Abstract
Dopamine (DA) plays a critical role in motor control, addiction and reward-seeking behaviors, and its release dynamics have traditionally been linked to changes in midbrain dopamine neuron activity. Here, we report that selective endogenous cholinergic activation achieved via in vitro optogenetic stimulation of nucleus accumbens (NAc), a terminal field of dopaminergic neurons, elicits real-time DA release. This mechanism occurs via direct actions on DA terminals, does not require changes in neuron firing within the midbrain and is dependent on glutamatergic receptor activity. More importantly, we demonstrate that in vivo selective activation of cholinergic interneurons (CINs) is sufficient to elicit DA release in the NAc. Therefore, the control of accumbal extracellular DA levels by endogenous cholinergic activity results from a complex convergence of neurotransmitter/neuromodulator systems that may ultimately synergize to drive motivated behavior.
Introduction
The mesolimbic DA system, comprised mainly of the rostral dopaminergic projection from the ventral tegmental area (VTA) to the NAc, (Wise, 2004; Sulzer, 2011), is crucial for decision making, motivated behaviors and addiction. Patterns and levels of DA in NAc are traditionally determined by the combination of firing of VTA neurons and the dynamics of DA release from the axon terminals.
Although cholinergic interneurons (CINs) are 2-5% of all striatal neurons, they establish an extensive arrangement of axons and form a diffuse neurotransmission system (Descarries et al., 1997; Descarries and Mechawar, 2000). Therefore, cholinergic activity in the striatum has long been hypothesized to play a role in the modulation of DA release (Giorguieff et al., 1976; Zhou et al., 2001; Zhang and Sulzer, 2004; Threlfell et al., 2010). Genetic deletion or pharmacological manipulation studies of nicotinic cholinergic (nAChRs) as well as muscarinic (mAChRs) receptors, have shown that they modulate electrically-evoked DA release in the striatum (Exley et al., 2012; Zhou et al., 2001; Exley et al., 2008, 2011; Zhang et al., 2009; Threlfell et al., 2010). Moreover, nAChR-targeted drugs differentially alter DA release in a frequency-dependent manner. This finding has led to the notion that a “high-pass” filter dependent on antagonism of nAChRs or nicotine, facilitates burst release of DA (Exley and Cragg, 2008), an activity pattern observed mainly following the presentation of reward and reward-predicting cues (Mirenowicz and Schultz, 1996; Nakahara et al., 2004; Roesch et al., 2007; Schultz, 2007; Apicella et al., 1991).
However, the precise role that endogenous cholinergic activity exerts on DA release in the NAc has not been explored. Endogenous release of acetylcholine (ACh) obtained by optogenetic control of CINs allows for the elucidation of cholinergic receptor action when activation occurs by the natural ligand. To examine the effect of endogenously released ACh on terminal DA release we used a combination of optogenetic techniques for selective stimulation of CINs, combined with electrophysiology, real-time detection of DA levels and pharmacology. Activation of CINs is sufficient to evoke DA release in the NAc, independently of contingent activation of the VTA, in vitro and in vivo. We further reveal that endogenous cholinergic control of DA release is not only mediated by nAChRs and modulated by mAChRs, but also results from actions at AMPA receptors. Hence, our results show that endogenous cholinergic activity exerts a powerful influence on accumbal DA release and that this phenomenon recruits a previously unrecognized convergence of neurotransmitter systems.
Results
Endogenous cholinergic activity elicits terminal dopamine release
Studies of nAChR dynamics (desensitization or antagonism) have long suggested that endogenous cholinergic tone may establish the baseline for the probability of DA release modulates DA release in the striatum (Giorguieff et al., 1976; Zhou et al., 2001; Zhang and Sulzer, 2004; Threlfell et al., 2010). This phenomenon has a presynaptic locus of action, as studied by application of exogenous acetylcholine (ACh) or ligands of the nAChR in striatal synaptosomes (Rapier et al., 1990; Wonnacott et al., 2000; Chéramy et al., 1996) or slices (Giorguieff et al., 1977; Wonnacott et al., 2000). However, is not known whether selective activation of CINs and subsequent release of endogenous ACh can directly control DA release. To test this hypothesis, we utilized optogenetic techniques to selectively activate CINs in the NAc. Briefly, we injected an adeno-associated virus encoding channelrhodopsin2 (ChR2) and enhanced yellow fluorescent protein (eYFP) into the NAc of mice expressing Cre-recombinase downstream of the choline-acetyltransferase (ChAT) promoter (ChAT-Cre mice) (see Methods). Four weeks after viral injection, studies were performed in coronal slices of the NAc.
