Abstract
The BALB/c mouse model of Niemann-Pick type C (NPC) disease exhibits neuropathological similarities to the human condition. There is an age-related cerebral atrophy, demyelination of the corpus callosum, and degeneration of cerebellar Purkinje cells in the NPC mouse. In human NPC, many cortical and subcortical neurons contain neurofibrillary tangles, which are thought by some investigators to play an important role in the neurodegenerative process. The purpose of the present study was to determine whether neurodegeneration occurs in the NPC mouse, in brain regions other than the cerebellum and whether the degeneration is related to the presence of neurofibrillary tangles. Using light microscopic methods with immunohistochemistry, electron microscopy, and cell counting methods, 11-week-old NPC+/+ and NPC−/− animals were examined. In the NPC−/− mice, there were 96% fewer Purkinje cells, 28% fewer neurons in the prefrontal cortex, 20% fewer neurons in the thalamus, and 63% fewer glial cells in the corpus callosum. On the other hand, previous studies indicate normal numbers of neurons and glial cells in these same neuroanatomical regions in young NPC−/− mice. There were normal numbers of cholinergic neurons in sections assessed in the striatum and basal forebrain in the 11-week-old animals and no evidence of neurofibrillary tangles within cells. The present data indicate that both neurons and glial cells die in the NPC mouse but that all cells are not equally vulnerable. There was no evidence for neurofibrillary tangles in the NPC mouse, and therefore the degenerative process in the mouse is unrelated to the neurofibrillary tangle.
Indexing terms: Purkinje cells, corpus callosum, prefrontal cortex, glial cells, thalamus, cholinergic neurons, stereology
Niemann-Pick type C (NPC) disease is a fatal neurovisceral disorder that is characterized by progressive enlargement of internal organs and the onset of degenerative neurological disease, often in late childhood. During the course of the disease schizophrenic-like symptoms are sometimes manifest (Turpin et al., 1991; Love et al. 1995), and dementia and ataxia are commonly observed (Love et al., 1995). Whereas types A and B Niemann-Pick disease are due to a defect in sphingomyelinase, type C disease is thought to be caused by a defect in the intracellular trafficking of cholesterol (Sokol et al., 1988; Blanchette-Mackie et al., 1988). This concept was further supported when a mutant gene was found to encode a 1278-amino acid protein, NPC1, that shares considerable homology with other proteins known to be important in regulating cholesterol balance across cells (Carstea et al., 1997). The cellular accumulation of unesterified cholesterol occurs within the lysosomal compartment (Blanchette-Mackie et al., 1988; Sokol et al., 1988), explaining the increase in lysosomal structures observed within neurons along with different types of intracellular inclusion bodies (e.g., myelin figures, multivesicular bodies; Higashi et al., 1993; Love et al., 1995).
The mechanisms responsible for cell death in NPC disease are unknown. Many cortical and subcortical neurons contain neurofibrillary tangles (NFTs) in NPC disease that are biochemically and morphologically similar to those found in Alzheimer’s disease (Auer et al., 1995; Love et al., 1995). The NFT has been implicated in cell death in Alzheimer’s disease (Saper et al., 1985; Goedert et al., 1998; Hardy et al., 1998) and in neurodegeneration in other diseases involving mutations in the tau protein (Hong et al., 2000), In the cerebellum of patients with NPC disease, for example, there is degeneration of Purkinje cells (Gilbert et al., Love et al., 1995), and NFTs can be found in the cerebellum (Love et al., 1995). Furthermore, NPC disease is characterized by cerebral atrophy and demyelination of fiber tracts (Palmeri et al., 1994; Love et al., 1995), which suggest that there is also significant degeneration of cortical neurons and glial cells, respectively.
