Abstract
The Rank Forum on Vitamin D was held on 2nd and 3rd July 2009 at the University of Surrey, Guildford, UK. The workshop consisted of a series of scene-setting presentations to address the current issues and challenges concerning vitamin D and health, and included an open discussion focusing on the identification of the concentrations of serum 25-hydroxyvitamin D (25(OH)D) (a marker of vitamin D status) that may be regarded as optimal, and the implications this process may have in the setting of future dietary reference values for vitamin D in the UK. The Forum was in agreement with the fact that it is desirable for all of the population to have a serum 25(OH)D concentration above 25 nmol/l, but it discussed some uncertainty about the strength of evidence for the need to aim for substantially higher concentrations (25(OH)D concentrations > 75 nmol/l). Any discussion of ‘optimal’ concentration of serum 25(OH)D needs to define ‘optimal’ with care since it is important to consider the normal distribution of requirements and the vitamin D needs for a wide range of outcomes. Current UK reference values concentrate on the requirements of particular subgroups of the population; this differs from the approaches used in other European countries where a wider range of age groups tend to be covered. With the re-emergence of rickets and the public health burden of low vitamin D status being already apparent, there is a need for urgent action from policy makers and risk managers. The Forum highlighted concerns regarding the failure of implementation of existing strategies in the UK for achieving current vitamin D recommendations.
Keywords: Vitamin D, 25-Hydroxyvitamin D, Sunlight exposure, Deficiency, Insufficiency, Recommendations
The Rank Forum on Vitamin D was held on 2nd and 3rd July 2009 at the University of Surrey, Guildford, UK. The workshop consisted of a series of scene-setting presentations to address the current issues and challenges concerning vitamin D and health, and then an open discussion followed. The discussion focused on the identification of the concentrations of plasma 25-hydroxyvitamin D (25(OH)D), a marker of vitamin D status, that may be regarded as optimal, and the implications this process may have in the setting of future dietary reference values (DRV) for vitamin D in the UK. The implications of any changes in the present recommendations on vitamin D were also considered. The sessions were co-chaired by S. A. L.-N. of the University of Surrey and J. L. B. of the British Nutrition Foundation. L. M. M. from the British Nutrition Foundation was the Forum Rapporteur. Professor C. M. W. (University of Reading and Rank Prize Fund Committee) acted as overall Forum Chair.
Background
Vitamin D can be obtained from dietary sources in two main forms, namely ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). Vitamin D is also produced photochemically in the skin in the form of vitamin D3. The action of sunlight (UV radiation of wavelength 290–310 nm) converts 7-dehydrocholesterol to vitamin D3 (via its precursor). The liver enzyme 25-hydroxylase converts endogenously synthesised vitamin D3 and diet-derived D2 and D3 to 25(OH)D. In the kidney, 25(OH)D can be converted to 1,25-dihydroxyvitamin D, the active hormone which acts in concert with parathyroid hormone and calcitonin to maintain plasma Ca concentrations through homoeostatic regulation. 25(OH)D is the major circulating metabolite of vitamin D, with a half-life of several weeks, and therefore, it is considered to be a valid indicator of vitamin D status. Serum 1,25-dihydroxyvitamin D is tightly regulated, only falling in extreme deficiency. Traditionally, a serum 25(OH)D concentration below 25 nmol/l has been regarded as an index of increased risk of overt bone disease and hence as vitamin D ‘deficiency’, and this has been used as the criterion for determining adequacy of vitamin D supply and for setting DRV in the UK. Prolonged deficiency of vitamin D results in osteomalacia in adults and in rickets in children.
There are very few dietary sources of vitamin D, with oily fish being the richest source of the nutrient. However, in the UK, only 27 % of the population are consumers of oily fish(1). Other dietary sources include eggs, meat and fortified products such as margarine, reduced fat spreads and some breakfast cereals. Since 1940, there has been mandatory fortification of margarine with vitamin D in the UK (to bring the concentration of vitamin D to that of butter); many reduced fat spreads are also voluntarily fortified with vitamin D. Dietary vitamin D can be present as either vitamin D2 or vitamin D3.
Fig. 1 shows that low vitamin D status is prevalent in the UK, and that it is particularly marked in young and older adults and in ethnic minorities(2,3). Although once thought of as a disease of the past, the re-emergence of rickets is evident in some subgroups of the population in the UK(4), predominantly in those of African–Caribbean and South Asian origins. Government advice(4–6) is for Asian children and women to take supplementary vitamin D. The high prevalence of vitamin D insufficiency among people of African–Caribbean and South Asian origin, especially children, adolescents and women, is likely to be due to a combination of factors including consumption of a vegetarian diet poor in vitamin D, low Ca intake and limited sunlight exposure(4). In a study of UK pregnant women from ethnic minorities, Datta et al.(7) reported that > 50% had a serum 25(OH)D concentration < 25 nmol/l. In southern England, 18% of pregnant White women had a serum 25(OH)D concentration < 25 nmol/l, and 31% had serum 25(OH)D concentration < 50 nmol/l(8), showing that the problem is also present in the white Caucasian population(9). In addition, the UK Government’s Scientific Advisory Committee on Nutrition (SACN) highlighted that young women of childbearing age throughout the population may have low vitamin D stores during the initial stages of pregnancy, and that many older people may have poor vitamin D status especially those in institutional care. Current government advice is for pregnant women, infants, young children and those over 65 years of age to supplement their diet with vitamin D(4–6). However, as will be discussed later, compliance with this advice is poor.
Fig. 1.
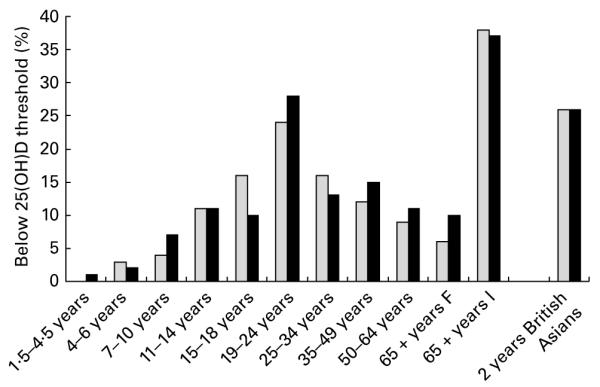
Prevalence of vitamin D deficiency (25-hydroxyvitamin D, 25(OH)D concentrations < 25 nmol/l) in people in the UK(2,3). F, free-living; I, institutionalised. British Asians were defined as those of South Asian origin (Pakistani, Indian and Bangladeshi). Source(11,62,64). Light grey shaded square, Males; dark grey shaded square, Females.
In recent years, the link between sunlight exposure and skin cancer risk has been recognised and, as a result, regular use of sunscreen has been advised and groups at particular risk such as young children and older people have been advised to ‘cover up’. This public health advice may have influenced skin synthesis of vitamin D adversely. Few studies have attempted to quantify skin UVB exposure typical of the UK population, but several studies funded by the Food Standards Agency (FSA) are now underway (see Ireland (Cork), Aberdeen and Surrey studies for more details.)
The current UK DRV for vitamin D are summarised in Table 1. These were first derived in 1991 by the Committee on Medical Aspects of Food and Nutrition Policy (COMA)(5), and have since been endorsed by a 1998 COMA report on nutrition and bone health(6). These were reviewed by the SACN in their position statement in 2007(4), which considered that there was insufficient evidence to change the DRV at that time. The high prevalence of low vitamin D status in the UK has led to speculation about the appropriateness of the current UK DRV and, in particular, about the absence of a reference nutrient intake (RNI) for people aged between 4 and 65 years in the general population, other than for those at a specific risk of limited UVB skin exposure.
Table 1.
| Age | Males | Females |
|---|---|---|
| 0–6 months | 8·5 | 8·5 |
| 7 months to 3 years | 7 | 7 |
| 4 years to 65 years | 0* | 0 |
| 65 + years | 10 | 10 |
| Pregnancy | – | 10 |
| Lactation, 0–4 months | – | 10 |
| Lactation, 4 + months | – | 10 |
10μg/d for individuals who are at risk of inadequate UVB sunshine exposure.
