Abstract
Purpose
To evaluate the efficacy and tolerability of mycophenolate mofetil (MMF) in patients with treatment refractory interstitial cystitis/painful bladder syndrome (IC/PBS).
Materials and Methods
Two hundred and ten patients with IC/PBS were to be randomized into a multicenter, placebo-controlled trial using a 2:1 randomization. Participants who had failed at least three IC/PBS-specific treatments and had at least moderately severe symptoms were enrolled in a 12-week treatment study. The primary study endpoint was the Global Response Assessment (GRA). Secondary endpoints included general and disease-specific symptom questionnaires and voiding diaries.
Results
Only 58 subjects were randomized before a black box warning regarding MMF safety was issued by the manufacturer in October 2007. The trial was halted, and an interim analysis was performed and presented to an independent Data and Safety Monitoring Board. Of the subjects randomized at the time of study cessation, 6/39 (15%) were considered responders for MMF, compared to 3/19 (16%) of controls (p=0.67). Secondary outcome measures reflected more improvement among controls.
Conclusion
In a randomized placebo-controlled trial that was prematurely halted, MMF demonstrated efficacy similar to placebo in treating symptoms of refractory IC/PBS. The results of this limited study cannot be used to confirm or refute the hypothesis that immunosuppressive therapy may be beneficial to at least a subgroup of IC\PBS patients. Despite study termination, there are lessons that can be gleaned to inform future investigations.
INTRODUCTION
Interstitial cystitis/painful bladder syndrome (IC/PBS) is a syndrome characterized by debilitating pain, pressure or discomfort related to bladder filling, usually accompanied by urinary frequency (to relieve the pain) and urge to void. Treatments to date have been empiric and inadequate, and there remains a pressing need for an effective oral treatment for IC/PBS. The pathogenesis of the disorder is still undefined, and a number of theories based on clinical and experimental observations have been advanced. A case for immune dysregulation in at least a subset of IC/PBS patients can be made based on epidemiologic, histopathologic, and clinical response criteria1–6.
If the etiology of IC/PBS is in part due to an induced autoimmune/inflammatory disorder, immunosuppressant therapy is a reasonable consideration for a treatment trial. Methotrexate used in a small open label study showed no significant effect on voiding patterns7, but two open label trials from Finland showed that Cyclosporin-A (CyA) produced short term8 and long-term9 pain resolution, reductions in frequency and increased voided volumes. A subsequent randomized study suggested that CyA was well tolerated and was significantly more effective than pentosanpolysulfate sodium (active control)10 in controlling the symptoms of patients with severe IC/PBS. Mycophenolate mofetil (MMF, Cellcept®) is commonly used in transplant recipients as an antirejection agent in combination with CyA and corticosteroids. While it has not been used in IC/PBS, there are many published reports of its use in inflammatory and autoimmune disorders, such as inflammatory uveitis11, systemic lupus erythematosis12 and lupus nephritis13, and Wegener’s granulomatosis14.
We conducted a randomized, double-blind, placebo-controlled clinical trial of MMF in patients with IC/PBS who failed previous therapy for this syndrome. The agent was chosen because it was an immunosuppressant drug with a reasonable safety profile; also, CyA was not available for a randomized trial due to the unavailability of a placebo. The primary objectives of the trial were to compare MMF 2 grams daily to placebo for effects on overall IC/PBS symptoms and well being in patients with refractory IC/PBS, and to assess the medication’s safety profile.
METHODS
Participants & Study Design
Men and women over the age of 18 were recruited from 11 urology/ urogynecology clinics in the United States and Canada. All study sites obtained local institutional review board (IRB) approval. Eligibility required fulfillment of all of the following: (a) persistent symptoms of both urinary frequency and pain rated at least 4 on a scale of 0 to 10, (b) failure of at least 24 weeks of active treatment with a minimum of 3 standard forms of therapy or combination of therapies for IC/PBS, (c) a cystoscopic diagnosis of IC/PBS in the past with the findings of glomerulations and/or ulcerations, and (d) a screening cystoscopy within the 24 weeks prior to entry, to check for unevaluated pathology. Additional exclusion criteria are shown in the Supplementary Table (posted at URL). Except for medications listed in the Exclusion Criteria, subjects were allowed to continue on their current medication regimen.
