Abstract
Background
Although nocturnal awakenings help categorize asthma severity and control, their clinical significance has not been thoroughly studied.
Objective
To determine the clinical consequences of nocturnal asthma symptom(s) requiring albuterol in children with mild-to-moderate persistent asthma outside of periods when oral corticosteroids were used for worsening asthma symptoms.
Methods
285 children ages 6 to 14 years with mild-to-moderate persistent asthma were randomized to receive one of three controller regimens and completed daily symptom diaries for 48 weeks. Diary responses were analyzed for the frequency and consequences of nocturnal asthma symptoms requiring albuterol.
Results
Nocturnal asthma symptoms requiring albuterol occurred in 72.2% of participants at least once and in 24.3% ≥13 times. 81.3% of nocturnal symptoms occurred outside of exacerbation periods and were associated the next day with the following events: albuterol use (56.9% of days preceded by nocturnal symptoms versus 18.1% of days not preceded by nocturnal symptoms, Relative Risk (RR) 2.3, 95%CI: 2.2,2.4), school absence (5.0% versus 0.3%, RR 10.6, 95%CI: 7.8,14.4), and doctor contact (3.7% versus 0.2%, RR 8.8, 95%CI:6.1,12.5). Similar findings were noted during exacerbation periods (RR 1.7 for albuterol use, 5.5 for school absence, and 4.9 for doctor contact). Nocturnal symptoms did not predict the onset of exacerbations.
Conclusion
Nocturnal symptoms requiring albuterol in children with mild-to-moderate persistent asthma receiving controller therapy occurred predominantly outside of exacerbation periods. Despite being poor predictors of exacerbations, they were associated with increases in albuterol use, school absences, and doctor contacts the day after nocturnal symptom occurrences.
Keywords: asthma, nocturnal symptoms, exacerbation
INTRODUCTION
The frequency of nocturnal awakenings is used to categorize asthma severity and control based on expert opinion consensus1. Although these nocturnal events are considered important in childhood asthma, their significance remains unclear due to a paucity of literature regarding this topic, in part due to the fact that most publications investigating nocturnal asthma have focused on adults (e.g., 2–5). Studies in children have examined the prevalence of nocturnal asthma6–11, associations with nocturnal asthma and sleep quality10 and other factors associated with nocturnal asthma9, 12–15, but all of these studies were of short duration (≤7 days) or cross-sectional. One cross-sectional study examined the consequences of nocturnal asthma on school attendance, school performance, and parents’ work attendance, using parent report for both awakening and morbidity in the 4 weeks before interviews. The authors concluded that nocturnal awakening may affect these outcomes, but were not able to examine associations over time due to the cross-sectional design 12. Fuhlbrigge and coworkers have also shown that the number of nocturnal awakenings over a 4-week period was associated with future asthma-related events (emergency department visits, hospitalizations or oral steroid bursts) 16.
The Pediatric Asthma Controller Trial (PACT) was a 48-week long randomized clinical trial involving 6–14 year old children with mild to moderate persistent asthma that incorporated measures to enhance and monitor medication adherence 17. It provides the opportunity to identify the prevalence of, and factors related to, nocturnal asthma symptom(s) requiring albuterol (NASRA), as well as clinically important events linked to these symptoms. To our knowledge, this report is the first examination of prospective data of extended duration to determine associations of NASRA in children with asthma with the ability to discriminate the consequences of nocturnal asthma symptoms that occur both within and outside periods of exacerbation. Further, it defines the impact of these symptoms on morbidity under study conditions with medications provided and monitored.
METHODS
The details of the trial have been previously described 17. Briefly, 285 subjects ages 6 to <14 years with mild to moderate persistent asthma were randomized. Inclusion criteria were physician-diagnosed asthma, PC20 methacholine FEV1 ≤12.5mg/ml, and no personal smoking in the past year. Exclusion criteria were albuterol use (more than 8 rescue puffs per day on average) and/or symptoms (night awakenings more than 2 days per week on average) consistent with severe persistent disease during run-in, FEV1 <80% predicted at screening or <70% predicted at randomization, ≥ 2 asthma hospitalizations in the past year, ≥4 courses of systemic corticosteroids in the past year, history of life-threatening asthma exacerbation, <75% adherence of doses of placebo capsules and dry powder inhaler during the 2 week run-in period, and respiratory tract infection, asthma exacerbation, or systemic corticosteroid use within 4 weeks.
