Summary
In neurons transmembrane proteins are targeted to dendrites in vesicles that traffic solely within the somatodendritic compartment. How these vesicles are retained within the somatodendritic domain is unknown. Here we use a novel pulse chase system, which allows synchronous release of exogenous transmembrane proteins from the endoplasmic reticulum, to follow movements of post-Golgi transport vesicles. Surprisingly, we found that post-Golgi vesicles carrying dendritic proteins were equally likely to enter axons and dendrites. However, once such vesicles entered the axon they very rarely moved beyond the axon initial segment, but instead either halted or reversed direction in an actin and Myosin Va-dependent manner. In contrast, vesicles carrying either an axonal or a nonspecifically localized protein only rarely halted or reversed and instead generally proceeded to the distal axon. Thus, our results are consistent with the axon initial segment behaving as a vesicle filter that mediates the differential trafficking of transport vesicles.
Introduction
Differential targeting of neuronal proteins to either the axonal or somatodendritic compartment is essential for the establishment and maintenance of neuronal structure and function (Horton and Ehlers, 2003b). During synthesis in the secretory pathway axonal and dendritic transmembrane proteins are sorted into distinct vesicles at the trans-Golgi membrane through the interaction of peptide motifs, clathrin adaptor proteins and vesicular coat proteins (Matsuda et al., 2008). Subsequent to this loading process, post-Golgi transport vesicles carrying dendritic proteins traffic solely within the somatodendritic compartment (Burack et al., 2000; Silverman et al., 2001). Although the mechanism by which such vesicles are confined to the somatodendritic domain remains poorly understood, other aspects of dendritic protein trafficking have been clarified by recent experiments. For instance, dendritic transport vesicles are likely carried by kinesin motors, as blocking kinesin function restricts dendritic proteins to the cell body and proximal dendrites (Chu et al., 2006; Guillaud et al., 2003; Setou et al., 2002).
Many kinesin motors that carry dendritic proteins, such as Kif17, are found only in the somatodendritic compartment, which would suggest that they preferentially bind to microtubules that project to dendrites and avoid those that project to the axon (Setou et al., 2000). Surprisingly though, a Kif17 mutant that lacks a tail domain, so that it works in an autonomous manner, travels to both axons and dendrites, indicating that it has no preference for dendritically projecting microtubules (Nakata and Hirokawa, 2003). This conclusion is corroborated by results from experiments in COS cells showing that Kif17 moves both on stable microtubules, which predominate in axons, and unstable microtubules, which are more abundant in dendrites (Cai et al., 2009). Thus, the dendritic kinesin Kif17 cannot autonomously distinguish between axonal and dendritic microtubules, which suggests that Kif17 likely works in concert with additional proteins that cause it to interact preferentially with microtubules within the somatodendritic compartment.
Several experiments suggest that actin and myosin are involved in directing proteins to either the axonal or the somatodendritic compartments. Interaction with Myosin Va, a plus end-directed motor, is both necessary and sufficient for dendritic localization of transmembrane proteins, while Myosin VI, a minus end-directed motor, is similarly important for the localization of proteins to the surface of the axon (Lewis et al., 2009; Lewis et al., 2011). Disruption of actin filaments results in the nonspecific localization of both axonal and dendritic proteins. Actin-based structures within the axon initial segment (AIS) restrict the diffusion of surface proteins between the axonal and dendritic compartments (Winckler et al., 1999) and when large molecules are injected into the cell body their diffusion into the axon is limited in an actin-dependent fashion (Song et al., 2009). Despite this evidence that actin and myosin are involved in protein localization, the specific mechanisms by which myosin motors and actin might work to direct vesicles to the somatodendritic compartment are not understood.
In this paper we use a novel pulse-chase system based on the protein FKBP12 (Rivera et al., 2000) to synchronize the behavior of proteins moving through the secretory pathway, allowing us an unobstructed view of vesicles as they move between the Golgi and the plasma membrane. Remarkably, we found that vesicles carrying dendritic proteins do not preferentially enter the somatodendritic compartment as compared to the axonal compartment. However, once they enter the axon a majority of those vesicles either stop moving or reverse direction in an actin- and Myosin Va-dependent fashion. In contrast, vesicles that carry either an axonal or a nonspecifically localized protein move beyond the AIS into the distal axon. All three types of vesicles behave in a similar manner within the dendrites. Thus, our findings are consistent with the presence of an actin-based vesicle filter in the AIS that differentially modulates the trafficking of transport vesicles depending on their cargos.
Results
A pulse/chase system based on FKBP12 and Shield-1
Expressing exogenous proteins tagged with GFP has allowed the localization and trafficking of transmembrane proteins to be visualized in live cells. However, it is often difficult to identify the type of vesicle being observed as there are vesicles moving simultaneously from ER to Golgi, Golgi to plasma membrane, plasma membrane to endosome, and endosome to lysosome. To overcome this obstacle we have developed a method that allows exogenous, tagged transmembrane proteins to gradually accumulate in the ER and then to be simultaneously released causing them to proceed along the secretory pathway in a synchronous manner.
This pulse/chase paradigm was adapted from a method based on the unique properties of a mutated version of the human FKBP12 protein. A single mutation within FKBP12 causes the resulting protein, FM, to bind to itself at moderate affinity and to rapamycin analogs at much higher affinity, but not to endogenous FKBP12 (Clackson et al., 1998). When four FM domains (FM4) are expressed in tandem they aggregate together under control conditions, but the aggregate is very quickly dispersed upon addition of the small molecule (Keenan et al., 1998; Rollins et al., 2000). When FM4-fused secreted proteins are expressed in cells the aggregated protein is retained within the endoplasmic reticulum, but can be released from the ER upon addition of the small molecule (Rivera et al., 2000).
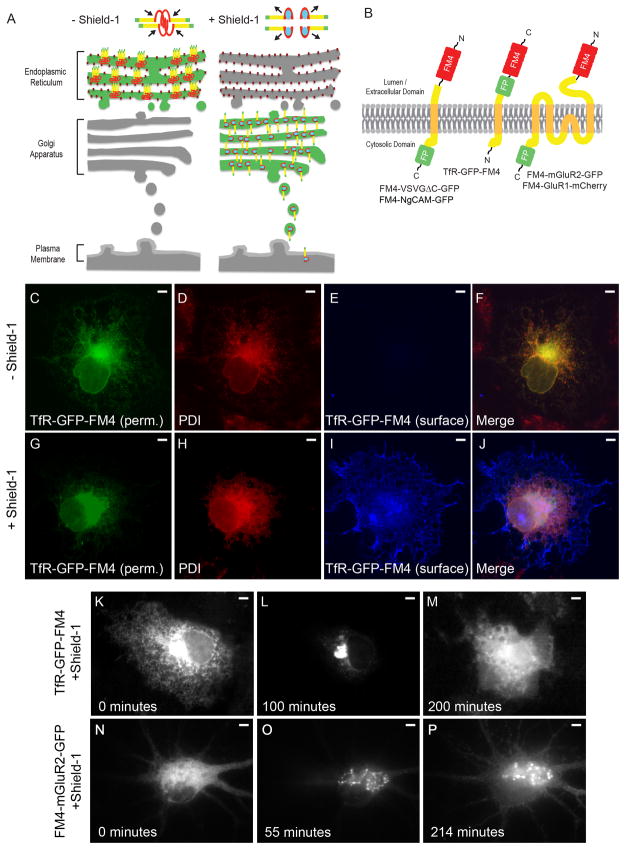
In order to test whether transmembrane proteins could be similarly sequestered within the ER and then released upon the addition of a small molecule binder of FM (Figure 1A), we fused 4 FM domains to the ectodomain of the Transferrin Receptor (TfR) tagged with EGFP and expressed the resulting fusion (TfR-GFP-FM4, Figure 1B) in COS cells. Note that TfR was chosen because it targets to dendrites in a robust fashion and is transported in large, easily visualized vesicles (Burack et al., 2000; Gu et al., 2003; Rivera et al., 2005; Silverman et al., 2001; West et al., 1997). Following expression in COS cells for 12–16 hours TfR-GFP-FM4 localized in a reticular pattern (Figure 1C, F) that colocalized with the endogenous ER resident protein PDI (Figure 1D, F) and was absent from the cell surface (Figure 1E, F). In contrast, when the small molecule Shield-1, a very efficient binder of FM, was added to these cultures, the protein translocated to the cell surface (Figure 1G–J) (Banaszynski et al., 2006). Live imaging revealed that FM4-fused dendritic proteins moved in a synchronized fashion from ER to Golgi and then to the cell surface in both COS cells and in neurons (Figure 1K–P), similar to the translocation of the temperature sensitive mutant of VSVG (tsVSVG) following the change from a restrictive to a permissive temperature (Horton and Ehlers, 2003a; Presley et al., 1997). Thus, as with tsVSVG, the FM4/Shield-1 paradigm allows proteins in a specific phase of the secretory pathway to be observed without interference from proteins in other phases.
