Abstract
Salivary glands from hematophagous animals express a notable diversity of negative modulators of platelet function. Triplatin is an inhibitor of collagen-induced platelet aggregation which has been described as an antagonist of glycoprotein VI (GPVI). Because triplatin displays sequence homology to members of the lipocalin family of proteins, we investigated whether triplatin mechanism of action could be explained by interaction with pro-hemostatic prostaglandins. Our results demonstrate that triplatin inhibits platelet aggregation induced by low doses of collagen, thromboxane A2 (TXA2) mimetic (U46619), and arachidonic acid (AA). On the other hand, it does not inhibit platelet aggregation by convulxin, PMA, or low-dose ADP. Isothermal titration calorimetry (ITC) revealed that triplatin binds AA, cTXA2, TXB2, U46619 or PGH2 mimetic (U51605). Consistent with its ligand specificity, triplatin induces relaxation of rat aorta contracted with U46619. Triplatin also interacts with PGF2α and PGJ2, but not with leukotrienes, AA or biogenic amines. Surface plasmon resonance experiments failed to demonstrate interaction of triplatin with GPVI; it also did to inhibit platelet adhesion to fibrillar or soluble collagen. Because triplatin displays sequence similarity to apolipoprotein D (ApoD)—a lipocalin associated with HDL, it was tested as a putative TXA2-binding molecule. ITC failed to demonstrate binding of ApoD to all prostanoids described above, or to AA. Furthermore, ApoD was devoid of inhibitory properties towards platelets activation by AA, collagen, or U46619. In conclusion, Triplatin mechanism of action has been elucidate without ambiguity as a novel TXA2- and PGF2α- binding protein. It conceivably blocks platelet aggregation and vasoconstriction, thus contributing to successful blood feeding at the vector-host interface.
Introduction
Platelets have a central role in hemostasis. Platelet initially interacts with the exposed extracellular matrix, which contains macromolecules such as collagens and fibronectin. Under conditions of high shear present in small arteries and arterioles, the initial tethering of platelets to the matrix is mediated by interaction between the platelet receptor glycoprotein (GP)Ib and vWF bound to collagen (1). However, the binding of GPIb to vWF dissociates rapidly and is insufficient to mediate stable adhesion but rather maintains the platelet in close contact with the exposed surface. This interaction allows the collagen receptor GPVI to bind to collagen (2), triggering the conformational change of integrins (e.g., α2β1) to a high-affinity state, thereby enabling them to mediate firm adhesion to collagen, and also promoting the release of TXA2 and adenosine diphosphate (ADP) (3, 4). Both ADP and TXA2 are particularly important for completion of platelet aggregation under physiologic conditions but may also contribute to thrombus formation under pathologic events (5). This explains why blockade of TXA2 generation (e.g., aspirin) and antagonism of purinergic ADP receptors (e.g., clopidogrel) have been used to treat ischemic disorders (6–9).
Platelets are also target of exogenous secretions from a variety of sources such as hematophagous animals which have evolved various strategies to counteract the host hemostatic system (10–15). The salivary glands (SGs) of these animals express molecules named sialogenins (from the Greek sialo, saliva; gen, origin, source; and ins for proteins)(12), which target the host response to injury, modulate immune response, prevent pain, and block several aspects of hemostasis (10–15). With respect to platelet aggregation, 11 different mechanisms have been identified, including enzyme inhibition, small ligand binding properties, disintegrins, release of antiplatelet nitric oxide, among others (12). Reports on carefully performed experiments demonstrated that identification of salivary protein function is not necessarily trivial, and the use of adequate concentrations of ligands was shown to be critical to correctly identify the target of a given platelet inhibitor. For example, pallidipin was initially described as a specific collagen inhibitor (16) but is in actuality a TXA2-binding protein (17). Moubatin was also initially described as a specific collagen inhibitor (18) but then identified as a ligand for TXA2 (19). A protein named chrysoptin was found to block platelet aggregation by a mechanism that was claimed to be due to a disintegrin activity; however, chrysoptin exhibits extensive sequence homology to apyrase, suggesting that degradation of ADP by an enzyme conceivably explains its inhibitory activity toward ADP-induced platelet aggregation (20). More recently, triplatin a lipocalin from Triatoma infestans salivary gland was suggested to affect collagen-mediated platelet responses through antagonism of GPVI (21). Because triplatin shares sequence homology to DPTL, a TXA2-binding protein (17), we verified whether its mechanism of action could be explained by mopping up TXA2 and other pharmacologically relevant prostanoids involved in platelet aggregation and vasoconstriction. We have also investigated whether apolipoprotein D (ApoD), a plasma lipocalin constituent of HDL — a lipoprotein known to inhibit platelet aggregation and marker of protection for cardiovascular diseases (22) — could also bind to pro-aggregatory prostaglandins and modulate platelet function.
Methods
Horse tendon insoluble Horm fibrillar collagen was from Chrono-Log Corp. (Haverstown, PA, USA). Soluble collagen type I was from BD biosciences. PGD2, PGE2, PGF2α, PGH2 endoperoxide mimetic (U-51605), PGJ2, cTXA2, TXA2-mimetic (U-46619), TXB2, LTC4, 5(S)-HETE, 9(S)-HETE, 15(S)-HETE, and 20-HETE were obtained from Cayman Chemicals (Ann Arbor, MI, USA). ADP, norepinephrine, epinephrine, serotonin, indometacin and histamine were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Sensor CM5, amine coupling reagents, and buffers were purchased from GE-Healthcare (Piscataway, NJ, USA). Convulxin was purified as described (23) and GPVI was expressed as reported (24).
Sequence analysis
Sequence similarity searches were performed using BLAST. Cleavage site predictions of the mature proteins used the SignalP program. The molar extinction coefficient (ε280 nm) of mature triplatin at 280 nm was obtained at http://www.expasy.ch/cgi-bin/protparam, yielding for mature triplatin a value of ε280 nm = 18825 M−1.cm−1; A0.1%280 nm/cm (1 mg/ml) = 1.008, predicted molecular weight 18,672.7 (164 amino acids, including 6xHis-tag), and pI 8.02.
Expression of triplatin in E. coli
Synthetic cDNA for triplatin was produced by BioBasic Inc. (Markham, ON, Canada). The sequence displays an N-terminal NdeI and a C-terminal XhoI restriction site. The NdeI site adds a 5′-methionine codon to all sequences that acts as start codon in the bacterial expression system, whereas the XhoI site was incorporated after the 6xhis tag and stop codon. pET 17b constructs were confirmed before transformation of E. coli strain BL21(DE3)pLysS cells. For recombinant protein production, a protocol similar to that described was employed (17). Briefly, 30 ml of Luria Bertani broth (with 34 μg/ml chloramphenicol and 100 μg/ml ampicillin added) was inoculated and grown overnight for a maximum of 16 h. Luria Bertani broth (1 liter with 34 μg/ml chloramphenicol and 100 μg/ml ampicillin added) was inoculated with 10 ml of the overnight culture and grown at 37°C with continuous shaking (250 rpm) until an optical density of 0.6–0.8 (A600 nm) was reached (~3 h). Isopropyl-1-thio-b-D-galactopy-ranoside (1 mM) was added to induce expression, and the flask was shaken for 3 h under the same conditions; cells were harvested by centrifugation (6000×g; 20 min) and washed once in 20 mM Tris-HCl, pH 8.0, before the cell pellet was frozen and stored until use. Refolding procedure and concentration of the sample were carried out essentially as described (17).
