewicz et al., 2006), and spatially blurred with a 6 mm full-width at half-maximum Gaussian filter. Mean AUC estimates were extracted from Talairach-defined region-of-interest (ROI) in the bilateral amygdala and entered into a paired t-test to test the effect of valence (negative-maintain vs. neutral-maintain) and into a repeated measures ANOVA to test the effects of regulation (negative pictures: enhance, maintain, suppress).
To quantify regulation success, difference scores were computed as enhance – maintain and suppress – maintain for both cEMG activity and amygdala ROI estimates. A higher number in enhance – maintain indicates a better ability to up-regulate, whereas a lower number in suppress – maintain indicates a better ability to down-regulate negative emotion. To examine whether the cEMG index of regulatory success predicted amygdala index of regulatory success 1.3 years later, Pearson correlations were computed between cEMG and amygdala differences scores. Finally, functional connectivity analysis was preformed using psychophysiological interaction (PPI) method (Friston et al., 1997). Timeseries from Talairach-defined bilateral amygdala (the same ROI as above) was extracted as a physiological seed, and regulation contrast (suppress > maintain) was used as a psychological context, in order to create the psychophysiological interaction term (PPI). This interaction term was entered into a voxelwise regression, with the covariates of raw amygdala timeseries, six motion parameters and a second-order polynomial, and all original regressors of each regulation condition, in order to account for variance explained by the PPI over and above main effects of regulation conditions or amgydala activity. The resulting PPI parameter estimates (z-transformed betas) denoted the strength of functional coupling between the amygdala and the remainder of the brain during suppress relative to maintain trials. To examine the extent to which individual differences in down-regulation ability predicted this connectivity, cEMG difference scores (suppress – maintain) were entered into a voxelwise regression as a predictor of the PPI map. All statistical maps were thresholded at P < 0.01, and corrected for multiple comparisons using cluster-size thresholding (k > 80) based on whole-brain Monte Carlo simulation.
Horizontal pupil diameter was continuously acquired (60 Hz) using a remote eye-tracking device (SensoMotoric Instruments, Teltow, Germany). Pupil data from 14 participants were not usable due to technical problems. Data were processed using algorithms written by Siegle et al. (2002, unpublished Matlab code) and modified in our laboratory. Blinks were eliminated, missing points were linearly interpolated, and signals were smoothed with a 5-sample rolling average. Trials were removed for >50% interpolation during the regulation period and corrected for outliers (±3 SD). Data were aggregated into 0.5-s bins, baseline-corrected (0.5-s pre-instruction), and computed for the mean proportional change averaged across 8-s of the regulation period. Pupil values were analyzed using GLM to test for the regulation effects.
Results
First, we verified that the intended negative emotion was elicited by the pictures. In session 1, cEMG activity was greater for negative versus neutral pictures during the initial 4-s period prior to regulation instructions (t55 = 6.66, P < 0.001). In session 2, we confirmed the presence of picture-induced negative emotion by showing that amygdala activation was greater for negative-maintain versus neural-maintain trials (t55 = 2.41, P = 0.02).4
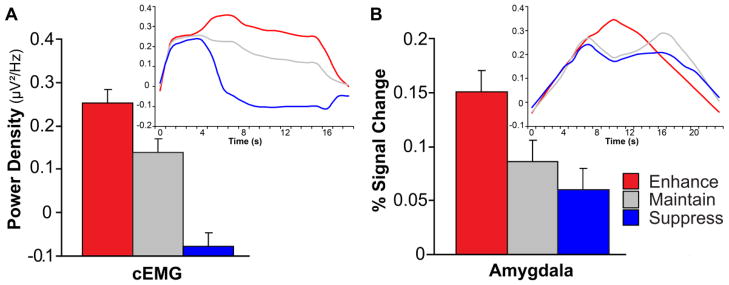
Next, we examined the effects of cognitive regulation of negative emotion. In session 1, replicating previous findings (Jackson et al., 2000; Lee et al., 2009; Ray et al., 2010), cEMG activity was modulated according to regulation instructions (enhance > maintain > suppress; F(2,110) = 51.54, P < 0.001, pair-wise Ps < 0.001; Figure 2A). In session 2, consistent with prior reports (Eippert et al., 2007; Ochsner et al., 2004; Urry et al., 2006; van Reekum et al., 2007), amygdala activation was modulated by the regulation instructions (enhance > maintain > suppress; F(2,110) = 10.63, P < 0.001, pair-wise Psone-tailed < 0.045; Figure 2B). We additionally confirmed that the intended effort was expended following regulation attempts as evidenced by pupil dilation (enhance > suppress > maintain; F(2,82) = 51.75, P < .001, pair-wise Ps < .001). Thus, in both sessions, participants as a group were able to regulate negative emotion as instructed.