Figure 1.
An FM4/Shield-1-based pulse chase system allows for synchronization of transmembrane proteins in the secretory pathway. (A) In the absence of Shield-1 transmembrane proteins (yellow) fused to FM4 domains (red) and to fluorescent proteins (green) multimerize causing clustering of the expressed fusion protein and retention within the ER. Binding of Shield-1 (cyan) to the FM4 domain causes disaggregation of the expressed transmembrane fusion protein, releasing it from the ER and allowing it to proceed through the secretory pathway. (B) Constructs contain 4 FM domains in tandem on the extracellular domain and a fluorescent protein fused to transmembrane proteins. (C, F) Following expression in COS cells and without exposure to Shield-1, TfR-GFP-FM4 (green) localizes in a reticular pattern that colocalizes with the endogenous ER markerPDI (red, D, F). A lack of TfR-GFP-FM4 on the surface (blue, E, F) is consistent with it being retained within the ER. In contrast, in cells exposed to Shield-1 TfR-GFP-FM4 (G, J) only partially colocalizes with PDI (H, J ) and TfR-GFP-FM4 expresses on the cell surface (I, J). Live imaging confirms that when TfR-GFP-FM4 is expressed in a COS cell for 12–16 hours it localizes in a reticular manner, suggesting that it is retained in the ER (K). Following addition of Shield-1 it becomes concentrated in a pattern consistent with Golgi localization (L). Subsequently, it localizes in a more diffuse pattern consistent with expression on the cell surface (M). Similarly, when FM4-mGluR2-GFP is expressed in a neuron for 16 hours, it localizes in a reticular manner consistent with retention in the ER (N). After the addition of Shield-1, FM4-mGluR2-GFP localizes in a perinuclear pattern, suggesting it had migrated to the Golgi (O). Afterwards, FM4-mGluR2-GFP shows a diffuse pattern of labeling consistent with presence on the plasma membrane (P). Scale bar is 5 μm.
Vesicles carrying dendritic proteins do not preferentially enter dendrites
The FM4/Shield-1 paradigm allowed us to observe vesicles carrying either dendritic or nonspecifically-localized proteins in neurons following release from the Golgi. To test whether these manipulations might disrupt dendritic targeting we expressed TfR-GFP-FM4 in dissociated cortical neurons at 11–14 days in vitro in the presence of Shield-1. We found that it localized specifically to dendrites with an axon to dendrite ratio (ADR, see methods) of 0.22 +/− 0.03 (n = 10; Figure 2A, B), a value consistent with previous results (Lewis et al., 2009). As a control we expressed an FM4- and GFP-tagged version of VSVG with its C-terminus deleted (FM4-VSVGΔC-GFP, Figure 1B) in the presence of Shield-1 and found that it localized nonspecifically with an ADR of 1.1 +/− 0.2 (n = 10; Figure 2C, D).
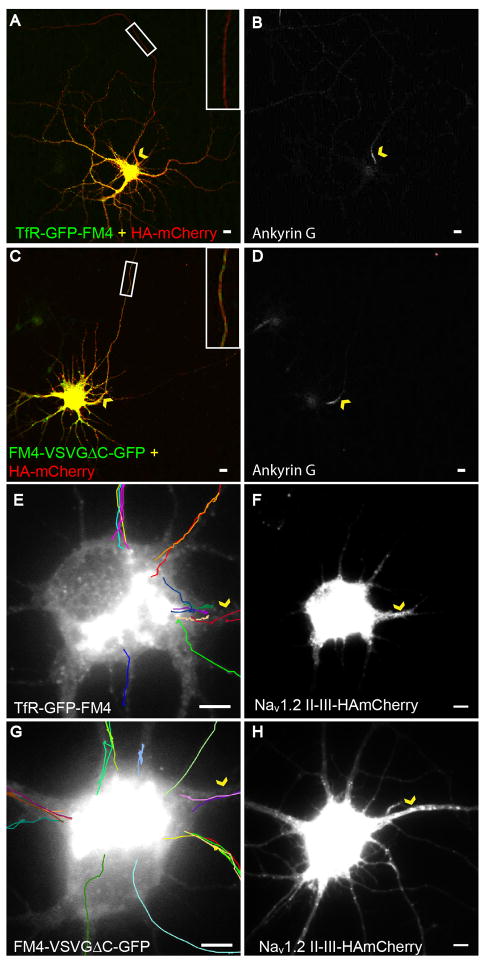
Figure 2.
Vesicles carrying TfR-GFP-FM4 enter axonal and dendritic processes with equal probability following exit from the Golgi (see also Figure S1, Movie 1). (A) In cortical neurons in dissociated culture that are exposed to Shield-1, TfR-GFP-FM4 (green) localizes in the somatodendritic compartment and is relatively absent from the axon as compared with HAmCherry (red), which localizes nonspecifically. Inset shows axonal region within the white box at higher magnification. (B) Ankyrin G staining labels the axonal process of the cell. (C) In contrast, both HAmCherry (red) and FM4-VSVGΔC-GFP (green) localize nonspecifically. Inset shows axonal region within the white box at higher magnification. (D) AnkyrinG staining labels the axonal process of the cell. (E) Tracks made by vesicles carrying TfR-GFP-FM4 following exit from the Golgi are consistent with these vesicles entering the closest process regardless of whether it is an axon or dendrite. (F) Nav1.2 II-III-HAmCherry co-expression labels the axonal process of the cell. (G) Vesicles containing FM4-VSVGΔC-GFP enter dendrites and axons with frequencies similar to that of vesicles carrying TfR-GFP-FM4 following exit from the Golgi. (H) Nav1.2 II-III-HAmCherry co-expression labels the axonal process of the cell. Arrowheads (yellow) point to the axon initial segment in each panel. Scale bar is 5 μm.
When this pulse/chase paradigm was applied to dissociated neurons expressing TfR-GFP-FM4, vesicles began to emerge from the Golgi at between 30 and 80 (Figure 1O) minutes after exposure to Shield-1. Within this time period we chose cells in which GFP fluorescence was concentrated within the Golgi and imaged them every 1–1.5 second for approximately 2 minutes. Following processing of these movies to improve their contrast and definition (see methods), we traced the paths of vesicles as they emerged from the Golgi and entered into the neuronal processes. We explored vesicle behavior in two separate phases: entry into neuronal processes and trafficking within processes. Surprisingly, we found that vesicles carrying TfR-GFP-FM4 did not preferentially enter dendritic processes, but rather entered the closest process regardless of whether it was an axon or dendrite. Approximately 20% of TfR-GFP-FM4-containing vesicles entered the axon (Figure 2E, F, Movie 1; n = 31), which is similar to the fraction of total processes that are axonal (17%; n = 70). Vesicles containing FM4-VSVGΔC-GFP behaved in a comparable manner with 21% entering axons (Figure 2G, H; n = 28; p > 0.5, Chi-square). These results suggest that following exit from the Golgi vesicles containing either protein associate with the closest microtubule regardless of the type of process to which it projects. Note that Nav1.2 II-III-HAmCherry, a marker of the AIS (Garrido et al., 2003), was co-expressed along with either TfR-GFP-FM4 or FM4-VSVGΔC-GFP, allowing the axon to be identified (see methods, Figures 2, F, H, S1).