Protein purification
Triplatin (8 ml) was loaded into a HiPrep 16/60 Sephacryl S-100 HR (GE Healthcare, Piscataway, NJ, USA) column equilibrated in 20 mM Tris-HCl, NaCl 0.15M, pH 9.3, with a flow of 1 ml/min and connected to an AKTA purifier system (Amersham Biosciences, Piscataway, NJ, USA). Protein was detected by peak absorbance at 280 and 220 nm, and fractions containing triplatin (estimated by SDS/PAGE) were combined (4 ml) and concentrated in centricon (10-kDa cut-off). To remove bacterial DNA potentially contaminating triplatin, the active fraction was dialyzed against 20 mM Na2HPO4- NaH2PO4, pH 6.0, centrifuged, and loaded into cation-exchange chromatography using a HiPrep 16/10 SP FF column (Amersham Biosciences). Proteins were eluted with a linear gradient of NaCl (0–1 M) over 60 min at a flow rate of 0.5 ml/min. Fractions containing triplatin were combined and dialyzed against TBS, pH 7.4. Samples were loaded in a gel filtration column (Superdex 75 HR10/30; Amersham Biotech) equilibrated in TBS, pH 7.4. Elution was carried out at a flow rate of 1 ml/min, and fractions containing triplatin were combined. Active fractions were exhaustively dialyzed against PBS, pH 7.4. PAGE and Edman degradation were performed essentially as described (17).
Isothermal titration calorimetry (ITC)
Prostanoids (in ethanol or methyl acetate) were placed in glass vials and the vehicle evaporated under nitrogen atmosphere; the dried material was then resuspended in appropriate concentrations in 20 mM Tris-HCl, 0.15 M NaCl, pH 7.4, sonicated, and vortexed. Calorimetric assays for measuring triplatin binding to a number of ligands were performed using a VP-ITC microcalorimeter (Microcal, Northampton, MA, USA) at 30°C. Titration experiments were performed by making successive injections of 10 μl each of 40 μM ligand into the 1.34-ml sample cell containing 4 μM triplatin until near saturation was achieved. Prior to the run, the proteins were dialyzed against 20 mM Tris-HCl, 0.15 M NaCl, pH 7.4, for binding experiments. The calorimetric enthalpy (ΔHcal) for each injection was calculated after correction for the heat of triplatin dilution obtained in control experiments performed by titrating triplatin into buffer. The binding isotherms were fitted according to a model for a single set of identical binding sites by nonlinear squares analysis using Microcal Origin software (OriginLab, Northampton, MA).
SPR experiments
All SPR experiments were carried out in a T100 instrument (Biacore AB, Uppsala, Sweden) following the manufacturer’s instructions. HBS-P (10 mM Hepes, pH 7.4, 150 mM NaCl, and 0.005% (v/v) P20 surfactant) was used as the running buffer for all SPR experiments. Recombinant GPVI (25 μg/ml) (24) in acetate pH 4.5 buffer was immobilized on a CM5 sensor with a final surface density of 1290.8 RU. A blank flow cell was used to subtract any effect of buffer in the refractory index change. Then triplatin, DPTL or convulxin in HBS-P buffer was injected over immobilized GPVI for 120 seconds at 30 μl/minute. Complex dissociation was monitored for 400 seconds. Sensor surface was regenerated between runs with by a 30-second pulse of HCl (10 mM).
Expression and purification of ApoD
Synthetic gene for ApoD was synthesized by BioBasics (Ontario, Canada). The sequence displays an N-terminal BamHI and a C-terminal containing a sequence coding for 6xHis, a stop codon, and a BamHI restriction site. The sequence was cloned in a VR1020 digested with BamHI for both ends. The plasmid was diluted in 20 μl TE buffer and used to transform TOP10 cells by heat-shock procedure and generation of a glycerol stock. Luria Bertani broth (1 liter in the presence of 100 μg/ml ampicillin) was inoculated with a few μl of the glycerol stock, and the plasmid was purified using a purification kit (Qiagen, Valencia, CA, USA). 293 C18 cells (ATCC CRL-10852) were grown/maintained in a 50:50 media mix, consisting of FreeStyle 293 medium (Invitrogen) and SFM4HEK293 medium (HyClone), supplemented with 50 μg/ml Geneticin (Invitrogen). Cells were grown in suspension at 37°C with 5% CO2. Transfection was carried out with PEI (Polyethylenimine, linear, MW-25,000 from Polysciences, Inc.) prepared at 1mg/ml in 18Ω water, sterile filtered and quantitated for transfection efficiency ratio of PEI:DNA solution which was added to the cells and incubated for 4 hours at 37°C/5% CO2. After 72 hours cells were centrifuged at 1776.4 × g for 13 min and supernatant collected. Purification of ApoD was carried in Ni-NTA column (GE-Pharmacia) with an 0–1 M Imidazol gradient in Tris 5 mM, pH 8.3, followed by a purification step in Sephadex 75 HR10/30, equilibrated in PBS pH 7.4, with a flow rate of 0.5 ml/min. The fractions corresponding to ApoD were loaded in a C18 Vydac reversed-phase column, and eluted with a gradient of acetonitrile (0–100%), 0.1% TFA at a flow rate of 1 ml/min. ApoD was extensively dialysed against PBS and tested for platelet aggregation assays.
Platelet aggregation
Platelet-rich plasma was obtained from medication-free platelet donors under informed consent participating in an NIH IRB-approved protocol of the Department of Transfusion Medicine, NIH Blood Bank. Platelet aggregation assays were performed essentially as described (17). Briefly, platelet-rich plasma (100 μL) was added to 200 μL of Tyrode’s buffer (137 mM NaCl, 27 mM KCl, 12 mM NaHCO3, 0.42 mM NaH2PO4, 1 mM MgCl2, 5.55 mM Hepes, 0.25% bovine serum albumin, pH 7.4) giving a final concentration of 200,000 platelets/μL. Platelets aggregation was estimated using a Lumi-aggregometer (Chrono-Log Corp). For platelet adhesion assays, washed platelets were incubated with calcein-AM (2 μM) for 30 min at RT, centrifuged at in the presence of EDTA (5 mM), and apyrase (0.2 U/ml). Platelet was resuspended (200,000 platelets/μL) in Tyrode’s buffer (no additions).
Platelet adhesion
Inhibition of platelet adhesion to immobilized collagen was examined by fluorometry. Microfluor black microtiter 96-well plates (ThermoLabsystems, Franklin, MA) were coated with (50 ul, 1 μg/well, in PBS) of fibrillar (Horm) or soluble collagen overnight at 4 °C in PBS, pH 7.2. Wells were washed and blocked with 2% BSA. Calcein-labeled platelets (2 × 105/μl) were incubated with Triplatin or EDTA and 50 μl of platelets was added to each well. After 1 hour, wells were washed 3–4 times with Tyrode-BSA and adhesion estimated by fluorescence (485 nm/530 nm) (17).
Contraction of rat aorta
Contraction of rat aortic ring preparations by U-46619 was measured isometrically and recorded with transducers from Harvard Apparatus Inc. (Holliston, MA) as reported (17). Aortic rings in a 0.5-ml bath kept at 30°C and pre-constricted by 100 nM U-46619 followed by addition of DPTL (1 μM).
Preparation of indometacin
Indometacin (Sigma, 1.4 mg) was diluted in 1 ml of 0.1 M Na2CO3 and vortexed. Then, it was neutralized with 5.8 μl of acetic acid (glacial, 17.5 M), followed by addition of Tyrode (4 ml) to adjust concentration to 0.8 mM. Indometacin was kept on ice, and discard at the end of the experiment.
Statistical analysis
Results are expressed as mean ± SEM (GraphPad Software, Inc., San Diego, CA, USA).
