Abstract
Background
Consent to participate in research is an important component of the conduct of ethical clinical trials. Current consent practices are largely policy-driven. This study was conducted to assess comprehension of study information and satisfaction with the consent form between subjects randomized to concise or to standard informed consent forms as one approach to developing evidence-based consent practices.
Methods
Participants (N=111) who enrolled into two Phase I investigational influenza vaccine protocols (VRC 306 and VRC 307) at the NIH Clinical Center were randomized to one of two IRB-approved consents; either a standard or concise form. Concise consents had an average of 63% fewer words. All other aspects of the consent process were the same. Questionnaires about the study and the consent process were completed at enrollment and at the last visit in both studies.
Results
Subjects using concise consent forms scored as well as those using standard length consents in measures of comprehension (7 versus 7, p=0.79 and 20 versus 21, p=0.13), however, the trend was for the concise consent group to report feeling better informed. Both groups thought the length and detail of the consent form was appropriate.
Conclusions
Randomization of study subjects to different length IRB-approved consents forms as one method for developing evidence-based consent practices, resulted in no differences in study comprehension or satisfaction with the consent form. A concise consent form may be used ethically in the context of a consent process conducted by well-trained staff with opportunities for discussion and education throughout the study.
Keywords: clinical trial, informed consent, Institutional Review Board, comprehension, research subject
INTRODUCTION
It is standard practice and an ethical requirement to obtain the informed consent of research participants in clinical trials. Although consent involves more than signing a form, the consent form itself is a key element [1] and must be approved by the Institutional Review Board (IRB) overseeing the study. The protocol team and regulatory agencies reviewing the consent form look for elements required by regulations and institutional policies. A common practice among IRBs is to have required template language for key topics, such as the voluntary nature of research participation, sample storage, HIV testing, genetic testing, and other concerns that are not unique to the protocol. Consent form templates may be long and complex even before the specific goals, risks, and benefits of the particular protocol are incorporated. Common experience, including ours, is for the regulatory review process to result in recommendations to add text more often than to remove text [2, 3].
Prior to the initiation of this consent form study, the Vaccine Research Center (VRC) Clinic (Bethesda, MD) had used a standard consent form template provided by the NIAID Intramural IRB from 2002 through 2008 for the enrollment of subjects into 20 investigational vaccine studies. Consent forms ranged from 9 to17 pages, depending upon study complexity and issues raised by various regulatory agencies throughout the review process. The average length was 12 pages of single-spaced text with 10 point font using the IRB’s standard template form.
While much emphasis is put on the form itself during the protocol regulatory reviews, in practice a significant component of the consent process is verbal. Clinic staff actively educate subjects about the procedures and potential consequences of the protocol and provide opportunities for dialogue when volunteers ask additional clarifying questions. Personnel conducting the consent process assess the study volunteer's cognitive ability, educational needs, and understanding of the clinical trial.
The NIH Clinical Center Department of Bioethics has studied the informed consent process [4–6] as well as the ethics of vaccine research [7]. To study whether or not standard consent forms can be simplified while still ensuring adequate informed consent requires research to support evidence-based practices. Prior to the study reported here, the Department of Bioethics collaborated with a Clinical Research Unit in New Haven, CT to evaluate randomization to standard and concise consent forms in a bioequivalence study of a marketed drug [6]. An initial pilot consent substudy was conducted with the NIAID Intramural program for a protocol [8] in which the avian influenza vaccine under study was one approved for use in the National Stockpile. When the pilot study was first proposed, the NIAID IRB was concerned that a concise form could expose participants to the risk of inadequate study information. Ultimately, they approved the substudy agreeing that there was insufficient evidence of risk and studies of this type would help to provide needed evidence. The ethical pros and cons of this methodology were subsequently published in a commentary [5]. We report here the results from the next step in the larger Consent Project initiative, in which randomization to standard and concise informed consent forms was included in the protocols for two similar investigational influenza vaccine studies conducted by the VRC, NIAID.
The hypothesis stated in advance was: In the context of a full consent process as regularly conducted in accordance with VRC Clinic standard policies (i.e., including discussion with clinic staff and Assessment of Understanding) a concise consent document will result in no difference in understanding compared to a standard consent document. The complexity of consent and the controversial nature of randomizing subjects to different consent forms has required a stepwise approach to addressing this hypothesis; a single small study cannot definitively resolve the question. Larger studies in diverse settings and different types of trial populations are needed. However, the current study is an important step in evaluating the implications and methods of comparing consent forms of different lengths within the same trial and encouraging and facilitating evidence-based consent practices.
