Abstract
Objective
An AIDS epidemic among older children and adolescents is clinically apparent in Southern Africa. We estimated the likely scale and time course of the epidemic in older survivors of vertical HIV infection.
Design
We modelled demographic, HIV prevalence, mother-to-child transmission (MTCT) and child survival data to project HIV burden among older children in two Southern African countries at different stages of severe HIV epidemics. Using measured survival data for children, we estimate that 64% of HIV-infected infants are fast-progressors with median survival=0.64 years and 36% are slow-progressors with median survival=16.0 years. We confirmed model validity by comparing model predictions to available epidemiological data.
Findings
Without treatment, HIV prevalence among 10-year olds in South Africa is expected to increase from 2.1% in 2008 to 3.3% in 2020 while in Zimbabwe, it will decrease from 3.2% in 2008 to 1.6% in 2020. Deaths among untreated slow-progressors will increase in South Africa from 7000/year in 2008 to 23,000/year in 2030, and in Zimbabwe from 8000/year in 2008 to peak at 9700/year in 2014. Drugs to prevent MTCT could reduce death rate in 2030 to 8700/year in South Africa and to 2800/year in Zimbabwe in 2014.
Conclusions
A substantial epidemic of HIV/AIDS in older survivors of MTCT is emerging in Southern Africa. The lack of direct observations of survival in slow-progressors has resulted in failure to anticipate the magnitude of the epidemic and to adequately address the clinical needs of HIV-infected older children and adolescents. Better HIV diagnostic and care services for this age-group are urgently required.
Keywords: HIV, Africa, Adolescent, vertical transmission, modelling
Introduction
Sub-Saharan Africa, especially East and Southern Africa, has the highest burden of HIV infection in the world.[1] Substantial progress has been made in reducing the gap between those who need and those who receive antiretroviral therapy (ART), and in the coverage of interventions to prevent mother-to-child transmission (PMTCT) of HIV. In Southern Africa, ART coverage among adults increased by 10% to 32% in 2007 and PMTCT coverage was 43%.[2] For HIV-infected children, however, the treatment gap remains disproportionately high throughout the developing world.[2]
HIV is less well characterized in children and adolescents (defined by the World Health Organization as children aged between 10 to 19 years) than in adults and the number of, and prognosis for, those with untreated paediatric HIV remains uncertain. In the absence of drug treatment, about one third of infants born to HIV-infected mothers will be infected during pregnancy, birth, or breastfeeding (vertical or mother-to-child transmission: MTCT).[3] Until recently, it was assumed that few HIV-positive vertically-infected children survived beyond the age of 5 years.[4] However, a pooled cohort analysis estimated that at least 13% of such children will survive to age 10 years,[5] and, more recent projections suggest that 17% will survive to age 15 years.[6]
Most population-based HIV prevalence surveys excluded children between the age of 5 and 15 years although they have been included in recent surveys in Zimbabwe,[7] South Africa,[8, 9] Botswana,[10, 11] and Swaziland,[12] and in all of these countries a substantial burden of HIV has been reported in this age-group. In Zimbabwe, in particular, increasing numbers of HIV-positive adolescents are presenting with severe stunting, pubertal delay, and a history of chronic ill-health, consistent with long-standing infection but with little to suggest risk of transmission through routes other than MTCT.[13] Reports from other countries in the region confirm that considerable numbers of HIV-infected older children are presenting for care.[14] There has, however, been little planning or provision of HIV testing or treatment services for this age group.
We use data on time trends in the prevalence of HIV among women attending ante-natal clinics and on survival in children to estimate the prevalence of infection in older survivors of MTCT and compare our estimates with data on the prevalence of HIV in children in population surveys, on the age-distribution of children receiving ART in South Africa and the age-distribution of HIV-infected children in clinical care in Zimbabwe.
Methods and data sources
Demographic data
Population data were taken from the Population Division of the United Nations (UNPOP) database.[15] The data, given in five-year age bands, were interpolated to one-year age bands up to 1980 using cubic-splines. We used crude birth and death rates from the UNPOP database up to the start of the HIV epidemic[15] and use our model to project birth and death rates forward in time.