Vesicles carrying dendritic proteins halt or reverse in the AIS
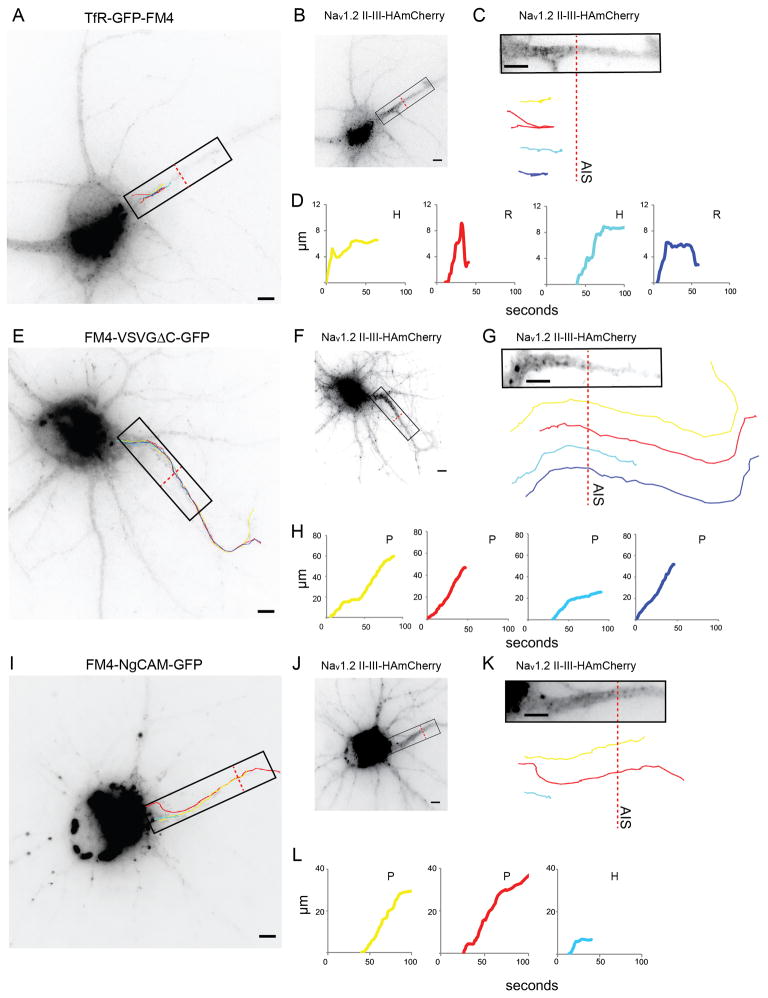
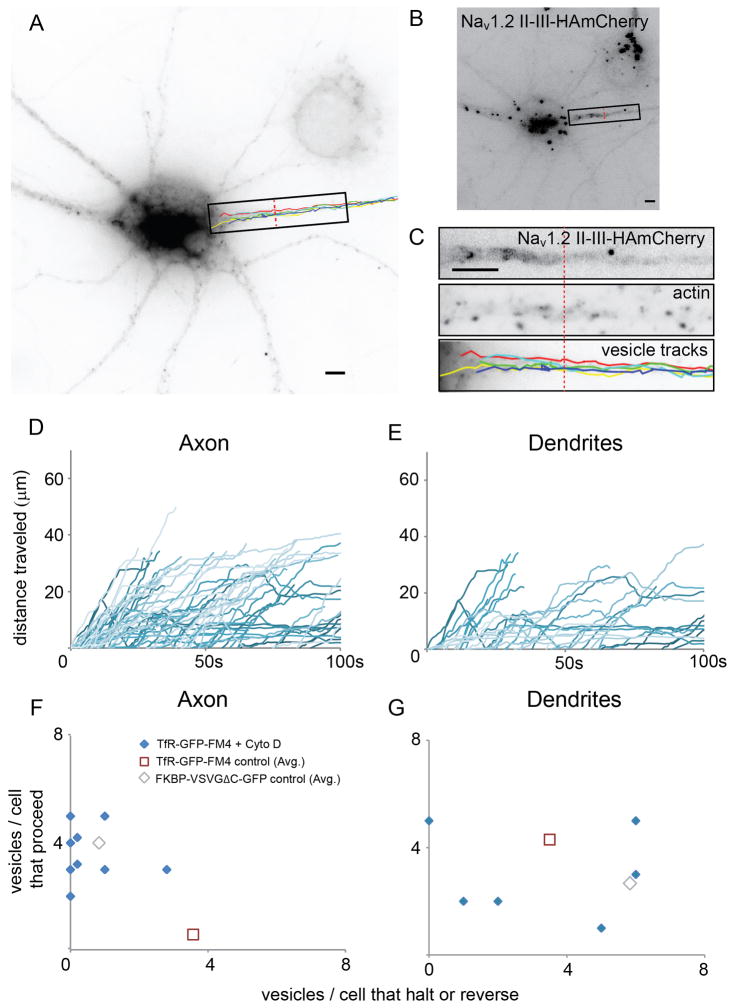
Since vesicles were equally likely to enter the axon and the dendrites regardless of their contents, we asked whether trafficking within the axon differed depending on a vesicle’s contents. Strikingly, we found that vesicles that entered the axon carrying TfR-GFP-FM4 (Figures 3A, S2, Movie 2) traveled a much shorter distance than those carrying FM4-VSVGΔC-GFP (Figure 3E, S2, Movie 3). The tracks taken by vesicles (Figures 3C, G, S2) as well as corresponding plots of distance from the cell body versus time (Figures 3D, H, 4A, B) showed that, in general, the trafficking of vesicles could be categorized as follows: (1) Proceed, where vesicles proceeded beyond the end of the AIS. (2) Halt, where vesicles stopped (moved less than 1 μm) for at least 5 seconds at the end of their tracks. (3) Reverse, where vesicles returned at least 1 μm towards the cell body following the cessation of forward motion. Note that the AIS was defined using the distribution of Nav1.2 II-III-HAmCherry (Figures 3C, G, K, S1). Axonal vesicles containing TfR-GFP-FM4 were far more likely to halt or reverse than to proceed beyond the AIS, whereas vesicles carrying FM4-VSVGΔC-GFP were far more likely to proceed than to halt or reverse within the AIS (Figure 4G).
Figure 3.
Vesicles carrying TfR-GFP-FM4 tend to haltand reverse within the AIS, whereas vesicles carrying FM4-VSVGΔC-GFP or FM4-NgCAM-GFPproceed beyond the end of the AIS (see also Figure S2 and Movies 2, 3). (A) Paths taken by vesicles carrying TfR-GFP-FM4 following release from the Golgi. (B) Localization pattern of Nav1.2 II-III-HAmCherry, which exhibits enrichment at the AIS. (C) Distribution of Nav1.2 II-III-HAmCherry in the AIS within the region shown in (A) compared with vesicle paths. (D) Graphs of distance traveled versus time for vesicle paths shown in (A) and (C). Note that all vesicles either halt or reverse before reaching the end of the AIS. (E) Paths taken by vesicles carrying FM4-VSVGΔC-GFP following release from the Golgi. (F) Localization pattern of Nav1.2 II-III-HAmCherry. (G) Distribution of Nav1.2 II-III-HAmCherry within the region shown in (E) compared with vesicle paths. (H) Graphs of distance versus time for vesicle paths shown in (E) and (G). Note that all vesicles proceed beyond the end of the AIS. (I) Paths taken by vesicles carrying FM4-NgCAM-GFP following release from the Golgi. ( J) Localization pattern of Nav1.2 II-III-HAmCherry. (K) Distribution of Nav1.2 II-III-HAmCherry within the region shown in (I).( L) Graphs of distance versus time for vesicle paths shown in (I) and(K ). Note that 2 vesicles proceed beyond the end of the AIS, while 1 vesicle halts. P refers to proceed, H to halt, R to reverse. Dashed red line marks the border of the AIS. Scale bar is 5 μm.