METHODS
1. Setting and Participants
The consent form study was conducted at the National Institutes of Health Clinical Center (Bethesda, MD) through IRB-approved substudies included in the VRC 306 [9] and VRC 307 investigational vaccine clinical trials. All applicable regulatory requirements were met and conduct of the study was consistent with the Declaration of Helsinki. Those authorized to obtain consent in the research reported here had an average of 10 years of experience in clinical research and obtaining informed consent. Before being authorized to obtain consent from participants for any study, new VRC staff members receive 3 months of mentored training in the consenting process. All potential study participants for these protocols were recruited and screened through a single screening protocol (VRC 300). As is routinely the case for all VRC studies, volunteers expressing interest in a vaccine study had access to an IRB-approved one page study summary and to public information, such as the clinicaltrials.gov study summary, in advance of a screening visit. If after initial screening, the volunteer appeared to be an eligible, healthy adult who was willing to participate in a vaccine study, he or she was randomized to a consent form through a function built into the electronic data capture (EDC) system provided by the contract research organization (CRO), EMMES, Inc. (Rockville, MD). Randomization to the standard consent form (Form A) or the concise consent form (Form B) occurred in a 1:1 ratio for each of the two vaccine protocols; both forms were IRB-approved and each included a statement to inform subjects that the consent process was also being studied. A comparison of the two consent forms for each study in terms of pages, word count overall and by section, and reading level is provided in Table 1. The concise consent form was developed by eliminating repetition, using active voice, and simplifying words and sentences used in the standard form. The Form A standard consents had an average of 5023 words, while the Form B concise consents had an average of 1853 words for an overall 63% reduction in number of words. The Flesch-Kincaid Reading Grade level was slightly higher for the standard consent, but both were higher than the recommended 8th grade level, at about grades 10.2 and 9.4, respectively. All routine practices of the consent process were followed; including allowing volunteers to read and review the appropriate consent form at home in advance of the vaccine study enrollment visit and encouraging discussion with staff regarding questions or concerns. Prior to signing a vaccine study consent, an IRB-approved, 21-item, true/false “Assessment of Understanding” (AoU) was administered and the number of correct answers was recorded. The VRC AoU is a standard instrument used in all consenting interactions and is comprised of both questions about the nature of research used for all vaccine trials as well as questions specific to the particular protocol. Any incorrect answers were reviewed by the study clinician to confirm the subject’s understanding of the study followed by the volunteer’s verbalization of his or her own interpretation. Both the volunteer and clinician then initialed and dated the correction on the AoU to indicate that the correct answers are now understood by the volunteer. The volunteer signed the Informed Consent form only after the clinician was satisfied that the volunteer understood the study. The enrollment was then completed by the usual procedure of signing the informed consent form and completion of the enrollment procedure in the EDC.
Table 1.
Length and Complexity Comparison for Standard and Concise Consent Forms
| Format A Standard |
Format B Concise |
Reduction | |||
|---|---|---|---|---|---|
| VRC 306 | VRC 307 | VRC 306 | VRC 307 | percentage | |
| Total Pages (when in 10 pt font) | 10 | 10 | 5 | 5 | 50 |
| Total Word Count | 5134 | 4912 | 1790 | 1916 | 63 |
| Words: Intro, Purpose, *Study Vaccines | 920 | 721 | 249 | 240 | 70 |
| Words: Procedures and Monitoring/What Happens in the Study | 925 | 973 | 372 | 477 | 55 |
| Words: Genetic Tests/HLA/Stored Samples | 981 | 981 | 166 | 165 | 83 |
| Words: Study Risks/Birth Control | 1271 | 1191 | 535 | 507 | 58 |
| Words: Study Benefits/Alternatives | 65 | 88 | 83 | 100 | (13) |
| Words: Costs and Compensation | 166 | 169 | 57 | 67 | 60 |
| Words: Reasons for Removal from Study | 199 | 194 | 68 | 65 | 66 |
| Words: Conflict of Interest/Other Information | 607 | 595 | 260 | 295 | 54 |
| Flesch-Kincaid Reading Grade Level | 10.1 | 10.2 | 9.5 | 9.2 | <1 grade |
| *Example of standard and concise text selected from VRC 306 regarding the DNA Vaccine | |||||
|