Trends in the adult prevalence of HIV
Data from antenatal HIV prevalence surveys in South Africa,[16] Botswana,[17] Swaziland [18] and from National HIV and AIDS Estimates for Zimbabwe[19] were used to estimate the number of HIV-positive pregnant women in each country. In order to estimate the HIV prevalence and mortality in adults, which we do only to estimate the relative size of the epidemic in adults and in children, we scale the data to fit UNAIDS national estimates of HIV in adults.[1]
Survival of vertically infected children
The model predictions depend mainly on the prevalence of HIV in pregnant women and the survival distribution of vertically infected children. Since cohort studies of children infected by their mothers have not been continued for more that five years we combine these data[5, 6] with data from the CASCADE cohort, a meta-analysis of 38 studies in developed countries which determined survival as a function of age and included children infected before the age of five years (Figure 1).[20] We fitted the data to an exponential survivor function for the fast progressors and a Weibull survival function for the slow progressors so that the survival, S(t), is given by
| 1 |
where the proportion of fast progressors is α, and of slow progressors is 1 −α. The least-squares parameter estimates are α = 0.36, β = 1.08/year, μ = 16.0 years and s = 2.7.
Figure 1.
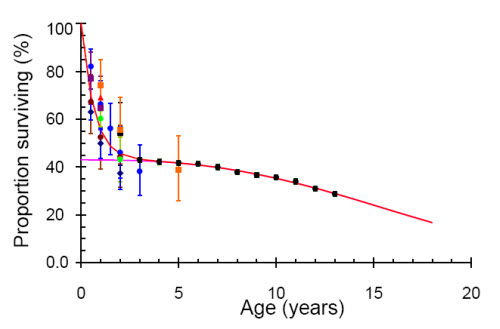
Proportion of children surviving to different ages. Data from Rakai, red triangle (no error bars); Kampala, blue dots; Abidjan, brown dots; Nairobi, black square; Durban, purple square; and Kigali, orange square [8]; Harare, dark blue diamond [41]; Africa, light green dots [42]; developed countries, black dots [26]. The fitted (red line) line is the sum of an exponential for the fast progressors and a Weibull (pink line) for the slow progressors as described in the text.
Modelling adult incidence, prevalence and mortality
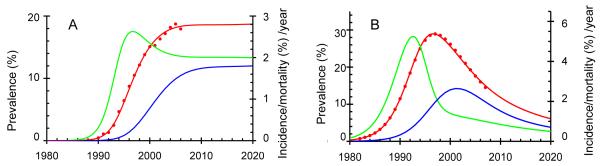
We fitted a compartmental model to time trends in the prevalence of HIV among adults (Figure 2) and used the model to estimate time trends in adult incidence and mortality in order to be able to compare the predictions for children with those for adults. Details of the model of adult transmission have been published elsewhere.[21]
Figure 2.
The prevalence (red), incidence (green) and mortality (blue) of HIV among adults in South Africa (A) and Zimbabwe (B). Data from ante-natal clinic surveys (red dots) scaled to give the prevalence in all adults.
Age specific prevalence and mortality rates for infants and older children
The birth rate combined with HIV prevalence among adult women was used to estimate the number of children born to HIV positive mothers over time for different options for PMTCT interventions. This, with the survival distribution (Equation 1), determines the prevalence and mortality, of fast and slow progressors.
PMTCT interventions
We assume that the probability of MTCT will be reduced from 35% (mixed breastfeeding) to 16% with single-dose nevirapine and exclusive breastfeeding, and to 2% with highly active antiretroviral therapy (HAART) plus exclusive breastfeeding.[6] We estimate the impact of increasing the coverage of these two PMTCT interventions starting in 2000, reaching 40% by 2005 and levelling off at 80% in 2010, and assuming that the interventions affect fast and slow progressors equally.