Participants demonstrated symptoms consistent with mild to moderate persistent asthma during the 2 week run-in period by diary symptoms, rescue albuterol use, or peak expiratory flow (PEF) <80% calculated from the mean of morning and evening PEFs obtained during the final week of the run-in period at least 3 times per week while not receiving controller medication for at least 2 weeks prior to randomization. Participants were randomized to one of three treatment groups for 48 weeks: (1) fluticasone propionate Diskus 100µg BID and oral placebo QPM; (2) combination fluticasone propionate 100µg/salmeterol 50µg Diskus QAM and salmeterol Diskus 50µg QPM (PACT combination) and oral placebo QPM; (3) placebo Diskus BID and oral montelukast 5mg QPM. PEF, symptom scores, and albuterol use for rescue were recorded by a parent or caregiver on diaries twice daily. Older children were permitted to complete diaries with help from a parent or caregiver. Cough and wheeze were scored on a 0–3 scale: 0= no symptoms, 1= mild (awareness of symptoms that were easily tolerated), 2= moderate (symptoms with some discomfort, causing some interference of sleep or daily activities), 3= severe (symptoms which led to inability to sleep or perform daily activities). Each morning parents were asked to record if “albuterol was used for asthma during the night”. An affirmative response to this question identified a NASRA.
During the trial, oral prednisone was initiated if 1) the participant used >12 puffs of albuterol in 24 hours and had a diary card symptom code of 3 or PEF <70% of personal best before each albuterol use, 2) the subject had a symptom code of 3 for ≥48 hours, 3) PEF dropped to <50% of personal best despite albuterol treatment, or 4) by physician discretion. An Asthma Control Day (ACD) was defined as a day without albuterol rescue use, use of oral corticosteroids, use of non-study asthma medications, PEF <80% of personal best, daytime symptoms, night-time awakenings, unscheduled heathcare visits, emergency department visits, or hospitalizations for asthma and school absenteeism for asthma.
The protocol and consents were approved by the institutional review boards at all participating centers. Parents provided written informed consent and participants provided written assent. The Childhood Asthma Research and Education Network Data and Safety Monitoring Board monitored the trial.
Statistical Analyses
Data analyses were performed using diary data. One of the subjects did not have diary data recorded after randomization, so data from 284 subjects were analyzed. The primary outcome in PACT was the percent improvement in ACDs. This percent improvement was defined by comparing the 48-week treatment period to the run-in, placebo period. A full description of the primary statistical analysis plan and sample size justification was previously reported17.
Determination of NASRA
88.4% subjects were followed for 48 weeks, as 35 participants withdrew from the study before 48 weeks (no difference in rates between treatment groups). 16 participants were lost to followup, 7 withdrew consent, and 9 withdrew for other reasons. Participants provided data to this analysis if they completed any diary cards during the study. Only 4 participants had less than 28 days of diary data, and 32 participants had less than 100 days of diary data. Due to the varying amount of diary card data obtained, participant withdrawal, or treatment failure, an annualized frequency of NASRA was determined as the total number of NASRA recorded divided by the number of days for which diary data were available, multiplied by 365 days. Subjects were stratified into three groups based on the annualized frequency of NASRA during the treatment period: 0, 1–12, and 13 or more per year. The 13 annualized NASRA cutpoint was chosen since ≤1 nocturnal awakening per month represents well-controlled asthma among children 5–11 years of age per the NAEPP/EPR3 Guidelines 1. These three groups were compared with respect to baseline characteristics using Analysis of Variance for continuous measures such as BMI, age and lung function parameters, and Chi-square tests for categorical assessments such as race, gender, and medication history.
Association Studies
Longitudinal associations between NASRA and exacerbations and other diary-based symptom parameters, including cough, wheeze, albuterol use, low PEFs, school absenteeism and doctor contacts, were analyzed using the Cochran-Mantel-Haenszel test stratified by subject. Relative risks and associated 95% confidence intervals were also calculated. Associations were also examined stratified by treatment group. All analyses were carried out using the SAS statistical software system version 9.1 (SAS Institute Inc, Cary, NC). Significance was established at the two-sided level with p<0.05.