Results
Purification of triplatin
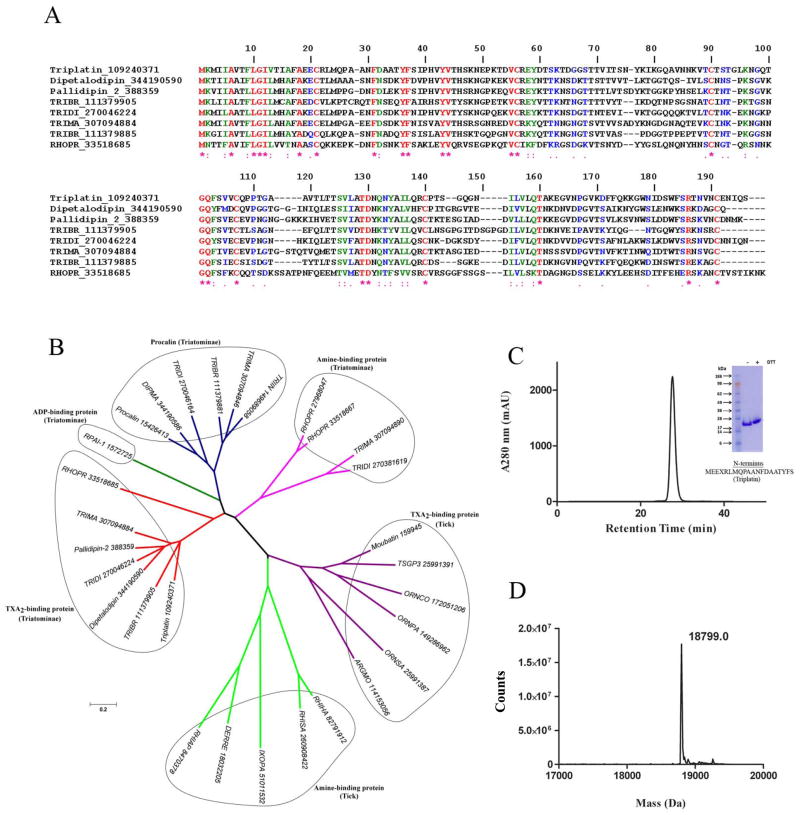
Figure 1A shows the Clustal alignment for triplatin and several other salivary lipocalins which reportedly bind TXA2, including dipetalodipin (17), pallidipin (16), and several other homologues cloned from Triatomines. A high degree of conservation can be observed among different members of this sub-family of lipocalins, and when triplatin is compared to dipetalodipin and pallidipin identical amino acids were 41.9% and 50.6%, respectively.
Fig. 1. Characterization of triplatin.
(A) Clustal alignment of triplatin (gi109240370) and DPTL (gi344190590), pallidipin-2 (gi388359), RPAI-1 (gi1572725), triabin (gi1122389) and several other triatomine putative TXA2-binding proteins. (B) Phylogenetic tree of triplatin and related salivary lipocalins. Tree was generated using the neighbor-joining method after 10,000 bootstraps. The numbers in the phylogram nodes indicates percent bootstrap support for the phylogeny. The bar at the bottom indicates 20% amino acid divergence in sequences. (C) Triplatin was loaded in a Sephadex G75 gel-filtration column and eluted at 1 ml/min in TBS, pH 7.4. Inset, gel electrophoresis of triplatin (reducing [+] and non-reducing [−] conditions) was carried out in a 4–12% NuPAGE gel. N-terminus was performed by Edman degradation. (D) Mass spectrometry of triplatin reveals a mass of 18,799 Da, which is in agreement with a theoretical mass of 18,672.7 Da with an extra methionine and 6xhis.
Next, a Clustal alignment for triplatin and several other salivary lipocalins targeting prostanoids (e.g. TXA2), adenine nucleotides (e.g. ADP), and bioagenic amines (e.g. histamine, serotonine) was performed (not shown) and the results used to generate a phylogenetic tree. Figure 1B demonstrates that Triplatin clades with pallidipin and dipetalodipin and several homologues which also likely bind TXA2. In contrast, RPAI-1 — the only lipocalin known to bind ADP (25) clades independently of other lipocalins. Members of the procalin family of lipocalin (26) whose function remains unknown form another independent clade. Likewise, triatomine lipocalins which bind bioagenic amines (27), or tick salivary lipocalins with specificity for TXA2 (19) or bioagenic amine (28) also clades independently. This philogenetic analysis suggested that triplatin, a previously reported GPVI antagonist (21) could alternatively operate as a TXA2-binding protein.
In an attempt to test this hypothesis the cDNA for triplatin (gi 109240370) was synthesized and cloned in a Pet17b vector. Expression was carried out in Escherichia coli after induction with IPTG as described in Methods. Purification was performed in an S-100 gel-filtration column, followed by cation-exchange chromatography; the last step was carried out in a gel-filtration G-75 column (Figure 1C). The inset shows SDS/PAGE for triplatin under reducing and denaturing conditions. The N-terminus was identified by Edman degradation and matches the N-terminus predicted by the cDNA. Figure 1D depicts the mass spectrometry for triplatin; the calculated mass of 18,799 Da is in reasonable agreement with the theoretical mass of 18,672 Da predicted by the cDNA in addition to an extra methionine and a tag of 6xhis.
Effects of triplatin on platelets
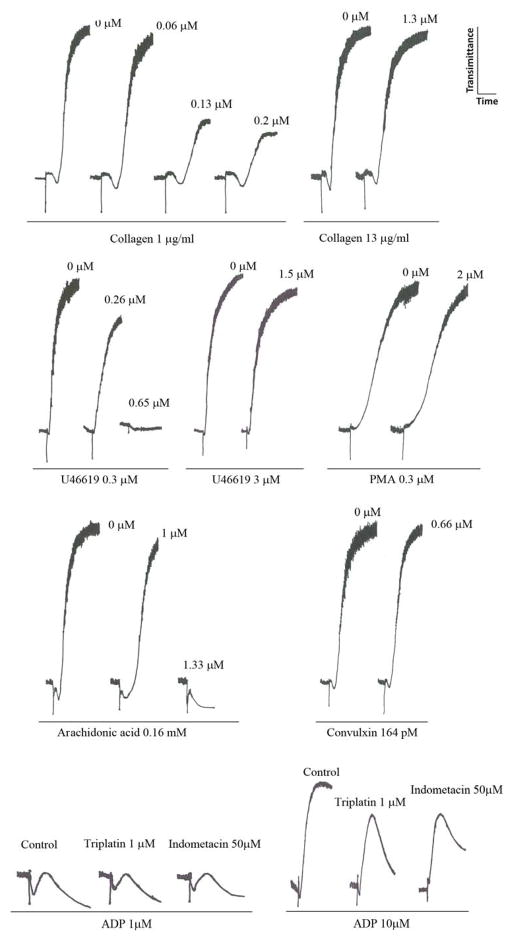
Next, the effects of triplatin on platelets were tested. Figure 2 demonstrates that triplatin dose-dependently attenuates low doses (1 μg/ml) of collagen-induced platelet aggregation. Inhibition was characterized by a delay in the shape change and in the initiation of the platelet response leading to a decrease in maximum amplitude of platelet response. In contrast, triplatin at the highest concentrations tested (2 μM) was ineffective when collagen was tested at 13 μg/ml which induces platelet activation through PLCγ activation pathway which bypasses the need for TXA2 (5). Triplatin also attenuated U46619 (0.34 μM)- or arachidonic acid (AA, 0.16 mM)-induced platelet aggregation, but high concentrations of either U466419 (3 μM) or AA (1 mM, not shown) were no longer inhibitable by triplatin. No inhibition of platelet aggregation was observed when appropriate concentrations of strong agonists were employed, including convulxin (29–32), or PMA. In the case of ADP, low doses (1 μM) which trigger shape change and reversible platelet aggregation without granule release or TXA2 generation (33–36) were not affected by triplatin or indometacin. In contrast, increasing the dose of ADP (10 μM), which reportedly induces irreversible aggregation and TXA2 production (33–36) was attenuated by triplatin and also by indometacin. These experiments suggested that the TXA2 pathway of platelet activation was a potential target for triplatin, and excluded ADP as a target for the inhibitor.