Other data sources
To investigate the validity of the model we use age-specific data on individuals in HIV care nationally in Zimbabwe,[22] and from a cohort of individuals registered at HIV care clinics in South Africa run by the Catholic Bishops Conference (CBC), which provides ART to resource-poor communities in South Africa.[23] We also compare the predicted prevalence of HIV to data from national prevalence surveys in three countries, Botswana,[10] South Africa,[9] and Swaziland.[12] We assume that the specificity was 99.5% for blood-based HIV-tests and 98% for oral mucosal transudate-based tests.[24]
Results
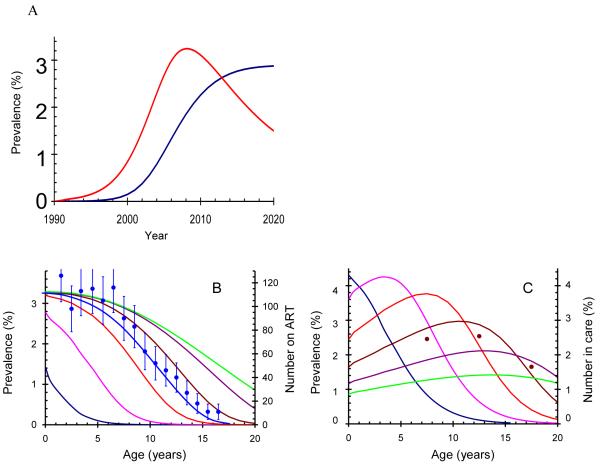
We estimated the age-specific incidence, prevalence and mortality for vertically-infected slow progressors and for adults and examined the potential impact of PMTCT on these outcomes. Prevalence of HIV in South Africa and Zimbabwe among slow progressors without PMTCT We predict substantial epidemics of HIV in adolescent survivors in both countries. In South Africa, the prevalence of vertically acquired HIV among ten year olds has probably increased from less than 0.2% in 2000 to 2.1% in 2008, and is expected to reach 3.3% by 2020 if no PMTCT is provided. In Zimbabwe, where adult HIV prevalence peaked in the late 1990s and has since declined,[19] the corresponding prevalence reached a peak of 3.2% in 2008, and is projected to fall to 1.6% in 2020 (Figure 3A).
Figure 3.
A: Projected prevalence of HIV among vertically-infected individuals aged 10 years in South Africa (blue) and Zimbabwe (red). Figure 3B and C: Prevalence of vertically-infected HIV-positive children by time and age in South Africa (B) and Zimbabwe (C). Lines at five year intervals: blue 1995; pink 2000; orange 2005; brown 2010; purple 2015; green 2020. In B the age-distribution of children receiving ART from the Catholic Bishops in South Africa is given by the blue dots and line, scaled vertically to match the value at age zero. In C the age-distribution of 16,588 children in care between the ages of 5 and 20 years and infected with HIV is given by the brown dots scaled to match the distribution for 2008. The assumption is that individuals received no PMTCT or ART.
The age-specific prevalence of HIV-positive slow progressors at 5 year intervals from 1995 to 2020 is shown in Figure 3B and 3C. In South Africa, where the adult HIV epidemic is about ten years behind that in Zimbabwe, the prevalence of slow-progressors increases until 2020 (Figure 3B). In 1995 the adult epidemic of HIV in South Africa was still relatively new and the few infected slow progressors had to have been infected in the previous five years. By 2020, assuming that the epidemic stabilizes (Figure 2), the age-specific prevalence reflects the Weibull survival curve (Equation 1). In Zimbabwe, the prevalence of slow-progressors increases up to 2013 and declines thereafter but the mean age of the slow progressors increases steadily as the adult epidemic falls giving rise to fewer new infections among slow progressors (Figure 3C).
AIDS deaths attributable in South Africa and Zimbabwe without PMTCT
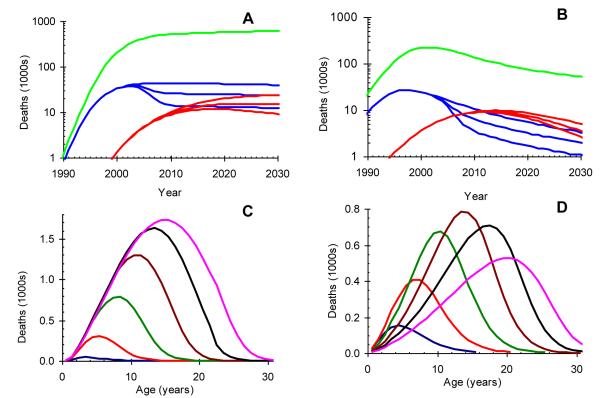
As shown in Figures 4A and 4B, the high median survival in slow progressors implies a substantial time-lag between the epidemics of AIDS deaths in adults and slow progressors. In South Africa, without treatment, deaths in vertically-infected slow progressors will increase from 7 thousand per year in 2008 to 23000 per year in 2030 (Figure 4A) when the mean age of those dying will be 15 years (Figure 4C). In Zimbabwe these deaths will increase from 8000 per year in 2008 to a peak at 9700 per year in 2014 (Figure 4B), when the mean age of those dying will be 16 years (Figure 4D), and will decline to 7500 per year in 2030 when the mean age will be 18 years.