RESULTS
Prevalence and frequency of NASRA and the effect of controller therapy
205/284 (72.2%) subjects had at least one NASRA over the 48 week duration of the trial. 136 subjects (47.9%) had 1–12 NASRA, while 69 subjects (24.3%) had 13 or more NASRA. 31.2% of the subjects experienced at least one NASRA during the 2 week run-in (maximum 4 nocturnal awakenings permitted during run-in for randomization). The majority (73.5%) of NASRA occurred on isolated nights without a NASRA occurring on the following night, whereas 14.8% of NASRAs occurred on 2 consecutive nights, and 11.7% of NASRAs occurred on 3 or more consecutive nights. The frequency of NASRA was greatest in the fall season, with nearly double the frequency of NASRA occurring during fall compared with summer (31.5% vs. 16.8%, p<0.0001), with the frequencies during spring (24%) and winter (28%) being intermediate. The frequency of NASRA was lowest in the fluticasone group (F, median = 2.0 NASRA/year, 1st quartile=0, 3rd quartile=7), followed by the PACT combination group (C, median = 3.0 NASRA/year, 1st quartile=0, 3rd quartile=13), and highest in the montelukast group (M, median = 6.5 NASRA/year, 1st quartile=1, 3rd quartile=18) (overall: p=0.014, F vs. M: p=0.005, F vs. C: p=0.11, M vs. C: p=0.16).
Characteristics of subjects with NASRA
Subjects with ≥13 NASRA were more likely to be non-Caucasian than subjects with <13 NASRA (p=0.03) (Table 1). The number of NASRA during the trial was directly associated with the number of NASRA during the run-in period (p<0.0001) and inversely related to the number of ACDs experienced during the run-in period (p=0.008). The number of NASRA (0, 1–12, ≥13) did not differ by other baseline characteristics, such as age, gender, controller medication use in the prior year, peripheral blood eosinophils, serum IgE, skin test positivity, PC20, PEF variability during the run-in period, and exhaled nitric oxide at baseline. Only 30 parents reported smoking at study entry and there was no evidence of association of smoke exposure to NASRA.
Table 1.
Baseline characteristics relating to frequency of NASRA
| Characteristic: | Number of annualized NASRA | P value | ||
|---|---|---|---|---|
| 0 NASRA (79 subjects) |
1–12 NASRA (136 subjects) |
≥13 NASRA (69 subjects) |
||
| Male gender, n (%) | 50 (63.3) | 88 (64.7) | 36 (52.5) | NS |
| BMI | 19.5 ± 4.0 | 20.1 ± 4.8 | 20.3 ± 5.8 | NS |
| Non-Caucasian race*, n (%) | 13 (16.5) | 23 (16.9) | 22 (31.2) | 0.03 |
| Age at randomization (years) | 10.0 ± 2.2 | 9.8 ± 2.3 | 9.7 ± 2.0 | NS |
| Age at onset of asthma (years) | 3.6 ± 2.8 | 3.1 ± 2.7 | 3.0 ± 2.7 | NS |
| Number of NASRA/subject during the 14 day run-in period | 0.10 ± 0.3 | 0.58 ± 1.1 | 1.3 ± 1.5 | <0.0001 |
| Number of asthma-free days/subject during the 14-day run-in period | 4.7 ± 3.7 | 3.5 ± 3.0 | 3.4 ± 3.0 | 0.008 |
| AM PEF during run-in period (L/min) | 252.0 ± 72.0 | 244.8 ± 71.8 | 248.6 ± 58.4 | NS |
| PM PEF during run-in period (L/min) | 256.8 ± 69.8 | 251.9 ± 74.1 | 254.7 ± 59.1 | NS |
| PEF variability during run-in period (%) | 9.3 ± 4.6 | 10.2 ± 5.8 | 9.8 ± 4.7 | NS |
| Pre-BD FEV1 (% predicted) | 98.8 ± 11.2 | 96.3 ± 12.8 | 98.3 ± 12.4 | NS |
| Pre-BD FEV1/FVC (%) | 80.9 ± 7.5 | 78.8 ± 7.7 | 81.3 ± 7.8 | NS |
| Maximum BD response (%) | 9.9 ± 7.3 | 10.9 ± 7.5 | 11.1 ± 8.3 | NS |
| Peripheral Blood Eosinophils (%) | 5.4 ± 3.6 | 6.1 ± 4.6 | 6.4 ± 3.8 | NS |
| PC20 (mg/mL), median (Quartile 1, Quartile 3) | 1.1 (0.4, 2.6) | 0.8 (0.3, 2.5) | 0.8 (2, 2.9) | NS |
| FeNO (ppb), median (Quartile 1, Quartile 3) | 24.8 (9.7, 50.5) | 22.7 (12.8, 51.3) | 31.9 (12.6, 58.0) | NS |
| IgE (IU/mL), median (Quartile 1, Quartile 3) | 129.0 (40.9, 363.0) | 164 (61.7, 363.0) | 176.0 (69.0, 415.0) | NS |
| At least 1 positive skin test, n (%) | 58 (73.4) | 109 (80.2) | 55 (79.7) | NS |
| Medication use in prior year: | ||||
| Inhaled or nebulized corticosteroids, n (%) | 44 (55.7) | 80 (58.5) | 36 (52.2) | NS |
| Fluticasone/salmeterol combination, n (%) | 10 (12.7) | 15 (11.4) | 10 (13.7) | NS |
| Montelukast, n (%) | 28 (35.4) | 52 (38.2) | 15 (21.7) | NS |
Data represent mean ± SD except as noted.