Figure 2.
Effects of emotion regulation on (A) cEMG activity (session 1) and (B) amygdala BOLD signal (session 2). For both sessions, participants regulated their responses to negative pictures according to instructions. Error bars indicate the SEMdifference. Inset figures illustrate the time series of cEMG and amygdala activity.
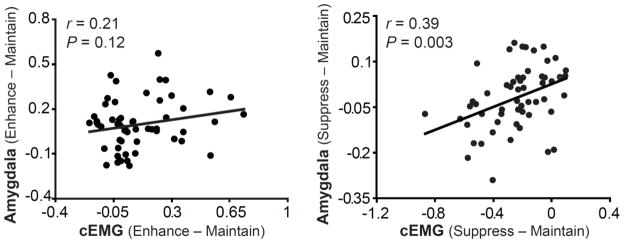
Further, to quantify regulation success difference scores were computed (i.e., suppress – maintain; enhance – maintain) for both cEMG and amygdala. The ability to down-regulate negative emotion (i.e., suppress – maintain) as measured by cEMG in session 1 was predictive of the amygdala BOLD signal in session 2 about 1.3 years later (r = 0.39, P = 0.003). The ability to up-regulate negative emotion (i.e., enhance – maintain) was positively correlated across sessions but not statistically significant (r = 0.21, P = 0.12; Figure 3).5
Figure 3.
Stability of emotion regulatory success across sessions. The ability to down-regulate negative emotion assessed using cEMG (μV2/Hz) and amygdala activity (% signal change) were moderately correlated over the 1.3-year interval. However, the ability to up-regulate negative emotion was not significantly correlated.
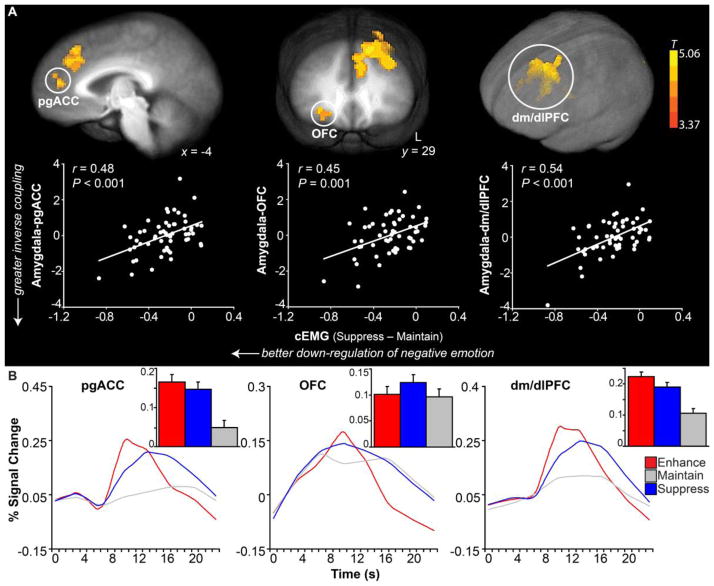
Finally, to determine the extent to which individual differences in down-regulatory ability predicted amygdala-PFC connectivity, cEMG difference scores of suppress – maintain were regressed voxel-wise on the functional connectivity of the amygdala during suppress versus maintain trials (Friston et al., 1997). Results suggested that individuals with greater capacity for reducing negative emotion (as measured with cEMG) exhibited greater inverse functional coupling between the amygdala and several regions of the PFC including the pregenual anterior cingulate cortex (pgACC), orbitofrontal cortex (OFC), and dorso-medial/lateral PFC (dm/dlPFC) when down-regulating negative emotion (Figure 4A; see Table 1 for the complete list of regions). Conversely, unsuccessful regulators showed more positive coupling between the amygdala and these PFC regions. Among these PFC regions, when examining the main effects of regulatory goal, OFC was not modulated by regulation instructions (F(2,110) = 1.81, P = 0.17) whereas pgACC and dm/dlPFC showed significant regulation effects (Fs(2,110) > 21.75, Ps < 0.001; enhance = suppress Ps > 0.01, suppress > maintain Ps < 0.001) (Figure 4B).