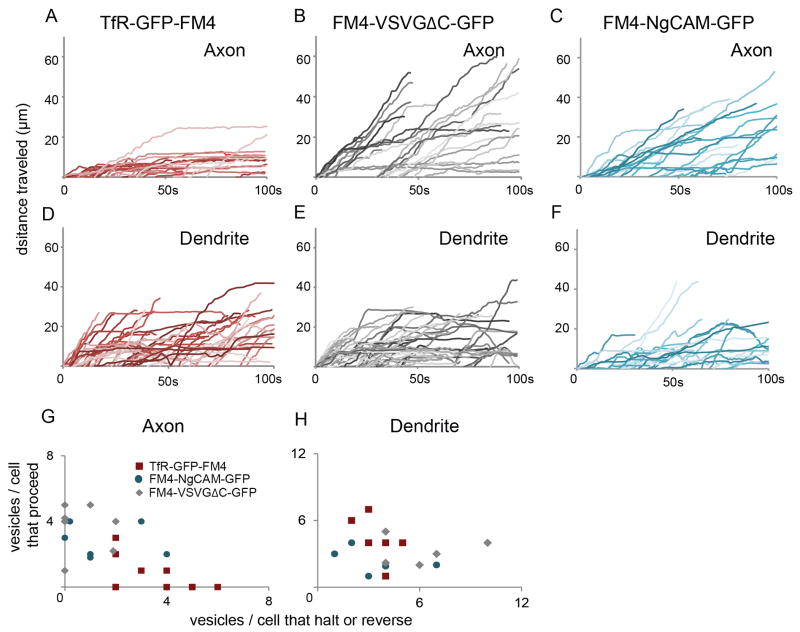
Figure 4.
Vesicles carrying TfR-GFP-FM4 behave differently from those carrying FM4-VSVGΔC-GFP or FM4-NgCAM-GFP in axons, but similarly in dendrites (see also Figure S3 and Movies, 4, 5). (A) Distance traveled versus time plots for vesicles carrying TfR-GFP-FM4 in the axon reveal that most vesicles did not move more than 10 μm beyond the cell body following release from the Golgi. In dramatic contrast, similar plots for vesicles carrying FM4-VSVGΔC-GFP (B) reveal that these vesicles proceeded up to 60 μm into the axon. Similarly, plots for vesicles carrying FM4-NgCAM-GFP (C) reveal that these vesicles proceeded up to 50 μm into the axon. (D, E, F) Plots of distance traveled versus time for vesicles containing TfR-GFP-FM4 or FM4-VSVGΔC-GFP or FM4-NgCAM-GFP in dendrites indicate that all three types of vesicles behave in a similar manner in this compartment.( G, H) Axon and dendrite scatter plots showing the ratio of the number of vesicles that proceed versus the number that halt or reverse for individual cells. Note that each data point refers to a single cell. Vesicles carrying TfR-GFP-FM4 behave differently from those carrying FM-VSVGΔC-GFP or FM4-NgCAM-GFP in axons, but similarly in dendrites.
Only 14% of vesicles carrying TfR-GFP-FM4 proceeded beyond the distal end of the AIS (Figures 4A, G, S2). Instead, most either halted (62%) or reversed (24%; n = 29 vesicles, 7 cells). In contrast, the majority of FM4-VSVGΔC-GFP-containing vesicles proceeded beyond the AIS (85%) while a much smaller number halted (9%) or reversed (6%; Figures 4B, G, S2; n = 33 vesicles, 8 cells). Thus, although the vesicles containing TfR-GFP-FM4 were roughly as likely to enter the axon as those containing FM4-VSVGΔC-GFP, their behavior was significantly different within the AIS (Figure 4A, B, G; p < 0.0001 Chi-square). In contrast to the movements of vesicles entering the axon, the vesicles that entered the dendrites carrying TfR-GFP-FM4 were more likely to proceed than were those carrying FM4-VSVGΔC-GFP, although the difference was not significant (Figures 4D, E, H, S2, see Supplemental Methods). 55% of vesicles carrying TfR-GFP-FM4 proceeded into the dendrites, a distance equivalent to the length of the AIS without halting or reversing while 23% of similar vesicles halted and 21% reversed (n = 47 vesicles), whereas 31% of vesicles carrying FM4-VSVGΔC-GFP proceeded, 35% halted and 33% reversed (Figure 4H; n = 51 vesicles, 6 cells; p > 0.05, Chi-square).
To further explore axonal trafficking behavior, we examined vesicles carrying FM4-fused NgCAM, an axonal protein (Burack et al., 2000). FM4-NgCAM-GFP containing vesicles were more likely to proceed than to halt or reverse within the axon initial segment (Figures 3I–L, S2, Movie 4). Roughly 66% of axonal vesicles carrying FM4-NgCAM-GFP proceeded, while 24% halted and 10% reversed (Figures 4C, G, S2; n = 26 vesicles, 6 cells). These movements were significantly different from those of vesicles carrying TfR-GFP-FM4 (p < 0.0001, Chi-square), but not those carrying FM4-VSVGΔC-GFP (p > 0.1, Chi-square). In contrast, in dendrites 41% of vesicles carrying FM4-NgCAM-GFP proceeded, while 31% halted and 28% reversed (Figures 4F, H, S2; n = 29 vesicles), which was not significantly different from the movements within dendrites of vesicles containing either TfR-GFP-FM4 or FM4-VSVGΔC-GFP (Figure 4F, H; p > 0.1, Chi-square).
To determine whether other dendritic proteins are trafficked in a manner similar to that of TfR, we expressed FM4-fused versions of GluR1 and mGluR2 (Craig et al., 1993; Stowell and Craig, 1999). Following addition of Shield-1 12% of vesicles carrying FM4-GluR1-mCherry and 6% of vesicles carrying FM4-mGluR2-GFP proceeded, 56% and 83% halted, and 32% and 11% reversed, respectively (Figure S3, Movie 5; n = 28, 23 vesicles; 7, 6 cells), proportions that are not significantly different from those associated with vesicles containing TfR (p > 0.2, Chi-square). The behavior of vesicles carrying either FM4-GluR1-mCherry or FM4-mGluR2-GFP within dendrites was also similar to that of vesicles carrying TfR-GFP-FM4 (Figure S3; p ≥ 0.05, Chi-square). Taken together, our results suggest that vesicles carrying dendritic proteins in the AIS are subject to specific forces that either halt vesicles or cause them to retreat to the cell body. These forces are much less likely to act on vesicles entering the axon that carry a nonspecifically-localized or an axonally-localized protein. Finally, vesicles entering the dendrites tend to behave similarly regardless of their cargos.
Actin filaments are necessary for vesicle halting and reversing
Results from static localization experiments showed that the presence of intact actin filaments is necessary for the localization of dendritic proteins (Lewis et al., 2009). Accordingly, we tested whether intact actin filaments are necessary for the halting and reversing of vesicles carrying dendritic proteins within the AIS. We exposed cortical neurons in culture to 4 μM Cytochalasin D, an actin depolymerizer, and then observed vesicles carrying TfR-GFP-FM4 at the AIS (Figures 5A, B, S4, Movie 6). To confirm that actin had, in fact, been disrupted we stained the live-imaged cells with phalloidin, a marker of actin filaments, and looked for cells with a punctate phalloidin staining pattern within the AIS (Figure 5C). We found that in fixed cells exposed to similar conditions TfR-GFP-FM4 localized nonspecifically, but the distributions of Ankyrin G or Nav1.2 II-III-HAmCherry were similar to those in control cells (Figures S5, S6) indicating that AIS had likely not undergone a major disruption. Strikingly, we found that in live cells with disrupted actin filaments a high percentage of vesicles carrying TfR-GFP-FM4 proceeded beyond the AIS (85%) versus a small minority (15%) that reversed and none that halted (Figure 5D, F, S4; n = 29 vesicles, 8 cells), which is significantly different from the movements of similar vesicles in control cells (p < 0.0001, Chi-square). In contrast, in dendrites of cells exposed to Cytochalasin D 47% of vesicles containing TfR-GFP-FM4 proceeded, while 24% halted and 29% reversed, which is not significantly different from the behavior of similar vesicles in control cells (Figure 5E, G, S4; n = 38 vesicles; p > 0.5, Chi-square). These results suggest that halting and reversing events in dendrites are not dependent on actin filaments. Thus, intact actin filaments are necessary for halting and reversing of vesicles carrying dendritic proteins in the AIS, but not in dendrites.