Figure 4.
Projected number and age profile of deaths due to HIV infection by time and age in South Africa and Zimbabwe. A and C: South Africa; B and D: Zimbabwe. A and B: deaths in thousands, summed over all ages, on a logarithmic scale among fast-progressors (blue), slow-progressors (red) and adults (green). For fast and slow progressors the upper line assumes no PMTCT, the middle line assumes 80% coverage of single-dose nevirapine for PMTCT by 2010, and the lower line assumes 80% coverage of ART for PMTCT by 2010. C and D: deaths (per year of age and time) in thousands among vertically-infected slow progressors assuming no PMTCT or ART. Lines given at five year intervals: blue 1995; red 2000; green 2005; brown 2010; black 2015; pink 2020.
In South Africa, infant AIDS deaths among rapid progressors will continue to outnumber those in slow progressors (Figure 4A) while in Zimbabwe adolescent slow-progressors will outnumber infant fast-progressors after 2012 (Figure 4B).
In our model, deaths among slow-progressors do occur in infancy although infrequently. In the early years of the adult HIV epidemic all slow progressors will still be young, so deaths among slow progressors can only be at a young age. AIDS deaths among slow progressors may still be less apparent clinically than AIDS deaths among fast progressors in 2011, despite being almost equal in number, because slow progressors span a much wider age range.
The potential impact of PMTCT interventions on AIDS deaths in South Africa and Zimbabwe
HIV prevalence and deaths among fast progressors begin to fall very soon after PMTCT interventions are implemented (Figures 4A and B), but much more slowly among adolescents, reflecting their longer survival. The most potent intervention (ART for mothers and babies plus breast feeding) will halve HIV related mortality by 2010 in 5 year olds, and by 2020 in 15 year olds in both Zimbabwe and South Africa. The number of deaths among slow progressors in 2030 could be reduced from 23000 to 8700 per year in South Africa and from 7500 to 2800 per year in Zimbabwe. Nevirapine with exclusive breast feeding, will only reduce mortality by about 57% as much.
Potential role of clinical and national survey data for providing estimates of median survival of slow-progressors
Figure 3B shows the age distribution of children in the South African Catholic Bishops Cohort [25] superimposed on our estimates of HIV prevalence, and figure 3C shows the age-distribution of children in HIV care in Zimbabwe. The age distribution of children in both countries is close to the estimated age-specific prevalence predicted by our model.
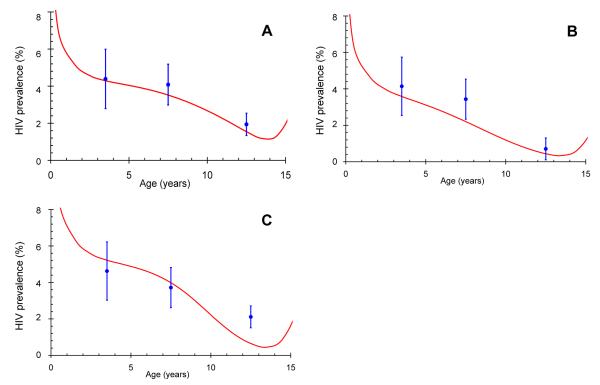
In Figure 5 we use our model to predict the prevalence of HIV in children and adolescents in Botswana, Swaziland and South Africa. It is important to note that the model is not fitted to the data, so that the comparison is direct, and the only adjustment to the data is for the assumed specificity of the HIV-tests. The agreement between model estimates and observed data give support for the model and underlying assumptions and suggests ways in which the validity of the model can be explored further. Finally, we can now use these data to examine the sensitivity of the results to changes in the survival distribution of slow-progressors by varying their median survival and determining the lower limit that is consistent with the data in Figure 5. The lower 95% confidence limits are then 12.2 years for Botswana, 7.4 years for South Africa and 12.4 years for Swaziland giving strong support to the assumption in Figure 1 that the long-term survival follows the distribution found for those infected before the age of four years in developed countries.
Figure 5.
Observed prevalence (blue dots and 95% confidence limits) of HIV from national prevalence surveys in Botswana 2004 (A), South Africa 2005 (B) and Swaziland 2006 (C) and predicted prevalence (red line) from the model described in the text, assuming a median survival of 16 years. The different shapes of the projected curves reflect differences in the timing and magnitude of the respective adult epidemics in the years preceding the survey data.