Figure 4.
Amygdala-PFC connectivity supporting down-regulatory success. Individual differences in down-regulatory ability predicted amygdala-PFC functional connectivity 1.3 years later. (A) Top panel depicts PFC clusters showing functional connectivity with amygdala during suppress versus maintain as predicted by cEMG difference scores (Suppress – Maintain). Bottom panel illustrates scatterplots between amygdala-PFC connectivity (y-axis; standardized mean beta) for each identified region above, and cEMG regulation success (x-axis; μV2/Hz) obtained 1.3 years earlier. Individuals who were more successful at down-regulating negative emotion (more negative cEMG scores) exhibited greater inverse amygdala-PFC coupling during down-regulation of negative emotion (more negative betas), while individuals who were worse at regulation showed more positive coupling. (B) BOLD signal changes by regulatory instruction over time in the PFC regions implicated in this functional connectivity analysis. Inset figures represent the main effects of regulation. Error bars indicate the SEMdifference.
Table 1.
Regions where voxelwise regression of cEMG regulation success (Suppress – Maintain) significantly predicted functional connectivity of amygdala (Suppress > Maintain).
| Brain region (Brodmann area) | Size (mm3) | Max T | Location of Max T | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Middle, superior, medial frontal gyrus (BA 8, 9, 6) | 17784 | 5.06 | −15 | 27 | 46 |
| Caudate, thalamus | 3304 | 4.64 | −11 | 15 | 18 |
| Culmen | 2424 | 3.56 | −27 | −45 | −28 |
| Inferior, middle temporal gyrus (BA 20, 37) | 2248 | 3.96 | −53 | −33 | −18 |
| Anterior cingulate, medial/superior frontal gyrus (BA 32, 9, 10) | 1768 | 3.48 | −17 | 49 | 24 |
| Cuneus (BA19,18) | 1600 | 4.30 | −23 | −81 | 24 |
| Lentiform nucleus | 1352 | 4.39 | −23 | −9 | −8 |
| Inferior semi-lunar lobule, cerebellar tonsil, pyramis | 944 | 3.49 | 35 | −67 | −34 |
| Inferior frontal gyrus (BA 47, 11) | 688 | 3.37 | 27 | 31 | −4 |
Note: Corrected cluster for multiple comparisons at P < 0.01. Coordinates of the location of the cluster’s maximum T are in Talairach space.