For those groups for whom RNI exist (Table 1), food consumption surveys indicate that 97% of free-living older people and 99% of institutionalised older people, for example, have dietary vitamin D intakes below the RNI(10).
Low vitamin D status as defined by a cut-off value of 25 nmol/l for circulating 25(OH)D concentration is now a major public health problem in the UK, and there have been many calls for urgent action, including a revision of DRV, revised advice regarding the risks to health linked to sun exposure, and implementation of fortification and supplementation programmes(11–13). However, at the same time, a controversy has emerged regarding the optimal range of serum 25(OH)D concentrations and the threshold concentration of 25(OH)D below which there are increased risks to health. To date the cut-off value used is 25 nmol/l, which is based on the risk of (or the absence of) rickets and osteomalacia. Proponents of setting this threshold at a higher concentration than 25 nmol/l base this on the potential for benefits in relation to a number of chronic diseases, including osteoporosis, diabetes, CVD and some cancers, and for ‘optimising’ immune function. For example, following a meta-analysis of observational studies, the International Association for Research on Cancer(14) has concluded that an increased risk of colorectal cancer and colorectal adenoma is associated with serum 25(OH)D concentrations below 40 nmol/l. Therefore, the emergence of evidence for additional health benefits associated with higher concentrations of plasma 25(OH)D raises a number of issues that challenge the perceptions about the current general health of the population. There is already widespread evidence of poor vitamin D status in the UK on the basis of the 25 nmol/l cut-off value (see Fig. 1), and if the threshold value for 25(OH)D sufficiency were to be raised above 25 nmol/l, for example to 40 nmol/l or higher, the proportion of the population described as vitamin D deficient would increase substantially. For example, data obtained from the 1958 British birth cohort show evidence of a high prevalence of low vitamin D status in adults aged 45 years. Using the 25 nmol/l cut-off value, the prevalence (winter and spring combined) in 2003 was 15·5 %, but this increased to 46·6 % at a cut-off value < 40 nmol/l and to 87·1 % at a cut-off value < 75 nmol/l(15).
The Rank Forum on Vitamin D aimed to facilitate an open discussion about the current controversies surrounding vitamin D and health by bringing together forty scientists and health professionals, who were either actively engaged in vitamin D research or had a particular interest in the vitamin D field. The ultimate aim was to try to identify specific strategies and areas of common agreement with a view to moving the field forward, recognising that there are conflicting views and differing conclusions regarding the strength of the evidence for the role of vitamin D in the prevention of various chronic diseases.
An overview of vitamin D: controversial issues
B. J. B. (Queen Mary University of London, UK) outlined some of the current controversies regarding hypovitaminosis D and ill health, such as the variability in the assessment of repletion and sufficiency of vitamin D, and indicated that much of this variability is likely to be due to the wide range of functions that vitamin D supports in the body, often through local activation systems in target tissues that themselves contain vitamin D receptors. It is also clear that although there are known differences and similarities between vitamin D2 and D3, there are also areas of uncertainty regarding the functional differences of the two forms. B. J. B. also discussed the many different factors contributing to the widespread problem of low vitamin D status in the population, and highlighted the fact that assessing vitamin D status can be particularly challenging in pregnancy because early increases in decidual and placental vitamin D 1-α hydroxylase activity lower maternal 25(OH)D concentrations. Questions about the possible adverse effects of being in the highest part of the range of naturally occurring vitamin D status and about the most appropriate doses of vitamin D to be used in randomised controlled trials (RCT) were also raised, together with the likelihood of variation in vitamin D requirements with ethnicity and various genetic factors.
Vitamin D and the Finnish experience
C. L.-A. (University of Helsinki, Finland) initiated a discussion regarding vitamin D fortification by presenting, as an example, the Finnish experience of voluntary milk fortification. C. L.-A. summarised the Nordic dietary vitamin D recommendations (7·5μg/d for those aged 3–60 years and 10 μg/d for those aged < 3 and > 60 years) and the problems of low vitamin D status among the Finnish population. She discussed the simulation calculations for different fortification options that had been considered in Finland; these included fortification of milk, bread, spread and cheese products. Consequently, the Finnish Ministry of Trade and Industry launched a new decree on optional (voluntary) fortification, which came into operation in February 2003. This allowed all fluid milk products to be fortified with 0·5 μg vitamin D3/100 ml and all spreads to be fortified with 10 μg vitamin D3/100 g. C. L.-A. also presented the results of the DESE study, which compared vitamin D status of the Finnish population in 2002 and 2004. It was shown that vitamin D status improved markedly in those using fortified fluid milk products. Among individuals not using vitamin D supplements, use of fortified foods resulted in the percentage of individuals with 25(OH)D concentrations < 40 nmol/l falling from 44·8 to 27·7 %, and the percentage of individuals with serum 25(OH)D concentrations < 25 nmol/l falling from 2·4 to 0·4 %(16). Overall, vitamin D status improved in all groups of the population, but it did not reach the recommended targets in all subgroups of the population.
Dietary calcium, vitamin D status and fracture
C. N. (Deakin University, Australia) reviewed and presented the evidence on dietary Ca, vitamin D and fracture risk, with a focus on older people. The majority of the RCT that have intervened using oral vitamin D to assess the risk of fracture also included supplementary Ca as part of the supplementation regimen. C. N. presented data to show that there is sufficient evidence to support the effectiveness of either vitamin D or Ca supplementation in the reduction of the risk of fractures in older women. A meta-analysis of twelve RCT showed that a 20 μg dose of vitamin D per day is required to maintain vitamin D status and reduce the risk of fractures, and that this effect is independent of additional Ca supplementation(17). A further meta-analysis of seventeen RCT(18) investigating the effect of supplementation of Ca alone and combined supplementation of Ca and vitamin D on the risk of fractures showed a 12% decrease in risk (all studies combined). Subgroup analyses showed that there was no difference in risk reduction in the studies in which only Ca was supplemented and those in which Ca and vitamin D were supplemented together, suggesting that it is the Ca (at a dose of > 1000 mg/d) that is driving the reduction in the risk of fracture. On the other hand, other work(19,20) has suggested that vitamin D dosage was usually too low (<700 IU/d; <17·5 μg/d); also, since most studies used the combined supplementation of vitamin D and Ca, it is unclear to what extent the beneficial effects of vitamin D supplementation on falls and fracture may reflect the specific effect of a relatively high intake of dietary Ca or, alternatively, the dependence of vitamin D on adequate Ca intake for it to be effective. It should also be noted that compliance with Ca supplementation is known to be challenging, which has been a confounding factor in many such trials.
Global vitamin D requirements
K. D. C. (University College Cork, Ireland) gave an overview of vitamin D requirements from a global perspective. Most recommendations in relation to vitamin D are based on promoting ‘health’ and preventing deficiency as assessed based on serum 25(OH)D concentration. He argued that summer sunlight is a much more potent source of vitamin D than the diet, but that diet takes on an increasing importance during winter at latitudes greater than 40°N or S due to the unavailability of UVB radiation of sufficient strength to stimulate dermal synthesis of the vitamin. According to the FAO/WHO(21), it is clear that at about equatorial latitudes (42°N–42°S), sun exposure to the face and arms for 30 min/d is the most efficient way of maintaining adequate vitamin D status. However, outside these latitudes, exposure for 30 min/d is only effective in summertime for the reasons mentioned above, and furthermore, the dermal capacity of skin to synthesise vitamin D is impacted upon by a range of factors other than latitude such as ageing, skin pigmentation, the use of sunscreen, cloud cover, sun avoidance and various degrees of cover from clothing. Despite these considerations, many countries still make an assumption that sun exposure in summer will provide an amount sufficient for adequate vitamin D status all year round. Dietary vitamin D recommendations are variable across Europe (see Table 2) and also globally. Most countries have no official specific recommendations for ethnic minority groups. In many countries, dietary intakes of vitamin D are far lower than the national/regional recommendations(22).