Fig. 2. Triplatin inhibits platelet aggregation.
Triplatin was incubated for 1 min with platelet-rich plasma (2 × 105/μl) followed by addition of collagen, U46619, and arachidonic acid, PMA, convulxin and ADP. Triplatin and agonist concentrations are indicated. In some experiments, platelets were treated with indometacin (50 μM) for 3 minuets before addition of the agonist. Typical experiments are shown (n = 3).
Isothermal titration calorimetry (ITC) experiments
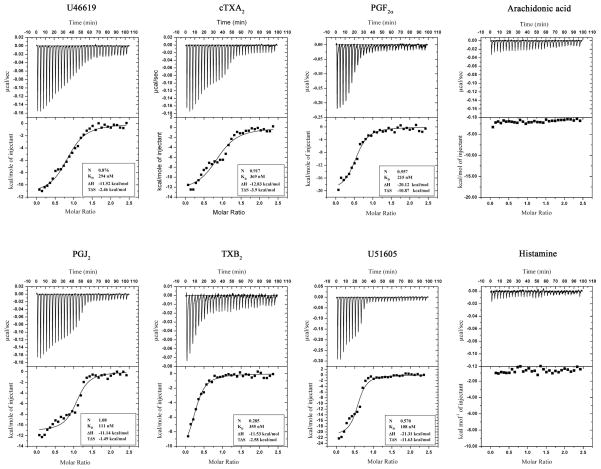
As TXA2 was a candidate to explain the effects of triplatin, ITC experiments were performed in an attempt to detect direct interaction between the reactants. Figure 3 demonstrates that triplatin binds to U46619, cTXA2, PGF2α, PGJ2, TXB2, and U51605. Binding was typically exothermic, stoichiometric, and of high affinity (KD 100–300 nM). Binding was not observed for a number of ligands including AA and histamine (Figure 3) in addition to PGE1, 5(S)-HETE, 12(S)-HETE, 20-HETE, NE, EPI, 5-HT LTC4 and ADP (not shown). Table 1 summarizes our findings including such thermodynamic parameters as enthalpy (ΔH), calculated free energy (ΔG), and entropy (TΔS).
Fig. 3. Isothermal titration calorimetry.
Upper panels: base line-adjusted heats per injection of different ligands (40 μM) into triplatin (4.0 μM). The lower panels indicate the molar enthalpies per injection for ligand interaction with triplatin. Filled squares measured enthalpies; solid line, fit of experimental data to a single site binding model. Thermodynamic parameters: ΔH in kcal/mol, TΔS in kcal/mol, and KD are indicated in the inset for each ligand. Experiments were repeated at least 2 times for each ligand.
Table 1.
Affinity, stoichiometry, and thermodynamic parameters of Triplatin interaction with different ligands
| Interaction was detected by ITC as described in Fig. 3.
| |||||
|---|---|---|---|---|---|
| Ligand | Na | ΔHb | TΔSb | KD (nM) | ΔGb |
| U46619 | 0.876 | −11.52±0.38 | −2.46 | 294±51 | −9.06 |
| PGF2α | 0.557 | −20.12±0.85 | −10.87 | 215±10 | −9.25 |
| cTXA2 | 0.917 | −12.83±0.73 | −3.90 | 369±103 | −8.93 |
| U51605 | 0.570 | −21.31±0.95 | −11.63 | 108±3.3 | −9.68 |
| PGJ2 | 1.080 | −11.14±0.38 | −1.49 | 111±3.4 | −9.65 |
| TXB2 | 0.285 | −11.53±0.92 | −2.58 | 355±6.7 | −8.95 |
| 15s-HETE | -c | - | - | >1 μM | - |
| 9s-HETE | - | - | - | >1 μM | - |
| PGE1 | - | - | - | >1 μM | - |
| PGD2 | n.d.d | n.d. | n.d. | n.d. | n.d. |
| PGE2 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 5s-HETE | n.d. | n.d. | n.d. | n.d. | n.d. |
| 20-HETE | n.d. | n.d. | n.d. | n.d. | n.d. |
| Arachidonic acid | n.d. | n.d. | n.d. | n.d. | n.d. |
| LTC4 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Epinephrine | n.d. | n.d. | n.d. | n.d. | n.d. |
| Norepinephrine | n.d. | n.d. | n.d. | n.d. | n.d. |
| Histamine | n.d. | n.d. | n.d. | n.d. | n.d. |
| Serotonin | n.d. | n.d. | n.d. | n.d. | n.d. |
| ADP | n.d. | n.d. | n.d. | n.d. | n.d. |
Values indicate the stoichiometry of ligand binding.
Values are given as kcal/mol. S.E. represents the deviation of the experimental data from the fitted data.
Parameters are not calculated.
n.d., no binding detected at 4μM protein and 40 μM ligand.
Vasoconstriction inhibition
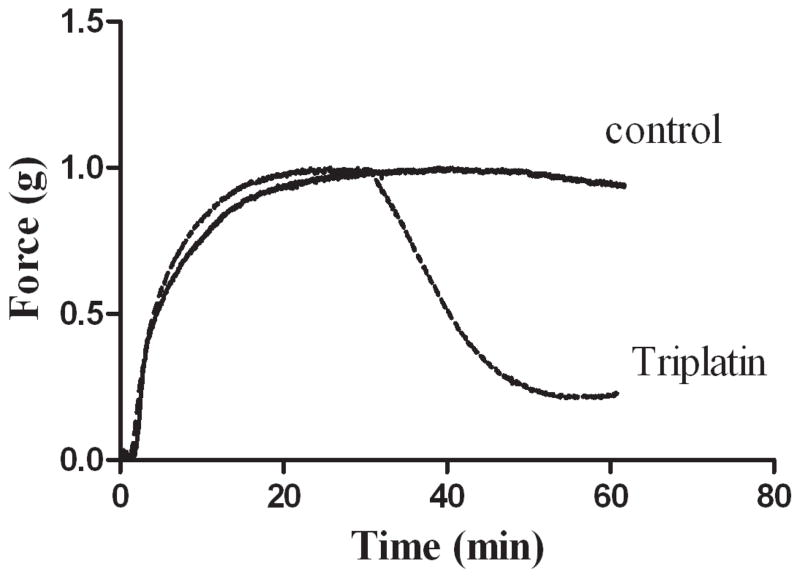
We verified whether triplatin also operates as an inhibitor of contraction of rat aortic ring which was measured isometrically. Figure 4 demonstrates that triplatin induced relaxation of vessels pre-contracted with U46619. Therefore, binding to U46619 translates to inhibition of its pharmacologic property in the vessel.
Fig. 4. Inhibition of vasoconstriction by triplatin.
Rat aorta was placed in a chamber and contraction induced by U-46619 (0.1 μM). After stabilization, triplatin was added and relaxation recorded isometrically. Triplatin or buffer was injected at 30 min. Representative experiment is shown.