Discussion
The present study adds to the growing literature on emotion regulation by having an independent assessment of an objective and trait-like index of emotion regulatory ability from a large number of individuals. To our knowledge, our study is the first to correlate individual differences in BOLD response and functional connectivity during emotion regulation with a cEMG measure of regulatory ability. Our data provide new evidence that the trait-like ability to regulate negative emotion is associated with modulation of the amygdala activity as well as with amygdala-PFC functional connectivity. Specifically, we found that individuals who were better able to down-regulate negative emotion as indexed by cEMG at session 1 showed not only more attenuated amygdala signal but also greater inverse functional connectivity between the amygdala and specific areas of the PFC, notably pgACC, OFC and dm/dlPFC, while down-regulating negative emotion at session 2. These PFC regions have previously been shown to exert regulatory influences on the amygdala—pgACC inhibits amygdala activity in the resolution of emotional conflicts (Egner et al., 2008; Etkin et al., 2006); OFC, through its extensive anatomical connections (Ghashghaei and Barbas 2002; Ongur and Price 2000), modulates the amygdala in the reappraisal of contextual value (Dolan, 2007); and lateral and dorsal PFC regions have also been found to influence amygdala function, possibly mediated via the OFC/vmPFC, in reducing negative emotion (Johnstone et al., 2007; Ochsner et al., 2002; Urry et al., 2006; Wager et al., 2008). Accordingly, our results suggest that the individual variations in emotion regulation skills are reflected in this amygdala-PFC circuit, in which PFC regions have an inverse functional connectivity with the amygdala in promoting adaptive regulation of negative emotion.
While previous research has primarily focused on between-subjects findings, our individual differences analyses using cEMG and functional connectivity revealed a new set of large prefrontal clusters that do not overlap with the previously-reported ventromedial/-lateral areas tied to amygdala activation (Johnstone et al., 2007; Ochsner et al., 2002; Urry et al., 2006; Wager et al., 2008). It is also notable that our OFC region as identified by the individual difference connectivity analysis, unlike pgACC and dm/dlPFC regions, did not reveal statistically significant regulation effects by the group-mean analysis. This finding suggests that the OFC was recruited in individuals who were particularly successful in decreasing both amygdala and cEMG activity, and contrasts a more generic circuitry of reappraisal comprising lateral and dorsomedial regions (Kalisch, 2009) with the OFC-like regions usually found in individual difference analyses (Banks et al., 2007; Johnstone et al., 2007; Urry et al., 2006; Wager et al., 2008). Our finding, however, was in the opposite direction from the only published study conducting the same type of connectivity analysis during reappraisal (Banks et al., 2007). Although Banks et al. located similar prefrontal regions, such as subgenual ACC, OFC, dmPFC, and dlPFC, better regulators showed the more positive amygdala-PFC connectivity. The reason for conflicting results can be in part attributable to the fact that Banks et al. used self-reported intensity of negative emotion on a restricted-range scale of 1–5 to index regulatory success in a small sample (N=14), whereas we used cEMG to capture a continuous and a much wider range of regulation ability in a large sample (N=56). Furthermore, Banks et al. did not include the self-reported index of regulatory success in their connectivity analysis, which may have biased the findings towards positive PFC-amygdala coupling. This discrepancy between the results showcases the impact of methodology in individual differences research, and could be resolved in future work by directly comparing the psychophysiological and self-report measures in the effectiveness and validity of representing trait-like regulatory ability.
Our results also showed that despite the long temporal interval between assessments individual differences in the capacity to volitionally down-regulate negative emotion were stable. Given the imperfect coherence among different emotional systems (Mauss et al., 2005), it is notable that this stability was observed across peripheral and central output systems. Moreover, for a subset of the current participants (n=17), the ability to regulate emotion predicted the ability to regulate pain three years later (Lapate et al., 2011). These findings underscore the trait-like quality of individual differences in emotion regulation and suggest that such individual differences may be an important target for understanding normal variation in temperament (Thompson, 1994) and for determining risk for psychopathology (Davidson, 2004; Phillips et al., 2008; Taylor and Liberzon, 2007).
In contrast to our findings for down-regulation, we did not find a stable association between cEMG and amygdala activation during the up-regulation of negative emotion. There are two plausible explanations: First, given prior finding that men show lower cEMG activity to negative pictures as compared to women (Bradley et al., 2001), our male-only sample might have a more limited range to increase cEMG activity above and beyond that already activated in response to negative stimuli, which subsequently constrained our ability to detect the significant correlation with amygdala activity. Indeed, our participants showed significantly less mean changes in cEMG activity when increasing (M = .10, SD = .22) as compared to decreasing negative emotion (M = .24, SD = .21), t55 = 3.93, P < .001. Second, differences in stimulus duration between sessions might have differentially affected our ability to detect the predicted correlation; for example, increasing negative emotion might have become easier in the fMRI session as participants had more time to elaborate on the negative pictures. In fact, previous work suggests that regulating emotion while the picture is on produce more pronounced effects of regulation as compared to regulating emotion beyond the picture offset (Dichter et al., 2002; Jackson et al., 2000; Lee et al., 2009). It should also be noted that while relations with amygdala activity might not be present, there may well be associations with other regions such as those in the PFC for the ability to increase negative emotion.