Table 2.
Overview of the recommendations, by age, for dietary intake of vitamin D (μg/d) for selected population groups in Europe (males and females)(61)*
| Population groups (age) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | Country | 3 months | 9 months | 5 years | 10 years | 15 years | 25 years | 50 years | 70 years |
| 2005 | Albania | 5 | 5 | 5 | 5 | 5 | 10 | 10 | |
| 2006 | Belgium | 12·5 | 12·5 | 7·5 | 6·3 | 6·3 | 6·3 | 6·3 | 10 |
| 2005 | Bulgaria | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 10 |
| 2004 | DACH countries† | 10 | 10 | 5 | 5 | 5 | 5 | 5 | 5 |
| 2006 | Estonia | 10 | 7·5 | 7·5 | 7·5 | 7·5 | 7·5 | 10 | |
| 2001 | France | 22·5 | 22·5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 2005 | Hungary | 10 | 10 | 10 | 10 | 10 | 5 | 5 | 5 |
| 2006 | Iceland | 10 | 10 | 10 | 10 | 10 | 10 | 15 | |
| 1999 | Ireland | 8·5 | 7 | 5 | 5 | 7·5 | 5 | 5 | 10 |
| 1996 | Italy | 17·5 | 5 | 5 | 7·5 | 5 | 5 | 10 | |
| 2001 | Latvia | 10 | 10 | 10 | 10 | 10 | 5 | 5 | 5 |
| 1999 | Lithuania | 10 | 10 | 5 | 5 | 5 | 5 | 5 | 5 |
| 2000 | Netherlands | 5 | 5 | 2·5 | 2·5 | 2·5 | 2·5 | 2·5 | 7·5 |
| 2004 | Nordic countries‡ | 10 | 7·5 | 7·5 | 7·5 | 7·5 | 7·5 | 10 | |
| 1996 | Poland | 10 | 10 | 10 | 10 | 10 | 5 | ||
| 1990 | Romania | 10 | 10 | 10 | 10 | 7·5 | 5 | 5 | 5 |
| 1991 | Russian Federation | 10 | 10 | 2·5 | 2·5 | 2·5 | 2·5 | 2·5 | 2·5 |
| 1994 | Serbia | 10 | 10 | ||||||
| 1997 | Slovakia | 7·5 | 10 | 7·5 | 7·5 | 10 | 7·5 | 5·8 | 5 |
| 2007 | Spain | 10 | 10 | 10 | 5 | 5 | 5 | 10 | 15 |
| 2001 | The former Yugoslav Republic of Macedonia | 7·5 | 10 | 10 | 10 | 10 | 5 | 5 | 5 |
| 1991 | United Kingdom | 8·5 | 7 | 0§ | 0§ | 0§ | 0§ | 0§ | 10 |
| 1993 | European Commission | 17·5 | 5 | 5 | 7·5 | 5 | 5 | 10 | |
| 2004 | WHO/FAO | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 15 |
Croatia, Federation of Bosnia and Herzegovina and Republic of Srpska (entities of Bosnia and Herzegovina) and Montenegro are excluded from the table because no recommendation report was available to the author, and the Czech Republic is excluded due to the lack of a published data source.
DACH countries are Austria, Germany and Switzerland.
Nordic countries are Denmark, Finland, Iceland, Norway and Sweden.
10μg/d for those at risk of limited UVB skin exposure.
The UK Scientific Advisory Committee on Nutrition position on vitamin D
E. M. S. (UK FSA) presented the view of SACN regarding vitamin D recommendations and requirements. SACN has succeeded COMA as the group of scientific experts charged with advising the UK government on scientific risk assessment regarding nutrition. In 1991, COMA published its DRV(5), which included DRV for vitamin D (see Table 1). These values were endorsed by a second COMA report on nutrition and bone health published in 1998(6), and in 2007, SACN published a position statement entitled An update on vitamin D(4). This update assessed whether there was sufficient accumulating new evidence to support a need for a full review of DRV for vitamin D. However, SACN’s update did not set out to provide a systematic review of the evidence on vitamin D and health. SACN concluded that there was insufficient evidence at that time to warrant a full review of UK DRV for vitamin D, but reiterated the previous COMA recommendations. SACN also highlighted the continued need for a clear public health strategy and guidance on vitamin D supplementation targeted at health professionals and at-risk groups. The Committee also acknowledged the accumulating, but as yet insufficient, evidence for an inverse association between vitamin D and chronic disease risk. SACN is now awaiting the results of a series of FSA-funded research projects on vitamin D, in particular, those investigating the relative importance of sunlight and diet in the determination of vitamin D status of the population. These were discussed at an FSA Workshop on Vitamin D held in November 2009(23).
Vitamin D and bone health
A. P. (MRC-Human Nutrition Research, UK) gave a presentation on vitamin D and bone health. A. P. described the deficiency states of vitamin D: rickets in children and osteomalacia in children and adults. Until its recent re-emergence in some subgroups of the UK population (particularly ethnic minorities), rickets has been regarded as a disease of the past. Traditionally, the threshold for identifying poor vitamin D status has been identified using the cut-off values for 25(OH)D above which rickets and osteomalacia would not be expected (>25 nmol/l in the UK). Prevalence figures for this degree of low vitamin D status in the UK were presented (see Fig. 1). Younger and older adults and British Asians show the lowest vitamin D status. Many ethnic groups across the world also show evidence of poor vitamin D status in pregnancy(24). The main dietary sources of vitamin D in the UK were also presented (see Table 3). The differences and similarities between vitamin D2 and D3 were highlighted (for example, similarities in absorption from the gastrointestinal tract and differences in the rates of disappearance from the circulation and in their metabolism and breakdown) as well as their usefulness in treating clinical vitamin D deficiency. However, uncertainties about their relative efficacies remain because of inconsistencies in the evidence. For example, Trang et al.(25) reported that vitamin D3 is more effective than vitamin D2 at raising total 25(OH)D, but Holick et al.(26) have suggested that there is little difference in their biological efficacy in this regard. There is also some debate about the use of biomarkers as measures of vitamin D status. A. P. concluded that serum 25(OH)D is a useful biomarker of supply to target tissues, though many factors influence its use as a biomarker of function and, most importantly, that the ongoing debate on the optimal level of serum 25(OH)D should not hinder progress towards the introduction of steps to combat vitamin D deficiency as laid down in current guidelines.
Table 3.
Dietary sources of vitamin D in the UK(62)
| Source | Contribution to dietary intakes in women (%) |
Contribution to dietary intakes in men (%) |
|---|---|---|
| Cereal and cereal products | 22 | 20 |
| Milk and milk products | 3 | 2 |
| Egg and egg dishes | 9 | 10 |
| Fat spreads (including fortified margarine) |
15 | 19 |
| Meat and meat products | 18 | 24 |
| Fish and fish dishes | 30 | 21 |
Significance of dietary intake and sunlight in determination of vitamin D status
M. K. (University College Cork, Ireland) put the new data she was presenting into context by reviewing a seminal study done by Heaney et al.(27), which showed that healthy men relied substantially on tissue stores of cholecalciferol from previous sun exposure to meet their wintertime vitamin D requirement, and that an additional minimum of 12·5 μg/d vitamin D3 would be needed to maintain autumn concentrations of serum 25(OH)D throughout winter. However, the slope of the relationship between cholecalciferol dose and serum 25(OH)D was approximately 0·7 nmol/l for each microgram of vitamin D3 consumed, indicating an average requirement of approximately 114 μg/d to achieve a 25(OH)D level of 80 nmol/l. In the context of these data and the current SACN recommendations for vitamin D in UK adults aged below 65 years, M. K. reported the results of two studies funded by the FSA investigating the hypothesis that there is a dietary requirement for vitamin D to prevent deficiency during wintertime in adults. The studies aimed to determine the total daily vitamin D intake needed to prevent vitamin D deficiency (serum 25(OH)D concentration < 25 nmol/l), and to provide data on the relative importance of diet and sunlight in determining vitamin D status in adults. These were two double-blind RCT involving healthy adults aged 20–40 (n 238) and > 64 (n 225) years who were supplemented with 5, 10 or 15 μg/d vitamin D3 during two successive winters; the control group did not receive supplemental vitamin D3. The studies were designed to produce data showing a distribution of serum 25(OH)D levels at endpoint arising from inter-individual variation in dose, habitual diet and sun exposure during the preceding summer, from which the relationship between vitamin D3 intake and early spring status could be examined. The data confirmed the original hypothesis, and showed that 8·7 μg/d (adults aged 20–40 years) and 8·6 μg/d (adults aged > 64 years) of dietary vitamin D3 were needed to maintain 97·5% of the population (i.e. RNI) above a threshold of 25 nmol/l of 25(OH)D through the winter(28,29). An individual’s preference for sun exposure had a major effect on these results; 20–40 year olds who reported avoiding the sun had an RNI of 12·3 μg/d and those aged > 64 years who reported receiving < 15 min of sun exposure/d during summer had an RNI of 11·4 μg/d. The data were also used to predict intakes of vitamin D3 that would maintain wintertime 25(OH)D at higher thresholds, including 50 and 80 nmol/l, although M. K. recommended the implementation of a new study using a similar design with higher doses of vitamin D to confirm these predictions, particularly for the 80 nmol/l cut-off value.