Non-Caucasian race was 73% Black, 17% American Indian, 3% Asian and 7% Pacific Islander.
NASRA and asthma exacerbations
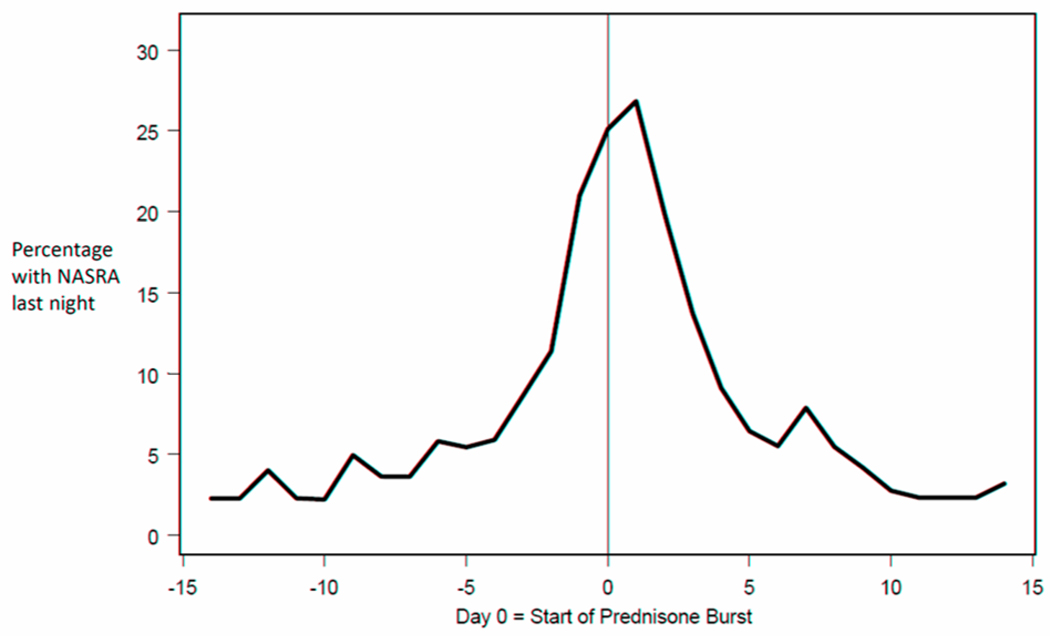
The majority (81.3%) of NASRA occurred outside of exacerbation periods, which were comprised of intervals including the 7 days before the oral corticosteroid start date, the duration of oral corticosteorid use (typically 4 days), and the 7 days after completion of oral corticosteroid. Only 38.4% of exacerbation periods included ≥1 NASRA, and when NASRA(s) were present during the 7 days preceding initiation of prednisone, the frequency was low until the day before prednisone was started (Figure 1). The occurrence of a NASRA was neither a specific nor sensitive indicator of an exacerbation requiring oral corticosteroid (Positive Predictive Value 0.12).
Figure 1. NASRA occurrence during exacerbation periods.
The horizontal axis is centered on the day prednisone treatment was initiated. For 21% of the asthma exacerbations which occurred during the course of the trial, a NASRA was reported the night before prednisone was initiated. The frequency of NASRA remained low until the third day prior to prednisone initiation and returned to pre-exacerbation levels within 5 days.