A mouse model for NPC disease has been identified that provides a powerful tool for exploring the pathophysiology of this disease in quantitative terms. Like the human, this animal manifests a gene mutation in NPC1 (Loftus et al., 1997), enlargement of the liver and spleen, liver function abnormalities, and progressive neurodegeneration (Xie et al., 1999a). Virtually every organ manifests the accumulation of unesterified cholesterol so that the whole-body pool of sterol increases almost threefold by the time these animals are 11 weeks of age (Xie et al., 1999a). This cholesterol represents the sequestration of sterol in late endosomes and lysosomes that are derived from the uptake of chylomicron remnants and low-density lipoproteins through the clathrin-coated pit pathway in every cell (Xie et al., 1999b). Many neurons in the central nervous system show similar cholesterol-rich inclusions, as evidenced by positive fluorescence in filipin-stained sections of the brain (Wu et al., 1999). As in the human disease, these animals also show an age-related loss of Purkinje cells (Tanaka et al., 1988; Higashi et al., 1993) and marked demyelination of the corpus callosum (Weintraub et al., 1987).
In the NPC mouse, only Purkinje cell numbers have been examined quantitatively, and there has been no characterization of cell content in such regions as the cerebral cortex or thalamus. The purpose of the present study, therefore, was to 1) quantify neuronal and glial cell numbers in the NPC mouse in brain regions other than the cerebellum, and 2) determine whether NFTs exist in brain regions exhibiting neurodegeneration. Thus, cell-counting methods were employed to quantify Purkinje cells, neurons in the prefrontal cortex and thalamus, and glial cells in the corpus callosum. In addition, cholinergic neurons in the striatum and basal forebrain were counted because they have been implicated in schizophrenia (Holt et al., 1999) and dementia (Whitehouse et al., 1982), respectively, symptoms that are also present in NPC patients.
MATERIALS AND METHODS
BALB/c mice carrying the mutation in NPC1 protein were originally obtained from the Pentchev laboratory at NIH (Loftus et al., 1997). Control (NPC+/+) and homozygous (NPC−/−) animals of both sexes were used in the present study. Preliminary studies revealed no phenotype in the heterozygous animals (Xie et al., 1999a). The NPC−/− animals have normal food intake and weight gain up to approximately 7 weeks of age, beyond which they develop progressive ataxia, decreased food intake, and weight loss (Xie et al., 1999a). Shortly after 11 weeks of age the animals die. Unless otherwise stated, NPC−/− and NPC+/+ mice were used at 11 weeks of age. The studies described were approved by the University of Texas Southwestern Medical Center’s Institutional Animal Care and Research Advisory Committee.
Mice were deeply anesthetized with flurothane and decapitated. The brains were removed from the skull and placed in 10% neutral buffered formalin (pH 7.0) for 3–4 days before being placed in formalin containing 20% sucrose (as a cryoprotectant). The brains were cut sagitally, and the left hemisphere was sectioned at 40 µm thickness and the right hemisphere sectioned at 20 µm. Every 10th 40-µm-thick section in the left hemisphere was stained with cresyl violet and used for stereological cell counting. The 20-µm-thick sections, from the right hemisphere, were used for immunostaining.
Histochemistry
The standard peroxidase anti-peroxidase method was used for immunohistochemical localization (Liang et al., 1993). Briefly, the thin frozen sections were treated as follows: 1) incubation in 10% normal sheep serum in phosphate-buffered saline (PBS)-T solution (PBS with 0.3% Triton X-100, pH 7.0) for 30 minutes; 2) incubation in rabbit polyclonal antibody against the cholinergic enzyme choline acetyltransferase (ChAT; 1:500; Chemicon, Temecula, CA) in PBS-TSS (PBS-T with 1% normal sheep serum) for 18–24 hours at room temperature (with agitation on a shaker table for all incubation and rinse steps); 3) incubation in 1:100 sheep anti-rabbit IgG in PBS-T for 30 minutes; and 4) incubation in 1:300 rabbit peroxidase anti-peroxidase (Jackson ImmunoResearch, West Grove, PA) in PBS-T for 60 minutes. Following steps 2, 3, and 4, the sections were rinsed thoroughly in PBS (3 × 10 minutes). The sections were then treated with nickel diaminobenzidine (DAB)/hydrogen peroxide solution (2.5 g nickel ammonium sulfate, 50 mg DAB, 33 µl 30% H2O2, 100 ml acetate buffer, pH 6.0). The sections were rinsed, mounted on gelatin-coated slides, and coverslipped. Control sections were processed in PBS instead of the primary antibody solutions, and no immunostaining was observed.