Eligible participants were randomized to either MMF or matching placebo in a 2:1 ratio. For the first 14 days subjects were instructed to take MMF 1 gram by mouth daily. Subjects discontinuing study drug during this Introduction/Tolerability phase were followed until the primary endpoint visit at 12 weeks. After successful completion of this phase, the Full Dose phase (2 grams/day, in 2 divided doses) was continued for 10 more weeks. Laboratory values (complete blood counts, liver enzymes) and physical symptoms were closely monitored for adverse events at regular intervals throughout the study. All subjects were treated and followed for a period of up to 16 weeks after randomization, including 12 weeks of study treatment and 4 weeks post treatment. Participants withdrawing prior to completion of the intervention phase were asked to complete all outcome measures.
Outcome measures
The primary efficacy outcome measure was the Global Response Assessment (GRA) at 12 weeks. The GRA queries “As compared to when you started the current study, how would you rate your overall pelvic symptoms now?”, with 7 response categories. Participants who indicated they were “moderately” or “markedly” improved were considered intervention responders. Participants with missing GRAs were considered non-responders, and included in the denominator for the assessment of response rates.
Secondary measures obtained at baseline and 12 weeks included a 24-hour voiding diary, ratings of pain and frequency on a 10-point scale, and responses to the following validated symptom questionnaires: the McGill Pain Questionnaire15, the O’Leary-Sant Interstitial Cystitis Symptom and Problem Indexes16, the SF-12 Health Status Questionnaire, with separate calculation of the Physical Component Score [PCS] and the Mental Component Score [MCS]17, the Female Sexual Function Index [FSFI]18 or Sexual Health Inventory for Men [SHIM]19 as appropriate, and the Hospital Anxiety and Depression Scale [HADS]20.
Statistical Methods
The study was powered to detect a difference in GRA response rates between 20% for placebo to 45% for MMF (difference of 25%). Based on previous IC/PBS studies, the placebo response was estimated at 20%21. In order to achieve 90% power to detect this 25% difference, using a 2:1 randomization and two-sided p-value of 0.05, a minimum of 180 participants was required. The sample size was increased by 15% to account for clinical center variation (n=210, 140 MMF and 70 placebo). Stratified permuted block randomization, with variable block sizes by clinical site, was conducted to ensure balance across treatment groups.
Standard descriptive statistics were used to summarize baseline characteristics and study outcome measures at each follow-up visit, both overall, and within each treatment group. The balance of baseline measures across the three treatment groups was compared using appropriate k-sample tests, including Kruskal-Wallis tests and Fisher’s exact tests. The primary analysis compared GRA response rates using the exact conditional test version of the Mantel-Haenszel test to control for clustering by clinical center22. The pooled rate difference and 95% confidence interval across clinical centers were calculated using the “metan” routine within Stata®v 10 (StataCorp LP, College Station, TX, 2008)23. For secondary efficacy outcomes, changes from baseline to 12 weeks were calculated for those subjects with data at both time points, not representing intention to treat analyses. Comparisons of these changes between treatment groups are also presented via 95% confidence intervals calculated using standard normal-based methods, not adjusting for clustering by clinical center.
RESULTS
Recruitment for the study began in April 2007. A black box warning for MMF was issued by Roche Pharmaceuticals on October 31, 200724. The warning addressed the change of the drug classification by the United States Food and Drug Administration from a Pregnancy Category C (Risk of Fetal Harm Cannot Be Ruled Out) to Category D (Positive Evidence of Fetal Risk). The recommendation from Roche was for female subjects of child bearing potential to be contacted immediately, and that all subjects be re-counseled and re-consented with specific attention to the requirements for two methods of effective contraceptive use, beginning 4 weeks prior to the study, use throughout the study, and for 6 weeks after stopping MMF. The black box warning also addressed the risk of susceptibility to infection and the possible development of lymphoma. As a result of these warnings, study drug delivery and all subject enrollment were suspended on November 15, 2007, to remain in place until an amended protocol and consent form were approved by each study site’s IRB.