Discussion
The starting point for this study was the increasing evidence of frequent, prolonged survival among untreated vertically-infected HIV-positive children.[8, 13, 14] Available data suggest that about one-third of infected African infants are slow progressors with a median life-expectancy of about 16 years,[5, 6] and this analysis provides strong support for a substantial and prolonged epidemic of HIV-infected older children, with a considerable number of survivors reaching late adolescence.
The epidemic of slow progressors emerges slowly and leads to a persistent epidemic of HIV-infected survivors, with numbers of older survivors continuing to increase for ten to twenty years after the HIV epidemic in adults has peaked and peak mortality occurring even later. These dynamics contrast sharply with the much more immediately obvious epidemic of infant deaths among fast-progressors, and may help to explain why the scale of the epidemic in long term survivors of MTCT has only recently been recognised.
An additional factor is that children infected postnatally through breast feeding may be more likely to be slow progressors than those infected in utero or intrapartum.[26, 27] The immune system undergoes major changes in the post-partum period, shifting away from active acquisition of self-tolerance in utero towards defensive responses to antigens encountered postnatally [28], and the timing of HIV infection could have a critical impact on the anti-HIV immune response. Infants infected after the first few weeks of life have significantly lower peak viral loads and lower viral set-points than those infected earlier, and have substantially lower mortality rates.[26, 29, 30]
If timing of infection affects the likelihood of slow progression, then the emerging African epidemic may have a natural history that is different from those in developed countries because of differences in infant feeding practices. In developed countries formula-feeding is almost universal among HIV infected mothers,[31] while in Africa most mothers breastfeed their babies even if they know that they are infected with HIV.[8] Breastfeeding approximately doubles the risk of vertical HIV transmission, with a third of transmission events from breast milk occurring after 6 months.[32] Thus, in addition to the much higher HIV prevalence among infants in Africa, a higher proportion of these may be slow progressors.
Both South Africa and Zimbabwe are now scaling up PMTCT programs, with the aim of achieving universal coverage by 2010. However, coverage of PMTCT was only 57% in South Africa and 29% in Zimbabwe in 2007,[2] and PMTCT will not affect the current cohort of surviving children who have slowly progressing HIV infection that was acquired before the recent scale-up of services. Potentially, the scale-up of PMTCT programmes may further increase the proportion of vertical HIV infections that are acquired after the first few weeks of life, although reducing the overall burden of infected infants.
In spite of the increasingly apparent epidemic of slow progressors in Southern Africa, surviving older children and adolescents are still very poorly served by routine HIV testing and care services.[33] As well as systematic under-appreciation of the burden of HIV in this age group, the need for parental or guardian consent present considerable barriers to HIV testing in minors. Older survivors of vertical HIV infection are mostly orphaned and often impoverished by parental ill-health.[34] Routine diagnosis is usually made only after several years of increasingly poor health, by which time children have lost years of schooling and are at high risk of avoidable but irreversible chronic complications of HIV.[13] Added to this is an expanding cohort of children with more rapidly progressing HIV who would have died but are now likely to survive to adolescence because of ART.[35] Furthermore, as increasing numbers of children enter adolescence and become sexually active, it is likely that onward horizontal HIV transmission will contribute to ongoing adult HIV epidemics.
The study is limited by the lack of reliable age-specific cohort data on which to base projections, highlighting the need for much better monitoring and for more comprehensive data pertaining to HIV among older children. The estimated median survival of 16 years for slow progressors was based on data from the CASCADE cohort.[20] However, these children were infected mainly from contaminated blood products and, because they were all from developed countries, their survival may be different from the survival of vertically infected slow progressors in Africa. The national HIV prevalence surveys often have low participation rates and the specificity of the HIV tests used in the surveys is uncertain. The data reported by the Catholic Bishops in South Africa, used for comparison with model projections, may over-represent older children and adolescents due to the programme’s special focus on orphans, and provision of HIV care would improve survival.
The median survival of slow progressors plays a critical role in determining the magnitude and duration of the HIV epidemic in older survivors of MTCT but cohort studies to estimate this parameter would require a very long follow-up that would not now be feasible given the increasing availability of ART. However, it may be possible to obtain indirect evidence of the distribution of survival through much more extensive prevalence surveys than have so far been carried out, through data from health facilities implementing provider-initiated or routine HIV testing and from data on the age distribution of patients entering clinical care with HIV.