Symptom changes prior to occurrence of NASRA
While the likelihood of a NASRA increased as symptom severity (cough and wheeze) and use of albuterol for rescue increased, NASRAs were still infrequent following days with significant asthma symptoms: a NASRA occurred during only 19.0, 17.8, and 20.5% of nights following daytime reports of severe cough, severe wheeze, or albuterol use ≥7 puffs, respectively (Table 2). The likelihood of a NASRA was related to the PEF obtained the evening of the NASRA, with a red zone PEF being followed by a NASRA on 5.9% of nights whereas a green zone PEF was followed by a NASRA on 1.4% of nights (Table 2, also see Online Repository).
Table 2.
Occurrence and severity of cough and wheeze scores preceding NASRA
| 2a. Occurrence and severity of cough during the day preceding a NASRA | |||
|---|---|---|---|
| Report of NASRA that night | |||
| Cough score during day | No NASRA n (%) |
NASRA n (%) |
|
| 0 (no symptoms) | 57080 (98.6) | 802 (1.4) | P<0.0001 |
| 1 (mild) | 14635 (96.4) | 540 (3.5) | |
| 2 (moderate) | 3414 (87.8) | 477 (12.3) | |
| 3 (severe) | 592 (81.0) | 141 (19.0) | |
| 2b. Occurrence and severity of wheeze during the day preceding a NASRA | |||
|---|---|---|---|
| Report of NASRA that night | |||
| Wheeze score during day | No NASRA n (%) |
NASRA n (%) |
|
| 0 (no symptoms) | 65719 (98.3) | 1170 (1.7) | P<0.0001 |
| 1 (mild) | 7607 (94.4) | 448 (5.6) | |
| 2 (moderate) | 2114 (88.3) | 281 (11.7) | |
| 3 (severe) | 281 (82.2) | 61 (17.8) | |
| 2c. Occurrence and amount of albuterol use during the day preceding a NASRA | |||
|---|---|---|---|
| Report of NASRA that night | |||
| Puffs of albuterol in previous 24 hours |
No NASRA n (%) |
NASRA n (%) |
|
| 0 | 61020 (98.6) | 851 (1.4) | P<0.0001 |
| 1–2 | 9663 (95.0) | 499 (5.0) | |
| 3–4 | 3296 (91.9) | 291 (8.1) | |
| 5–6 | 1006 (88.6) | 129 (11.4) | |
| ≥7 | 736 (79.5) | 190 (20.5) | |
| 2d. Evening PEF and the occurrence of a NASRA that night | |||
|---|---|---|---|
| Report of NASRA that night | |||
| PM PEF Zone | No NASR n (%) |
NASRA n (%) |
|
| Green | 60259 (98.6) | 858 (1.4) | P<0.0001 |
| Yellow | 10680 (96.1) | 437 (3.9) | |
| Red | 286 (94.1) | 18 (5.9) | |
n=number of events for all participants
Effect of NASRA on school attendance and doctor contact was greater outside of exacerbations than during exacerbation periods
Albuterol use, school absence, doctor contact, significant wheeze (score of 2 or 3), and significant cough (score of 2 or 3) all occurred significantly more frequently on days after a NASRA than on days preceding a NASRA (Table 3). Overall, the effect of a NASRA was greatest for school absence the following day [RR 14.9 (95%CI:12.2,18.4) overall] and least for albuterol rescue use the following day [RR 2.4 (95%CI:2.3,2.5) overall] when compared to these events on days that preceded a NASRA. Associations between NASRA and these indicators of morbidity were present both during and outside exacerbations, with relative risks for the effects of NASRA being greater outside than during exacerbations. 309/527 (58.6%) school absences occurred outside of exacerbation periods. Of these 309 absences, 26.2% reported NASRA the prior night, whereas 43.6% of the absences during exacerbation periods were preceded by a NASRA. Similarly, 237/441 (53.7%) doctor contacts occurred outside exacerbation periods. Of these 237 contacts, 25.3% reported NASRA the prior night, whereas 39.5% of doctor contacts during exacerbation periods were preceded by a NASRA.
Table 3.