Standard: Vaccines are substances used to try to create resistance (or immunity) to a disease and to prevent an infection. You cannot get an avian flu infection from either study vaccine because neither of the study vaccines contain avian influenza virus that can cause avian flu infection or disease. The investigators will then see if the body creates resistance or immunity to an influenza protein called “hemagglutinin 5”. This is also called H5 or H5 protein. In this consent the two vaccines will be called “DNA H5 Vaccine” and “Inactivated H5N1 Vaccine”. The hope is that an immune response to H5 protein may protect against avian influenza virus infection. It is not known if the study vaccines work to protect against avian influenza. DNA H5 Vaccine: Most vaccines are made of proteins and injected into a muscle. Proteins are natural substances that the body uses as building blocks. DNA serves as nature’s code (instructions) for protein production in the body. A new kind of vaccine being tested in this study is made from the DNA that is the code for an influenza H5 protein. It is made from just one small part of the code for avian influenza H5 virus protein. In this study, the DNA will be injected into a muscle. It will instruct the body to make a small amount of the H5 protein, which is based on the protein from the avian influenza virus. The dose of the DNA H5 vaccine in this study has been tested in 15 people in an earlier study. Smaller doses of the DNA H5 vaccine have been tested in 58 people… |
Simplified: Although vaccines are used to boost the immune system to prevent infection, we do not know if either of the two experimental study vaccines will prevent bird flu. However, you cannot get bird flu from the study vaccines. One study vaccine is made from DNA that will cause your body to make a protein called H5. The same dose of the vaccine used in this study (4 mg.) was tested in 15 people, and a lower dose was tested in 58 people… |
||||
Word counts and reading levels were obtained using Microsoft Word 2007®. The percent reduction in size or score was calculated using the mean of the two standard documents and mean of the two simplified documents. Parentheses indicate a percentage increase.
2. Data Collection Method
Following enrollment, typically before administration of the first study injection (but with allowance for up to 48 hours afterwards), a questionnaire (survey) tool developed for the consent investigation was completed by the subject directly into the EDC independently or on paper (infrequently) and entered into the database by the data management CRO. Clinic staff were not permitted to see the subject’s responses or access the cumulative questionnaire results until after the consent substudy data collection was completed. An additional 5-question survey focused on satisfaction was completed at the last study visit, which varied from 24 to 48 weeks after enrollment, depending upon the vaccine protocol. The initial consent surveys were completed from November 17, 2008 through November 16, 2009 and the final visit surveys from September 2, 2009 through October 18, 2010.
3. Instruments
The self-administered, initial questionnaire measured 4 domains (1) socio-demographics, (2) motivations for participating and decision making (3) comprehension of study information and (4) satisfaction with the consent form and process. The questionnaire format was previously piloted in consent studies conducted in collaboration with the NIH Clinical Center Department of Bioethics as described above. Questions measuring comprehension were similar in format, but study-specific. The majority of questions were multiple choice and some allowed for free text comments.
4. Statistical Analysis
For statistical analyses, our goal was to explore all possible differences between the two forms. The analysis outcomes were exploratory and the comparison p values were reported without adjustment for multiplicity. A p value ≥ 0.05 in the answers to a question indicated no statistically significant difference between forms.
For the six demographics questions and the fifteen satisfaction or motivation questions, distribution of answers to each question was compared by Chi-square test, and percentages of participants under each answer were calculated and compared by Fisher’s exact test. Two different measures of comprehension were: 1) the number of correct responses to the 21 True/False Assessment of Understanding and 2) the number of correct answers to the 8 multiple choice questions that had a single correct answer. For both measures, the mean and median were calculated and the median scores were compared by Wilcoxon test between the two consent groups (i.e., standard Form A and concise Form B groups). Data were analyzed using the statistical software SAS.