Triplatin does not interact with GPVI and its activity is lost in the presence of indometacin
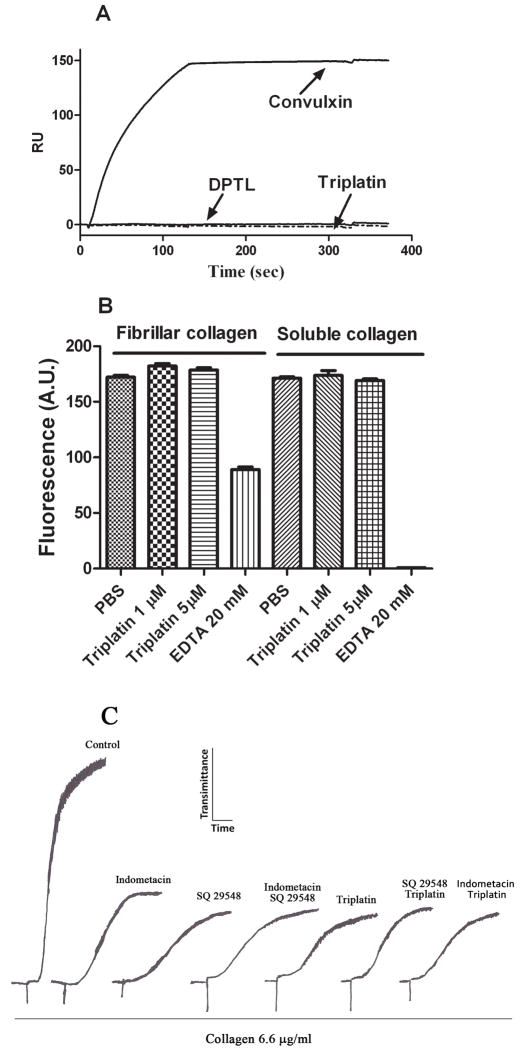
As triplatin was initially described as a GPVI antagonist (21), we tested whether it could bind recombinant GPVI directly by surface plasmon resonance (SPR) experiments. GPVI tested in our experiments has previously been confirmed to bind collagen and to inhibit platelet aggregation (24) confirming its suitability for further experimentation. SPR results show that Cvx interacts with immobilized GPVI (23, 24) which was characterized by an extremely slow dissociation phase, confirming its high affinity for GPVI which has been calculated according to ligand binding experiments (32). In contrast, sensorgrams demonstrates that no resonance signal was detectable when triplatin or DPTL were used as an analyte (Figure 5A). Triplatin also did not interfere with platelet adhesion to fibrillar or soluble collagen type I, excluding integrin α2β1 or GPVI as a target for the inhibitor (Figure 5B). EDTA was used as control to discriminate platelet adhesion to GPVI (Ca2+-independent adhesion to fibrillar collagen), or to integrin α2β1 (Ca2+-dependent adhesion to soluble collagen)(37, 38).
Fig. 5. Triplatin does not interact with GPVI and its activity is loss in the presence of indometacin.
(A) Convulxin (10 nM), triplatin (1 μM), or DPTL (1 μM) was injected for 180 sec over immobilized GPVI using HBSP as a buffer. Dissociation was carried out for 5 min followed by regeneration of CM5 chip with HCl (10 mM). As a control, Cvx was injected again without loss of interaction with GPVI. (B) Calcein-labeled human platelets (2 × 105/μl) were incubated with fibrillar or soluble collagen for 1 h at indicated concentrations of triplatin. EDTA was used to discriminate GPVI- or integrin α2β1-mediated adhesion. Absolute fluorescence values are reported. Platelet adhesion BSA-blocked wells was negligible (n=6). (C) platelet-rich plasma (2 × 105/μl) was incubated with 50 μM indometacin, or 0.2 μM SQ29548 for 3 min alone, or with and without triplatin (1 μM) followed by addition of collagen (6.6 μg/ml).
Next, platelets were activated by moderate concentration of collagen (6.6 μg/ml) which promotes platelet aggregation by a mechanism that is partially dependent on TXA2 (39). Figure 5C demonstrates control platelets activated by collagen. When platelets were incubated with indometacin (50 μM) or SQ29548 (0.2 μM) alone, partial inhibition of platelet aggregation was attained, which was similar to the inhibition observed in the presence of triplatin alone. In addition, triplatin did not enhance inhibition of platelet aggregation produced by indometacin or SQ29548 suggesting interference with a common pathway of platelet aggregation, i.e., TXA2.
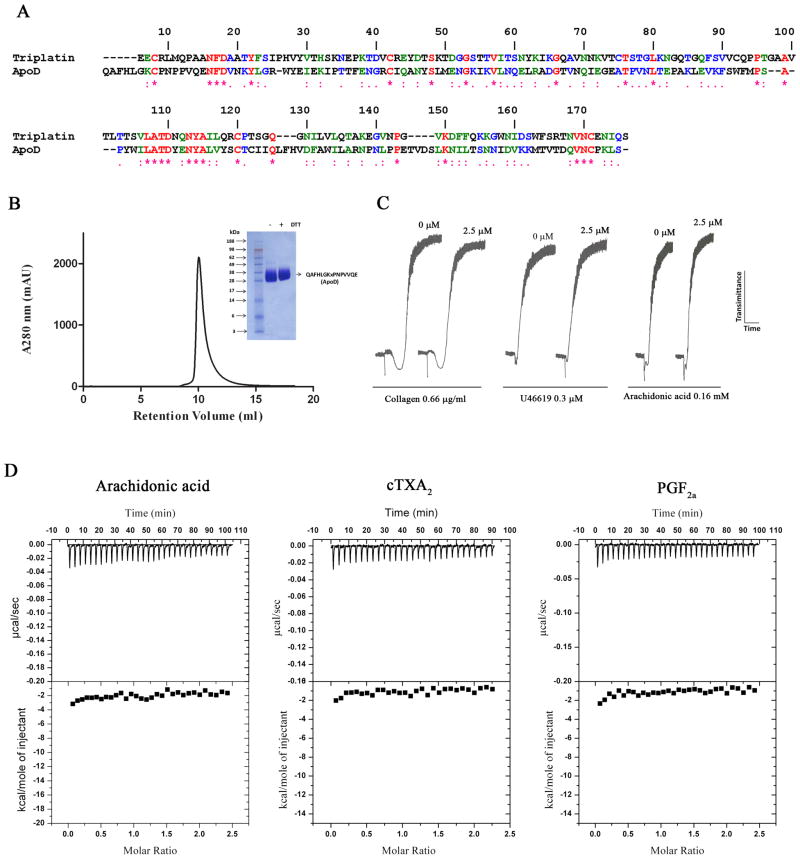
ApoD does not modulate platelet function
Triplatin displays high sequence homology to ApoD (Figure 6A). Because ApoD is part of the HDL, a lipoprotein which reportedly inhibits platelet aggregation (22), and because ApoD reportedly binds AA (40), it was hypothesized that ApoD could be a scavenger of AA and thus interfere with platelet aggregation. ApoD was expressed in HEK293 cells, and purified to homogeneity using a Ni-NTA column, gel-filtration chromatography and reverse-phase column to remove cell culture-derived ligands potentially bound to ApoD. Figure 6B shows a chromatogram, PAGE, and the confirmatory N-terminus of purified ApoD. Figure 6C demonstrates that ApoD did not inhibit platelet aggregation triggered by low doses of collagen, U46619 or AA. Figure 6D also shows the results of ITC experiments that failed to detect binding of ApoD to AA, cTXA2, and PGF2α.
Fig. 6. Apolipoprotein D (ApoD) does not inhibit platelet aggregation.
(A) Alignment of ApoD and triplatin. (B) Purification of ApoD, PAGE and N-terminus identification were carried out as described in Methods. (C) ApoD was incubated for 1 min with platelets (2 × 105/μl) followed by addition of collagen, U46619, and arachidonic acid. (D) Isothermal titration calorimetry for ApoD interaction with AA, cTXA2α and PGF2 is shown.
Discussion
Several hematophagous salivary (sialogenins) inhibitors of platelet aggregation have been identified. The chemical nature of sialogenins varies remarkably, and include a gas (e.g. nitric oxide), enzymes (e.g., apyrases), prostaglandins (e.g., PGI2), nucleotides (e.g., adenosine), disintegrins (e.g., tablysin), and binders of small ligands such as biogenic amines and prostaglandins (12, 41, 42). Appropriate identification of these inhibitors may be difficult and choosing the right agonist has proven to be a critical step in correct functional characterization. This is particularly important because platelets respond differently to distinct agonists and their concentrations (37). The other confounding that should be particularly understood when dealing with small-ligand binders is that the mopping property of these inhibitors is rapidly saturated by pro-aggregatory molecules released by platelets, because the interaction usually occurs stoichiometrically. Therefore, their inhibitory function may remain unidentified unless they are tested at sufficiently high concentrations, even when the agonist has been correctly employed at low doses. For example, the nucleotide-binding protein RPAI-1 (1 μM) partially inhibits platelet aggregation by ADP (< 0.5 μM) but is ineffective if ADP concentration increases above 1 μM (25). In other words, it is usually not possible to infer functional identification for some anti-platelet sialogenins using a single dose of a platelet aggregation inducer.