Thioflavin-S histochemistry was used to detect NFTs in both postmortem Alzheimer’s disease brain (79-year-old male) and in the NPC+/+ and NPC−/− mouse. Briefly, slide-mounted tissue sections were immersed in a 1% solution of thioflavin S (Sigma, St. Louis, MO) in distilled water for 8 minutes, rinsed in distilled water for 1 minute, differentiated in 80% ethanol for 1 minute, rinsed twice in distilled water for 1 minute each, and then cover-slipped with glycerin (Schwartz, 1972).
Antibodies
In addition to ChAT, other antibodies were used to define the NPC phenotype, by using methods like those described above. Anti-calbindin-D28k (SWant; 1:10,000) was used to examine Purkinje cell morphology (German et al., 1997), and glial fibrillary acidic protein (GFAP) antibody (Chemicon; 1:1,000) was used to identify astrocytes. For both antibodies the staining method was similar to that described for ChAT.
Electron microscopy
Standard electron microscopy (EM) procedures were employed, as previously used in this laboratory (Liang et al., 1993) to look for the presence of NFTs, Briefly, 11-week-old NPC+/+ and NPC−/− mice were anesthetized with Nembutal (65 mg/kg, intraperitoneal) and perfused transcardially with 2 ml heparinized saline, 50 ml of 4% paraformaldehyde, and 0.1% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were blocked in the coronal plane, and tissues containing cerebellum and pre-frontal cortex were postfixed in the same fixative for 2 hours and cut at 40 µm and 20 µm thickness alternately on a Vibratome. The 20-µm sections were mounted onto gelatin-coated slides and stained with cresyl violet. The 40-µm sections were rinsed in PBS, fixed with 1% osmium tetroxide for 60 minutes, dehydrated, and flat-embedded in Poly-bed 812 (Polysciences, Warrington, PA) between two Aclar plastic sheets. Then, the brain regions were located in the Nissl-stained thin sections, and the corresponding regions in the thick sections were cut out and embedded in Epon. Ultrathin sections were cut, picked up on copper grids, and counterstained with 2% uranyl acetate and lead citrate. The EM analyses of the regions of interest were systematically scanned and photographed at an initial magnification of 10,000–20,000, in a JEOL 100 CX electron microscope.
Stereology
Stereological methods were employed, as previously described (West, 1993). Every 10th section, for a total of 6 sections, was used from medial to lateral through the nucleus/region of interest (the prefrontal cortex, thalamus, cerebellum, and corpus callosum). The first of the six sections was 0.5 mm from the midline, and the last section was 2.5 mm from the midline. This 2-mm tissue block encompasses 75% of the thalamus, 80% of the prefrontal cortex, 66% of a cerebellar hemisphere, and 80% of the corpus callosum. Four mice were studied in each of the two groups.