Longitudinal study of diet and sunlight interactions in north-east Scotland
H. M. M. and A. M. (University of Aberdeen, UK) highlighted some issues surrounding poor vitamin D status and seasonality from a Scottish perspective. They presented preliminary results obtained from a 15-month longitudinal study of diet and sunlight interactions funded by the FSA and conducted in Aberdeen (Aberdeen Nutrition, Sunlight and Vitamin D Study), with W. D. F. (University of Liverpool, UK) measuring 25(OH)D for the study. In order to determine whether post-menopausal women in the north of the UK have worse vitamin D status than their counterparts living in the south of the country, and to assess whether the sunlight and dietary contributions are different according to latitudinal residential position, the related FSA-funded study by the Surrey group (see later) used the same study design. This allowed a direct comparison across the same year (summer 2006 to spring 2007). An additional measurement of 25(OH)D was made in spring 2008 to determine whether the poor summer of 2007 had any impact on vitamin D status in the following spring.
Vitamin D, food intake, nutrition and exposure to sunlight in Southern England (Vitamin D, Food Intake, Nutrition and Exposure to Sunlight) study
S. A. L.-N. and A. L. D. (University of Surrey, UK) presented some preliminary findings from the Vitamin D, Food Intake, Nutrition and Exposure to Sunlight study in Southern England funded by the FSA. This study was conducted in collaboration with the University of Manchester, and it investigated the effects of ethnicity and menopausal status on vitamin D status and on the relative contributions of dietary intake of vitamin D and UV sunlight exposure to vitamin D status. The impact of vitamin D status on functional markers of bone health and the relative contribution of diet and sunlight exposure to the vitamin D status of ethnic groups were also determined. A total of eighty-six Asian women and 270 Caucasian women aged 18–69 years (pre- and post-menopausal) were recruited, with data being collected in each season for one 12-month period (summer 2006 to spring 2007). Full data were collected on seventy Asian women and 223 Caucasian women.
Vitamin D methodology project
M. A. (Ashwell Associates, UK) gave an overview of a new study funded by the FSA, entitled How can we standardise the measurement of serum 25(OH)D in national surveys? This work was commissioned in response to a recommendation by SACN(4) to urgently resolve the lack of standardisation between laboratories and methodologies regarding measurement of serum 25(OH)D. A comprehensive, critical review of all recent publications on the assays used for 25(OH)D was completed, and the most robust method was recommended for use in the next UK National Diet and Nutrition Survey. The results of the comprehensive review were discussed at the FSA Workshop on Vitamin D in November 2009(30,31).
Vitamin D and diabetes
E. H. (Institute of Child Health, UK) presented the latest evidence on vitamin D and diabetes. It has been purported that vitamin D can reduce the risk of both type 1 and type 2 diabetes(32). It is thought that the risk of type 1 diabetes can be influenced by vitamin D through immunomodulation (via vitamin D receptors in macrophages and monocytes), and the risk of type 2 diabetes can be influenced by increased production of insulin (via vitamin D receptors in the pancreas) or because 1,25-dihydroxyvitamin D produced in the kidney enters the circulation and can down-regulate renin production in the kidney and stimulate insulin secretion in the islet β-cells of the pancreas. E. H. reviewed the scientific evidence to support these hypotheses, and highlighted several gaps and limitations. Overall, she concluded that there is support for an inverse association between vitamin D and type 1 diabetes(33,34), with the strengths of the evidence base including temporal relevance, evidence of a dose–response effect, biological plausibility and fair consistency across studies. However, causality for the role of vitamin D in type 1 diabetes has not been demonstrated. For type 2 diabetes, the main gap is the lack of well-controlled experiments; randomised trials of the effects of vitamin D on glycaemic control or type 2 diabetes prevention have provided inconsistent evidence, generally reporting no effect(35,36). There are some cross-sectional data to support an association between vitamin D and type 2 diabetes/related phenotypes(37), and some longitudinal studies also offer support(38), though these are often limited by the lack of ability to fully adjust for strong confounders such as adiposity.
Vitamin D and immune function
A. R. M. (Queen Mary University of London, UK) presented evidence on vitamin D and immune function. Much of the evidence is based on the association between vitamin D deficiency and susceptibility to active tuberculosis(39); indeed, vitamin D was used to treat tuberculosis in the pre-antibiotic era. It has been reported that calcitriol (1,25-dihydroxyvitamin D3) enhances the ability of leucocytes to suppress the growth of mycobacteria in vitro, and that this is associated with the induction of cathelicidin LL-37, which possesses anti-tuberculous activity(40,41). A clinical trial has shown that a single oral dose of 2·5 mg vitamin D2 enhances the ability of whole blood taken from tuberculosis contacts to restrict mycobacterial growth in vitro (42), but that it was insufficient to maintain vitamin D sufficiency for 8 weeks in tuberculosis patients(43). A. R. M. concluded by commenting that a number of clinical trials of vitamin D supplementation for the prevention and treatment of various respiratory infections are underway, and that findings from these studies will be important for the this area of research to progress.
The concentration of circulating 25-hydroxyvitamin D that can be regarded as optimal
The final two presentations by R. V. (University of Toronto, Canada) and R. M. F. (University of Newcastle, UK) set the scene for an open discussion. These presentations focused on the identification of the serum concentration of 25(OH)D that should be selected as the criterion for judging the adequacy of vitamin D supply in the UK, and highlighted the various considerations that need to be taken into account.
R. V. showed a series of data obtained from cross-sectional studies that indicated an association between 25(OH)D and bone mass and bone mineral density/content in girls. He also showed results obtained from a study investigating the long-term efficacy and safety of high vitamin D intakes delivered via fortified bread to older adults. In this study, Mocanu et al.(44) reported that serum 25(OH)D increased with 5000 IU/d (125 μg/d) vitamin D, which was also associated with a significant improvement in hip bone mineral density. Several observational studies have reported an association between low 25(OH)D status and increased CVD risk. Many of these studies have been cross-sectional, but there is some prospective evidence showing an association between low 25(OH)D status and higher risk of myocardial infarction(45). Data were also presented showing some inconsistencies regarding vitamin D and prostate cancer risk, indicating that both low and high 25(OH)D statuses are associated with an increased risk(46). These findings have been linked to a hypothesis that cycles of rising and falling 25(OH)D concentrations contribute to cancer risk. R. V. highlighted some limitations of the RECORD trial(47), in which no decrease in falls, fractures or mortality in older men and women with a low trauma fracture was found with vitamin D supplementation, in particular, poor compliance with the intervention. He also argued that other studies show more positive results for vitamin D and fracture risk.