Figure 5.
Actin filaments are necessary for halting and reversing of vesicles carrying TfR-GFP-FM4 in the AIS (see also Figure S4 and Movie 6). (A) Paths taken by vesicles carrying TfR-GFP-FM4 in the axons of cortical neurons in culture that were exposed to 4 μM cytochalasin D for 80 minutes. (B) AIS is defined by staining of Nav1.2 II-III-HAmCherry. (C) High power view of boxed region from (A) showing Nav1.2 II-III-HAmCherry, Phalloidin staining in AIS consistent with disrupted actin filaments, and vesicle tracks. (D) Plots of distance traveled versus time for vesicles in axons containing TfR-GFP-FM4 in cells exposed to cytochalasin D are consistent with those vesicles moving beyond the AIS into the distal axon. (E) Plots of distance versus time for vesicles in dendrites containing TfR-GFP-FM4 in cells exposed to cytochalasin D are similar to comparable plots of similar vesicles in control cells. (F, G) Axon and dendrite scatter plots showing the ratio of the number of vesicles carrying TfR-GFP-FM4 that proceed versus the number that halt or reverse for cells exposed to cytochalasin D. Note that each data point refers to a single cell. In axons such vesicles behave differently from similar vesicles in control cells, but similarly to those carrying FM4-VSVGΔC-GFP. In dendrites, vesicles carrying TfR-GFP-FM4 in cells exposed to cytochalasin D do not behave in a noticeably different manner from similar vesicles in control cells. Scale bar is 5 μm.
Interaction with Myosin Va is necessary for vesicle halting and reversing
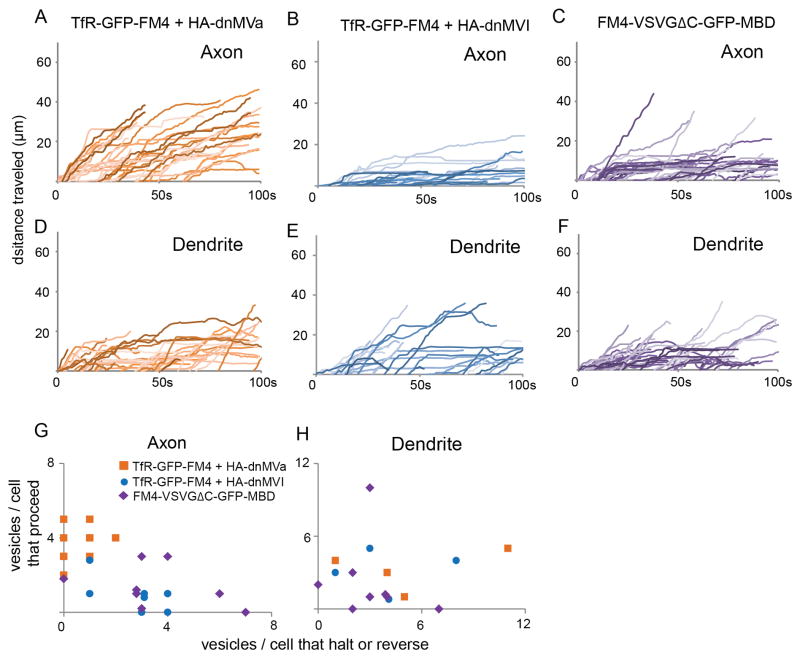
In our previous study we found that Myosin Va is required for localization of proteins to the dendrites (Lewis et al., 2009), a result that we confirmed with TfR-GFP-FM4 (Figure S6). To test whether Myosin Va might be involved in post-Golgi vesicle trafficking we examined the effect of blocking its function on the movement of vesicles within the AIS. Indeed, when co-expressed with a dominant negative version of Myosin Va consisting only of the globular tail region (HA-dnMVa), a majority of vesicles carrying TfR-GFP-FM4 proceeded to the distal axon (87%, n = 38 vesicles, 9 cells; Figures 6A, G, S4, Movie 7) compared with 11% that halted and 3% that reversed. These results are significantly different from those obtained with vesicles carrying TfR in control cells (p < 0.0001, Chi-square), but not from those carrying FM4-VSVGΔC-GFP (p > 0.5, Chi-square). In dendrites of cells expressing HA-dnMVa the movements of vesicles carrying TfR-GFP-FM4 (37% proceed, 30% halt, 33% reverse, n = 27 vesicles) were not significantly different from those of vesicles carrying TfR-GFP-FM4 in both control cells (p > 0.2, Chi-square) and in cells exposed to Cytochalasin D (Figures 6D, H, S4; n = 38 vesicles; p > 0.5, Chi-square). Note that disrupting the function of Myosin Va does not disrupt the distribution of either Ankyrin G or Nav1.2 II-III-HAmCherry at the axon initial segment (Figure S5), indicating that the effects on vesicular trafficking are likely not due to gross changes in the structure of the AIS. Thus, our results demonstrate that interaction with functional Myosin Va is necessary for both halting and reversing of vesicles carrying TfR-GFP-FM4 in the AIS.
Figure 6.
Interaction with Myosin Va, but not Myosin VI, is necessary for vesicle halting and reversing, whereas interaction with Myosin Va is sufficient to cause halting of vesicles at the AIS. (see also Figure S5 and Movies 7, 8). (A) Plots of distance traveled versus time for vesicles in axons containing TfR-GFP-FM4 in cells co-expressing HA-dnMVa are consistent with those vesicles moving beyond the AIS into the distal axon. In contrast, similar plots for vesicles in the axon containing TfR-GFP-FM4 in cells coexpressing HA-dnMVI (B) indicate that these vesicles are likely to halt or reverse while in the AIS. Note that this pattern is similar to that of cells expressing TfR-GFP-FM4 only. Similarly, plots of distance traveled versus time for vesicles in axons carrying FM4-VSVGΔC-GFP-MBD (C) indicate that those vesicles are more likely to halt within the AIS than vesicles carrying FM4-VSVGΔC-GFP. Plots of distance versus time show that vesicles in dendrites containing TfR-GFP-FM4 behave similarly in cells co-expressing HA-dnMVa (D) or HA-dnMVI (E). In addition, both types of vesicles behave in a similar manner to vesicles carrying FM4-VSVGΔC-GFP-MBD (F) in dendrites of control cells. (G, H) Axon and dendrite scatter plots showing the ratio of the number of vesicles carrying TfR-GFP-FM4 that proceed versus the number that halt or reverse in cells co-expressing HA-dnMVa or HA-dnMVI and a similar ratio for vesicles carrying FM4-VSVGΔC-GFP-MBD in control cells. Note that each data point refers to a single cell. In axons the vesicles carrying TfR-GFP-FM4 in cells co-expressing HA-dnMVI and vesicles carrying FM4-VSVGDC-GFP-MBD in control cells behave similarly to each other, but differently from those carrying TfR-GFP-FM4 in cells co-expressing HA-dnMVa. All three types of vesicles behave similarly in the dendrites.