Two limitations of the current study warrant future research. First, the causal influence of prefrontal regions on the amygdala, or vice versa, cannot be determined with the functional connectivity analysis. This is a shortcoming of all correlative neuroimaging research and more invasive techniques would be required to examine the causative nature of these relationships. Second, given prior evidence that men are less emotionally reactive in expressive measure to aversive stimulation (e.g., Bradley et al., 2001) and based on our finding that men showed a truncation of range for increasing negative emotion, caution is warranted when generalizing our results to women. Given gender differences in the prevalence of affective disorders (Kessler et al., 2004), future research with adequate sample sizes of each gender would be required to systematically address this issue.
In sum, this study complements and extends the extant group-based research by adopting a rigorous individual-differences approach (Braver et al., 2010; Kosslyn et al., 2002) and integrating psychophysiology and neuroimaging (Davidson, 2003). Our data suggest that successful emotion regulators exhibit inverse functional connectivity between the amygdala and PFC during down-regulating negative emotion. Such connectivity patterns have been implicated in affective disorders (Phillips et al., 2008), and could be targeted for clinical assessment of reappraisal success or training. More broadly, our data underscore the importance of examining stable individual differences to provide further insights into the neural bases of emotion regulation. Future research should examine the extent to which regulatory ability is plastic and the extent to which interventions designed to reduce negative emotion and promote well-being modulate amygdala-prefrontal circuitry.
Acknowledgments
This work was funded by National Institute of Mental Health (R01-MH43454, P50-MH069315) to R.J.D. Representative photos in Figure 1 were retrieved from http://commons.wikimedia.org. We thank L. Greischar, M. Anderle, L. Angelos, and R. Fisher for help with data collection and processing; R. Lapate and A. Shackman for commenting on the manuscript draft; and the staff at the Waisman Laboratory for Brain Imaging and Behavior for administrative and technical support.
Footnotes
This finding was assessed with eyeblink startle EMG (Lee et al., 2009). Because this study had initially intended to collect both startle and corrugator EMG, we limited our sample to men.
The IAPS numbers used were the following: Set 1: negative (1050, 1275, 2053, 2120, 2205, 2206, 2276, 2490, 2681, 2691, 2692, 2730, 2753, 2900.1, 3010, 3051, 3053, 3061, 3063, 3064, 3071, 3140, 3160, 3168, 3181, 3230, 3261, 3266, 3280, 3300, 3530, 3550, 4621, 4664.2, 6210, 6243, 6250.1, 6260, 6300, 6313, 6510, 6530, 6550, 6560, 6821, 6838, 7380, 9000, 9006, 9045, 9046, 9050, 9080, 9102, 9110, 9160, 9180, 9181, 9250, 9252, 9253, 9280, 9320, 9331, 9340, 9373, 9405, 9417, 9420, 9421, 9430, 9433, 9452, 9520, 9561, 9570, 9592, 9600, 9611, 9621, 9800, 9910, 9920, 9921) and neutral (1450, 2190, 2210, 2270, 2320, 2495, 2620, 2630, 2840, 5120, 5390, 5510, 5520, 5530, 5532, 5731, 5740, 7000, 7002, 7004, 7009, 7010, 7031, 7060, 7090, 7110, 7175, 7182, 7184, 7185, 7186, 7190, 7205, 7207, 7233, 7234, 7235, 7490, 7500, 7595, 7700, 7705). Set 2: negative (1111, 1220, 1274, 2110, 2312, 2352.2, 2590, 2661, 2682, 2710, 2750, 2751, 2800, 3000, 3015, 3030, 3060, 3062, 3080, 3102, 3120, 3130, 3150, 3170, 3180, 3220, 3250, 3301, 3350, 3400, 3500, 3550.1, 6200, 6212, 6213, 6230, 6242, 6244, 6312, 6314, 6350, 6360, 6370, 6540, 6561, 6570.1, 6571, 6830, 6831, 6834, 9001, 9007, 9008, 9010, 9040, 9041, 9042, 9090, 9101, 9120, 9140, 9182, 9220, 9265, 9290, 9300, 9330, 9390, 9400, 9410, 9415, 9432, 9470, 9530, 9560, 9571, 9584, 9620, 9622, 9630, 9810, 9830, 9911, 9912) and neutral (1670, 2200, 2214, 2240, 2372, 2440, 2480, 2570, 2580, 2880, 5030, 5130, 5500, 5531, 5533, 5534, 5535, 5720, 5800, 6150, 7006, 7020, 7025, 7030, 7034, 7035, 7040, 7050, 7080, 7100, 7130, 7140, 7150, 7170, 7180, 7183, 7187, 7211, 7217, 7224, 7491, 7950).