R. V. indicated that several authors have estimated different optimal serum 25(OH)D concentrations in relation to individual or multiple health outcomes. Dawson-Hughes et al.(48) generated a consensus statement suggesting a lower threshold vitamin D3 status of about 75 nmol/l 25(OH)D (equivalent to an intake of 800–1000 IU/d or 20–25 μg/d).
In terms of potential toxicity, R. V. proposed that an intake of up to 10 000 IU/d (250 μg/d) of vitamin D3 is physiological and safe because it matches the effects of exposure to natural UVB in sunlight on 25(OH)D concentrations. He described a series of potential toxicities in which vitamin D had been implicated, and produced evidence to dispute these data. Hathcock et al.(49) have also reported the absence of toxicity in trials involving normal adults using vitamin D dosages at and above a level of 250 μg/d (10000 IU vitamin D3). These data were used to argue about the selection of this value as the upper level that could be taken without the risk of toxic exposures. Furthermore, R. V. considered there to be no evidence of an adverse effect of serum 25(OH)D up to 400 nmol/l, and on this basis, he suggested that supplements of 4000 IU/d (100 μg/d) could be considered as safe.
R. M. F. highlighted the difficulty in determining what constitutes optimal vitamin D status for bone health. For example, though there is an inverse relationship between serum 25(OH)D and parathyroid hormone, there is no threshold of 25(OH)D above which parathyroid hormone reaches a plateau. He also discussed the findings of the RECORD study(47) and the results of a recent Cochrane review(50) that showed that vitamin D alone has no significant effect on hip fracture (nine trials), but that combined vitamin D and Ca supplementation (eight trials) reduced hip fractures, especially in institutionalised older people. Overall, R. M. F. concluded that there is evidence that combined Ca and vitamin D supplementation decreases fracture risk in institutionalised older people, a group in whom vitamin D deficiency (serum 25(OH)D concentrations < 25 nmol/l) is common. Nevertheless, he acknowledged that a number of authors have advocated higher serum 25(OH)D concentrations for optimal bone health, ranging from 50 to 80 nmol/l. He also recognised that there may be skeletal and non-skeletal benefits of increasing serum 25(OH)D concentrations above 75 nmol/l, but felt that this is still unproven, and expressed concerns about the lack of data on the long-term safety of high-dose vitamin D. He recommended focusing attention on targeting groups at the highest risk of vitamin D deficiency in order to ensure that serum 25(OH)D is kept at least above the 25 nmol/l level.
Discussion
A number of themes emerged during the open discussion, which are summarised below.
Our evolutionary past
It has been considered by many that humans evolved for an outdoor lifestyle, and so the common problems caused due to a poor vitamin D status may be a feature of modern lifestyles as they diverge from those of our evolutionary past(51). Now, the demands of Western Society seem to dictate a lifestyle that involves large amounts of time spent indoors, and for many being sedentary is the norm, i.e. the majority of occupations are now office based or in the service sector rather than manual work conducted outdoors. Furthermore, risks associated with skin cancer also mean that the public is now increasingly aware of the dangers of excessive sun exposure. People with outdoor lifestyles, such as lifeguards, tend to have higher serum 25(OH)D concentrations(51), and therefore, the sunlight exposure that might be a ‘normal’ level for an outdoor worker may differ from that for an older person who spends a great deal of time indoors.
Optimal concentrations of serum 25-hydroxyvitamin D
The group was in agreement with the fact that it is desirable for all the population to have a serum 25(OH)D concentration above 25 mmol/l. There was considered to be some uncertainty about the strength of evidence for the need to aim for substantially higher conentrations (25(OH)D concentrations >75 nmol/l). Much of the data that are used to support a higher target level of 25(OH)D are based on cross-sectional or, at best, observational cohort data, and there is a need for further evidence from RCT. The majority of this observational evidence that is related to health outcomes other than bone is epidemiological, and is thus unable to establish causality directly, especially given the major problems caused by confounding in many such studies. A further limitation of science in this area is the inappropriate extrapolation of the study results obtained from one country to another, when they lie at different latitudes or altitudes and have different customary styles of dress and lifestyle and are therefore exposed to different levels of sunlight and UVB.
It was agreed that any discussion of ‘optimal’ concentration of serum 25(OH)D needs to define ‘optimal’ with care since it is important to consider the normal distribution of requirements and the requirements for a wide range of outcomes. For population health, the aim is to identify targets that 97·5% of the population should achieve. If 25(OH)D concentration ≤ 25 nmol/l defines the bottom 2·5 percentile of the normal distribution, then an important consideration is to determine what the median and 97·5 percentile values should be. Future discussions regarding ‘optimal’ levels of serum 25(OH)D require clarification on the definition of ‘optimal’ in terms of the normal distribution of requirements; currently, consensus is only available on the lower 2·5 % value (25 nmol/l), though this is an important cut-off value as it defines overt bone risk and it has been shown that supplementation at the population level can raise the majority of the population above this cut-off value. Furthermore, DRV are designed for use in monitoring the dietary adequacy of populations and not for gauging individual risk. When serum 25(OH)D concentration is used to assess individual vitamin D status, other considerations also come into play, such as the period of time over which the concentration has been at the measured level, individual variability in vitamin D requirements and whether there are physiological factors that affect the interpretation of plasma concentrations of vitamin D.
Considerations for potential supplementation and fortification programmes
A number of issues need to be considered before any (mass) supplementation or population-level fortification programme could be implemented. It is known that compliance/concordance with oral vitamin D (especially when given with Ca) supplements is poor in the clinical setting, particularly in older people, and there are also some uncertainties that remain regarding potential adverse effects of high doses. High-dose (4000 IU/d; 100 μg/d) supplements have not been used in the UK; thus, there are no compliance data available to assess their use. The presentation by C. L.-A. highlighted a number of issues that are to be considered before a voluntary fortification programme is implemented (for example, choice of fortificant, bioavailability, technical issues related to adding vitamin D to foods and current intake levels of fortificant). In Finland, more comprehensive dietary recommendations for vitamin D were also available than those that currently exist in the UK. It was considered that if the UK were eventually to pursue a route of fortification, further planning, modelling analyses and testing of systems for supplementation would be needed before there could be implementation across the population. There is also a need to look at the long-term safety of any proposed fortification programme. For example, in the context of population-based fortification programmes, R. M. F. referred again to the issue of the potential for adverse effects in older people with undetected primary hyperparathyroidism and in younger people with unrecognised sarcoidosis.
Inter-individual variability in response to vitamin D
Further research is needed to help understand possible inter-individual differences in the metabolism of vitamin D. Genetic polymorphisms affect vitamin D metabolism, and may underlie inter-individual variability in status. It is also possible that requirements for vitamin D intake are affected by genetic variations, as many of the metabolic effects of hormonal vitamin D are mediated via genomic pathways; vitamin D receptors are located throughout the body and a large number of other genes have vitamin D response elements in their promoter regions. The possibility of establishing a reference range for serum 25(OH)D concentrations based on a threshold at which serum parathyroid hormone concentration starts to rise has been precluded by the large variation between individuals and the multitude of factors affecting the circulating concentrations of the analytes(52). It is important to note that population-based considerations, such as DRV, are designed to cover the needs of the general population at large, and genetic variations are therefore not relevant for policy setting unless an approach that is different from that currently being used to set requirements is used.
Body stores of vitamin D and seasonality in ‘status’
Seasonal fluctuation in vitamin D status is found in most non-tropical populations. Therefore, it is important to understand fully the mechanisms by which vitamin D is stored in the body, so that it is possible to determine whether stores of vitamin D derived from summer sunlight are adequate to maintain the desired status throughout the winter. It is known that vitamin D is stored in the liver, adipose and muscle tissues, but whether these stores act as a genuine reserve for vitamin D in the winter months, whether the vitamin D in adipose and liver tissues is fully labile and whether the speed of its release from stores is a factor that determines the length of time that stores in various individuals can help to maintain adequate status are not known; for example, fat stores are thought to sequester vitamin D, lowering circulating 25(OH)D concentrations, but what happens over the seasons or with weight loss in such subjects is ill-defined.