Service provision has been adversely affected by the under-appreciation of the numbers of surviving older children and adolescents living with HIV in Africa. The need to consider HIV as a cause of ill-health in older children has not been emphasised to health providers, and little or no provision has been made for the special needs of this age group. While awaiting more precise projections there is an urgent need to develop and rapidly implement policies and programmes aimed at providing early diagnosis, treatment and care including secondary prevention services to the expanding numbers of children and adolescents who are growing up with HIV.
Acknowledgements
ELC and BGW conceived the idea of modelling the epidemic in older children and BGW did the modelling. RAF wrote the first draft of the paper and contributed the Harare Adolescent Morbidity Study data and the Zimbabwe HIV Clinics data, and RW contributed the Catholic Bishops Conference data. EG did the comparison of the model and National Survey data. All authors were involved in discussions on the models and projections and contributed to the writing of the paper. All authors have seen and approved the final version.
BGW had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
RAF and ELC are funded by the Wellcome Trust. The Wellcome Trust had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.2008 Report on the Global HIV/AIDS epidemic. UNAIDS; Geneva, Switzerland: 2008. [Google Scholar]
- 2.WHO/UNAIDS/UNICEF . Progress Report 2008. Geneva, Switzerland: 2008. Towards Universal Access. Scaling up priority HIV/AIDS interventions in the health sector. [Google Scholar]
- 3.De Cock KM, Fowler MG, Mercier E, de Vincenzi I, Saba J, Hoff E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. Jama. 2000;283:1175–1182. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 4.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 5.Marston M, Zaba B, Salomon JA, Brahmbhatt H, Bagenda D. Estimating the net effect of HIV on child mortality in African populations affected by generalized HIV epidemics. J Acquir Immune Defic Syndr. 2005;38:219–227. doi: 10.1097/00126334-200502010-00015. [DOI] [PubMed] [Google Scholar]
- 6.Stover J, Walker N, Grassly NC, Marston M. Projecting the demographic impact of AIDS and the number of people in need of treatment: updates to the Spectrum projection package. Sex Transm Infect. 2006;82(Suppl 3):iii45–50. doi: 10.1136/sti.2006.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomo E, Rusakaniko S, Mashange W, Mutswanga J, Chandiwana B, Munyati S. Household survey of HIV-prevalence and behaviour in Chimanimani District, Zimbabwe. Human Social Research Council; Cape Town, South Africa: 2005. [Google Scholar]
- 8.Shisana O, Methtar S. HIV risk exposure among young children. A study of 2 to 9 year olds served by the public health facilities in the Free State, South Africa. Human Social Research Council; Cape Town, South Africa: 2005. [Google Scholar]
- 9.Shisana O, Rehle T, Simbayi LC, Parker W, Ziuma K, Bhana A, et al. South African national HIV prevalence, HIV incidence, behaviour and communication survey, 2005. Human Social Research Council; Cape Town, South Africa: 2005. [Google Scholar]
- 10.Botswana AIDS Impact Survey II. National AIDS Coordinating Agency and Central Statistics Office; Botswana: 2005. [Google Scholar]
- 11.Tsheko G, Odirile L, Bainame K, Segwabe M, Nair P, Ntshebe O. Household Survey of Behavioural Risks and HIV Sero-Status in two districts in Botswana. Human Social Research Council; Cape Town, South Africa: 2007. [Google Scholar]
- 12.Central Statistical Office (CSO) [Swaziland] Macro International Inc. Swaziland Demographic and Health Survey 2006-07. Central Statistical Office and Macro International Inc.; Mbabane, Swaziland: 2008. [Google Scholar]
- 13.Ferrand RA, Mafukidze A, Mangeya N, Bandason T, Bwakura T, Nathoo K, et al. Causes of Acute Hospitalization During Adolescence: The Burden and Spectrum of HIV-Related Morbidity in a Country with an Early and Severe HIV Epidemic. Conference of Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 14.Walker AS, Mulenga V, Sinyinza F, Lishimpi K, Nunn A, Chintu C, Gibb DM. Determinants of survival without antiretroviral therapy after infancy in HIV-1-infected Zambian children in the CHAP Trial. J Acquir Immune Defic Syndr. 2006;42:637–645. doi: 10.1097/01.qai.0000226334.34717.dc. [DOI] [PubMed] [Google Scholar]
- 15.Population data. World Population Prospects: The 2004 Revision. 2005.