RESULTS
1. Study Population
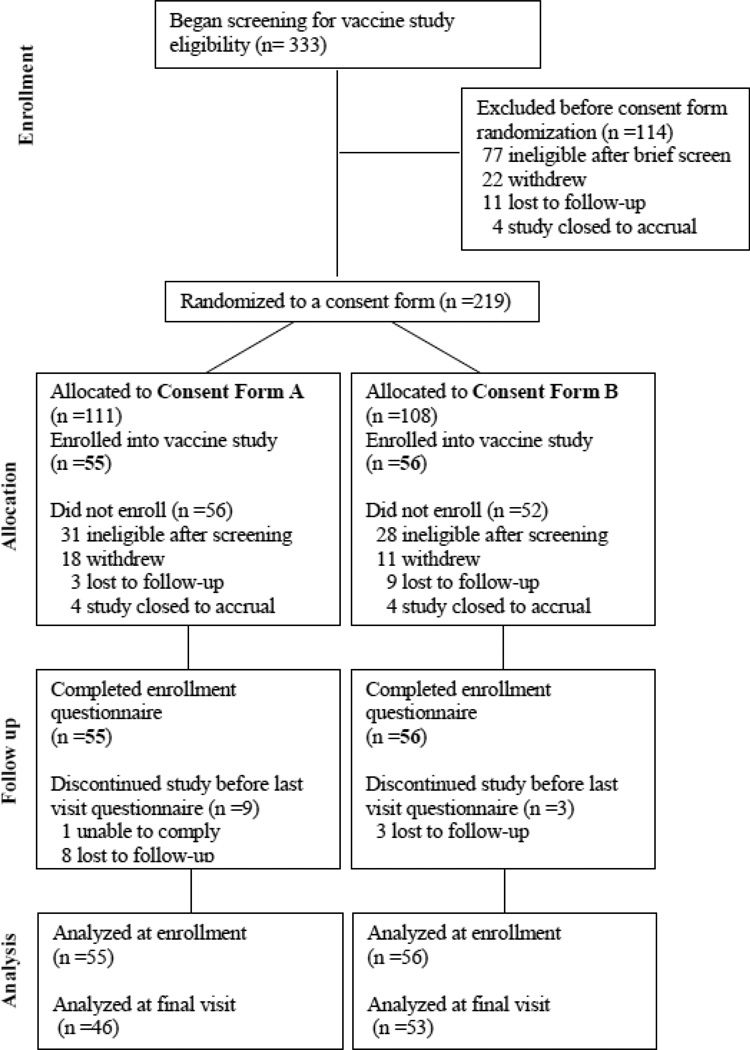
A total of 111 subjects enrolled into the vaccine studies and all completed the initial questionnaire. A flow diagram showing numbers of subjects from screening and randomization through analysis is provided in Figure 1. Overall, 51% were male and 49% female; the mean age was 34.5 years; 91% had college or advanced degrees; 66% were white, 21% were black or African American, 10% were Asian and 3% were all other races combined; and 5% of all participants identified themselves as Hispanic or Latino. Demographic characteristics analyzed statistically by consent randomization group are shown in Table 2. There were no statistically significant differences by gender (percentage of female: 46% versus 52%, p=0.5), age (median: 29 versus 31.5, p=0.09), education (p=0.38), employment status (p=0.07) or prior participation in a research study (p=0.75) between groups randomized to consent form A (standard) and consent form B (concise).
Figure 1.
Consent Study Disposition Flow Diagram
Table 2.
Statistical Comparison of Characteristics by Consent Form Groups
| Consent Form A (n=55) |
Consent Form B (n=56) |
Comparison P value |
|
|---|---|---|---|
| AGE in years: mean (median) | 32.8 (29) | 36.1 (31.5) | 0.09 |
| percentage | |||
| GENDER | 0.50 | ||
| Male | 54.5 | 48.2 | |
| Female | 45.5 | 51.8 | |
| HIGHEST EDUCATION | 0.38 | ||
| Primary school | 0 | 0 | |
| Some high school | 0 | 0 | |
| Graduated from high school | 7.3 | 1.8 | |
| Some college or university | 18.2 | 17.9 | |
| Graduated from college or university | 38.2 | 32.1 | |
| Post-graduate | 36.4 | 48.2 | |
| CURRENT EMPLOYMENT | 0.07 | ||
| No answer | 1.8 | 0 | |
| Employed full time | 63.6 | 55.4 | |
| Employed part time | 3.6 | 17.9 | |
| A student | 20.0 | 17.9 | |
| Retired | 0 | 3.6 | |
| Unemployed | 10.9 | 5.4 | |
| PRIOR RESEARCH STUDY PARTICIPATION | 0.75 | ||
| Never before | 41.8 | 48.2 | |
| 1–2 previous studies | 38.2 | 35.7 | |
| 3–10 previous studies | 18.2 | 12.5 | |
| More than 10 previous studies | 1.8 | 3.6 | |
| REFER TO CONSENT TO ANSWER QUESTIONS? | 0.21 | ||
| No | 76.4 | 85.7 | |
| Yes | 23.6 | 14.3 | |
2. Comprehension and Feeling Well Informed at Enrollment
No statistically significant differences were seen in the overall understanding scores between the two consent form groups (median: 7 versus 7, p=0.79), as shown in Table 3. The mean understanding score on the enrollment consent survey was 6.5 out of 8 multiple choice comprehension questions and the mean AoU score for each group was 20 out of 21 true or false statements. A statistically significant difference was seen between the two groups on responses to only one comprehension question for which more individuals in consent group A chose a wrong answer than group B (see question #17). The question asked who in the study would receive the inactivated influenza vaccine, 58.2 and 67.9% of individuals (groups A and B, respectively) chose the correct answer (“everyone”), but more of group A than B (30.9% vs. 14.3%, p=0.04) chose an incorrect response (“half of those who join”).