Slow recruitment of study participants up to this point prompted the study’s data and safety monitoring board (DSMB) to request an interim analysis in January 2008, which identified an observed reduced efficacy of MMF compared to placebo. Due to a lack of treatment efficacy, increased safety concerns due to the black box warning, and difficulty recruiting eligible study participants, the DSMB recommended to the study sponsors (NIDDK) early termination of the study. This recommendation was accepted and the study was terminated on February 4, 2008. The final analysis reported here was performed in April 2008.
The CONSORT diagram is shown in Figure 1. Baseline demographic data by treatment arm for all randomized subjects (n=58) are shown in Table 1. There were no differences in the distribution of demographic characteristics between the two treatment arms. Table 1 also shows selected baseline symptom scores. Overall, baseline symptoms were moderate to severe: 72% of subjects presented with severe (7–10) pain and 79% with severe frequency. Treatment groups were comparable across all baseline measures evaluated.
Figure 1.
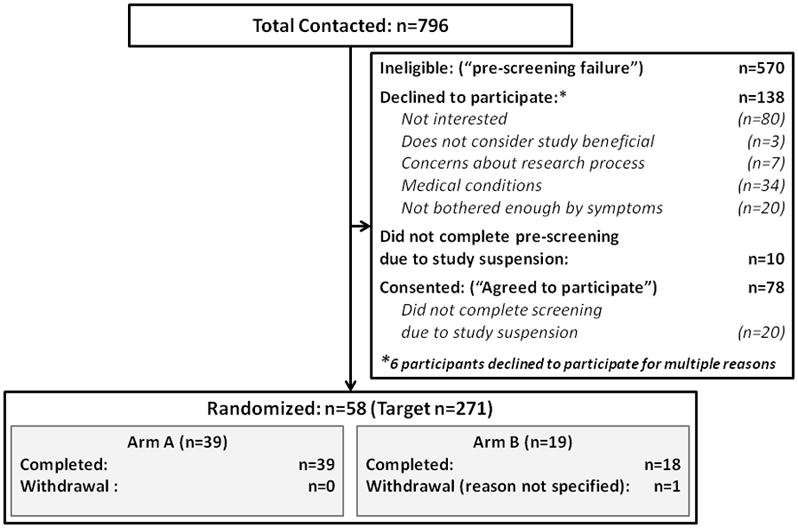
Consort Diagram for Flow of Subjects Through Study Phases, by Treatment Arm
Table 1.
Baseline Characteristics by Treatment Group*
| MMF | Placebo | |
|---|---|---|
| Number of Subjects Randomized† | 39 | 19 |
| # (%) Female | 33 (85%) | 15 (79%) |
| Mean ± s.d. Age | 51.3 ± 10.3 | 51.8 ± 11.6 |
| Race | ||
| # (%) White/Caucasian | 35 (90%) | 17 (89%) |
| # (%) Black/African-American | 1 (3%) | 1 (5%) |
| # (%) Multi-race, Asian, Other | 3 (8%) | 1 (5%) |
| # (%) Ever Diagnosed with IC/PBS | 38 (97%) | 19 (100%) |
| Mean ± s.d. Years Since Diagnosis (if applicable) | 6.4 ± 4.1 | 6.3 ± 5.9 |
| Mean ± s.d. Years Since Onset of Initial Symptoms | 13.8 ± 12.0 | 14.4 ± 14.4 |
| Mean ± s.d. Pain Score (0–10) | 6.9 +/− 1.5 | 7.2 +/− 1.2 |
| Mean ± s.d. Urgency Score (0–10) | 7.1 +/− 1.8 | 7.1 +/− 1.7 |
| Mean ± s.d. Frequency Score (0–10) | 7.7 +/− 1.4 | 7.4 +/− 1.8 |
| Mean ± s.d. IC Symptom Index | 15.7 +/− 3 | 15.1 +/− 3 |
| Mean ± s.d. IC Problem Index | 12.9 +/− 3 | 12.8 +/− 3 |
| Mean ± s.d. SF-12 PCS (38,18) | 34.9 +/− 10 | 34.8 +/− 11 |
| Mean ± s.d. SF-12 MCS (38,18) | 42.6 +/− 11 | 38.3 +/− 13 |
| Mean ± s.d. FSFI total (30,12) | 14.0 +/− 10 | 11.5 +/− 9 |
| Mean ± s.d. McGill total (37,16) | 21.5 +/− 10.4 | 21.5 +/− 11.3 |
For the symptom scores shown, the measurements at the two baseline visits were averaged to give the overall baseline score.