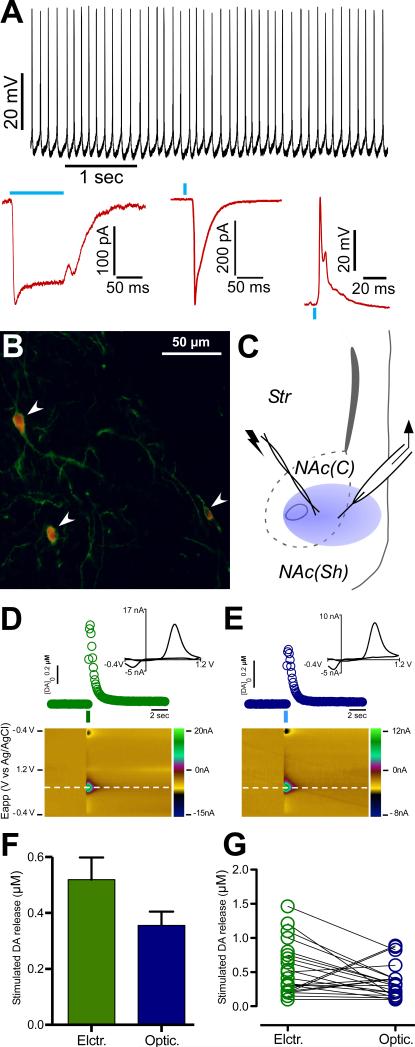
First, we tested the functionality of CINs in our in vitro preparation by performing whole-cell recordings. Under the current-clamp configuration, YFP-positive neurons (putative CINs) displayed the characteristic spontaneous, tonic firing at a rate of ~10 Hz [the average of the membrane potential value cycles between action potentials was -28 mV (Fig. 1A)]. Under voltage clamp, delivery of a single optical (100 ms) or a brief (4 ms) pulse, elicited an inward current that lasted for the length of the pulse (n = 6, Fig. 1A). Furthermore, delivery of a 4 msec blue light pulse under current clamp conditions induced a single action potential on YFP-positive cells (Fig. 1A). Mean latency between the start of the light pulse and the start of the action potential was 5.0 ± 0.36 msec (n = 5, confirming that optical excitation of ChR2 reliably drives generation of action potentials on accumbal CINs). Additionally, histological analyses confirmed expression of ChR2-eYFP (Fig. 1B).
Figure 1. Selective optical stimulation of ChR2-expressing CINs elicits accumbal dopamine release.
A. Top: Trace from a whole-cell recording of a YFP positive neuron (putative CIN). YFP-positive neurons displayed spontaneous, tonic firing at a rate of ~10 Hz. Bottom: Under whole-cell voltage-clamp recording from a YFP-positive neuron, blue light exposure (100 msec, left or 4 msec, middle) induced a ChR2-mediated inward current. Under whole-cell current-clamp recordings of YFP-positive neurons, delivery of a blue light pulse (4 msec, right) induced firing of a single action potential.
B. Image of eYFP-positive (green) cell bodies (arrowheads) counterstained for ChAT (red) and processes from the NAc of a ChAT-Cre mouse transfected with a ChR2-eYFP viral vector.
C. Scheme of the recording arrangement from coronal NAc striatal slices. DA levels were measured by FSCV through a carbon fiber microelectrode (right) while performing electrical stimulation (left) and/or optical stimulation (blue circle) delivered by an optical fiber in apposition with the tissue.
D. Concentration trace (top) and color plot (bottom) for DA release triggered by electrical stimulation of the NAc. Top: Representative trace shows concentration of DA (nM) over time in response to electrical stimulation (indicated by green line). Inset shows characteristic DA voltammogram. Bottom: Corresponding color plot depicts the voltammetric data with time on the X axis, applied scan potential (Eapp) on the Y axis and background-subtracted faradaic current shown on the z-axis in pseudocolor. DA can be identified by an oxidation peak (green) at +0.6V and a smaller reduction peak (yellow) at -0.2V.
E. Concentration trace (top) and color plot (bottom) for DA release triggered by optical stimulation of CINs. Top: As in D, representative trace shows concentration of DA (nM) over time in response to optical stimulation (indicated by blue line) Inset shows characteristic DA voltammogram. Bottom: Corresponding color plot of voltammetric data.
F. Bar graph represents peak values of accumbal DA release obtained by electrical and optical stimulation.
G. Dispersion plot indicating peak values for all the experiments performed under electrical (green circles) and optical stimulation (blue circles).
Error bars represent Standard Error of the Mean (SEM).
To verify if selective stimulation of CINs is sufficient to evoke accumbal DA release, optical stimulation was delivered by a 125 μm optical fiber placed on the surface of the slice, while real-time changes in DA release were measured with fast-scan cyclic voltammetry (FSCV) using a glass-encased carbon fiber placed 100 μm away from the optical fiber (Fig. 1C). Single pulse optical stimulation (4 ms duration square, 10 mW) in coronal slices of NAc from ChR2-expressing ChAT-Cre mice elicited an immediate and robust increase in DA levels, which was comparable to single pulse electrical stimulation (400 mA; 4 ms duration square; 23 paired measurements made in 12 animals) (Figure 1D-G). This result confirms that endogenous ACh actions on DA terminals are sufficient to elicit DA release in the NAc.