Table 3.
Comprehension and Feeling Well Informed at Enrollment
| Form A (n=55) |
Form B (n=56) |
Comparison P value |
||
|---|---|---|---|---|
| Mean (median) | ||||
| Understanding Score; number correct out of 8 multiple choice questions | 6.5 (7) | 6.5 (7) | 0.79 | |
| Assessment of Understanding Quiz: number correct out of 21 true or false statements | 20.4 (21) | 20.1 (20) | 0.13 | |
| Survey Item # | Answer | percentage | ||
| Item #2 | 0.03(*) | |||
| How well informed do you feel right now about this study? | No answer | 0 | 1.8 | 0.50 |
| 1-Not informed at all | 0 | 1.8 | 0.50 | |
| 2-Slightly informed | 0 | 0 | ||
| 3-moderately well informed | 14.5 | 1.8 | 0.02 (*) | |
| 4-very well informed | 85.5 | 94.6 | 0.12 | |
| Item #13 | 0.01(*) | |||
| How well did the research staff (doctors, nurse, others) explain the medical research study to you? | 1-Very well | 89.1 | 100 | 0.01 |
| 2-Fairly well | 10.9 | 0 | 0.01 | |
| 3-Not very well | 0 | 0 | ||
| 4-No one explained the study | 0 | 0 | ||
| 5-I don’t remember | 0 | 0 | ||
| Item #17 | 0.11 | |||
| In this study who will get the [inactivated flu] vaccine? | 1-Everybody in the study | 58.2 | 67.9 | 0.33 |
| 2-Half of those who join | 30.9 | 14.3 | 0.04(*) | |
| 3-Only people assigned to Group 1 | 7.3 | 16.1 | 0.24 | |
| 4-I was not given that information | 3.6 | 1.8 | 0.62 | |
Asterisk (*) indicates statistically significant difference between consent form groups.
Note that not all questions related to understanding are shown in this table.
Some statistically significant differences were noted regarding how informed participants felt and how well they felt the staff had explained the study. Group A individuals were more likely to choose feeling “moderately well informed” than group B (14.5% vs. 1.8%; p=0.02); overall group B had a higher, though not statistically significant, rate (94.6% vs. 85.5%; p=0.12) of answering “very well informed.” Similarly, when asked how well the staff explained the study, group A subjects were more likely to choose “fairly well” than group B (p=0.01); 100% of group B reported that the staff explained the study “very well.” The trend is towards the concise consent group feeling better informed.
3. Satisfaction and Motivation at Enrollment
No significant differences between groups were noted on measures of satisfaction or motivation (Table 4). The majority of both Groups A and B said they found the form they were given was about the right length (80.0% and 85.7%, p=0.46) and detail (87.3% and 92.9%, p=0.36). Few people in either group (10.9% vs 7.1%, p=0.53) chose “reading the consent form” as having a major impact on their decision to join the study. The chosen “main reason” for joining the study was not statistically different between groups (p=0.66) and followed the same order of frequency in selection in Groups A and B. The most common answer was “because I wanted the money” (45.5% vs 32.1%, p=0.18) and the next most common motivation being helping find ways to prevent flu; few people were seeking “protection from flu.”
Table 4.