Approximately nine subjects on each arm were missing data on voiding frequency, and eight subjects on each arm were missing data on years since symptoms began. At most one subject was missing data for all other measures.
The GRA response rates in the MMF and placebo groups were 15.4% and 15.8%, respectively (p=0.67). The confidence interval for the difference in response rates, adjusting for center variability, is also displayed in Table 2. Consistent with intention-to-treat analytic strategies, participants who did not provide data at 12 weeks (only one subject, on placebo) were considered treatment non-responders. Given the single subject who did not complete the study, additional “non-completer” analyses would not have changed the conclusions. Fourteen MMF and 8 placebo subjects were not on drug at primary endpoint due to study suspension (see below). If these are excluded from the analysis (i.e., removed from the intention-to-treat process), the responder rates are 2/25(8%) and 3/11(27.3%) for MMF and placebo respectively.
Table 2.
Primary and Selected Secondary Symptom Outcomes
| MMF Placebo | Difference (95% Confidence Interval) | ||
|---|---|---|---|
| Number of Subjects Randomized | 39 | 19 | 58 |
| Primary Endpoint: Response Rate at 12 weeks* | 6 (15%) | 3 (16%) | −0.5% (−32%, 22%) |
| Secondary Endpoints: Change from Baseline to 12 weeks | |||
| Number of Subjects Analyzed | 39 | 18 | 57† |
| Pain Score | −0.5 +/− 2.0 | −1.9 +/− 2.1 | 1.4 (0.2, 2.5) |
| Urgency | −0.7 +/− 2.0 | −1.8 +/− 1.9 | 1.1 (0.02, 2.2) |
| Frequency | −1.0 +/− 2.1 | −1.9 +/− 2.1 | 0.9 (−0.3, 2.1) |
| IC Symptom Index | −0.8 +/− 3 | −1.8 +/− 4 | 1.0 (−0.7, 2.6) |
| IC Problem Index | −0.7 +/− 3 | −1.7 +/− 4 | 0.9 (−0.9, 2.7) |
| SF-12 PCS (n=37,17) | −2.5 +/− 8 | 0.6 +/− 10 | −3.1 (−8.2, 2.1) |
| SF-12 MCS (n=37,17) | 0.0 +/− 10 | −4.6 +/− 10 | 4.6 (−1.3, 10.5) |
| FSFI total (n=26,10) | −0.9 +/− 6 | 2.9 +/− 6 | −3.8 (−8.5, 0.9) |
| McGill total (n=34,14) | −1.4 +/− 8.8 | 6.7 +/− 11.4 | 5.4 (−0.8, 11.5) |
| HADS (n=39,18) | 0.0 +/− 6 | −4.9 +/− 7 | 4.9 (1.5, 8.3) |
For the primary response endpoint, responders are defined as those reporting “Markedly Improved” or “Moderately Improved” on the GRA. Patients for whom the GRA value is missing are considered non-responders and included in the denominator for the assessment of response rates using an intention-to-treat analysis.
A positive number in this column indicates that the improvement was larger in the placebo group.
Table 2 also summarizes changes in selected symptom outcomes from baseline to 12 weeks by treatment arm, and provides confidence intervals for these differences. These do not strictly represent an intention-to-treat analysis, since they are based only on those subjects with complete data. None of the comparisons for these secondary endpoints were statistically significant at a more stringent p=0.01 level typically used for statistical comparisons of secondary endpoints.