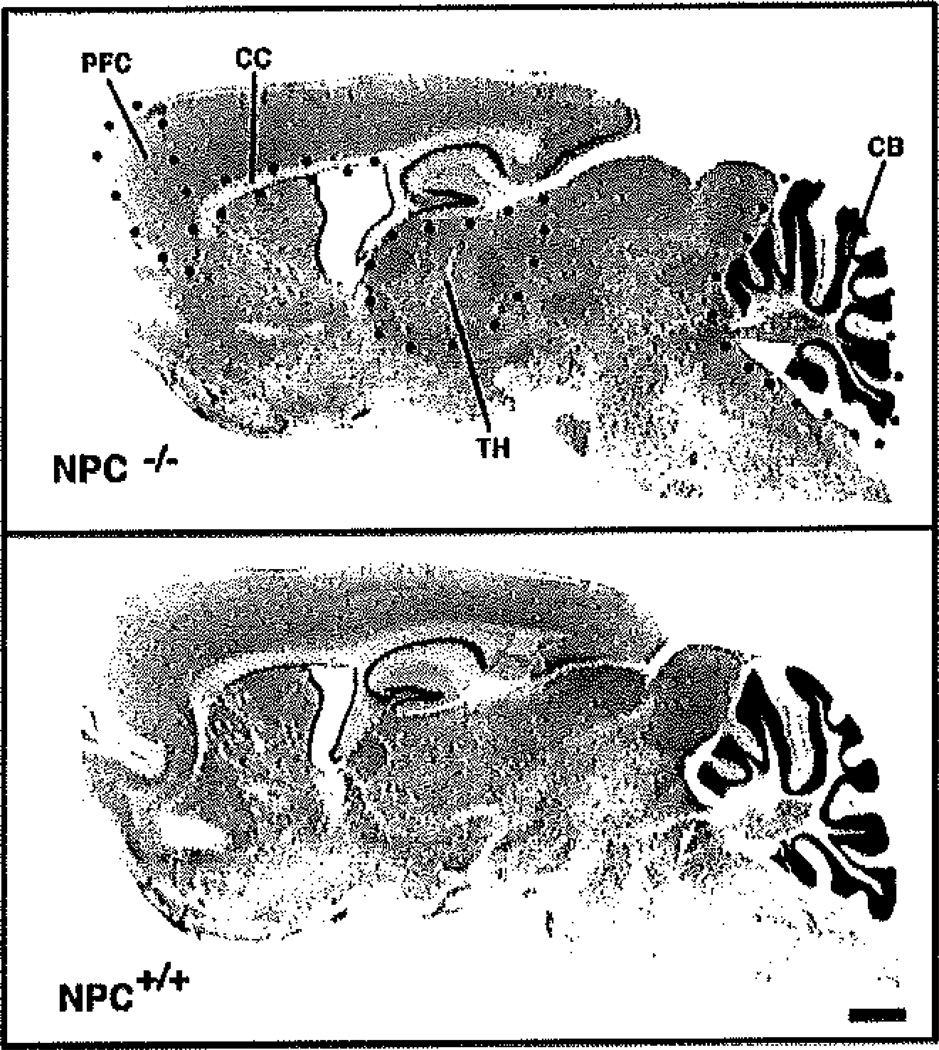
Figure 1 illustrates the neuroanatomical borders used to define the four brain regions in which cell counts were taken. The brain regions were outlined with the person (E.M.Q.) blind as to the experimental condition of the animal. The prefrontal cortex was denned as the cortical region that extends rostral to the forceps minor of the corpus callosum, through the 2-mm medial-lateral extent of the cerebral cortex. The prefrontal cortex region was outlined for cell counting purposes by drawing a straight line on the dorsal surface of the corpus callosum, and a second line was drawn perpendicular at the rostral border of the corpus callosum. The prefrontal cortex was defined as the tissue rostral to the perpendicular line. The thalamus was outlined according to the atlas of Paxinos and Watson (1986), which provides illustrations of sagittal sections. All thalamic nuclei were included for cell counting purposes from the reticular nucleus rostrally, to the border with the tectum caudally, to the zona incerta ventrally. The cerebellar hemisphere was outlined for volume measurements, and Purkinje cells were counted within the Purkinje cell layer. Glial cells were counted within the rostral half of the corpus callosum, because the only somata in this structure are those of glial cells. The corpus callosum was outlined from the forceps minor rostrally, to a caudal border at the level of the CA3 region of the hippocampus.
Fig. 1.
Nissl-stained sections of the 11-week-old NPC+/+ and NPC−/− mouse brain illustrating the four regions where stereological cell counts were made. Sagittal sections illustrate the neuroanatomical boundaries used to demarcate the prefrontal cortex (PFC), thalamus (TH), rostral portion of the corpus callosum (CC), and cerebellar hemisphere (CB) where Purkinje cells were counted. Notice that the NPC−/− brain is smaller than the NPC+/+ brain, and that the corpus callosum is markedly shrunken in the NPC−/− brain. Scale bar = 750 µm.