Frequency-dependence of cholinergic control of dopamine release
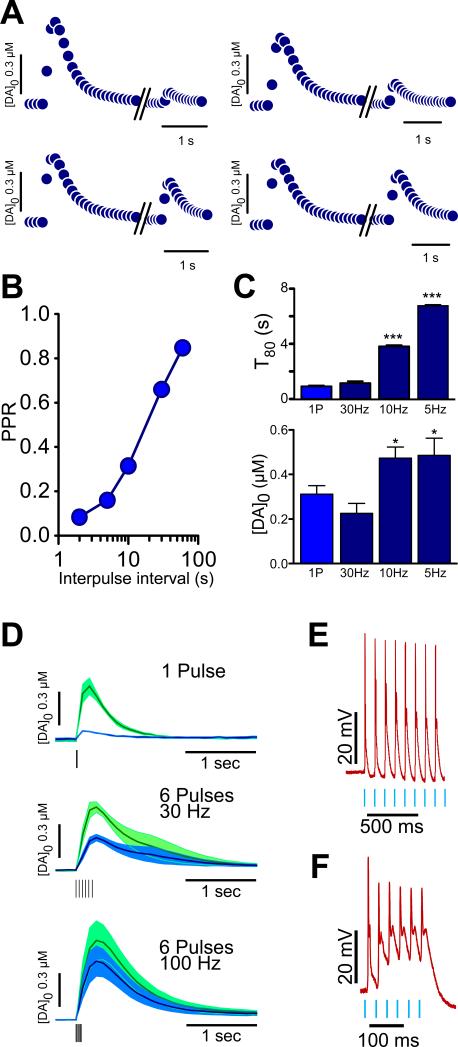
Because of cholinergic receptor agonists modulate the release of DA, we hypothesized that changes in CIN firing rate should correspondingly enhance DA release. To test this, we measured DA following application of different patterns of CIN optical stimulation. First, we monitored DA levels in response to paired pulse optical stimulations at different intervals. When two pulses of blue light (10 mW, 4 msec duration) were delivered at an interval of 2 sec, the second pulse triggered a second peak of DA, which was 8.3 ± 1.6 % (n=4), of the first peak (Fig 2A). Subsequent tests showed lessened reduction in the second peak down of 16 ± 2.1% at 5 s intervals (n=4), 31.5 ± 3.1% at 10 s (n=4), 66.0 ± 3.0% at 30 s (n=4) and 84.75 ± 1.1% at 60 sec interval (n=4) (Fig. 2A,B). This suggests that when the conditions for endogenous ACh release are met, a frequency-dependent limiting factor for ACh/DA release exists, most probably resulting from activation of DA or/and ACh auto-receptors. Next, we monitored DA levels following different patterns of optical stimulation to determine the optimal range of CIN activity resulting in enhanced DA release. When compared to single pulse stimulation (n=17), analysis of T80 values (see supplemental materials) of DA concentration versus time curves showed a significant T80 increase following stimulation at 5Hz (p < 0.0001, n= 15) and at 10Hz (p < 0.0001, n=16) but not at 30 Hz (n=10) (Fig. 2C, top). DA peak values were also significantly different when triggered by 5Hz (p < 0.05) and 10Hz (p < 0.001), when compared to single pulse stimulation (Fig. 2C, bottom) This suggests that CIN firing rates between ~5-10 Hz (frequencies well within the normal range of CIN firing) preferentially control release mechanisms. Application of TTX completely abolished DA release triggered by optical activation of CINs with a single pulse or 10 Hz trains (n=2; data not shown), confirming that the control of CIN activity over DA release is action potential-dependent.
Figure 2. Frequency-dependent response of DA release evoked by stimulation of CINs.
A. Average traces of DA levels evoked by paired pulses of optical stimulation of CINs delivered at 5, 10, 30 and 60 seconds intervals.
B. Summary plot of DA peak amplitudes in response to paired pulse ratio stimulation of CINs.
C. Top: Summary bar graph showing the average T80 decay values for DA release evoked by 5, 10, 30 Hz and single pulse stimulation. Bottom: Summary graph of peak values of DA release evoked by 5, 10, 30 Hz and single pulse stimulation.
D. Average traces of DA levels triggered by different patterns of electrical stimulation, while sustained optical stimulation of CINs was being performed
E. Representative traces from whole-cell recordings under current clamp of CIN neurons showing responsiveness to 10Hz optical stimulation.
F. As in E., representative trace depicting CIN responsiveness to 30 Hz optical stimulation.
Error shadows or bars represent SEM.
Application of nicotine acts as a “high-pass” filter facilitating burst release of DA (Exley and Cragg, 2008). Specifically, electrical stimulation frequencies ≤10 Hz elicit lower DA levels compared to control, while stimulation frequencies ≥25 Hz elicit higher DA levels compared to control (Rice and Cragg, 2004). We tested if this filtering phenomenon occurs following sustained CIN activation. To do this, we applied brief electrical stimulation at different frequencies, while long optical stimulation trains to CINs (50 pulses of 4 ms duration delivered at 5 Hz, 10 mW) were applied. Under these conditions, all of the tested protocols of electrical stimulation (1 pulse, 6 pulses at 30Hz and 6 pulses of 100Hz) evoked lower peak levels of DA release, compared to peak levels obtained in absence of optical stimulation (17.6 % at 1 pulse, p < 0.0001; 51.3% at 30Hz, p < 0.0001, 74.1% at 100Hz, p > 0.05; n= 3-6) (Fig. 2D). Therefore, endogenous cholinergic activity under the present experimental conditions does not produce the high-pass filtering elicited by bath application of nicotine. To confirm whether CINs follow high frequency patterns of optical stimulation, we performed intracellular electrophysiological recordings. Under whole-cell current clamp, optical stimulation of CINs at 10 Hz elicited a corresponding sequence of action potentials at 10 Hz (Fig. 2E). However, optical stimulation at 30 Hz elicited a sequence of action potentials where only the first action potential reached full amplitude (Fig. 2F), suggesting that CINs optimally follow optical stimulation at a rate of ~10 Hz. However, CIN responsiveness is limited at higher stimulation frequencies (although this may also result from ChR2 channel dynamics).