At primary endpoint 50% (n=19) of subjects in the treatment arm were not taking study drug due to the study suspension (n=14) or drug tolerability (n=5).
The cumulative numbers of participants with at least one adverse event, subdivided by the primary body system or specific itemized categories, are also given for each treatment arm in Table 3. Overall, 28% (16/58) of study participants reported at least one mild, 26% (15/58) at least one moderate, and 28% (16/58) at least one severe adverse event; thus, 81% (47/58) of participants reported at least one adverse event. There was no statistically significant difference in the overall adverse event rates between treatment arms (MMF 87% vs. control 68%)(p=0.09). There were two reported serious adverse events (SAEs) in the treatment arm. Neither event required unmasking of the treatment, and both subjects continued on study. One event was determined to be unrelated to treatment (narcotic withdrawal), and one event was determined to be possibly related to treatment (asthma exacerbation).
Table 3.
Significant, Cumulative Adverse Events by Body System and Treatment Group
| MMF(%)* | Placebo(%)* | |
|---|---|---|
| Number of Subjects Randomized | 39 | 19 |
| Number of subjects with at least one adverse event | 34 (87%) | 13 (68%) |
| Blood/Bone marrow | 2 (5%) | 1 (5%) |
| Constitutional Symptoms (primarily fatigue, malaise) | 14 (36%) | 6 (32%) |
| Dermatology/Skin | 7 (18%) | 3 (16%) |
| Gastrointestinal (primarily nausea, constipation, diarrhea) | 27 (69%) | 8 (42%) |
| Hemorrhage | 2 ( 5%) | 0 ( 0%) |
| Infectious/febrile | 7 (18%) | 2 (11%) |
| Lymphatics | 0 ( 0%) | 1 (5%) |
| Metabolic/Laboratory | 4 (10%) | 0 ( 0%) |
| Musculoskeletal | 1 (3%) | 2 (11%) |
| Neurologic (primarily dizziness, anxiety) | 8 (21%) | 4 (21%) |
| Ocular, Visual | 2 (5%) | 1 (5%) |
| Pain (primarily headache) | 21 (54%) | 11 (58%) |
| Pulmonary | 2 (5%) | 2 (11%) |
| Renal/Genitourinary | 9 (23%) | 2 (11%) |
| Sexual/Reproductive function | 1 ( 3%) | 1 (5%) |
| Benign Viral Syndromes | 2 (5%) | 1 (5%) |
| Vascular | 0 (0%) | 1 (5%) |
Subject may be in more than 1 category.
DISCUSSION
The etiology and pathophysiology of IC/PBS remains obscure. The rationale for selecting MMF for this trial was based on evidence that immune system dysregulation/autoimmunity plays a role in the perpetuation of IC/PBS symptoms. The evidence includes: the age and sex distribution of IC/PBS patients (which is similar to those of known autoimmune diseases)25, the clinical concordance of IC/PBS with other established autoimmune diseases26, and the efficacy of immunosuppressive drugs in IC/PBS7, 10, 27, mostly in small case series. Scientific investigations of bladder tissue have failed to produce any strong indication, however, that autoimmune complexes are consistently associated with IC/PBS.
Sairanen and colleagues conducted a randomized controlled trial showing that 75% of patients responded to cyclosporine, compared to only 19% with pentosanpolysulfate10. This compelling data led to our interest to assess immunotherapy in a well-designed, placebo controlled study. The investigators felt that the risks of immunosuppressive therapy were outweighed by the morbidity experienced by patients with a severe, albeit benign, disease. We tried to carry out such an investigation with CyA, but were unable to have placebo pills made. Ultimately, MMF was chosen based on strongly positive data presented as an abstract (although never published) at a 2003 NIH Interstitial Cystitis research meeting.