Stratified relative risks for morbidity on days following and not following a NASRA
| Morbidity | % Days with NASRA last night | % Days without NASRA last night | Stratified Relative Risk (95% CI) | |
|---|---|---|---|---|
| Used albuterol | Overall | 58.9% (1175/1996) | 19.2% (14882/77476) | RR=2.4 (2.3,2.5) |
| During exacerbation | 67.6% (252/373) | 47.0% (1345/2862) | RR=1.7 (1.5,1.8) | |
| Outside exacerbation | 56.9% (923/1623) | 18.1% (13637/74614) | RR=2.3 (2.2,2.4) | |
| School Absence | Overall | 8.8% (176/1996) | 0.5% (351/77476) | RR=14.9 (12.1,18.4) |
| During exacerbation | 25.5% (95/373) | 4.3% (123/2862) | RR=5.5 (4.0,7.4) | |
| Outside exacerbation | 5.0% (81/1623) | 0.3% (228/74614) | RR=10.6 (7.8,14.4) | |
| Contacted Doctor for Asthma | Overall | 7.1% (141/1996) | 0.4% (300/77476) | RR=13.3 (10.5,16.7) |
| During exacerbation | 21.7% (81/373) | 4.3% (124/2862) | RR=4.9 (3.6,6.7) | |
| Outside exacerbation | 3.7% (60/1623) | 0.2% (177/74614) | RR=8.8 (6.1,12.5) | |
| Wheeze Score of 2 or 3 | Overall | 20.4% (407/1996) | 3.1% (2400/77476) | RR=58 (5.2,6.4) |
| During exacerbation | 36.2% (135/373) | 10.5% (301/2862) | RR=3.5 (2.9,4.2) | |
| Outside exacerbation | 16.8% (272/1623) | 2.8% (2099/74614) | RR=5.1 (4.5,5.8) | |
| Cough Score of 2 or 3 | Overall | 33.8% (675/1996) | 5.2% (4040/77476) | RR=6.0 (5.6,6.5) |
| During exacerbation | 61.7% (230/373) | 20.6% (590/2862) | RR=2.9 (2.6,3.3) | |
| Outside exacerbation | 27.4% (445/1623) | 4.6% (3450/74614) | RR=5.3 (4.8,4.9) |
DISCUSSION
Over the 48-week treatment period in the PACT trial, during which continuous therapy with controller medication was provided by the study, the vast majority (81.3%) of NASRA occurred in periods outside of exacerbation. Nights with asthma symptoms requiring albuterol use were often followed the next day by indicators of asthma morbidity, including increased albuterol use, school absence, doctor contact, and occurrence of severe wheeze and/or cough. The relative risks of occurrence of these indicators of morbidity due to NASRA outside of exacerbations were greater than the corresponding relative risks within periods of exacerbation, indicating that nocturnal asthma symptoms have substantial clinical impact on patient’s lives even during periods of relative asthma stability (i.e. not during exacerbation). To our knowledge, such a pattern of nocturnal asthma symptom morbidity has not previously been reported, in part due to the cross-sectional approaches of prior studies12,15.
Nearly a quarter of participants with mild to moderate persistent asthma receiving daily controller therapy experienced a NASRA on at least a monthly basis, with the distribution of NASRA similar to the seasonal distribution of exacerbations (fall greatest, summer lowest) 18. Treatment group assignment significantly affected the occurrence of NASRA, with the fluticasone group having a significantly lower rate of NASRA than the montelukast group. The superiority of fluticasone in reducing NASRA is consistent with the overall effect of fluticasone in terms of asthma control days reported in a previous PACT publication 17. Similar to the findings in terms of asthma control days, the PACT combination of salmeterol 50µg twice daily with fluticasone 100µg once daily in the morning was not associated with significantly fewer NASRA than fluticasone twice daily, despite the use of a long acting β-agonist in the evening. This finding may have been due to the once daily (morning) dosing of fluticasone during the trial in the combination group rather than the typical twice daily dosing of fluticasone. While salmeterol has been demonstrated to decrease nocturnal awakenings in adults with asthma19, the clinical efficacy of salmeterol on nocturnal asthma symptoms may differ in children. The inhaled corticosteroid dosing regimen in the combination group did not allow us to examine if the nocturnal administration of inhaled corticosteroid would have resulted in a greater effect on nocturnal symptoms.