The terms “enhance,” “maintain” and “suppress” were used based on prior instructions by Jackson et al. (2000). In this study, the terms “suppress” and “enhance” were used to instruct participants to decrease and increase their negative emotion, respectively, using cognitive reappraisal strategies, and our instruction should not be confused with the “suppress” instruction used by Gross (1998, 2002) which refers to inhibiting expression.
The correlation with amygdala and cEMG activity was r = .26, P = .05, suggesting the level of negative emotion elicited by the pictures, in the absence of active regulation, was positively related across sessions.
We found similar results using the statistical amygdala ROI. The ability to down-regulate negative emotion was still significant, r = .33, P = .01, and the ability to up-regulate negative emotion was not significant, r = .18, P = .18.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno GA, Papa A, Lalande K, Westphal M, Coifman K. The importance of being flexible: The ability to both enhance and suppress emotional expression predicts long-term adjustment. Psychological Science. 2004;15(7):482–487. doi: 10.1111/j.0956-7976.2004.00705.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001;1(3):300–319. [PubMed] [Google Scholar]
- Braver TS, Cole MW, Yarkoni T. Vive les differences! individual variation in neural mechanisms of executive control. Current Opinion in Neurobiology. 2010;20(2):242–250. doi: 10.1016/j.conb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for the Study of Emotion and Attention [CSEA-NIMH] The international affective picture system: Digitized photographs. The Center for Research in Psychophysiology, University of Florida; Gainesville, FL: 1999. [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of handedness. Brain and Cognition. 1987;6(2):175–183. doi: 10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective neuroscience and psychophysiology: Toward a synthesis. Psychophysiology. 2003;40(5):655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Prolegomenon to the structure of emotion: Gleanings from neuropsychology. Cognition & Emotion. 1992;6(3):245–268. [Google Scholar]
- Davidson RJ. Well-being and affective style: Neural substrates and biobehavioural correlates. Philosophical Transactions: Biological Sciences. 2004;359(1449):1395–1411. doi: 10.1098/rstb.2004.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, LeDoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59(5):829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ, Baucom BR. Startle modulation before, during and after exposure to emotional stimuli. International Journal of Psychophysiology. 2002;43:191–6. doi: 10.1016/s0167-8760(01)00170-2. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. The human amygdala and orbital prefrontal cortex in behavioural regulation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362(1481):787–799. doi: 10.1098/rstb.2007.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral Cortex. 2008;18(6):1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28(5):409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, Lanius RA. Neuroimaging studies of psychological interventions for mood and anxiety disorders: Empirical and methodological review. Clinical Psychology Review. 2008;28(2):228–246. doi: 10.1016/j.cpr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39(3):281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Hamann S, Canli T. Individual differences in emotion processing. Current Opinion in Neurobiology. 2004;14(2):233–238. doi: 10.1016/j.conb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37(4):515–522. [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, Walsh KSO, Greischar LL, Alexander AL, Fox AS, Davidson RJ, Oakes TR. Motion correction and the use of motion covariates in multiple-subject fMRI analysis. Human Brain Mapping. 2006;27(10):779–788. doi: 10.1002/hbm.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: Time matters. Neuroscience & Biobehavioral Reviews. 2009;33(8):1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Koretz D, Merikangas KR, Wang PS. The epidemiology of adult mental disorders. In: Levin BL, Petrilia J, Hennessy KD, editors. Mental Health Services: A Public Health Perspective. 2. New York: Oxford University Press; 2004. [Google Scholar]