Unfortunately, as described above, the study was interrupted by unforeseeable events and then appropriately halted due to a futility analysis. It should be emphasized that 19 of the 39 subjects randomized to study drug were not taking it at primary endpoint (14 due to study suspension), strongly biasing the study toward a negative result, and limiting interpretation of tolerability and other data related to ingestion of the drug. The results in aggregate, however, do not indicate that further investigation of the specific drug MMF would be fruitful.
What lessons, then, can we take away from this effort that will inform future investigators? First, we observed that MMF was reasonably well tolerated. Risks of immunosuppressive therapy include general risks of cytopenia, infection, and promotion of malignancy, as well as specific risks unique to individual drugs. In total, 87% of subjects on the active agent suffered an adverse event compared to 68% for placebo; Grade 3 adverse events were seen in 31% vs. 21%, respectively. Importantly, there were no Grade 3 infectious events and no significant cytopenia. These data suggest that further research in immunosuppressive therapy is reasonably safe, with a need for specific precautions related to the drug to be studied. It should be noted that the long-term risks such as development of malignancies cannot be assessed in a short term trial.
Second, we observed that recruitment was very difficult. A much higher proportion of interested (and otherwise eligible) patients were excluded than in other randomized trials for IC/PBS. This was primarily due to the exclusion criteria mandated for prior malignancies or premalignant conditions (including cervical dysplasia, colon polyps, any skin cancer including basal cell) (see Supplementary Table). The projected risks are largely based on experience with these drugs in the transplant community where triple therapy including steroids is standard; there is inadequate data regarding the risk of immunosuppressive agents as monotherapy. It is conceivable that risks to subjects receiving monotherapy are much less. Our experience does suggest that it will continue to be difficult to investigate use of immunosuppressive therapy until such a time that other studies of the drugs used as monotherapy allow for relaxed exclusion criteria.
Finally, we should acknowledge that the rationale for selecting MMF was based on less than solid reasoning. Although an immunosuppressant agent (CyA) was effective for treating IC/PBS symptoms, it does not necessarily follow that another immunosuppressant drug (MMF) would also be effective. Both CyA and MMF inhibit T cell function, but through different mechanisms28. Medications for immunosuppression have multiple immune and non-immune effects, and these effects are not all defined for each agent. There is no compelling evidence that IC is a purely T cell mediated condition, and thus the immunosuppressant mechanism(s) of MMF may not be effective for the treatment of IC/PBS. However, even though MMF is not the ideal agent for IC/PBS, this should not preclude future trials of other immunosuppressant medications.
CONCLUSION
In a multicenter randomized placebo-controlled trial that was prematurely halted, MMF demonstrated efficacy similar to placebo in treating symptoms of refractory IC/PBS. The results of this limited study cannot be used to confirm or refute the hypothesis that immunosuppressive therapy may be beneficial to at least a subgroup of patients with IC/PBS. Despite the study termination, there are lessons that can be gleaned to inform future investigations.
Supplementary Material
Acknowledgments
Funding Sources: Supported by cooperative agreements U01 DK65209, U01DK65255, U01DK65213, U01DK65214, U01DK65215, U01DK65178, U01DK65190, U01DK65192, U01DK65255, U01DK65267, U01DK65271, U01DK65202 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
We thank the women and men who participated in this clinical trial. In addition to the authors, the Interstitial Cystitis Collaborative Research Network (ICCRN) Study Group includes the following institutions and individuals. The number of subjects randomized at each center is given in parentheses. University of Rochester Robert Mayer, Edward Messing, Elizabeth Betty Smith, Kay Rust, Jay Reeder (8); William Beaumont Hospital Eleanor Anton, Cheryl Wolfert, Loni Lampkins (8); Queen’s University Alvaro Morales, Laurel Emerson, Lesley Carr, Joseph Downey, Janet Clark-Pereira, Sylvia Robb (7); Stanford University Rajesh Shinghal, Rodney Anderson, Debra Clay, Anna Ramakrishnan (7); Loyola University Medical Center Linda Brubaker, Judy Senka, Lucia Radukanu, Janet Rindels, Grace Bucher (6); University of Pennsylvania Diane K. Newman, Sylvia Salazar, Jennifer Milado, Gia Deleon (6); University of Maryland Susan Keay, Rosanna Dinh, Rupali Sangrampurkar, Judith Murray, Lisa Radebaugh (4); University of Iowa Michael O’Donnell, Susan Lutgendorf, Mary Eno, Kelly O’Berry (4); University of Washington Jane Miller, Jean Kalhoff, Sharon Downing, Robert F. Bale Jr. (3); University of California, San Diego Charles Nager, Marianne Chenoweth (3); Henry Ford Hospital Kandis Rivers, Samina Romero, Michelle Peabody, Jill Sullivan (2); University of Pennsylvania School of Medicine (Data Coordinating Center) Keith Mickelberg, Ted Barrell, Shannon Chuai, Rosemary Madigan; The National Institute of Diabetes and Digestive and Kidney Diseases Christopher Mullins, Mary Harris; Interstitial Cystitis Association Vickie Ratner.