While Myosin Va, a plus end-directed motor, is necessary for localization of proteins to the dendrites, Myosin VI, a minus end-directed motor is not (Lewis et al., 2009; Lewis et al., 2011). To test whether Myosin VI may be involved in the halting and reversing events seen in the axon initial segment, we expressed TfR-GFP-FM4 in the presence of a dominant negative variant of Myosin VI consisting of the globular tail region (HA-dnMVI). We found that in the AIS of these cells very few vesicles carrying TfR-GFP-FM4 proceeded (27%), whereas 61% halted and 12% reversed (n = 26 vesicles, 7 cells), proportions that were not significantly different from those associated with vesicles carrying TfR-GFP-FM4 in the axons of control cells (Figures 6B, G, S4, Movie 7; p > 0.3, Chi-square). Similar proportions of vesicles that proceeded, halted or reversed were also seen in the dendrites of cells co-expressing HA-dnMVI (45% proceed, 24% halt, 31% reverse) versus control cells (Figures 6E, H, S4, n = 29; p > 0.3, Chi-square). Thus, while Myosin Va function is necessary for halting and reversing events within the AIS, Myosin VI function is not. Furthermore, because Myosin VI is the only known minus end-directed myosin motor (Hasson et al., 1994), our results suggest that actin-based movement that is plus end-directed is necessary for halting and reversing events within the AIS, but minus end-directed movements are not.
Interaction with Myosin Va facilitates halting of vesicles
As shown previously, vesicles carrying FM4-VSVGΔC-GFP, a protein that goes nonspecifically to both axons and dendrites, only rarely halted or reversed following entry into the AIS (Figures 3, 4, S2). However, if Myosin Va plays an instructive role in preventing vesicles from moving to the distal axon, it would be expected that forcing FM4-VSVGΔC-GFP to bind to Myosin Va would cause vesicles carrying it to halt and reverse. To test this hypothesis, we fused FM4-VSVGΔC-GFP to the Myosin Va binding site from Melanophilin to give FM4-VSVGΔC-GFP-MBD (Geething and Spudich, 2007; Lewis et al., 2009). This construct localized specifically to the somatodendritic compartment at steady state in fixed cells (Figure S6). When vesicle movements were examined following addition of Shield-1 in living neurons we found that FM4-VSVGΔC-GFP-MBD behaved in a markedly different manner from FM4-VSVGΔC-GFP. 66% of vesicles carrying FM4-VSVGΔC-GFP-MBD halted compared with 9% for vesicles carrying FM4-VSVGΔC-GFP (Figures 6C, G, S4, Movie 8; n = 33 vesicles, 8 cells), although the number of reversal events stayed constant (5% for both FM4-VSVGΔC-GFP-MBD and FM4-VSVGΔC-GFP). Overall, the number of vesicles proceeding, halting and reversing is significantly different for axonal vesicles carrying FM4-VSVGΔC-GFP-MBD versus FM4-VSVGΔC-GFP (p < 0.001, Chi-square). In contrast, behavior of FM4-VSVGΔC-GFP-MBD-containing vesicles in dendrites was comparable to that of vesicles containing FM4-VSVGΔC-GFP (Figures 6F, H, S4; n = 30; p > 0.1, Chi-square), suggesting that Myosin Va exerts its influence on post-Golgi dendritic cargo mainly within the AIS. Given the dramatic increase in halting events in the AIS with the addition of MBD, it is somewhat surprising that there was no change in reversing events. This could mean that halting and reversing are fundamentally different and that Myosin Va is not by itself sufficient to mediate reversing. Alternatively, it could simply mean that Myosin Va bound to the myosin binding domain does not work efficiently enough to cause reversal events within the relatively short time window that we are able to follow vesicles.
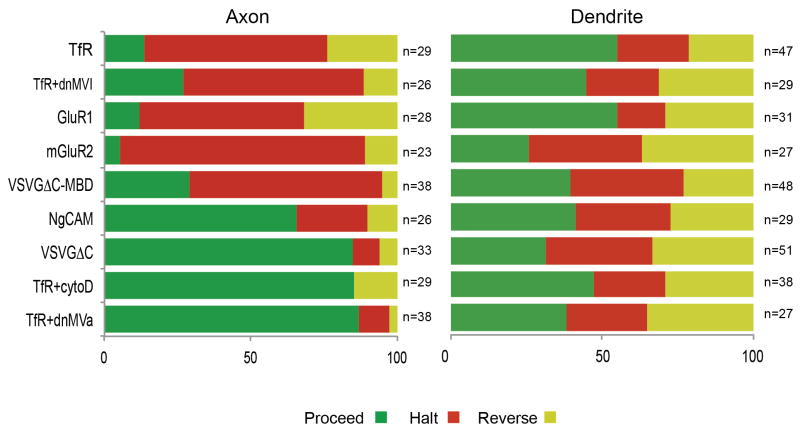
An overview of vesicle behavior indicates some clear properties of halting and reversing events (Figure 7). Both the presence of intact actin filaments and interaction with functional Myosin Va are necessary to cause vesicles to efficiently halt and reverse within the AIS, while interaction with Myosin Va is sufficient to dramatically increase halting events. Vesicles that preferentially halt and reverse carry proteins that localize to dendrites in fixed cells, while vesicles that preferentially proceed carry proteins that localize to the axon as well as the dendrites in fixed cells (Figures 7, S6). In contrast to the axon, the frequencies with which vesicles halt and reverse within the dendrites are largely independent of either the contents of the vesicle or of the presence of intact actin filaments and functional myosin motors. These results suggest that halting and reversing events in the dendrites are fundamentally different from those in the axon and that they are not involved in polarized trafficking of dendritic proteins.
Figure 7.
In axon and dendrites, the overall percentage of vesicles that proceeded (green), halted (red) or reversed (yellow) with different contents orunder different conditions (see also Figure S6).
Discussion
In both epithelial cells and neurons proteins that will eventually assume polarized distributions are sorted at the trans-Golgi membrane into distinct sets of vesicles through the interaction of coat proteins, adaptor proteins and peptide signals on the cargo (Fèolsch et al., 1999; Kirchhausen, 1999; Matsuda et al., 2008; Mostov et al., 2000). Loaded vesicles then traffic along specific pathways depending on the cargo that they are carrying (Burack et al., 2000; Kaether et al., 2000; Wisco et al., 2003). In this study, we trace the paths of vesicles containing different cargos after they emerge from the Golgi and enter processes of dissociated neurons in culture. Using a novel FM4-based system we produced tagged transmembrane proteins that accumulate in the endoplasmic reticulum following translation and that can be released simultaneously following the addition of Shield-1 to the medium. Thus, addition of Shield-1 had the effect of synchronizing vesicles as they moved along the secretory pathway, allowing them to be viewed as they moved from the Golgi to the plasma membrane without being obscured by vesicles in other phases of the secretory pathway. Using this system we found that within the AIS the paths of vesicles carrying dendritic proteins diverge from those of vesicles carrying either nonspecifically localized proteins or axonal proteins: the carriers of dendritic proteins almost all halt and/or reverse, whereas the carriers of either axonal or nonspecifically localized proteins move through the AIS to the distal axon. Remarkably, this appears to be the only place where the pathways diverge, as vesicles carrying both dendritic proteins and nonspecifically localized proteins enter axons and dendrites with equivalent frequencies and display similar behaviors in the dendrites. Thus, we conclude that the selective rerouting of dendritic protein carriers within the AIS is the main driver for their restriction to the somatodendritic compartment. This conclusion is consistent with work showing that AIS proteins such as Ankyrin G are critical for the maintenance of neuronal polarity (Hedstrom et al., 2008), although defining possible functions of such proteins in vesicle trafficking will require further investigation.