Satisfaction with the Consent Process and Motivation at Enrollment
| Form A (n=55) |
Form B (n=56) |
Comparison P value |
||
|---|---|---|---|---|
| Survey Item # | Answer | percentage | ||
| Item #1 | 0.31 | |||
| Overall, how satisfied are you with the consent process for this study? | 1-Very satisfied | 94.5 | 89.3 | 0.49 |
| 2-Somewhat satisfied | 5.5 | 10.7 | ||
| 3-Somewhat unsatisfied | 0 | 0 | ||
| 4-Not satisfied at all | 0 | 0 | ||
| Item #9 | 0.70 | |||
| Do you think the consent you were given for this research was… | 1-Much too long | 1.8 | 1.8 | |
| 2-Too long | 16.4 | 12.5 | ||
| 3-About right | 80.0 | 85.7 | 0.46 | |
| 4-Too short | 0 | 0 | ||
| 5-Much too short | 0 | 0 | ||
| 6-I didn’t read it | 1.8 | 0 | ||
| Item #10 | 0.37 | |||
| Do you think the consent form you were given for this research study was… | 1-Much too detailed | 0 | 0 | |
| 2-Too detailed | 10.9 | 5.4 | ||
| 3-About right | 87.3 | 92.9 | 0.36 | |
| 4-Too simple | 0 | 1.8 | ||
| 5-Much too simple | 0 | 0 | ||
| 6-I didn’t read it | 1.8 | 0 | ||
| Item #14 | 0.69 | |||
| Which source of information was more helpful to you? | 1-Reading the consent form | 7.3 | 3.6 | |
| 2-Talking to the research staff | 47.3 | 48.2 | >0.99 | |
| 3-Both were equally helpful | 45.5 | 48.2 | 0.85 | |
| 4-Neither was helpful | 0 | 0 | ||
| Item #4 | 0.66 | |||
| What was the main reason that you joined this study? | 1-Because I wanted the money | 45.5 | 32.1 | 0.18 |
| 2-Because I wanted to help scientists find a way to prevent flu | 29.1 | 30.4 | ||
| 3-Because I am interested in science | 20.0 | 28.6 | ||
| 4-Because I wanted protection from the flu | 1.8 | 3.6 | ||
| 5-Another reason (explain) | 3.6 | 3.6 | ||
| No answer | 0 | 1.8 | ||
Note: To explain selecting “another reason” 3 subjects wrote about helping others or society and 1 wrote “…to see what it was about.”
4. Satisfaction Questionnaire at Last Visit
Data from the last visit questionnaire are shown in Table 5. The rate of last visit questionnaire completion in the Form A group as compared to the Form B group was 83.6% vs 94.6%. The majority in both groups (84.8% vs 86.8%) reported being “very satisfied” and the remainder reported being “satisfied.” Although not of statistical significance the percent reporting the level of detail as “about right” was 78.3 vs 88.7 % for Form A and Form B, respectively.
Table 5.
Satisfaction with the Consent Process at Final Visit
| Form A (n=46) |
Form B (n=53) |
Comparison P value |
||
|---|---|---|---|---|
| Survey Item # | Answer | percentage | ||
| Item #1 | >0.99 | |||
| How satisfied were you with the consent process that occurred before you enrolled in the study? | 1-Very satisfied | 84.8 | 86.8 | 0.78 |
| 2-Satisfied | 15.2 | 13.2 | ||
| 3-Somewhat unsatisfied | 0 | 0 | ||
| 4-It could have been much better | 0 | 0 | ||
| Item #2 | 0.94 | |||
| Was there anything you experienced that you now feel should have been explained better before you enrolled in the study? | 1-No | 100 | 98.1 | >0.99 |
| 2-Yes | 0 | 1.8 | ||
| Item #4 | 0.37 | |||
| Do you think the consent form you were given for this research study was… | 1-Much too detailed | 4.3 | 3.8 | |
| 2-Too detailed | 15.2 | 7.5 | ||
| 3-About right | 78.3 | 88.7 | 0.18 | |
| 4-Too simple | 0 | 0 | ||
| 5-Much too simple | 0 | 0 | ||
| 6-I didn’t read it | 2.2 | 0 | ||
Note: 46 of 55 (83.6%) in the Form A group and 53 of 56 (94.6%) in the Form B group completed the final visit questionnaire. The final visit occurred at study week 24 or 48, depending upon the vaccine study in which the subject was participating.
DISCUSSION
Although the consent process for a clinical trial is intended to ensure that potential participants understand that they are participating in research, as well as the risks, benefits, study procedures and alternatives to enrollment in the study [4], data show that participants have variable levels of understanding of study information [10]. At the same time, clinical research consent forms have become increasingly long and complex [2, 3, 11]. The data from this study of consent forms indicate that the healthy adult participants in these vaccine trials had a similar level of understanding of study information and similar satisfaction with the length and complexity of the consent form regardless of whether they received a standard consent form or a concise form that had about a 63% reduction in the number of words. Those who received the concise form were more likely to report feeling very well informed and that the staff had explained the study to them very well. The evidence from this study is consistent with the hypothesis that a simpler more concise form will not affect comprehension of key study information. Of note, our goal was not to evaluate if a concise consent would improve comprehension. Vaccine study participants at this clinic routinely have a high level of understanding of the research, and the majority of healthy adults who volunteered had at least a college level education. The concern raised about research using a concise consent form was that subjects would be at risk of harm because of insufficient information about research study participation. Although it is logical to assume that the length and complexity of the consent form may affect participant comprehension of the study and satisfaction with the experience, the direction of that effect is unknown, and very few randomized studies have investigated the influence of the length and complexity of consent forms on understanding. It is also possible that the form itself has much less to do with comprehension than multiple discussions with research staff. We did not attempt to evaluate the contribution of discussion to consent comprehension and satisfaction since clearly all subjects must be allowed to ask questions and receive information from qualified clinical site staff.