NASRA were neither specific nor sensitive indicators nor antecedents of exacerbations requiring oral corticosteroid. Even when NASRA(s) did occur during the week preceding an exacerbation, they generally did not appear until a couple of nights prior to oral corticosteroid start (Figure 1). Similarly, peak expiratory flows, symptoms and albuterol use during the day were not good indicators of a NASRA that evening, as only about 6% of evenings with a red zone PEF were followed by a NASRA and only about 20% of days before a NASRA included severe cough or wheeze or ≥7 puffs of albuterol use. Only 38.4% of exacerbation periods included a report of at least one NASRA, suggesting that NASRAs are a sign of symptom escalation in a subgroup of subjects.
Parents appear to seek medical attention following some, but not all, NASRAs, and NASRAs that occur during exacerbations were more often followed by a doctor visit. This may be due to the cumulative nature of the symptoms that occur with the exacerbation and a higher likelihood that symptoms during exacerbations may be more severe or less responsive to therapy than those outside of exacerbation periods.
The CAMP trial of children with mild to moderate asthma reported the prevalence of at least one nocturnal awakening during the 28 day albuterol-only and exacerbation-free run-in period to be 33.7% 8, a finding very similar to the 31.2% of subjects who experienced at least one NASRA during the 2 week PACT albuterol-only run-in period, and not much dissimilar to the 24.3% who had 13 or more NASRA during PACT while taking a controller medication. The current analysis expands on the results from CAMP as it includes a longer period of observation (48 weeks vs. 4 weeks) as well as examining NASRAs that occur both outside and during asthma exacerbations and examining the effects of different treatment regimens on these events. To our knowledge, these observations have not been previously reported in either adults or children.
A limitation of this study is that the diary card did not include separate questions for nocturnal awakenings and nocturnal albuterol use for asthma symptoms. NASRA is likely a suitable surrogate measure of nocturnal awakenings since albuterol use requires being awake. However, our data may underestimate nocturnal awakenings since some nocturnal awakenings may not have been treated with albuterol. The study did not include a placebo group to establish the background frequency of NASRA. Since subjects with very frequent NASRA during the run-in period were excluded from the trial, the trial may have selected for children with fewer NASRA. The education provided by the coordinators and the support from the clinical center teams may have influenced clinical events occurring in days after NASRAs, most likely by decreasing event occurrence. It is possible that some patients experienced poor perception of airflow obstruction and thus did not experience a NASRA. Finally, as with all post hoc subgroup analyses, inadequate statistical power may have resulted in failure to detect significant associations with NASRA.
In conclusion, most NASRA occurred outside exacerbation periods and those NASRA observed during exacerbation periods did not appear to be a clinically useful antecedent of exacerbations. Despite the lack of association with exacerbations, NASRA were associated with morbidity in the form of symptom scores, additional albuterol use, and school absences and doctor contacts outside of exacerbation periods. NASRA frequency appears differentially responsive to controller therapy, with the fluticasone group experiencing the lowest frequency of NASRA and the montelukast group experiencing the greatest frequency. Since NASRA were associated with clinically relevant outcomes and treatment differences, inclusion of nocturnal symptoms as an important outcome measure in future clinical trials, along with further investigation into the etiologies and prevention of nocturnal asthma symptoms, may have substantial clinical implications.
Clinical Implications.
Nocturnal asthma symptoms primarily occurred outside of exacerbation periods and were associated with clinical morbidity. Investigation into the etiologies and prevention of childhood nocturnal symptoms may have substantial clinical implications.
Acknowledgments
Supported by: Grants 5U10HL064287, 5U10HL064288, 5U10HL064295, 5U10HL064307, 5U10HL064305, 5U10HL064313 from the National Heart, Lung, and Blood Institute. This study was carried out in part in the General Clinical Research Centers at Washington University School of Medicine (M01 RR00036) and National Jewish Medical and Research Center (M01 RR00051).