β2-containing nicotinic receptors mediate DA release evoked by endogenous cholinergic activity
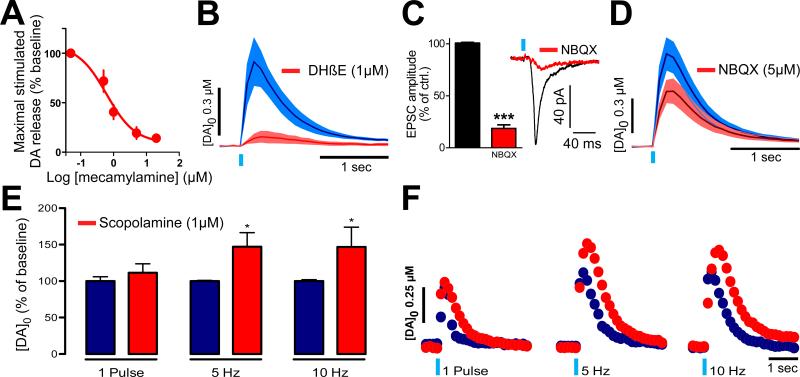
nAChRs are involved in the presynaptic control of DA release in the striatum (Hersch et al., 1994; Marshall et al., 2002; Rapier et al., 1990; Wonnacott et al., 2000). To confirm that CIN-driven enhancement of DA release is mediated by activation of nAChRs, we monitored DA levels while performing selective optical activation of CINs in the absence and presence of nAChR antagonists. Mecamylamine (n=6) decreased CIN stimulation-induced DA release with a half maximal inhibitory concentration (IC50) of 0.61 μM (Fig. 3A). The highest dose used (20 μM) decreased DA levels by 85.8 ± 3 % compared to pre-drug conditions.
Figure 3. Modulation of DA levels evoked by endogenous cholinergic activity.
A. Concentration-response plot showing the effect of increasing concentrations of the nAChR antagonist mecamylamine on DA peak levels evoked by single pulses of optical stimulation. IC = 0.61 μM.
B. Effect of the β2 subunit nAChR antagonist DHβE (1 μM) on DA levels evoked by single pulse (left) and train (right) optical stimulation of CINs.
C. Trace of an EPSC from a medium spiny neuron (MSN) under voltage clamp, elicited by single pulse optical stimulation of CINs. Effect of NBQX (5 μM) on the MSN EPSC.
D. Effect of the application of the AMPA receptor antagonist NBQX (5 μM) on DA levels evoked by single pulse optical stimulation of CINs.
E. Summary bar graph showing the effect of the mAChR antagonist scopolamine (1 μM) on DA peak levels evoked by single pulse, 5Hz and 10Hz optical stimulation of CINs, compared to pretreatment.
F. Representative traces of DA concentration transients triggered by single pulse, 5Hz and 10Hz optical stimulation of CINs in the presence of scopolamine, compared to pretreatment.
Error shadows or bars represent SEM.
In mammals, combinations of nAChR subunits from subfamilies II (α7) and III (α2–α6, β2–β4) result in the formation of functional hetero- and homo- pentamers (Le Novère et al., 2002). In striatal DA terminals, heteropentamers display two α/β pairs in the form of α4/β2 and/ or α6/β2 and/or α4/β4 (Champtiaux et al., 2003). Nicotinic control of striatal DA release depends on β2 subunit-containing nAChRs (Zhou et al., 2001; Exley et al., 2012). To verify the role of β2-containing receptors in the augmentation of accumbal DA release by selective activation of CINs, we examined the effect of the β2-containing nAChR antagonist dihydro-β-erythroidine (DHβE). Application of DHβE (1 μM) resulted in a potent reduction of DA peak levels elicited by optical stimulation (by 89.05 % relative to pretreatment values; p < 0.0001; n=4) (Fig. 3B).
Glutamate acting at AMPA receptors mediates DA release evoked by selective activation of CINs
Glutamate receptors also control DA release presynaptically (Desce et al., 1992; Krebs et al., 1991; Chéramy et al., 1986a, 1998, 1996) and CINs have recently been shown to release glutamate (Higley et al., 2011; Guzman et al., 2011). Moreover, several effects evoked by CIN activity are thought to be mediated by glutamate and not by ACh (Guzman et al., 2011). Because of our findings that endogenous cholinergic activity drives DA release in a nAChR-dependent fashion, we next examined if this occurred, at least in part, through activation of AMPA receptors. First, we confirmed that stimulation of CINs led to glutamate release. Under whole-cell voltage-clamp, single pulse optical stimulation of CINs evoked a single EPSC in medium spiny neurons (MSNs) (Fig. 3C). Furthermore, optical train stimulation failed to produce subsequent EPSCs following the initial pulse in the trains (5 pulses at 2 or 30 Hz; see supplemental material). Bath application of the AMPA receptor antagonist NBQX (5 μM) attenuated the EPSC amplitude to 18.6% ± 3.4% of the pretreatment value; p = 0.0002, n = 4. This confirms that selective optical stimulation of CINs drives AMPA receptor activation on MSNs (Higley et al., 2011). Next, we monitored DA release changes triggered by selective CIN optical stimulation following NBQX. When added to the bath solution, NBQX (5 μM) significantly decreased the peak amplitude of DA release by 40.3% p < 0.01, n = 7; Fig. 3D, confirming that AMPA receptors contribute to the control of DA release evoked by CIN activation in the NAc.
mAChR receptor activation modulates cholinergic-driven release of dopamine
mAChRs are also present in the striatum (Hersch et al., 1994) and the mAChR agonist oxotremorine decreases DA release evoked by single pulse electrical stimulation, but increases release evoked by high frequencies (Threlfell et al., 2010). Furthermore, genetic deletion of the muscarinic M4 subunit prevents this modulation of DA release in NAc (Threlfell et al., 2010). However, in contrast to nAChRs, there is no evidence of M4 expression on DA terminals, suggesting that mAChR activation preferentially inhibits ACh release in the NAc (Threlfell et al., 2010; Threlfell and Cragg, 2011).