Footnotes
Registration Number and Registry Name: ClinicalTrials.gov identifier: NCT00451867 “A Randomized Multicenter Double-Blind CT to Evaluate the Efficacy and Safety of Mycophenolate Mofetil (MMF) for Treatment of Refractory Interstitial Cystitis (IC) (ICCRN RCT2)”
Disclosures:
Dr. Nickel reports receiving consulting fees from Merck, Glaxo-Smith-Kline, Pfizer, Ortho Women’s Health, Farr Labs, Watson, Medtronic, NeurAxon, Genyous Biomed and research support from Merck, Glaxo-Smith Kline, Allergan, Watson, Pfizer and American Medical Systems. Dr. Hanno reports Astellas, Pfizer, Watson and Trillium. Dr. Chai reports Pfizer and Allergan. Dr. Kusek reports holding stock in deCode Genetics. Dr. Fitzgerald reports Astellas, and Ferring. Dr. Lukacz reports Pfizer, Novartis, Proctor & Gamble, Intuitive Surgical and Elsevier. No other potential conflict of interest relevant to this manuscript was reported.
References
- 1.Liebert M, Wedemeyer G, Stein JA, et al. Evidence for urothelial cell activation in interstitial cystitis. J Urol. 1993;149:470. doi: 10.1016/s0022-5347(17)36121-9. [DOI] [PubMed] [Google Scholar]
- 2.Oravisto KJ, Alfthan OS, Jokinen EJ. Interstitial cystitis. Clinical and immunological findings. Scan J Urol Nephrol. 1970;4:37. doi: 10.3109/00365597009136226. [DOI] [PubMed] [Google Scholar]
- 3.Meulders Q, Michel C, Marteau P, et al. Association of chronic interstitial cystitis, protein-losing enteropathy and paralytic ileus with seronegative systemic lupus erythematosus: case report and review of the literature. Clin Nephrol. 1992;37:239. [PubMed] [Google Scholar]
- 4.Ochs RL, Stein TW, Jr, Peebles CL, et al. Autoantibodies in interstitial cystitis. J Urol. 1994;151:587. doi: 10.1016/s0022-5347(17)35023-1. [DOI] [PubMed] [Google Scholar]
- 5.El-Mansoury M, Boucher W, Sant GR. Increased urine histamine and methylhistamine in interstitial cystitis. J Urol. 1994;152:350. doi: 10.1016/s0022-5347(17)32737-4. [DOI] [PubMed] [Google Scholar]
- 6.Letourneau R, Pang X, Sant GR, et al. Intragranular activation of bladder mast cells and their association with nerve processes in interstitial cystitis. Br J Urol. 1996;77:41. doi: 10.1046/j.1464-410x.1996.08178.x. [DOI] [PubMed] [Google Scholar]
- 7.Moran PA, Dwyer PL, Carey MP, et al. Oral methotrexate in the management of refractory interstitial cystitis. Aust N Z J Obstet Gynaecol. 1999;39:468. doi: 10.1111/j.1479-828x.1999.tb03135.x. [DOI] [PubMed] [Google Scholar]
- 8.Forsell T, Ruutu M, Isoniemi H, et al. Cyclosporine in severe interstitial cystitis. J Urol. 1996;155:1591. [PubMed] [Google Scholar]
- 9.Sairanen J, Forsell T, Ruutu MJ. Long-term outcome of patients with interstitial cystitis treated with low dose cyclosporine A. J Urol. 2004;171:2138. doi: 10.1097/01.ju.0000125139.91203.7a. [DOI] [PubMed] [Google Scholar]
- 10.Sairanen J, Tammela TL, Leppilahti M, et al. Cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol. 2005;174:2235. doi: 10.1097/01.ju.0000181808.45786.84. [DOI] [PubMed] [Google Scholar]