The rerouting that occurs in the AIS depends on Myosin Va, but not on Myosin VI, and on the presence of intact actin filaments. Furthermore, carriers of a nonspecific protein were made to behave in a manner similar to dendritic protein carriers when they were engineered to interact with Myosin Va. Although we showed previously that these molecules are important for localization of dendritic proteins (Lewis et al., 2009), this is the first evidence that they are directly involved in trafficking of vesicles carrying dendritic proteins. Recent experiments have suggested that the intrinsic properties of axonal versus dendritic microtubules in terms of stability and tyrosination could cause kinesins to preferentially traffic down microtubules that project to the axon versus the dendrites (Konishi and Setou, 2009). However, because interaction with functional Myosin Va is both necessary and sufficient for the unique trafficking of dendritic carriers within the AIS, our results would suggest that microtubule-based motors play a subordinate role in this process. Furthermore, our observation that vesicles carrying dendritic proteins enter axons and dendrites with equal frequency would suggest that kinesins carrying those vesicles associate with axonally- and dendritically-projecting microtubules with comparable efficiencies. Thus, our results suggest that vesicles carrying dendritic proteins are restricted to the somatodendritic compartment largely through an actin/myosin-based mechanism.
The hypothesis that Myosin Va motors mediate the polarized trafficking of transport vesicles by opposing microtubule-based transport is consistent with several reports that such motors impede microtubule-based movements of organelles. For example, it has been shown that in melanocytes Myosin Va actively works to impede microtubule-based movements resulting in the dispersion of melanosomes (Gross et al., 2002; Tuma et al., 1998). Furthermore, both Myosin Va and Myosin VI were found to impede the microtubule-dependent transport of mitochondria in the axons of Drosophila neurons in both directions (Pathak et al., 2010). Organelles accumulate in the distal regions of axons that lack Myosin Va, but are rich in dynamic microtubule endings (Bridgman, 1999), suggesting that Myosin Va opposes the distal movement of the organelles along microtubules. Interestingly though, Myosin Va has also been found to increase the motility of neurofilaments in axons, suggesting that it might also work in concert with microtubule based systems (Alami et al., 2009). However, the above results, which were obtained by observing events in the distal axon, suggest that myosin motors have complex behaviors that make the underlying patterns of actin filaments difficult to predict. In contrast, results from this paper suggest that the action of Myosin Va in the proximal axon is relatively simple (Figures 3–7). In general it causes vesicles carrying dendritic proteins to halt and reverse within the AIS, preventing their movement to the distal axon.
At present little is known about the specific structure of actin filaments in the AIS, however, our experiments would suggest that a unique structure not found in dendrites or the distal axon is present there. Accordingly, better definition of the cytoskeletal structure of the AIS, would likely shed light on the molecular mechanisms underlying vesicular trafficking. In addition, because targeted transport of transmembrane proteins directly affects neuronal structure, the establishment of a vesicle filter could play a major role in the development of neuronal polarity. A number of elegant studies have defined a cascade of kinases that initiate and propagate the events leading to establishment of cell polarity (Yi et al., 2010). However, thus far no clear functional end point of this cascade has been identified. The results of this paper suggest that, in fact, one end point of this cascade could be the concentration of actin filaments within the AIS, creating a vesicle filter that mediates polarized trafficking of transmembrane proteins thereby promoting the establishment and maintenance of two compartments with distinct structural and functional properties.
Experimental Procedures
Dissociated cultures
Dissociated cultures were made from cortical neurons from day 18 embryonic Sprague rats according to standard protocols (see supplementary methods). Experimental protocols were conducted according to the US National Institutes of Health guidelines for animal research and were approved by the Institutional Animal Care and Use Committee at the University of Southern California.
Transfection and incubation for live cell imaging
Dissociated cortical neurons were transiently transfected at 11–14 DIV with CalPhos (Clontech). GFP or HAmCherry-tagged versions of Nav1.2 II-III-loop were co-transfected to identify the AIS. After 12–16 hours of transient expression, coverslips were mounted on the RC-30 enclosed chamber (Warner Instruments) and incubated for 30–40 minutes in Shield-1-containing image buffer at 32°C.
Image acquisition
Live imaging experiments were performed on the IX71 wide-field epifluorescence microscope (Olympus) using a 100X (N.A. 1.4) oil immersible objective and an EMCCD camera (Hamamatsu). Cells were chosen for imaging that displayed GFP fluorescence that was highly concentrated within the Golgi. Prior to imaging the expression level of the GFP-tagged construct was assessed so that only cells with comparable expression levels were imaged (data not shown). Images were then collected at 1–1.5 frames/s for approximately 2 minutes.
Cytochalasin D experiments
In order to investigate the effects of disrupting actin 12–14 DIV neuron cultures were exposed to 4 μM Cytochalasin D in imaging buffer for 30–40 min prior to imaging.
Axon identification and distal border definition
To identify the axon in real time during acquisition a single image at optimum exposure was taken of the co-expressed Nav1.2 II-III-HAmCherry or Nav1.2 II-III-GFP (Garrido et al., 2001) prior to the start of the timelapse capture. To identify the distal border, the fluorescence associated with Nav1.2 II-III-HAmCherry or GFP was measured along a line drawn through the middle of the axon and the point at which it went below 50% of its maximal value was noted (Figure S1).
Image processing of timelapse data
To enhance timelapse images of vesicle movement background was subtracted, photobleaching was compensated for and contrast was enhanced (see supplemental methods for details.)
Vesicle tracking and analysis
High quality timelapses were selected solely on the basis of expression level, degree of localization to the Golgi, and visibility of vesicles during the detection period. After acquisition, timelapses were corrected for photobleaching and enhanced based on the image processing method described in supplemental methods. In axons and dendrites, vesicle paths were tracked frame by frame by naïve observers using ImageJ (Wayne Rasband, NIH) using the MTrack-J plugin (Erik Meijering). To study vesicle entry into a neuronal process the paths of all vesicles were traced that both exited from the Golgi and entered a process while being continuously visible. To study vesicle trafficking within a process the paths of all vesicles within 4 μm of the entrance of a particular process, that traveled at least 2 μm and were continuously visible for least 10 seconds were included. Vesicles were assigned a trajectory profile based on their fate upon process entry during the detection period. All analysis was performed by blinded observers.
Definitions for vesicle trajectory
Proceed, where vesicles proceeded beyond the end of the AIS.
Halt, where vesicles stopped (moved less than 1 μm) for at least 5 seconds at the end of their tracks.
Reverse, where vesicles returned at least 1 μm towards the cell body following the cessation of forward motion.
Supplementary Material
Highlights.