To identify the role of mAChRs on DA release evoked by endogenous cholinergic activity, we next tested the effect of the mAChR antagonist scopolamine on DA levels triggered by selective stimulation of CINs. When scopolamine (1 μM) was applied to the bath, DA peak levels evoked by a single pulse of selective CIN stimulation only modestly increased to 112 % of pretreatment values; p = 0.38. When CINs were optically stimulated by a train of 30 pulses at 10 or 5 Hz (10 mW, 4 msec duration per pulse), peak DA levels significantly increased to 146-148 %, p < 0.05) of pretreatment values (n=6-12) (Fig. 3E,F) suggesting that blocking mAChRs relieves the inhibition of ACh release and that this effect is preferentially seen when trains of action potentials drive mobilization of the ACh releasable pool.
In vivo selective stimulation of accumbal cholinergic interneurons induces dopamine release
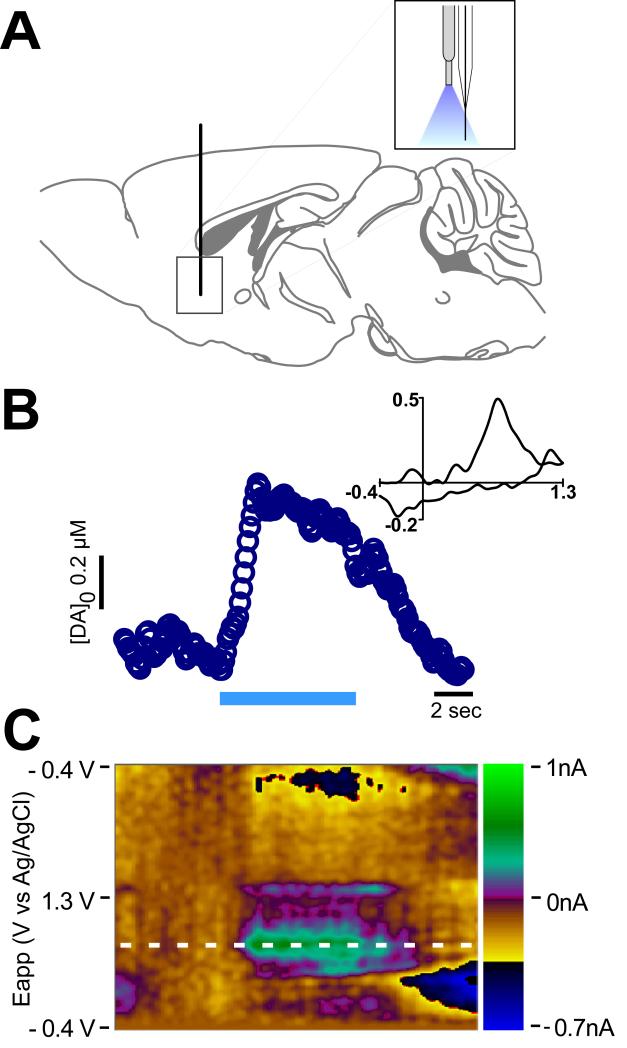
The present results suggest that termino-terminal endogenous cholinergic activity controls DA release and that this involves activation of glutamate receptors in vitro. However, it is not known if promoting DA release by selective activation of CINs occurs in vivo. To test this possibility, FSCV recordings combined with optical stimulation from adjacent sites (200 μm separation) were performed by implantation of an optical fiber/carbon fiber arrangement (optrode, see Methods, Fig. 4A) into the NAc of urethane-anesthetized mice. Because under these recording conditions the recording electrode cannot be optimally placed in the area of highest fluorescence under visual control, the conditions necessary to obtain CIN-evoked release were different from those used in the slice. Optical stimulation was reliably achieved by delivery of a train of blue light (473 nm wavelength, 10 mW, 4 msec duration/pulse, 150 pulses, 20 Hz) through the optical fiber of the implanted optrode. Selective activation of accumbal CINs triggered a significant increase in DA concentration (18.2 ± 2.1 nM), respect to baseline values; n= 3) (Fig. 4B,C), providing unambiguous in vivo evidence that CIN activity locally enhances DA release in the NAc.
Figure 4. In vivo selective stimulation of CINs evokes accumbal DA release.
A. Scheme depicting implantation of the optrode (optical fiber/carbon fiber arrangement) used to optically stimulate and record FSCV from a contiguous area in the NAc in vivo.
B. Concentration trace for DA release triggered by a 7 seconds-long (20 Hz, 150 pulses, 10 mW) optical stimulation of the NAc. Representative trace shows concentration of DA (nM) over time in response to optical stimulation (indicated by blue line). Inset shows characteristic DA voltammogram.
C. Corresponding color plot depicts the voltammetric data with time on the X axis, applied scan potential (Eapp) on the Y axis and background-subtracted faradaic current shown on the z-axis in pseudocolor.