- Kosslyn SM, Cacioppo JT, Davidson RJ, Hugdahl K, Lovallo WR, Spiegel D, Rose R. Bridging psychology and biology: The analysis of individuals in groups. American Psychologist. 2002;57(5):341. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. The Center for Research in Psychophysiology, University of Florida; Gainesville, FL: 1999. [Google Scholar]
- Lanteaume L, Khalfa S, Regis J, Marquis P, Chauvel P, Bartolomei F. Emotion induction after direct intracerebral stimulations of human amygdala. Cerebral Cortex. 2007;17(6):1307–1313. doi: 10.1093/cercor/bhl041. [DOI] [PubMed] [Google Scholar]
- Lapate RC, Lee H, Salomons TV, van Reekum CM, Greischar LL, Davidson RJ. Amygdalar function reflects common individual differences in emotion and pain regulation success. Journal of Cognitive Neuroscience. 2012;24:148–58. doi: 10.1162/jocn_a_00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson CL, Schaefer HS, Siegle GJ, Jackson CAB, Anderle MJ, Davidson RJ. Fear is fast in phobic individuals: Amygdala activation in response to fear-relevant stimuli. Biological Psychiatry. 2006;60(4):410–417. doi: 10.1016/j.biopsych.2006.03.079. [DOI] [PubMed] [Google Scholar]
- Lee H, Shackman AJ, Jackson DC, Davidson RJ. Test-retest reliability of voluntary emotion regulation. Psychophysiology. 2009;46:874–879. doi: 10.1111/j.1469-8986.2009.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gross JJ, Gabrieli JJD. Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Group Processes & Intergroup Relations. 2008;11(2):143–162. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nacewicz BM, Dalton KM, Johnstone T, Long MT, McAuliff EM, Oakes TR, Alexander AL, Davidson RJ. Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Archives of General Psychiatry. 2006;63(12):1417–1428. doi: 10.1001/archpsyc.63.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13(9):833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RD, McRae K, Ochsner KN, Gross JJ. Cognitive reappraisal of negative affect: Converging evidence from EMG and self-report. Emotion. 2010;10(4):587. doi: 10.1037/a0019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Ichikawa N, Steinhauer S. Blink before and after you think: Blinks occur prior to and following cognitive load indexed by pupillary responses. Psychophysiology. 2008;45(5):679–687. doi: 10.1111/j.1469-8986.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- Tassinary LG, Cacioppo JT, Green TR. A psychometric study of surface electrode placements for facial electromyographic recording: I. The brow and cheek muscle regions. Psychophysiology. 1989;26:1–6. doi: 10.1111/j.1469-8986.1989.tb03125.x. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends in Cognitive Sciences. 2007;11(10):413–418. doi: 10.1016/j.tics.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Thompson RA. Emotion regulation: A theme in search of definition. Monographs of the Society for Research in Child Development. 1994;59(2–3):25–52. 250–283. [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. NeuroImage. 2009;47(3):852–863. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26(16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boxtel A. Optimal signal bandwidth for the recording of surface EMG activity of facial, jaw, oral, and neck muscles. Psychophysiology. 2001;38(1):22–34. [PubMed] [Google Scholar]
- van Reekum CM, Johnstone T, Urry HL, Thurow ME, Schaefer HS, Alexander AL, Davidson RJ. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. NeuroImage. 2007;36(3):1041–1055. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]