Abbreviations
- ACD
Asthma Control Day
- BD
bronchodilator
- CAMP
Childhood Asthma Management Program
- FEV1
Forced Expiratory Volume in 1 second
- NASRA
Nocturnal Asthma Symptom(s) Requiring Albuterol
- PACT
Pediatric Asthma Controller Trial
- PC20
Provocation Challenge causing a 20% decrease in FEV1
- PEF
Peak Expiratory Flow
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
See Appendix
References
- 1.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Calhoun WJ. Nocturnal asthma. Chest. 2003;123:399S–405S. doi: 10.1378/chest.123.3_suppl.399s. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland ER. Nocturnal asthma. J Allergy Clin Immunol. 2005;116:1179–1186. doi: 10.1016/j.jaci.2005.09.028. quiz 87. [DOI] [PubMed] [Google Scholar]
- 4.Sutherland ER. Nocturnal asthma: underlying mechanisms and treatment. Curr Allergy Asthma Rep. 2005;5:161–167. doi: 10.1007/s11882-005-0091-z. [DOI] [PubMed] [Google Scholar]
- 5.Shigemitsu H, Afshar K. Nocturnal asthma. Curr Opin Pulm Med. 2007;13:49–55. doi: 10.1097/MCP.0b013e328010a890. [DOI] [PubMed] [Google Scholar]
- 6.Stores G, Ellis AJ, Wiggs L, Crawford C, Thomson A. Sleep and psychological disturbance in nocturnal asthma. Arch Dis Child. 1998;78:413–419. doi: 10.1136/adc.78.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadeh A, Horowitz I, Wolach-Benodis L, Wolach B. Sleep and pulmonary function in children with well-controlled, stable asthma. Sleep. 1998;21:379–384. doi: 10.1093/sleep/21.4.379. [DOI] [PubMed] [Google Scholar]
- 8.Strunk RC, Sternberg AL, Bacharier LB, Szefler SJ. Nocturnal awakening caused by asthma in children with mild-to-moderate asthma in the childhood asthma management program. J Allergy Clin Immunol. 2002;110:395–403. doi: 10.1067/mai.2002.127433. [DOI] [PubMed] [Google Scholar]
- 9.Ng Man Kwong G, Das C, Proctor AR, Whyte MK, Primhak RA. Diagnostic and treatment behaviour in children with chronic respiratory symptoms: relationship with socioeconomic factors. Thorax. 2002;57:701–704. doi: 10.1136/thorax.57.8.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chugh IM, Khanna P, Shah A. Nocturnal symptoms and sleep disturbances in clinically stable asthmatic children. Asian Pac J Allergy Immunol. 2006;24:135–142. [PubMed] [Google Scholar]
- 11.Mustafa G, Khan PA, Iqbal I. Nocturnal asthma in school children of south Punjab, Pakistan. J Ayub Med Coll Abbottabad. 2008;20:36–39. [PubMed] [Google Scholar]
- 12.Diette GB, Markson L, Skinner EA, Nguyen TT, Algatt-Bergstrom P, Wu AW. Nocturnal asthma in children affects school attendance, school performance, and parents' work attendance. Arch Pediatr Adolesc Med. 2000;154:923–928. doi: 10.1001/archpedi.154.9.923. [DOI] [PubMed] [Google Scholar]
- 13.Landstra AM, Postma DS, Boezen HM, van Aalderen WM. Role of serum cortisol levels in children with asthma. Am J Respir Crit Care Med. 2002;165:708–712. doi: 10.1164/ajrccm.165.5.2102115. [DOI] [PubMed] [Google Scholar]
- 14.Landstra AM, Boezen HM, Postma DS, van Aalderen WM. Effect of intravenous hydrocortisone on nocturnal airflow limitation in childhood asthma. Eur Respir J. 2003;21:627–632. doi: 10.1183/09031936.03.00085802. [DOI] [PubMed] [Google Scholar]
- 15.Fiese BH, Winter MA, Sliwinski M, Anbar RD. Nighttime waking in children with asthma: an exploratory study of daily fluctuations in family climate. J Fam Psychol. 2007;21:95–103. doi: 10.1037/0893-3200.21.1.95. [DOI] [PubMed] [Google Scholar]
- 16.Fuhlbrigge AL, Weiss ST, Kuntz KM, Paltiel AD. Forced expiratory volume in 1 second percentage improves the classification of severity among children with asthma. Pediatrics. 2006;118:e347–e355. doi: 10.1542/peds.2005-2962. [DOI] [PubMed] [Google Scholar]
- 17.Sorkness CA, Lemanske RF, Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007;119:64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 18.Covar RA, Szefler SJ, Zeiger RS, Sorkness CA, Moss M, Mauger DT, et al. Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children. J Allergy Clin Immunol. 2008;122:741–747. e4. doi: 10.1016/j.jaci.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraft M, Wenzel SE, Bettinger CM, Martin RJ. The effect of salmeterol on nocturnal symptoms, airway function, and inflammation in asthma. Chest. 1997;111:1249–1254. doi: 10.1378/chest.111.5.1249. [DOI] [PubMed] [Google Scholar]