Discussion
Cholinergic receptor activation potently controls striatal levels of DA, a neuromodulator crucial for the expression of coordinated motor activity and Pavlovian cue-reward associations (reviewed by Wise, 2004; Sulzer, 2011). In this report, we characterize the effects of selective CIN activation on accumbal DA levels. We find that, while having a relatively sparse distribution, CINs profoundly modulate DAergic output in NAc.
We show that selective optogenetic stimulation of CINs evokes DA release in a β2-containing nAChR-dependent manner. While electrophysiological studies have hypothesized that dopamine can be released in a manner that is not contingent upon ongoing activity in dopaminergic fibers (Ding et al., 2010), our data reveal previously unseen dynamics of this release process directly. Furthermore, we identify the convergence of different neurotransmitter systems participating in this phenomenon. Increased DA concentration during blockade of mAChRs suggests a critical role of these receptors in controlling ACh release. Consistent with recent reports demonstrating glutamate release from CIN terminals, interfering with AMPA receptor signaling weakens optically evoked DA release. More importantly, we determine that DA release can also be evoked by blue light activation of CINs in vivo.
Study of frequency-dependent relations between CIN stimulation and DA levels showed a clear paired-pulse depression, suggesting strong mechanisms of presynaptic control of release at either, or both, CIN and DA neuron terminals. Although this has been described separately at DA and ACh synapses, more detailed studies are necessary to demonstrate how interactions between these two sites of release interact into determining final DA levels. Moreover, we report that sustained optical stimulation of CINs does not mimic the nicotine-dependent high-pass filtering of electrically evoked DA release (Exley and Cragg, 2008). Together, these results point to a crucial role of mAChR activation in limiting the effects of persistent endogenous ACh activity on nAChRs. This feedback mechanism is absent under the effect of nicotine, which promotes desensitization of nAChRs, thought to be the main mechanism underlying nicotine-evoked high-pass filtering of DA release (Rice and Cragg, 2004; Exley and Cragg, 2008). In support of this notion, we confirmed that β2-containing nAChRs mediate ACh-evoked release of DA, and that mAChRs play a predominant role in limiting endogenous ACh release, because ACh-evoked DA release is enhanced (albeit modestly) following blockade of mAChRs.
Glutamate modulates DA release by acting on dopaminergic terminals (Chéramy et al., 1986b; Krebs et al., 1991; Chéramy et al., 1998, 1996) and because CINs mediate glutamatergic transmission (Guzman et al., 2011; Higley et al., 2011), we hypothesized that a fraction of the DA released by selective stimulation of CINs involves activation of glutamate receptors. Supporting this view, we found that CIN-evoked DA release relies –at least partially– on activation of AMPA receptors. This establishes even broader implications, given that glutamate released from CINs mediates not only excitation of MSNs, as previously described (Higley et al., 2011), but also shapes accumbal DA release.
The present experiments uncover a multiplicity of regulatory mechanisms that converge to control DA release elicited by the selective activation of CINs. In behaving animals, CINs encode reward-related events (Morris et al., 2004). While DA neurons increase or decrease their basal firing rate in response to the presentation or omission of reward, CINs respond with a brief pause independently of the outcome (Aosaki et al., 1994; Morris et al., 2004). This has been interpreted as the establishment of the appropriate temporal window for contingencies to be encoded, while DAergic responses are theorized to carry a learning signal about future outcomes (Morris et al., 2004). Here, we determined that in vivo DA release is in fact triggered by endogenous release of ACh. This allows new considerations to be taken into account for the way that CIN activity may set the stage for DA neuron activity to produce its postsynaptic effects. Reward-related activity of CINs consists of several phases (initial rise, pause, and second rise) (Morris et al., 2004; Aosaki et al., 1994; Shimo and Hikosaka, 2001; Apicella et al., 1991, 2011; Apicella, 2007). In response to reward, the peak of the initial phase coincides with the rise in DA neuron activity. We speculate that the initial rise phase of CIN firing rate and subsequent ACh-Glu release could act as a priming event, exciting MSN neurons and boosting DA release originating from the midbrain, whereas the transition to the pause in CIN activity may allow for the hypothesized contrast enhancement of the midbrain signal (Zhang and Sulzer, 2004; Cragg, 2006; Nicola et al., 2004). Moreover, activation of nAChRs promotes long-term depression of corticostriatal glutamatergic transmission via regulation of DA release (Partridge et al., 2002), and thus our findings provide evidence of a link between CIN activity and synaptic plasticity implicated in reinforcement learning. Our results generate a novel conceptual framework with which to interpret the regulation of accumbal DA release and its role in reward-directed behaviors.
Methods
Subjects
Male ChAT-Cre mice were used. They were single-housed in a room under 12 hour light/dark cycle and food/water was available ad libitum.
Stereotaxic virus injection
An AAV-ChR2 vector was injected bilaterally (500 nl/side) into the nucleus accumbens of mice that were allowed to recover for 4 - 8 weeks after virus injection, before any subsequent intervention.
Histology
At least 4 weeks after viral injection mice were perfused transcardially with 4% paraformaldehyde. Brains were extracted, sliced and processed with an anti-ChAT polyclonal antibody and a fluorescein-conjugated secondary antibody. Sections were then mounted and visualized by epifluorescence with a Leica DM LB microscope to identify ChAT-labeled and eYFP-positive neurons.