novel methodology can synchronize transport of protein through the secretory pathway
post-Golgi vesicles carrying dendritic proteins enter both axons and dendrites
following entry into the axon vesicles containing dendritic proteins halt and reverse
halting and reversing of vesicles depends on actin and Myosin Va, but not Myosin VI
Acknowledgments
We thank Lucas Griffin, Elizabeth Adabale and Terrence Liu for help analyzing data and Emily Liman and members of the Arnold lab for comments on the manuscript. This work was supported by NIH grants NS-041963 and MH-086381 to DBA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alami NH, Jung P, Brown A. Myosin Va increases the efficiency of neurofilament transport by decreasing the duration of long-term pauses. J Neurosci. 2009;29:6625–6634. doi: 10.1523/JNEUROSCI.3829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AG, Wandless TJ. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell. 2006;126:995–1004. doi: 10.1016/j.cell.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman PC. Myosin Va movements in normal and dilute-lethal axons provide support for a dual filament motor complex. J Cell Biol. 1999;146:1045–1060. doi: 10.1083/jcb.146.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burack MA, Silverman MA, Banker G. The role of selective transport in neuronal protein sorting. Neuron. 2000;26:465–472. doi: 10.1016/s0896-6273(00)81178-2. [DOI] [PubMed] [Google Scholar]
- Cai D, McEwen DP, Martens JR, Meyhofer E, Verhey KJ. Single molecule imaging reveals differences in microtubule track selection between Kinesin motors. PLoS Biol. 2009;7:e1000216. doi: 10.1371/journal.pbio.1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu PJ, Rivera JF, Arnold DB. A role for Kif17 in transport of Kv4.2. J Biol Chem. 2006;281:365–373. doi: 10.1074/jbc.M508897200. [DOI] [PubMed] [Google Scholar]
- Clackson T, Yang W, Rozamus LW, Hatada M, Amara JF, Rollins CT, Stevenson LF, Magari SR, Wood SA, Courage NL, et al. Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity. Proc Natl Acad Sci U S A. 1998;95:10437–10442. doi: 10.1073/pnas.95.18.10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, Blackstone CD, Huganir RL, Banker G. The distribution of glutamate receptors in cultured rat hippocampal neurons: postsynaptic clustering of AMPA-selective subunits. Neuron. 1993;10:1055–1068. doi: 10.1016/0896-6273(93)90054-u. [DOI] [PubMed] [Google Scholar]
- Fèolsch H, Ohno H, Bonifacino JS, Mellman I Department of Cell B of Ludwig Institute for Cancer Research YUSoMNHCUSA. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99(2):189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- Garrido JJ, Fernandes F, Giraud P, Mouret I, Pasqualini E, Fache MP, Jullien F, Dargent B. Identification of an axonal determinant in the C-terminus of the sodium channel Na(v)1.2. Embo J. 2001;20:5950–5961. doi: 10.1093/emboj/20.21.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido JJ, Giraud P, Carlier E, Fernandes F, Moussif A, Fache MP, Debanne D, Dargent B. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science. 2003;300:2091–2094. doi: 10.1126/science.1085167. [DOI] [PubMed] [Google Scholar]
- Geething NC, Spudich JA. Identification of a minimal myosin Va binding site within an intrinsically unstructured domain of melanophilin. J Biol Chem. 2007;282:21518–21528. doi: 10.1074/jbc.M701932200. [DOI] [PubMed] [Google Scholar]
- Gross SP, Tuma MC, Deacon SW, Serpinskaya AS, Reilein AR, Gelfand VI. Interactions and regulation of molecular motors in Xenopus melanophores. J Cell Biol. 2002;156:855–865. doi: 10.1083/jcb.200105055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Jan YN, Jan LY. A conserved domain in axonal targeting of Kv1 (Shaker) voltage-gated potassium channels. Science. 2003;301:646–649. doi: 10.1126/science.1086998. [DOI] [PubMed] [Google Scholar]
- Guillaud L, Setou M, Hirokawa N. KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons. J Neurosci. 2003;23:131–140. doi: 10.1523/JNEUROSCI.23-01-00131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson T, Mooseker MS Department of Biology, Y.U.N.H.C. Porcine myosin-VI: characterization of a new mammalian unconventional myosin. J Cell Biol. 1994;127(2):425–440. doi: 10.1083/jcb.127.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom KL, Ogawa Y, Rasband MN. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol. 2008;183(4):635–640. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci. 2003a;23:6188–6199. doi: 10.1523/JNEUROSCI.23-15-06188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD. Neuronal polarity and trafficking. Neuron. 2003b;40:277–295. doi: 10.1016/s0896-6273(03)00629-9. [DOI] [PubMed] [Google Scholar]
- Kaether C, Skehel P, Dotti CG. Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons. Mol Biol Cell. 2000;11:1213–1224. doi: 10.1091/mbc.11.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan T, Yaeger DR, Courage NL, Rollins CT, Pavone ME, Rivera VM, Yang W, Guo T, Amara JF, Clackson T, et al. Synthesis and activity of bivalent FKBP12 ligands for the regulated dimerization of proteins. Bioorg Med Chem. 1998;6:1309–1335. doi: 10.1016/s0968-0896(98)00125-4. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. Adaptors for clathrin-mediated traffic. Annu Rev Cell Dev Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Setou M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat Neurosci. 2009;12(5):559–567. doi: 10.1038/nn.2314. [DOI] [PubMed] [Google Scholar]
- Lewis TL, Jr, Mao T, Svoboda K, Arnold DB. Myosin-dependent targeting of transmembrane proteins to neuronal dendrites. Nat Neurosci. 2009;12(5):568–576. doi: 10.1038/nn.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, Mao T, Arnold DB. A Role for Myosin VI in the Localization of Axonal Proteins. PLoS Biol. 2011;9:e1001021. doi: 10.1371/journal.pbio.1001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Miura E, Matsuda K, Kakegawa W, Kohda K, Watanabe M, Yuzaki M. Accumulation of AMPA receptors in autophagosomes in neuronal axons lacking adaptor protein AP-4. Neuron. 2008;57:730–745. doi: 10.1016/j.neuron.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Mostov KE, Verges M, Altschuler Y. Membrane traffic in polarized epithelial cells. Curr Opin Cell Biol. 2000;12:483–490. doi: 10.1016/s0955-0674(00)00120-4. [DOI] [PubMed] [Google Scholar]
- Nakata T, Hirokawa N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J Cell Biol. 2003;162:1045–1055. doi: 10.1083/jcb.200302175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak D, Sepp KJ, Hollenbeck PJ. Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J Neurosci. 2010;30:8984–8992. doi: 10.1523/JNEUROSCI.1621-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Rivera JF, Chu PJ, Arnold DB. The T1 domain of Kv1.3 mediates intracellular targeting to axons. Eur J Neurosci. 2005;22:1853–1862. doi: 10.1111/j.1460-9568.2005.04384.x. [DOI] [PubMed] [Google Scholar]
- Rivera VM, Wang X, Wardwell S, Courage NL, Volchuk A, Keenan T, Holt DA, Gilman M, Orci L, Cerasoli F, Jr, et al. Regulation of protein secretion through controlled aggregation in the endoplasmic reticulum. Science. 2000;287(5454):826–830. doi: 10.1126/science.287.5454.826. [DOI] [PubMed] [Google Scholar]
- Rollins CT, Rivera VM, Woolfson DN, Keenan T, Hatada M, Adams SE, Andrade LJ, Yaeger D, van Schravendijk MR, Holt DA, et al. A ligand-reversible dimerization system for controlling protein-protein interactions. Proc Natl Acad Sci U S A. 2000;97:7096–7101. doi: 10.1073/pnas.100101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor- containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417:83–87. doi: 10.1038/nature743. [DOI] [PubMed] [Google Scholar]
- Silverman MA, Kaech S, Jareb M, Burack MA, Vogt L, Sonderegger P, Banker G. Sorting and directed transport of membrane proteins during development of hippocampal neurons in culture. Proc Natl Acad Sci U S A. 2001;98:7051–7057. doi: 10.1073/pnas.111146198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song AH, Wang D, Chen G, Li Y, Luo J, Duan S, Poo MM. A selective filter for cytoplasmic transport at the axon initial segment. Cell. 2009;136(6):1148–1160. doi: 10.1016/j.cell.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Stowell JN, Craig AM. Axon/dendrite targeting of metabotropic glutamate receptors by their cytoplasmic carboxy-terminal domains. Neuron. 1999;22:525–536. doi: 10.1016/s0896-6273(00)80707-2. [DOI] [PubMed] [Google Scholar]
- Tuma MC, Zill A, Le Bot N, Vernos I, Gelfand V. Heterotrimeric kinesin II is the microtubule motor protein responsible for pigment dispersion in Xenopus melanophores. J Cell Biol. 1998;143:1547–1558. doi: 10.1083/jcb.143.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AE, Neve RL, Buckley KM. Identification of a somatodendritic targeting signal in the cytoplasmic domain of the transferrin receptor. J Neurosci. 1997;17:6038–6047. doi: 10.1523/JNEUROSCI.17-16-06038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler B, Forscher P, Mellman I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 1999;397:698–701. doi: 10.1038/17806. [DOI] [PubMed] [Google Scholar]
- Wisco D, Anderson ED, Chang MC, Norden C, Boiko T, Folsch H, Winckler B. Uncovering multiple axonal targeting pathways in hippocampal neurons. J Cell Biol. 2003;162:1317–1328. doi: 10.1083/jcb.200307069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JJ, Barnes AP, Hand R, Polleux F, Ehlers MD. TGF-beta signaling specifies axons during brain development. Cell. 2010;142:144–157. doi: 10.1016/j.cell.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.