Safety of high doses of vitamin D
Research has not provided sufficient information to understand the potential toxicity of high doses of vitamin D. There is a clear need to distinguish between the risks from high doses of vitamin D derived from large exposures to sunlight (skin cancer) and those that might arise from taking vitamin D in fortified foods or as supplements, and to increase public understanding on these issues.
The human body is adapted such that it will not produce too much vitamin D as a result of sunlight exposure. Conversion of 7-dehydrocholesterol to previtamin D3 in the skin is regulated so that prolonged sunlight exposure does not lead to excess production. Production is shut off once a particular threshold is reached, when there is evidence of slight reddening of the skin. However, extensive sun exposure of skin increases the risk of skin cancer. Therefore, guidance on safe sun exposure in relation to both skin cancer and vitamin D status is a complex message to communicate to the public, and any advice needs to be latitude-specific. Some success has been achieved in Australia(53), and this could provide useful lessons for other countries. In the UK, Cancer Research UK no longer advocates total sun avoidance, but it does recommend sunburn avoidance.
Very high doses of oral vitamin D supplements have been found to have toxic effects in healthy people. Excessive vitamin D activity leads to hypercalcaemia with severe toxicity, which can lead to renal failure and cardiac arrest. The UK Expert Group on Vitamins and Minerals(54) identified safe upper limits for consumption of vitamins and minerals. They concluded that a level of 25 μg/d of supplementary vitamin D would not be expected to lead to adverse effects when consumed regularly over a long period. Around the same time, the EU Scientific Committee on Food(55) could not establish a ‘no observed effect level’ or a ‘lowest observed adverse effect level’ because of uncertainty in the data. However, a tolerable ‘upper intake level’ was established at 25 μg/d for infants and children aged 10 years or less and 50 μg/d for children aged over 11 years old and adults. The US Standing Committee on the Scientific Evaluation of Dietary Reference Intakes set an upper intake level of 25 μg/d for infants aged up to 12 months and an upper intake level of 50 μg/d for children aged 1–18 years and adults(56). More recent evidence indicates that adverse effects are not found until much higher doses are given, and that intakes of 100 μg/d are safe(57).
Availability of additional long-term safety data on vitamin D (trials of at least 2 years duration) would be valuable. Ethics committees have often used the tolerable upper level for vitamin D inappropriately, and this has hindered research on the safe use of long-term high doses of vitamin D. There are some signs that the situation is improving, but ethics committees should be issued with guidelines regarding the interpretation of safe upper limits of vitamins in the diet and the use of higher doses of vitamin D in trials. Further research is also needed to understand any physiological differences between dietary/supplementary vitamin D2 and D3 (if D2 has to be used rather than D3), and between dietary D3 and endogenous sunshine-derived D3. For example, mechanisms and rates of absorption might differ in the same way that half-times of clearance of 25(OH)D2 and 25(OH)D3 from the circulation differ(58), as might the rates of clearance of these metabolites into the tissues, although this requires further research. A careful balance is needed to ensure that prevalence of deficiency is reduced without creating concerns about toxicity.
Dietary reference values
As indicated in E. M. S.’s presentation, the SACN is awaiting the results of research studies, due to be reported by 2010, designed to quantify the relative contribution of sunlight and dietary sources to circulating concentrations of 25(OH)D. The recently published research by Cashman et al.(28,29) offers an excellent starting point for discussion. Nevertheless, current UK reference values concentrate on the requirements of large subgroups of the population (Table 1). These differ from those of other countries across Europe (Table 2), where the majority have recommendations to cover a wider range of age groups. DRI are a system of recommendations from the Institute of Medicine of the US National Academy of Sciences used by both the United States and Canada. DRI for vitamin D have been assessed assuming the absence of adequate exposure to sunlight, and thus differ from the UK DRV, where it is assumed that the general population has adequate exposure to UVB from sunshine during the summer months and no RNI is set; a separate RNI is provided for those with restricted UVB skin exposure. The recommendations for 2005 are summarised in Table 4. A new committee has now been established by the Institute of Medicine to set the new recommendations for 2010. In addition, the European Food Safety Authority is in the process of reviewing Europe-wide DRV for micronutrients, and plans to hold a consultation in the near future.
Table 4.
Dietary reference intakes – recommended intakes for individuals in the USA and Canada(63)
| Life stage group | Vitamin D (μg/d)*†‡ |
|---|---|
| Infants (months) | |
| 0–6 | 5 |
| 7–12 | 5 |
| Children (years) | |
| 1–3 | 5 |
| 4–8 | 5 |
| Males (years) | |
| 9–13 | 5 |
| 14–18 | 5 |
| 19–30 | 5 |
| 31–50 | 5 |
| 51–70 | 10 |
| > 70 | 15 |
| Females (Years) | |
| 9–13 | 5 |
| 14–18 | 5 |
| 19–30 | 5 |
| 31–50 | 5 |
| 51–70 | 10 |
| < 70 | 15 |
| Pregnancy (years) | |
| 14–18 | 5 |
| 19–30 | 5 |
| 31–50 | 5 |
| Lactation (years) | |
| 14–18 | 5 |
| 19–30 | 5 |
| 31–50 | 5 |
Values are based on adequate intakes which are believed to cover the needs of all individuals in the age group, but lack of data or uncertainty in the data prevents the specification of the percentage of individuals covered by this intake with confidence (other recommendations are RDA, which are set to meet the needs of almost all (97–98%) individuals in a group, or estimated average requirements, which are expected to meet the requirements of 50% in a group).
As cholecalciferol, 1μg cholecalciferol = 40 IU vitamin D.
In the absence of adequate exposure to sunlight.
Implementation of current guidelines on vitamin D supplementation
A very significant issue considered by the Forum was concern about the failure of current implementation strategies in the UK to achieve the current vitamin D recommendations for high-risk groups (pregnant and lactating women, infants, ethnic minority groups and older people). There is a lack of awareness of the need to take supplements among the relevant subgroups of the population, and health professionals’ knowledge in this area is considered poor. Health professionals have been slow to respond to the problem(59). There is an urgent need to assist health professionals in becoming better informed about, and motivated towards, the implementation of these recommendations. Currently, front-line health professionals do not routinely raise awareness about the importance of vitamin D status; and this argues for targeted training of health professionals in this area as well as a wide-reaching communication strategy from the Government. It is hoped that efforts from the Department of Health in connection with Healthy Start will help to raise awareness among pregnant women and those professionals who interact with them. However, this work is currently directed specifically towards the promotion of free supplements for pregnant women on income support (estimated to be about 20 % of the subpopulation) and their babies and young children aged under 4 years (see below); low vitamin D status is more widespread than this and does not, in fact, vary with social class(9).
With the re-emergence of rickets and a considerable public health burden of low vitamin D status being already apparent, there is a need for urgent action from policy makers and risk managers to implement the existing recommendations. Pregnant women are a recognised ‘high-risk’ group for vitamin D deficiency in the UK, and vitamin D supplements are recommended throughout pregnancy. Consideration should be given to providing recommendations to women of childbearing age about vitamin D supplementation because many have low vitamin D status before pregnancy begins, and many pregnancies are unplanned. In addition, there is a high prevalence of low vitamin D status in the general population of the UK, which is of concern: for example, 24% of the men and 28% of the women aged 19–24 years have serum 25(OH)D concentrations below 25 nmol/l(60), and there is a high prevalence among older people, which is greatly increased among those in institutional care(8).