In vitro optical stimulation and fast-scan cyclic voltammetry
4 weeks after virus injection, mice were killed by decapitation, the brain quickly removed and incubated in modified Krebs buffer. Coronal slices containing the NAc (250 μm thick) were obtained, incubated and then transferred to the recording chamber, perfused at 1 ml/min with 34°C oxygenated Krebs buffer.
Using a cylindrical carbon fiber, voltammetric recordings (versus an Ag/AgCl reference electrode) were performed using Demon Voltammetry and Analysis Software. Optical and electrical stimulation were delivered through an optical fiber in apposition with the brain slice and through a bipolar tungsten electrode in contact with the slice, respectively.
Whole-cell electrophysiological recordings
After incubation, brain striatal slices were transferred to the recording chamber, superfused with artificial cerebrospinal fluid and maintained at 30 ± 1° C. Current- and voltage- clamp recordings of CINs were performed in unmodified aCSF, while voltage clamp recordings of MSN EPSCs were performed in aCSF containing 50 μM picrotoxin to block GABAA-mediated currents. Optical stimulation was delivered via the epifluorescence light path. Clampex 10.3 software was used for data acquisition.
In vivo optical stimulation and fast-scan cyclic voltammetry
4 weeks after virus injection, access to the NAc was obtained under stereotaxic surgery. An optrode (carbon fiber/optical fiber) was implanted and voltammetric recordings (versus an Ag/AgCl reference electrode) performed using Tar Heel CV software.
Statistical Analysis
Unless otherwise indicated, 2-way ANOVA followed by Bonferroni corrections was performed using Prism (GraphPad Software, CA).
Supplementary Material
Highlights.
Selective cholinergic interneuron activation evokes accumbal dopamine release.
Cholinergic control of DA involves glutamatergic actions in vitro.
Accumbal regulation of DA release by cholinergic activity also occurs in vivo.
This interaction may be a novel target for the treatment of disorders of motivation.
Acknowledgements
This work was supported by NIDA grants DA022340 and DA025890 to JFC, and the Intramural Research Program at NIAAA (YM, BNM, DML) or NIDA (MM and HLW). The authors would like to acknowledge Dr. Garret Stuber for assistance with optogenetic techniques.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel A, Kimura M. Responses of tonically active neurons in the primate's striatum undergo systematic changes during behavioral sensorimotor conditioning. The Journal of neuroscience. 1994;14:3969–3984. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P. Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci. 2007;30:299–306. doi: 10.1016/j.tins.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Apicella P, Ravel S, Deffains M, Legallet E. The role of striatal tonically active neurons in reward prediction error signaling during instrumental task performance. J Neurosci. 2011;31:1507–1515. doi: 10.1523/JNEUROSCI.4880-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P, Scarnati E, Schultz W. Tonically discharging neurons of monkey striatum respond to preparatory and rewarding stimuli. Experimental Brain Research. Experimentelle Hirnforschung. Expérimentation Cérébrale. 1991;84:672–675. doi: 10.1007/BF00230981. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Léna C, Clementi F, Moretti M, Rossi FM, Le Novère N, et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chéramy A, Godeheu G, L'Hirondel M, Glowinski J. Cooperative contributions of cholinergic and NMDA receptors in the presynaptic control of dopamine release from synaptosomes of the rat striatum. J Pharmacol Exp Ther. 1996;276:616–625. [PubMed] [Google Scholar]
- Chéramy A, L'hirondel M, Godeheu G, Artaud F, Glowinski J. Direct and indirect presynaptic control of dopamine release by excitatory amino acids. Amino Acids. 1998;14:63–68. doi: 10.1007/BF01345244. [DOI] [PubMed] [Google Scholar]
- Chéramy A, Romo R, Godeheu G, Baruch P, Glowinski J. In vivo presynaptic control of dopamine release in the cat caudate nucleus--II. Facilitatory or inhibitory influence of L-glutamate. Neuroscience. 1986a;19:1081–1090. doi: 10.1016/0306-4522(86)90124-7. [DOI] [PubMed] [Google Scholar]
- Chéramy A, Romo R, Godeheu G, Baruch P, Glowinski J. In vivo presynaptic control of dopamine release in the cat caudate nucleus--II. Facilitatory or inhibitory influence of L-glutamate. Neuroscience. 1986b;19:1081–1090. doi: 10.1016/0306-4522(86)90124-7. [DOI] [PubMed] [Google Scholar]
- Costa E, Cheney DL, Murray TF. Levonantradol-induced inhibition of acetylcholine turnover in rat hippocampus and striatum. J Clin Pharmacol. 1981;21:256S–261S. doi: 10.1002/j.1552-4604.1981.tb02603.x. [DOI] [PubMed] [Google Scholar]
- Cragg SJ. Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci. 2006;29:125–131. doi: 10.1016/j.tins.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Descarries L, Gisiger V, Steriade M. Diffuse transmission by acetylcholine in the CNS. Prog Neurobiol. 1997;53:603–625. doi: 10.1016/s0301-0082(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Descarries L, Mechawar N. Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog Brain Res. 2000;125:27–47. doi: 10.1016/S0079-6123(00)25005-X. [DOI] [PubMed] [Google Scholar]