Not surprisingly, salivary inhibitors reported before as specific for collagen turn out to inhibit the TXA2 (or ADP) pathway of platelet aggregation, despite consistent and reliable experimentation. This is the case for moubatin (18) and pallidipin (16), which have now been identified as TXA2-binding molecules (17, 19). In fact, these inhibitors block low but not high doses of collagen-, TXA2-mimetics (e.g., U46619), and AA-induced platelet aggregation (17, 19). These inducers are particularly sensitive to TXA2 (and ADP) inhibition as both molecules provide additional signaling input for these agonists through interactions with specific receptors and transduction mechanisms needed for completion of platelet aggregation (5, 43). For example, TXA2 activates platelets through G proteins and thromboxane receptors and promotes shape change, Ca2+ mobilization and inositol phosphate production (39, 44); however, activation of this pathway is unable to induce sustained platelet aggregation in the absence of a Gi-coupled receptor agonist such as ADP (12). On the other hand, ADP binds to the Gq-protein-linked purinergic receptors on platelets, which causes a change in cell shape, mobilization of calcium, stimulation of PLC, and inhibition of adenylyl-cyclase-mediated cyclic AMP production (45). As a result, “inside-out” activation of integrin αIIbβ3 takes place and is accompanied by irreversible platelet aggregation (1).
In regard to triplatin, carefully performed experiments demonstrated that it inhibits platelet aggregation by collagen—and specific GPVI agonist CRP—without affecting platelet stimulation by other agonists tested at a single concentration (21). It also attenuates tyrosine phosphorylation of FcRγ chain, which occurs upon collagen binding to GPVI (5). These experiments suggested that triplatin interferes with collagen binding to GPVI although direct binding has not been demonstrated (21). Because of the strong sequence homology between DPTL (a prostaglandin binding protein)(17) and triplatin, and because these lipocalins clade together, we have hypothesized that the latter, like the former, could alternatively operate as a TXA2-binding protein. Platelet aggregation assays performed here demonstrate that triplatin inhibits platelet aggregation not only by collagen (1 μg/ml) but also by low doses of AA (160 μM) and U46619 (0.3 μM). In contrast, high doses of AA (1 mM) or U46619 (3 μM)(Figure 2) were not affected by triplatin, as reported previously (21). Triplatin also did not completely prevent shape change induced by collagen (Figure 2) or CRP (21), suggesting that it does not interact with platelets at a receptor level. Taken together, these finding are consistent with a mopping activity for triplatin, rather than collagen receptor antagonism.
Our results also demonstrate that agonists which trigger platelet aggregation independently of TXA2 were not inhibited by triplatin. For example, Cvx tested at moderate doses (164 pM) which consistently induces platelet aggregation independently of secondary mediators (29–32) was unaltered by triplatin, as it was aggregation triggered by the strong inducer PMA (Figure 2)(46). Of note, low doses of ADP which provoke shape change and reversible platelet aggregation without degranulation or TXA2 generation (33, 34, 36, 45) was insensitive to triplatin or indometacin. In contrast, higher doses of ADP (10 μM) which generates TXA2 (33, 34, 36, 45) was accompanied by disagregation in the presence of triplatin or indometacin (Fig. 2). Triplatin inhibition of CRP-induced platelet aggregation (21) is also consistent with scavenging of TXA2 since appropriate concentrations of CRP is attenuated by feedback inhibitors of platelet aggregation (37). Furthermore, inhibition of platelets by triplatin was abolished when they were incubated with indometacin or with thromboxane receptor antagonist SQ29548, suggesting inhibition of a common pathway of platelet aggregation. Triplatin, like DPTL (17), also did not block U46619-induced shape change as much as it blocked aggregation. This is consistent with the shape change being particularly sensitive to lower concentrations of U46619, while considerably higher concentrations are needed to promote platelet aggregation (39). Altogether, these data support the view that triplatin mechanism of action is explained by scavenging of TXA2. This assumption was confirmed by ITC experiments which demonstrated binding of triplatin to ligands involved in platelet aggregation and vasoconstriction, such as TXA2 and PGF2α with high affinity to interfere with hemostasis.
To examine the possibility of direct binding between recombinant GPVI and triplatin (or DPTL), SPR were performed. Control experiments demonstrate high affinity binding of GPVI to Cvx; these results attested for the functionality of our GPVI preparation for appropriate experimentation (24, 32). In contrast, SPR failed to demonstrate any interaction between GPVI and triplatin or DPTL. Also, GPVI- or integrin α2β1-mediated platelet adhesion to collagen was unaffected by triplatin, excluding platelet receptor as a target for the inhibitor. It is therefore clear that — under our experimental conditions — TXA2 but not GPVI is the target for triplatin; it also implies that native TXA2 generated by platelets is scavenged by the inhibitor. Finally, triplatin was found to relax rat aorta contracted by U46619, consistent with its specificity. It is evident that triplatin is a multifunctional protein that blocks platelet aggregation and vasoconstriction. It represents another notable example of molecular adaptations found in hematophagous SGs (10–15).
Because salivary proteins may mimic the function of endogenous proteins or serve as ligands for orphan receptors (10), it was of interest to verify whether a plasma glycoprotein—ApoD—could also bind to TXA2. ApoD was a relevant candidate among plasma lipocalins because it is found in HDL (47), a lipoprotein known to inhibit platelet aggregation by a mechanism involving specific receptors and blockade of signaling pathways (22). In addition, ApoD reportedly binds AA according to fluorescence spectroscopy experiments (40); presumably, it could modulate platelet function in vitro. ApoD was expressed in HEK293 cells and purified to homogeneity using a Ni-NTA column followed by reverse-phase chromatography to remove cell-derived ligands potentially bound to the recombinant protein. Our ITC experiments consistently failed to detect interaction of ApoD with PGF2α, TXA2 or AA. In addition, no inhibition of platelet aggregation triggered by low doses of different agonists including collagen, U46619, or AA was observed. The reason for discrepancy between our results and previous reports showing binding of AA to ApoD (40) is not clear. It could be due to the introduction of a 6xHis-tag in our preparation or due to specific glycosylations introduced by HEK293 cells which are not present in native or E. coli-expressed ApoD (48). It is also important to recognize that fluorescence spectroscopy employed previously (40, 48) is technically distinct from ITC experiments described herein. For example, solubility of AA or minimal enthalpy changes may have been a limitation for ITC measurements. Also, low affinity of ApoD for AA (~ 3 μM) may have been an issue for ITC (48). Nevertheless, it is coherent to say that modulation of platelet function in vitro is not evident when ApoD expressed by HEK293 cells is employed; however, it is not possible to extrapolate our results with those obtained with ApoD expressed in E. coli (48) or purified from breast fluid cysts (40). Structural features also corroborate with the view that ApoD is devoid of TXA2 binding properties. Accordingly, many of the residues in triplatin and DPTL predicted to lie in the putative binding pocket and which could be important in interactions with the hydrocarbon chain of eicosanoid ligands (e.g. conserved Arg39 and Gln135)(17)—are absent in ApoD.