Common policies and procedures related to consent form content and format that have developed over years within research institutions are the basis for standard practices in clinical research. These reflect an experience-based consensus about what constitutes an adequate consent process for participation in clinical research. To develop a truly evidence-based consensus about best practices for consent to clinical research, data evaluating outcomes of different aspects of the consent process in diverse groups of participants need to be collected and validated. The study reported here is part of a larger endeavor to investigate strategies of improving the informed consent process overall and evaluating consent forms in particular. One goal is to develop more concise and simpler consent forms that are acceptable to IRBs and clinical researchers and shown to be effective at informing study participants in the context of a properly conducted consent process.
As part of the larger consent project initiated through the NIH Clinical Center Department of Bioethics to study the length and complexity of consent forms, the study published by Stunkel, et al reported results from using different length consent forms in a low risk, bioequivalence study of a marketed drug in healthy volunteers in a different setting. Similar to the findings in our study, they reported no evidence of an effect on comprehension or satisfaction by using a concise consent. They also noted that their findings may not be generalizable to Phase I studies of investigational products [6]. Likewise, we recognize that the study results reported here may not be generalizable to other clinical trial circumstances. This comparative investigation of consent forms was conducted at a single site with only 111 healthy adult participants. The participants had high education levels and a little more than half had prior experience with research. It was not expected to be a definitive study, but rather to contribute to the development of approaches to studying consent forms and to the publicly available data on consent form comprehension and satisfaction. We recognize that highly experienced research staff routinely use consent practices that contribute to comprehension of the study apart from the consent form itself. However, despite being limited to a specific type of Phase I setting, our consent form study does serve as a next step in evaluating the consent process when standard and concise consents are used. Research by others, including a study comparing concise and standard consent forms for participants in a cancer research study, also found little difference in understanding when participants were given consent forms of different lengths, but found a participant preference for the concise form. This study is not directly comparable to ours as among other factors, the subjects were not randomized to the consent forms, the two consent reading levels (12th grade vs 5th grade) represented a greater difference in reading level, and the consent study participants were not actual candidates for the study for which the consents were written. [12]. Investigating the use of concise forms in an even greater diversity of clinical trials settings is important to fully address the study hypothesis.
Given these observations to date, one might ask why bother to make the consent form simpler and more concise if consent forms of different lengths result in similar levels of comprehension, as hypothesized. In our study, neither the length of time it took for subjects to read the consent form nor the reading ability of participants was measured, although the study participants overall had a high educational level. Other published data suggest that the concise consent at approximately 2000 words would likely take an adult with average reading skills 8–10 minutes to read, while the standard consent at approximately 5000 words would likely take 20–25 minutes to read [13]. Data collected in other spheres of research show that more people read and understand information that is presented in a shorter format [14]. It might be presumed that the less time it takes to read a consent form the more likely it is that it will be read and understood. The greatest potential benefit of a concise form is likely to be for participants with more limited reading abilities than our well-educated cohort. But everyone involved in the research process, including IRB staff, researchers, subjects of all educational and reading abilities, and regulatory agencies would benefit from shorter forms in terms of greater efficiency in review time, storage, printing and processing.
The Office for Human Research Protections has called for greater transparency of consent form content towards the goal of improving the quality of consent forms and the associated consent process [15]. Results reported here support continuing an evidence-based approach to evaluating both the consent form and the process. Continued investigation of strategies to improve research informed consent is critical and such research should be supported by IRBs towards widely shared goals of developing consistent and high quality consent forms for participation in clinical trials. The accumulated experience to date is that randomizing study participants to a standard or concise consent is an acceptable approach when both the study and the consent forms used are IRB-approved, and there is no evidence of harm by using a shorter consent form or from studying the consent process. Investigation of consent length in multicenter studies with larger numbers of participants, participants more likely to misunderstand aspects of the study, and with participants who have a greater diversity of educational levels are recommended as next steps, in conjunction with continuing exploration of other strategies to improve comprehension and the quality of consent to participate in clinical trials.