- 11.Thorne JE, Jabs DA, Qazi FA, et al. Mycophenolate mofetil therapy for inflammatory eye disease. Ophthalmology. 2005;112:1472. doi: 10.1016/j.ophtha.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Pisoni CN, Sanchez FJ, Karim Y, et al. Mycophenolate mofetil in systemic lupus erythematosus: efficacy and tolerability in 86 patients. J Rheumatol. 2005;32:1047. [PubMed] [Google Scholar]
- 13.Kapitsinou PP, Boletis JN, Skopouli FN, et al. Lupus nephritis: treatment with mycophenolate mofetil. Rheumatology (Oxford) 2004;43:377. doi: 10.1093/rheumatology/keh012. [DOI] [PubMed] [Google Scholar]
- 14.Langford CA, Talar-Williams C, Sneller MC. Mycophenolate mofetil for remission maintenance in the treatment of Wegener’s granulomatosis. Arthritis Rheum. 2004;51:278. doi: 10.1002/art.20240. [DOI] [PubMed] [Google Scholar]
- 15.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 16.Propert KJ, Mayer RD, Wang Y, et al. Responsiveness of symptom scales for interstitial cystitis. Urology. 2006;67:55. doi: 10.1016/j.urology.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE, Kosinski M, Dewey JE. How to Score Version Two of the SF-36 Health Survey. Lincoln, RI: QualityMetric; 2000. [Google Scholar]
- 18.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 19.Cappelleri JC, Rosen RC. The Sexual Health Inventory for Men (SHIM): a 5-year review of research and clinical experience. Int J Impot Res. 2005;17:307. doi: 10.1038/sj.ijir.3901327. [DOI] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 21.Hwang P, Auclair B, Beechinor D, et al. Efficacy of pentosan polysulfate in the treatment of interstitial cystitis: a meta-analysis. Urology. 1997;50:39. doi: 10.1016/S0090-4295(97)00110-6. [DOI] [PubMed] [Google Scholar]
- 22.Mehta C, Patel N. Proc-StatXact For SASR Users: Statistical Software for Exact Nonparametric Inference. Cambridge, MA 02139 USA: CYTEL Software Corporation; 1997. [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birgerson L. Important Drug Warning - Important Changes in the CellCept (mycophenolate mofetil) Prescribing Information; Roche: 2007. [Google Scholar]
- 25.van de Merwe JP. Interstitial cystitis and systemic autoimmune diseases. Nat Clin Pract Urol. 2007;4:484. doi: 10.1038/ncpuro0874. [DOI] [PubMed] [Google Scholar]
- 26.Alagiri M, Chottiner S, Ratner V, et al. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology. 1997;49:52. doi: 10.1016/s0090-4295(99)80332-x. [DOI] [PubMed] [Google Scholar]
- 27.Oravisto KJ, Alfthan OS. Treatment of interstitial cystitis with immunosuppression and chloroquine derivatives. Euro Urol. 1976;82 doi: 10.1159/000471967. [DOI] [PubMed] [Google Scholar]
- 28.Krensky AM, Vincenti F, Bennett WM. Chapter 52: Immunosuppressants, Toleragens, and Immunotimulants. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11. McGraw-Hill; 2006. [Accessed online May 31, 2010]. http://www.accessmedicine.com/content.aspx?aID=951722. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