In conclusion, triplatin mechanism of action has now been elucidated without ambiguity as a TXA2- and PGF2α-binding lipocalin. It adds up to a list of increasingly well characterized small ligand-binding sialogenins such as moubatin (19), RPAI-1 (25), pallidipin (16), DPTL (17), D7 family members (49), nitrophorins (50), ABP (27) and HBP (28). These sialogenins and several others (41, 42) conceivably inhibit platelet aggregation and vasoconstriction at sites of vascular injury and bloodfeeding, thus contributing to successful vector-host interactions.
What is known about this topic?
Triplatin inhibits collagen- and CRP-induced platelet aggregation. It also inhibits tyrosyl-phosphorylation of FcRγ chain. It has been suggested that Triplatin is an antagonist of platelet GPVI.
Apolipoprotein D, a plasma lipocalin constituent of the HDL, has been reported as an AA-binding protein; its effects on platelets have not been tested.
What does this paper add?
Triplatin inhibits platelet aggregation by collagen, and low doses of U46619 and AA. Calorimetry experiments demonstrate that Triplatin binds to prostanoids such as cTXA2 and PGF2α.
Surface plasmon resonance experiments failed to demonstrate triplatin interaction with recombinant GPVI.
It is concluded that triplatin is a high-affinity prostanoid-binding protein which modulates platelet function and vessel tonus through scavenging TXA2 and PGF2α, respectively.
Plasma apolipoprotein D does not bind to AA, TXB2 or PGF2 α, and it does not affect platelet aggregation by collagen, AA or U46619.
Acknowledgments
This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank NIAID intramural editor Brenda Rae Marshall for assistance. We thank Dr. Jean-Luc Villeval (INSERM, France) for providing recombinant GPVI, Drs. Glenn Nardone (NIAID) for mass spectrometry results and Mark Garfield (NIAID) for identification of the N-terminus of triplatin.
Because JA, JR and IF are government employees and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMedCentral for display and use by the public, and PubMedCentral may tag or modify the work consistent with its customary practices. You can establish rights outside of the U.S. subject to a government use license.
Abbreviations
- AA
arachidonic acid
- ADP
adenosine diphosphate
- ApoD
apolipoprotein D
- GP
glycoprotein
- ITC
isothermal titration calorimetry
- SG
salivary gland
- SPR
surface plasmon resonance
Footnotes
Statement
The authors declare they do not have direct or indirect conflicts of interest such as relationships with industry through investments, employment, consultancies, stock ownership, or honoraria.
References
- 1.Watson SP. Platelet activation by extracellular matrix proteins in haemostasis and thrombosis. Curr Pharm Des. 2009;15(12):1358–72. doi: 10.2174/138161209787846702. [DOI] [PubMed] [Google Scholar]
- 2.Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003 Jul 15;102(2):449–61. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 3.Jung SM, Moroi M. Activation of the platelet collagen receptor integrin alpha(2)beta(1): its mechanism and participation in the physiological functions of platelets. Trends Cardiovasc Med. 2000 Oct;10(7):285–92. doi: 10.1016/s1050-1738(01)00064-0. [DOI] [PubMed] [Google Scholar]
- 4.Cosemans JM, Iserbyt BF, Deckmyn H, et al. Multiple ways to switch platelet integrins on and off. J Thromb Haemost. 2008 Aug;6(8):1253–61. doi: 10.1111/j.1538-7836.2008.03041.x. [DOI] [PubMed] [Google Scholar]
- 5.Gibbins JM. Platelet adhesion signalling and the regulation of thrombus formation. J Cell Sci. 2004 Jul 15;117(Pt 16):3415–25. doi: 10.1242/jcs.01325. [DOI] [PubMed] [Google Scholar]
- 6.Jackson SP. The growing complexity of platelet aggregation. Blood. 2007 Jun 15;109(12):5087–95. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- 7.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008 Aug 28;359(9):938–49. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 8.Giannarelli C, Zafar MU, Badimon JJ. Prostanoid and TP-receptors in atherothrombosis: is there a role for their antagonism? Thromb Haemost. 2010 Nov;104(5):949–54. doi: 10.1160/TH10-03-0195. [DOI] [PubMed] [Google Scholar]
- 9.Jaipersad AS, Silverman SH, Lip GY. Anti-platelet therapy: is it all over in peripheral artery disease? Thromb Haemost. 2010 Apr;103(4):689–91. doi: 10.1160/TH10-01-0053. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro JM, Francischetti IM. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu Rev Entomol. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- 11.Fry BG, Roelants K, Champagne DE, et al. The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms. Annu Rev Genomics Hum Genet. 2009;10:483–511. doi: 10.1146/annurev.genom.9.081307.164356. [DOI] [PubMed] [Google Scholar]
- 12.Francischetti IM. Platelet aggregation inhibitors from hematophagous animals. Toxicon. 2010 Dec 15;56(7):1130–44. doi: 10.1016/j.toxicon.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen JF. Structure and mechanism in salivary proteins from blood-feeding arthropods. Toxicon. 2010 Dec 15;56(7):1120–9. doi: 10.1016/j.toxicon.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maritz-Olivier C, Stutzer C, Jongejan F, et al. Tick anti-hemostatics: targets for future vaccines and therapeutics. Trends Parasitol. 2007 Sep;23(9):397–407. doi: 10.1016/j.pt.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Steen NA, Barker SC, Alewood PF. Proteins in the saliva of the Ixodida (ticks): pharmacological features and biological significance. Toxicon. 2006 Jan;47(1):1–20. doi: 10.1016/j.toxicon.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Noeske-Jungblut C, Kratzschmar J, Haendler B, et al. An inhibitor of collagen-induced platelet aggregation from the saliva of Triatoma pallidipennis. J Biol Chem. 1994 Feb 18;269(7):5050–3. [PubMed] [Google Scholar]
- 17.Assumpcao TC, Alvarenga PH, Ribeiro JM, et al. Dipetalodipin, a novel multifunctional salivary lipocalin that inhibits platelet aggregation, vasoconstriction, and angiogenesis through unique binding specificity for TXA2, PGF2alpha, and 15(S)-HETE. J Biol Chem. 2010 Dec 10;285(50):39001–12. doi: 10.1074/jbc.M110.152835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waxman L, Connolly TM. Isolation of an inhibitor selective for collagen-stimulated platelet aggregation from the soft tick Ornithodoros moubata. J Biol Chem. 1993 Mar 15;268(8):5445–9. [PubMed] [Google Scholar]
- 19.Mans BJ, Ribeiro JM. Function, mechanism and evolution of the moubatin-clade of soft tick lipocalins. Insect Biochem Mol Biol. 2008 Sep;38(9):841–52. doi: 10.1016/j.ibmb.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy VB, Kounga K, Mariano F, et al. Chrysoptin is a potent glycoprotein IIb/IIIa fibrinogen receptor antagonist present in salivary gland extracts of the deerfly. J Biol Chem. 2000 May 26;275(21):15861–7. doi: 10.1074/jbc.275.21.15861. [DOI] [PubMed] [Google Scholar]
- 21.Morita A, Isawa H, Orito Y, et al. Identification and characterization of a collagen-induced platelet aggregation inhibitor, triplatin, from salivary glands of the assassin bug, Triatoma infestans. FEBS J. 2006 Jul;273(13):2955–62. doi: 10.1111/j.1742-4658.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 22.Nofer JR, Brodde MF, Kehrel BE. High-density lipoproteins, platelets and the pathogenesis of atherosclerosis. Clin Exp Pharmacol Physiol. 2010 Jul;37(7):726–35. doi: 10.1111/j.1440-1681.2010.05377.x. [DOI] [PubMed] [Google Scholar]