- 16.The National HIV and Syphilis Prevalence Survey, South Africa. Department of Health South Africa; 2007. [Google Scholar]
- 17.Botswana Second Generation HIV/AIDS Surveillance Technical Report. Botswana Department of HIV AIDS Prevention and Care; 2006. [Google Scholar]
- 18.Swaziland HIV Estimates and Projections: NERCHA. UNAIDS Worskshop Report. 2007.
- 19.Zimbabwe National HIV and AIDS Estimates 2007. Ministry of Health and Child Welfare; Harare, Zimbabwe: 2007. [Google Scholar]
- 20.Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Concerted Action on SeroConversion to AIDS and Death in Europe. Lancet. 2000;355:1131–1137. [PubMed] [Google Scholar]
- 21.Williams BG, Lloyd-Smith JO, Gouws E, Hankins C, Getz WM, Hargrove J, et al. The potential impact of male circumcision on HIV in Sub-Saharan Africa. PLoS Med. 2006;3:e262. doi: 10.1371/journal.pmed.0030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrand RA, Lowe S, Whande B, Munaiwa L, Langhaug L, Cowan FM, et al. Survey of children accessing HIV services in a high HIV prevalence setting: Time for HIV-infected Adolescents to Count? Bull World Health Organ. 2009 doi: 10.2471/BLT.09.066126. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.UNAIDS Best Practice Collection. UNAIDS; Geneva, Switzerland: 2006. A Faith-Based Response to HIV in Southern Africa: the Choose to Care Initiative. [Google Scholar]
- 24.World Health Organization . WHO Guidelines for HIV Testing Technologies. Geneva, Switzerland: 2007. [Google Scholar]
- 25.Wood R. Large Scale Implementation of Antiretroviral Therapy: Early Results from Faith-based Clinics in South Africa; South Africa Catholic Bishops Conference (SACBC) AIDS Office Publications; South Africa. 2007. [Google Scholar]
- 26.Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo KJ, Piwoz EG, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J. 2007;26:519–526. doi: 10.1097/01.inf.0000264527.69954.4c. [DOI] [PubMed] [Google Scholar]
- 27.Zijenah LS, Moulton LH, Iliff P, Nathoo K, Munjoma MW, Mutasa K, et al. Timing of mother-to-child transmission of HIV-1 and infant mortality in the first 6 months of life in Harare, Zimbabwe. Aids. 2004;18:273–280. doi: 10.1097/00002030-200401230-00017. [DOI] [PubMed] [Google Scholar]
- 28.Howard JG, Mitchison NA. Immunological tolerance. Prog Allergy. 1975;18:43–96. doi: 10.1159/000395256. [DOI] [PubMed] [Google Scholar]
- 29.Richardson BA, Mbori-Ngacha D, Lavreys L, John-Stewart GC, Nduati R, Panteleeff DD, et al. Comparison of human immunodeficiency virus type 1 viral loads in Kenyan women, men, and infants during primary and early infection. J Virol. 2003;77:7120–7123. doi: 10.1128/JVI.77.12.7120-7123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spira R, Lepage P, Msellati P, Van De Perre P, Leroy V, Simonon A, et al. Natural history of human immunodeficiency virus type 1 infection in children: a five-year prospective study in Rwanda. Mother-to-Child HIV-1 Transmission Study Group. Pediatrics. 1999;104:e56. doi: 10.1542/peds.104.5.e56. [DOI] [PubMed] [Google Scholar]
- 31.Achievements in public health. Reduction in perinatal transmission of HIV infection--United States, 1985-2005. MMWR Morb Mortal Wkly Rep. 2006;55:592–597. [PubMed] [Google Scholar]
- 32.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. Jama. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 33.Antiretroviral therapy of HIV infection in infants and children in resource-limited settings, towards universal access. World Health Organization; Geneva, Switzerland: 2006. [Google Scholar]
- 34.Andrews G, Skinner D, Zuma K. Epidemiology of health and vulnerability among children orphaned and made vulnerable by HIV/AIDS in sub-Saharan Africa. AIDS Care. 2006;18:269–276. doi: 10.1080/09540120500471861. [DOI] [PubMed] [Google Scholar]
- 35.Judd A, Doerholt K, Tookey PA, Sharland M, Riordan A, Menson E, et al. Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996-2006: planning for teenage and adult care. Clin Infect Dis. 2007;45:918–924. doi: 10.1086/521167. [DOI] [PubMed] [Google Scholar]