Acknowledgements
Funding Support: National Institutes of Health (NIH) through the Vaccine Research Center (VRC), National Institute of Allergy and Infectious Diseases (NIAID) and the Department of Bioethics, NIH Clinical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The VRC 306 and VRC 307 Consent Study Teams at VRC, NIAID, NIH also included Cynthia Starr Hendel, NP, LaSonji Holman, NP, Sarah Plummer, NP, Brenda Larkin, RN, Floreliz Mendoza, RN, Laura Novik, RN, M.A., Jamie Saunders, RN and Kathryn Zephir, RN, MS. We thank the research volunteers for their participation, Barney S. Graham, M.D., Ph.D. (VRC, NAIID, NIH, Bethesda, MD) for his contributions to the conduct of the study and review of the manuscript, the NIAID Intramural IRB for their support for this study, Phyllis Zaia, BS and Ly Diep, BA (EMMES Corp, Rockville, MD) and Olga Vasilenko, MS (Lockheed Martin, Bethesda, MD) for their data management contributions.
REFERENCES
- 1.Sloan J, Resnick GD. The consent form revisited. Arch Intern Med. 1993;153:1170–1173. [PubMed] [Google Scholar]
- 2.Beardsley E, Jefford M, Mileshkin L. Longer consent forms for clinical trials compromise patient understanding: so why are they lengthening? J Clin Oncol. 2007;25:e13–e14. doi: 10.1200/JCO.2006.10.3341. [DOI] [PubMed] [Google Scholar]
- 3.Albala I, Doyle M, Appelbaum PS. The evolution of consent forms for research: a quarter century of changes. IRB. 2010;32:7–11. [PubMed] [Google Scholar]
- 4.Wendler D, Grady C. What should research participants understand to understand they are participants in research? Bioethics. 2008;22:203–208. doi: 10.1111/j.1467-8519.2008.00632.x. [DOI] [PubMed] [Google Scholar]
- 5.Emanuel E, Grady C, Menikoff J. Is longer always better? Hastings Center Report. 2008;38:10–12. [PubMed] [Google Scholar]
- 6.Stunkel L, Benson M, McLellan L, Sinaii N, Bedarida G, Emanuel E, et al. Comprehension and informed consent: assessing the effect of a short consent form. IRB: Ethics & Human Research. 2010;32:1–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Grady C. Ethics of vaccine research. Nat Immunol. 2004;5:465–468. doi: 10.1038/ni0504-465. [DOI] [PubMed] [Google Scholar]
- 8.Beigel JH, Voell J, Huang CY, Burbelo PD, Lane HC. Safety and immunogenicity of multiple and higher doses of an inactivated influenza A/H5N1 vaccine. J Infect Dis. 2009;200:501–509. doi: 10.1086/599992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ledgerwood JE, Wei CJ, Hu Z, Gordon IJ, Enama ME, Hendel CS, et al. DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials. Lancet Infect Dis. 2011;11:916–924. doi: 10.1016/S1473-3099(11)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flory J, Wendler D, Emanuel EJ. Empirical Issues in Informed Consent for Research. In: Emanuel EJ, editor. The Oxford textbook of clinical research ethics. Oxford; New York: Oxford University Press; 2008. [Google Scholar]
- 11.Berger O, Gronberg BH, Sand K, Kaasa S, Loge JH. The length of consent documents in oncological trials is doubled in twenty years. Ann Oncol. 2009;20:379–385. doi: 10.1093/annonc/mdn623. [DOI] [PubMed] [Google Scholar]
- 12.Davis TC, Holcombe RF, Berkel HJ, Pramanik S, Divers SG. Informed consent for clinical trials: a comparative study of standard versus simplified forms. J Natl Cancer Inst. 1998;90:668–674. doi: 10.1093/jnci/90.9.668. [DOI] [PubMed] [Google Scholar]
- 13.Hochhauser M. How long does it take to read a consent form? SoCRA SOURCE. 2008:62–64. [Google Scholar]
- 14.Plaut VC, Bartlett RP., 3rd Blind Consent? A Social Psychological Investigation of Non-Readership of Click-Through Agreements. Law Hum Behav. 2011 doi: 10.1037/h0093969. [DOI] [PubMed] [Google Scholar]
- 15.Menikoff J. Making research consent transparent. JAMA. 2010;304:1713–1714. doi: 10.1001/jama.2010.1492. [DOI] [PubMed] [Google Scholar]