Accessibility to vitamin D supplements
A major obstacle to the implementation of the present dietary recommendations for high-risk subgroups of the population (i.e. use of supplementary vitamin D) is the lack of accessibility to affordable supplements. Under the UK Government’s Healthy Start scheme, pregnant women and children aged under 4 years are entitled to free supplements containing vitamin D if the mother is under the age of 18 years or on income support. Primary Care Trusts and Health Boards are responsible for making Healthy Start vitamin supplements available, and these can also be sold cheaply to those not eligible for free Healthy Start supplementation. However, several problems within the supply chain for these supplements have been reported. There have been problems with manufacture, and many National Health Service Trusts do not make the supplements generally available, and furthermore, not all mothers or indeed health professionals are aware of the need for vitamin D supplementation. Also, the supplements are not suitable for those who follow kosher or halal dietary patterns, and are not on general sale in pharmacies and retail outlets. Although most commercial multivitamins contain vitamin D, they are not appropriate for pregnant women or for older people as they often contain vitamin A. Furthermore, the available supplements of vitamin D commonly contain Ca, which often proves unacceptably constipating. Issues about the supply of supplements containing vitamin D without either Ca or vitamin A need to be resolved as a matter of urgency so that strategies to improve health professionals’ knowledge in this area and to improve provision of vitamin D to pregnant women can be implemented effectively across the UK as a whole.
Acknowledgments
Dedication The Rank Forum on Vitamin D Proceedings are dedicated to the late Professor Ian Macdonald, DSc (London), who encouraged S. A. L.-N. with great enthusiasm and foresight to organise the Rank Forum on Vitamin D, and who would have been so pleased with the meeting outcomes.
Acknowledgements
This Rank Forum on Vitamin D was funded by the Rank Prize Funds. Both S. A. L.-N. and J. L. B. express their sincere thanks to the Rank Prize Funds Committee for their financial support of this meeting and for their encouragement in its organisation. The views are those of the Forum delegates as expressed in their presentations and summarised in this document, and not those of the Rank Prize Funds Committee or Trustees. The paper is not a consensus statement, but is a summary of the discussions that took place at this Rank Forum on Vitamin D. S. A. L.-N. and J. L. B. thank Ms Angela Osborn (University of Southampton) and Ms Hannah Upton (University of Newcastle and Sainsbury’s/British Nutrition Foundation) for their considerable help with the organisation of this meeting, and Dr Helen Lambert (University of Surrey) for her help in final collation of the manuscript. E. H. holds a Department of Health (UK) Public Health Career Scientist Award.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- COMA
Committee on Medical Aspects of Food and Nutrition Policy
- DRV
dietary reference values
- FSA
Food Standards Agency
- RCT
randomised controlled trials
- RNI
reference nutrient intake
- SACN
Scientific Advisory Committee on Nutrition
Workshop attendees
S. A. L.-N., University of Surrey (Co-Chair)
J. L. B., British Nutrition Foundation (Co-Chair)
C. M. W., Rank Prize Funds Committee (Rank Chair)
Dr Khulood Alayha, Kuwait College, Kuwait
M. A. OBE, Ashwell Associates, Hertfordshire (Speaker)
J. L. B., University of Manchester (Co-Speaker)
B. J. B., Queen Mary University of London (Speaker)
K. D. C., University College Cork, Ireland (Speaker)
Dr Jane Coad, Massey University, New Zealand
Professor Robert D Cohen, University of London
A. L. D., University of Surrey (Speaker)
R. M. F., Newcastle University (Speaker)
W. D. F., University of Liverpool (Speaker)
Dr Oliver Gillie, Health Research Forum, London
Dr Gail Goldberg, MRC-Human Nutrition Research, Cambridge
Mrs Ohood Hakim, King Abdula-Aziz University, Saudi Arabia
Dr Tom Hill, University of Cork, Ireland
Dr Chris Holroyd, University of Southampton (representing C. C., not presented but provided data)
C. P. G. M. G. (University of Wageningen) (not presented but provided data)
Dr Anne de la Hunty, Ashwell Associates, Hertfordshire
E. H., Institute of Child Health, London (Speaker)
M. K., University College Cork, Ireland (Speaker)
C. L.-A., University of Helsinki, Finland (Speaker)
Dr Nicola Lowe, University of Central Lancashire
H. M. M., University of Aberdeen (Speaker)
Ms Rachel Marklew, Department of Health
A. R. M., Queen Mary’s University of London (Speaker)
A. M., University of Aberdeen (Co-Speaker)
L. M. Miles, British Nutrition Foundation (Rapporteur)
Professor Joe Millward, University of Surrey
Ms Judy Moore, Independent Dietitian, London
Dr Zulf Mughal, University of Manchester
T. M., University of Nottingham (not presented but provided data)
C. N., University of Deakin, Australia (Speaker)
Dr Jaana Nurmi-Lawton, University Hospital, Finland
A. P. OBE, MRC-Human Nutrition Research, Cambridge (Speaker)
Professor Margaret Rayman, University of Surrey
S. R., Department of Health (Co-Speaker)
Dr Inez Schoenmakers, MRC-Human Nutrition Research, Cambridge
E. M. S., FSA (Speaker)
R. V., University of Toronto, Canada (Speaker)
Dr Kate Ward, MRC-Human Nutrition Research, Cambridge.
References
- 1.Scientific Advisory Committee on Nutrition . Advice on Fish Consumption: Benefits & Risks. TSO; London: 2004. [Google Scholar]
- 2.Scientific Advisory Committee on Nutrition . The Nutritional Wellbeing of the British Population. TSO; London: 2008. [Google Scholar]
- 3.Lawson M, Thomas M. Vitamin D concentrations in Asian children aged 2 years living in England: population survey. Br Med J. 1999;318:28. doi: 10.1136/bmj.318.7175.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scientific Advisory Committee on Nutrition ACN Update on vitamin D: position statement by the Scientific Advisory Committee on Nutrition. 2007 http://www.sacn.gov.uk/pdfs/sacn_position_vitamin_d_2007_05_07.pdf 5.