- Desce JM, Godeheu G, Galli T, Artaud F, Chéramy A, Glowinski J. L-glutamate-evoked release of dopamine from synaptosomes of the rat striatum: involvement of AMPA and N-methyl-D-aspartate receptors. Neuroscience. 1992;47:333–339. doi: 10.1016/0306-4522(92)90249-2. [DOI] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–2166. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, Marti F, Threlfell S, Cazala P, McIntosh JM, et al. Distinct contributions of nicotinic acetylcholine receptor subunit alpha4 and subunit alpha6 to the reinforcing effects of nicotine. Proc Natl Acad Sci U S A. 2011;108:7577–7582. doi: 10.1073/pnas.1103000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, McIntosh JM, Marks MJ, Maskos U, Cragg SJ. Striatal 5 Nicotinic Receptor Subunit Regulates Dopamine Transmission in Dorsal Striatum. J Neurosci. 2012;32:2352–2356. doi: 10.1523/JNEUROSCI.4985-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008;153(Suppl):S283–S297. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorguieff MF, Le Floc'h ML, Glowinski J, Besson MJ. Involvement of cholinergic presynaptic receptors of nicotinic and muscarinic types in the control of the spontaneous release of dopamine from striatal dopaminergic terminals in the rat. J Pharmacol Exp Ther. 1977;200:535–544. [PubMed] [Google Scholar]
- Giorguieff MF, Le Floc'h ML, Westfall TC, Glowinski J, Besson MJ. Nicotinic effect of acetylcholine on the release of newly synthesized [3H]dopamine in rat striatal slices and cat caudate nucleus. Brain Research. 1976;106:117–131. doi: 10.1016/0006-8993(76)90077-9. [DOI] [PubMed] [Google Scholar]
- Guzman MS, De Jaeger X, Raulic S, Souza I. a, Li AX, Schmid S, Menon RS, Gainetdinov RR, Caron MG, Bartha R, et al. Elimination of the vesicular acetylcholine transporter in the striatum reveals regulation of behaviour by cholinergic-glutamatergic co-transmission. PLoS Biol. 2011;9:e1001194. doi: 10.1371/journal.pbio.1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch S, Gutekunst C, Rees H, Heilman C, Levey A. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype- specific antibodies. J. Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, Lowell BB, Kreitzer AC, Sabatini BL. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS One. 2011;6:e19155. doi: 10.1371/journal.pone.0019155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs MO, Desce JM, Kemel ML, Gauchy C, Godeheu G, Cheramy A, Glowinski J. Glutamatergic Control of Dopamine Release in the Rat Striatum: Evidence for Presynaptic N-Methyl-D-Aspartate Receptors on Dopaminergic Nerve Terminals. Journal of Neurochemistry. 1991;56:81–85. doi: 10.1111/j.1471-4159.1991.tb02565.x. [DOI] [PubMed] [Google Scholar]
- Marshall DL, Redfern PH, Wonnacott S. Presynaptic Nicotinic Modulation of Dopamine Release in the Three Ascending Pathways Studied by In Vivo Microdialysis: Comparison of Naive and Chronic Nicotine-Treated Rats. Journal of Neurochemistry. 2002;68:1511–1519. doi: 10.1046/j.1471-4159.1997.68041511.x. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Itoh H, Kawagoe R, Takikawa Y, Hikosaka O. Dopamine neurons can represent context-dependent prediction error. Neuron. 2004;41:269–280. doi: 10.1016/s0896-6273(03)00869-9. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Hopf FW, Hjelmstad GO. Contrast enhancement: a physiological effect of striatal dopamine? Cell Tissue Res. 2004;318:93–106. doi: 10.1007/s00441-004-0929-z. [DOI] [PubMed] [Google Scholar]
- Le Novère N, Corringer P-J, Changeux J-P. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol. 2002;53:447–456. doi: 10.1002/neu.10153. [DOI] [PubMed] [Google Scholar]
- Partridge JG, Apparsundaram S, Gerhardt GA, Ronesi J, Lovinger DM. Nicotinic acetylcholine receptors interact with dopamine in induction of striatal long-term depression. J Neurosci. 2002;22:2541–2549. doi: 10.1523/JNEUROSCI.22-07-02541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapier C, Lunt GG, Wonnacott S. Nicotinic modulation of [3H]dopamine release from striatal synaptosomes: pharmacological characterisation. J Neurochem. 1990;54:937–945. doi: 10.1111/j.1471-4159.1990.tb02341.x. [DOI] [PubMed] [Google Scholar]
- Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–170. doi: 10.1016/j.neuron.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Calu DJ, Schoenbaum G. Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards. Nat Neurosci. 2007;10:1615–1624. doi: 10.1038/nn2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Shimo Y, Hikosaka O. Role of tonically active neurons in primate caudate in reward-oriented saccadic eye movement. J Neurosci. 2001;21:7804–7814. doi: 10.1523/JNEUROSCI.21-19-07804.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Clements M. a, Khodai T, Pienaar IS, Exley R, Wess J, Cragg SJ. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 2010;30:3398–3408. doi: 10.1523/JNEUROSCI.5620-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Cragg SJ. Dopamine signaling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons. Front Syst Neurosci. 2011;5:1–10. doi: 10.3389/fnsys.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L, Jones IW. Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. European Journal of Pharmacology. 2000;393:51–58. doi: 10.1016/s0014-2999(00)00005-4. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zhang L, Liang Y, Siapas AG, Zhou F-M, Dani J. a. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci. 2009;29:4035–4043. doi: 10.1523/JNEUROSCI.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani J. a. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.