- 23.Francischetti IM, Saliou B, Leduc M, et al. Convulxin, a potent platelet-aggregating protein from Crotalus durissus terrificus venom, specifically binds to platelets. Toxicon. 1997 Aug;35(8):1217–28. doi: 10.1016/s0041-0101(97)00021-4. [DOI] [PubMed] [Google Scholar]
- 24.Jandrot-Perrus M, Busfield S, Lagrue AH, et al. Cloning, characterization, and functional studies of human and mouse glycoprotein VI: a platelet-specific collagen receptor from the immunoglobulin superfamily. Blood. 2000 Sep 1;96(5):1798–807. [PubMed] [Google Scholar]
- 25.Francischetti IM, Ribeiro JM, Champagne D, et al. Purification, cloning, expression, and mechanism of action of a novel platelet aggregation inhibitor from the salivary gland of the blood-sucking bug, Rhodnius prolixus. J Biol Chem. 2000 Apr 28;275(17):12639–50. doi: 10.1074/jbc.275.17.12639. [DOI] [PubMed] [Google Scholar]
- 26.Paddock CD, McKerrow JH, Hansell E, et al. Identification, cloning, and recombinant expression of procalin, a major triatomine allergen. J Immunol. 2001 Sep 1;167(5):2694–9. doi: 10.4049/jimmunol.167.5.2694. [DOI] [PubMed] [Google Scholar]
- 27.Andersen JF, Francischetti IM, Valenzuela JG, et al. Inhibition of hemostasis by a high affinity biogenic amine-binding protein from the saliva of a blood-feeding insect. J Biol Chem. 2003 Feb 14;278(7):4611–7. doi: 10.1074/jbc.M211438200. [DOI] [PubMed] [Google Scholar]
- 28.Paesen GC, Adams PL, Harlos K, et al. Tick histamine-binding proteins: isolation, cloning, and three-dimensional structure. Mol Cell. 1999 May;3(5):661–71. doi: 10.1016/s1097-2765(00)80359-7. [DOI] [PubMed] [Google Scholar]
- 29.Faili A, Randon J, Francischetti IM, et al. Convulxin-induced platelet aggregation is accompanied by a powerful activation of the phospholipase C pathway. Biochem J. 1994 Feb 15;298( Pt 1):87–91. doi: 10.1042/bj2980087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkinson BT, Stafford MJ, Pears CJ, et al. Signalling events underlying platelet aggregation induced by the glycoprotein VI agonist convulxin. Eur J Biochem. 2001 Oct;268(20):5242–8. doi: 10.1046/j.0014-2956.2001.02448.x. [DOI] [PubMed] [Google Scholar]
- 31.Vargaftig BB, Chignard M, Benveniste J. Present concepts on the mechanisms of platelet aggregation. Biochem Pharmacol. 1981 Feb 15;30(4):263–71. doi: 10.1016/0006-2952(81)90052-6. [DOI] [PubMed] [Google Scholar]
- 32.Francischetti IM, Ghazaleh FA, Reis RA, et al. Convulxin induces platelet activation by a tyrosine-kinase-dependent pathway and stimulates tyrosine phosphorylation of platelet proteins, including PLC gamma 2, independently of integrin alpha IIb beta 3. Arch Biochem Biophys. 1998 May 15;353(2):239–50. doi: 10.1006/abbi.1998.0598. [DOI] [PubMed] [Google Scholar]
- 33.Charo IF, Feinman RD, Detwiler TC. Interrelations of platelet aggregation and secretion. J Clin Invest. 1977 Oct;60(4):866–73. doi: 10.1172/JCI108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mustard JF, Perry DW, Kinlough-Rathbone RL, et al. Factors responsible for ADP-induced release reaction of human platelets. The American journal of physiology. 1975 Jun;228(6):1757–65. doi: 10.1152/ajplegacy.1975.228.6.1757. [DOI] [PubMed] [Google Scholar]
- 35.Kunapuli SP. P2 receptors and platelet activation. TheScientificWorldJournal. 2002 Feb 13;2:424–33. doi: 10.1100/tsw.2002.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Packham MA, Bryant NL, Guccione MA, et al. Effect of the concentration of Ca2+ in the suspending medium on the responses of human and rabbit platelets to aggregating agents. Thromb Haemost. 1989 Nov 24;62(3):968–76. [PubMed] [Google Scholar]
- 37.Jarvis GE, Atkinson BT, Snell DC, et al. Distinct roles of GPVI and integrin alpha(2)beta(1) in platelet shape change and aggregation induced by different collagens. Br J Pharmacol. 2002 Sep;137(1):107–17. doi: 10.1038/sj.bjp.0704834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura T, Jamieson GA, Okuma M, et al. Platelet adhesion to native type I collagen fibrils. Role of GPVI in divalent cation-dependent and -independent adhesion and thromboxane A2 generation. J Biol Chem. 1998 Feb 20;273(8):4338–44. doi: 10.1074/jbc.273.8.4338. [DOI] [PubMed] [Google Scholar]
- 39.Huang JS, Ramamurthy SK, Lin X, et al. Cell signalling through thromboxane A2 receptors. Cell Signal. 2004 May;16(5):521–33. doi: 10.1016/j.cellsig.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Morais Cabral JH, Atkins GL, Sanchez LM, et al. Arachidonic acid binds to apolipoprotein D: implications for the protein’s function. FEBS Lett. 1995 Jun 5;366(1):53–6. doi: 10.1016/0014-5793(95)00484-q. [DOI] [PubMed] [Google Scholar]
- 41.Ma D, Xu X, An S, et al. A novel family of RGD-containing disintegrins (Tablysin-15) from the salivary gland of the horsefly Tabanus yao targets alphaIIbbeta3 or alphaVbeta3 and inhibits platelet aggregation and angiogenesis. Thromb Haemost. 2011 Jun 6;105(6):1032–45. doi: 10.1160/TH11-01-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chmelar J, Oliveira CJ, Rezacova P, et al. A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation. Blood. 2011 Jan 13;117(2):736–44. doi: 10.1182/blood-2010-06-293241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Z, Delaney MK, O’Brien KA, et al. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010 Dec;30(12):2341–9. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feletou M, Vanhoutte PM, Verbeuren TJ. The thromboxane/endoperoxide receptor (TP): the common villain. J Cardiovasc Pharmacol. 2010 Apr;55(4):317–32. doi: 10.1097/fjc.0b013e3181d8bc8a. [DOI] [PubMed] [Google Scholar]
- 45.Kahner BN, Shankar H, Murugappan S, et al. Nucleotide receptor signaling in platelets. J Thromb Haemost. 2006 Nov;4(11):2317–26. doi: 10.1111/j.1538-7836.2006.02192.x. [DOI] [PubMed] [Google Scholar]
- 46.Stafford MJ, Atkinson BT, Watson SP, et al. Signalling components underlying platelet aggregation to a Ca2+ ionophore and a phorbol ester. Platelets. 2001 Dec;12(8):476–85. doi: 10.1080/095371001317126374. [DOI] [PubMed] [Google Scholar]
- 47.Weech PK, Camato R, Milne RW, et al. Apolipoprotein D and cross-reacting human plasma apolipoproteins identified using monoclonal antibodies. J Biol Chem. 1986 Jun 15;261(17):7941–51. [PubMed] [Google Scholar]
- 48.Vogt M, Skerra A. Bacterially produced apolipoprotein D binds progesterone and arachidonic acid, but not bilirubin or E-3M2H. J Mol Recognit. 2001 Jan-Feb;14(1):79–86. doi: 10.1002/1099-1352(200101/02)14:1<79::AID-JMR521>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 49.Alvarenga PH, Francischetti IM, Calvo E, et al. The function and three-dimensional structure of a thromboxane A2/cysteinyl leukotriene-binding protein from the saliva of a mosquito vector of the malaria parasite. PLoS Biol. 2010;8(11):e1000547. doi: 10.1371/journal.pbio.1000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ribeiro JM, Walker FA. High affinity histamine-binding and antihistaminic activity of the salivary nitric oxide-carrying heme protein (nitrophorin) of Rhodnius prolixus. J Exp Med. 1994 Dec 1;180(6):2251–7. doi: 10.1084/jem.180.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]