- 5.Department of Health . Dietary Reference Values for Food Energy and Nutrients for the United Kingdom. no.41. HMSO; London: 1991. [Google Scholar]
- 6.Department of Health . Nutrition and Bone Health: With Particular Reference to Calcium and Vitamin D. no. 49. The Stationary Office; London: 1998. [Google Scholar]
- 7.Datta S, Alfaham M, Davies DP, et al. Vitamin D deficiency in pregnant women from a non-European ethnic minority population – an interventional study. BJOG. 2002;109:905–908. doi: 10.1111/j.1471-0528.2002.01171.x. [DOI] [PubMed] [Google Scholar]
- 8.Javaid MK, Crozier SR, Harvey NC, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 9.Hyppönen E, Boucher BJ. Avoidance of vitamin D deficiency in pregnancy in the United Kingdom; the case for a unified approach in National policy. Br J Nutr. 2010;104:309–314. doi: 10.1017/S0007114510002436. [DOI] [PubMed] [Google Scholar]
- 10.Finch S, Doyle W, Lowe C, et al. National Diet and Nutrition Survey: People Aged 65 Years and Over. Report of the Diet and Nutrition Survey. vol. 1. The Stationery Office; London: 1998. [Google Scholar]
- 11.Mocanu V, Stitt PA, Costan AR, et al. Long-term effects of giving nursing home residents bread fortified with 125 μg (5000 IU) vitamin D3 per daily serving. Am J Clin Nutr. 2009;89:1132–1137. doi: 10.3945/ajcn.2008.26890. [DOI] [PubMed] [Google Scholar]
- 12.Schoenmakers I, Goldberg GR, Prentice A. Abundant sunshine and vitamin D deficiency. Br J Nutr. 2008;99:1171–1173. doi: 10.1017/S0007114508898662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hathcock JN, Shao A, Vieth R, et al. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 14.International Agency for Research on Cancer . Vitamin D and Cancer. Working Group Report. vol. 5. International Agency for Research on Cancer; Lyon: 2008. [Google Scholar]
- 15.Hyppönen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85:860–868. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 16.Lamberg-Allardt Vitamin D in foods and as supplements. Prog Biophys Mol Biol. 2006;92:33–38. doi: 10.1016/j.pbiomolbio.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169:551–561. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- 18.Tang BMP, Eslick GD, Nowson C, et al. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in older people: a meta-analysis. Lancet. 2007;370:657–666. doi: 10.1016/S0140-6736(07)61342-7. [DOI] [PubMed] [Google Scholar]
- 19.Avenell A, Gillespie W, Gillespie L, et al. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. The Cochrane Database of Systematic Reviews, 2005. 2005;(issue 3) doi: 10.1002/14651858.CD000227.pub2. CD000227. http://www.mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD000227/frame.html. [DOI] [PubMed] [Google Scholar]
- 20.Boonen S, Lips P, Bouillon R, et al. Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92:1415–1423. doi: 10.1210/jc.2006-1404. [DOI] [PubMed] [Google Scholar]
- 21.FAO/WHO . Human Vitamin and Mineral Requirements. FAO/WHO Non-series Publication. Report of a Joint FAO/WHO Expert Consultation. Food and Agriculture Organization; Rome: 2002. [Google Scholar]
- 22.Mosekilde L. Vitamin D requirement and setting recommendation levels: long-term perspectives. Nutr Rev. 2008;66:S170–S177. doi: 10.1111/j.1753-4887.2008.00103.x. [DOI] [PubMed] [Google Scholar]
- 23.Ashwell M, Stone E, Stolte H, et al. UK Food Standards Agency Workshop Report: an investigation of the relative contributions of diet and sunlight to vitamin D status. Br J Nutr. 2010 doi: 10.1017/S0007114510002138. (Epublication ahead of print version 4 June 2010) [DOI] [PubMed] [Google Scholar]
- 24.Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev. 2008;66:S153–S164. doi: 10.1111/j.1753-4887.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- 25.Trang HM, Cole DEC, Rubin LA, et al. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68:854–858. doi: 10.1093/ajcn/68.4.854. [DOI] [PubMed] [Google Scholar]
- 26.Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–681. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 28.Cashman KD, Hill TR, Lucey AJ, et al. Estimation of the dietary requirement for vitamin D in healthy adults. Am J Clin Nutr. 2008;88:1535–1542. doi: 10.3945/ajcn.2008.26594. [DOI] [PubMed] [Google Scholar]
- 29.Cashman KD, Wallace JMW, Horigan G, et al. Estimation of the dietary requirement for vitamin D in free-living adults 64 y of age. Am J Clin Nutr. 2009;89:1–9. doi: 10.3945/ajcn.2008.27334. [DOI] [PubMed] [Google Scholar]
- 30.Wallace A, Gibson S, de la Hunty A, et al. Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids. 2010;75:477–488. doi: 10.1016/j.steroids.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 31.de la Hunty A, Wallace M, Gibson S, et al. UK Food Standards Agency Workshop Consensus Report: the choice of method for measuring 25(OH)D to estimate vitamin D status for the UK National Diet and Nutrition Survey. Br J Nutr. 2010;104:612–619. doi: 10.1017/S000711451000214X. [DOI] [PubMed] [Google Scholar]
- 32.Holick M. Vitamin D deficiency. NEJM. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 33.Hyppönen E. Vitamin D and increasing incidence of type 1 diabetes – evidence for an association? Diabetes Obes Metab. 2010;12:737–743. doi: 10.1111/j.1463-1326.2010.01211.x. [DOI] [PubMed] [Google Scholar]
- 34.Hyppönen E, Läärä E, Reunanen A, Järvelin MR, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 35.De Boer IH, Tinker LF, Connelly S, et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care. 2008;31:701–707. doi: 10.2337/dc07-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jorde R, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur J Nutr. 2009;48:349–354. doi: 10.1007/s00394-009-0020-3. [DOI] [PubMed] [Google Scholar]
- 37.Pittas AG, Lau J, Hu FB, et al. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pittas AG, Dawson-Hughes B, Li T. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–656. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case–control study. Lancet. 2000;355:618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 40.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;312:1874–1875. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 41.Martineau AR, Wilkinson KA, Newton SM, et al. IFN-γ- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178:7190–7198. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 42.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 43.Martineau AR, Nanzer AM, Satkunam KR, et al. Influence of a single oral dose of vitamin D2 on serum 25-hydroxyvitamin D concentrations in tuberculosis patients. Int J Tuberc Lung Dis. 2009;13:119–125. [PubMed] [Google Scholar]
- 44.Mocanu V, Stitt PA, Costan AR, et al. Long-term effects of giving nursing home residents bread fortified with 125 μg (5000 IU) vitamin D3 per daily serving. Am J Clin Nutr. 2009;89:1132–1137. doi: 10.3945/ajcn.2008.26890. [DOI] [PubMed] [Google Scholar]
- 45.Giovannucci E, Liu Y, Hollis BW, et al. 25-Hydroxy-vitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tuohimaa P, Tenkanen L, Ahonen M, et al. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case–control study in the Nordic countries. Int J Cancer. 2004;108:104–108. doi: 10.1002/ijc.11375. [DOI] [PubMed] [Google Scholar]
- 47.Grant AM, Avenell A, Campbell MK, et al. Oral vitamin D3 and calcium for secondary prevention of lowtrauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365:1621–1628. doi: 10.1016/S0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]
- 48.Dawson-Hughes B, Heaney RP, Holick MF, et al. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 49.Hathcock JN, Shao A, Vieth R, et al. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 50.Avenell A, Gillespie WJ, Gillespie LD, et al. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. The Cochrane Database of Systematic Reviews. 2009;(issue 2) doi: 10.1002/14651858.CD000227.pub3. 2009. CD000227. [DOI] [PubMed] [Google Scholar]
- 51.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 52.Patel S, Hyer S, Barron J. Glomerular filtration rate is major determinant of the relationship between 25-hydroxy D and parathyroid hormone. Caclif Tissue Int. 2007;80:221–226. doi: 10.1007/s00223-007-9001-9. [DOI] [PubMed] [Google Scholar]
- 53.Cancer Council (Australia) How much sun is enough? 2008 http://www.cancer.org.au/File/Cancersmartlifestyle/Howmuchsunisenough.pdf.
- 54.Expert Group on Vitamins and Minerals . Safe Upper Levels for Vitamins and Minerals. Food Standards Agency; London: 2003. [Google Scholar]
- 55.Scientific Committee on Food . Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of vitamin D. European Commission; Brussels: 2002. [Google Scholar]
- 56.Institute of Medicine. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Food and Nutrition Board . Dietary Reference Intake for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academy Press; Washington, DC: 1997. Vitamin D, Chapter 7. [Google Scholar]
- 57.Vieth R, Chan PC, Macfarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001;73:288–294. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]
- 58.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–586S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 59.Pearce SH, Cheetham TD. Diagnosis and management of vitamin D deficiency. Br Med J. 2010;340:b5664. doi: 10.1136/bmj.b5664. [DOI] [PubMed] [Google Scholar]
- 60.Ruston D, Hoare J, Henderson L, et al. The National Diet and Nutrition Survey: Adults Aged 19 to 64 Years. The Stationery Office; London: 2004. [Google Scholar]
- 61.Doets EL, de Wit LS, Dhonukshe-Rutten RAM, et al. Current micronutrient recommendations in Europe with special focus on vitamin A and vitamin D. Eur J Nutr. 2008;47:17–40. doi: 10.1007/s00394-008-1003-5. [DOI] [PubMed] [Google Scholar]
- 62.Henderson L, Gregory J, Swan G, et al. The National Diet and Nutrition Survey: Adults Aged 19 to 64 years. The Stationery Office; London: 2002. [Google Scholar]
- 63.Institute of Medicine [accessed September 9 2009];Dietary Guidelines for Americans – Chapter 2. Adequate Nutrients Within Calorie Needs. 2005 http://www.health.gov/dietaryguidelines/dga2005/document/html/chapter2.htm.
- 64.Gregory J, Lowe S, Bates CJ, et al. National Diet and Nutrition Survey: Young People Aged 4–18 Years. Report of the Diet and Nutrition Survey. vol. 1. The Stationery Office; London: 2000. [Google Scholar]