StereoInvestigator-2000 software (v.4.01a; Micro-BrightField), using the optical fractionator, was used for cell counting. The counting grid dimensions were varied in each brain region to allow for sampling of more than 200 sites/brain regions of interest and resulted in counting more than 300 cells in each brain region. Studies conducted in this laboratory have confirmed the work of others (e.g., Harding et al., 1994) demonstrating that when more than 200 cells are counted, accurate cell number estimates are obtained. Cell counts were taken from Nissl-stained sections that measured on average 15 µm. (Sections were cut at 40-µm thickness.) A neuron or glial cell was defined in an unbiased manner as a cell with a clearly visible nucleolus within the 10-µm Z-plane of the counting frame. (Nucleoli that touched the bottom or left border of the counting frame were not counted.) Counts were made by using a 100× oil immersion objective (NA, 1.25). An upper buffer zone of 3 µm was used, and the lower buffer zone was 2 µm thick. Cell counting was performed with the counter “blind” as to the experimental condition of the animals. However, in the case of the Purkinje cells and glial cells in the corpus callosum, it was clear which animals were NPC−/− and therefore it was not possible for the counter to be blind. Volume determinations were made with StereoInvestigator software and represent Cavalieri’s estimate.
Choline acetyltransferase neuronal quantification
NeuroLucida software (v.3.18; MicroBrightField) was used to quantify ChAT-containing neurons in the striatum and in the basal forebrain in NPC+/+ and NPC−/− mice. Using four animals in each group (NPC+/+ and NPC−/−), four to six sections were stained for ChAT, and two sections were carefully matched from all brains in order to examine a similar portion of the striatum and basal fore-brain. For each animal the mean number of ChAT-containing neurons/section was determined for the basal forebrain, and for the striatum from two 20-µm thick sections. Stereological methods were not used to count the cholinergic neurons because we wished only to get a simple comparison of cell numbers/section in two cholinergic brain regions and not estimate the total number of neurons in each nucleus.
The size of the cholinergic neurons in the striatum was measured, to determine whether the NPC phenotype causes a change in soma size. The long axis of 40–60 ChAT-immunostained somata was measured in NPC+/+ (n = 3) and NPC−/− (n = 3) animals, with the person performing the measurements “blind” as to the experimental condition of the animal. Cells were measured, by using a 40× objective, with Neurolucida software. All cells within the region of interest that contained a clearly visible, unstained nucleus were measured.
Statistics
Student’s t-tests were used to quantify differences in cell numbers and volumes in the four brain regions (prefrontal cortex, thalamus, corpus callosum, and cerebellum) in the two groups of mice (NPC+/+ and NPC−/−). Statistical significance was defined by using a two-tailed test and P < 0.01. Welch’s t-tests were used to compare the number of cholinergic neurons in the striatum and basal forebrain in the two mouse groups. A two-tailed test of statistical significance was used, and significance was defined as P < 0.05.
Light microscopic images were taken with an Olympus DP11 digital camera. All images were sharpened (setting 10), and the contrast was enhanced (setting 15).
RESULTS
Selective degeneration
Stereology
To quantify the number of neurons and glial cell bodies in the NPC mouse, we estimated the total number of cells in four brain regions by using stereological counting methods. Neurons were counted in the prefrontal cortex and thalamus, as representations of cortical and subcortical brain regions, respectively. Purkinje cells were counted in the cerebellum because this region has been found to degenerate in an age-dependent manner in the NPC mouse (Tanaka et al., 1988). Glial cells were counted in the corpus callosum, because this structure contains only the cell bodies of glial cells. Demyelination has been imported previously for NPC mice (Weintraub et al., 1987) and in patients with NPC disease (Palmeri et al., 1994), probably reflecting a loss of oligodendrocytes in the corpus callosum. In all the brain regions/animals examined, the coefficient of error for the cell number and volume estimates (Scheaffer et al., 1996; Glaser and Wilson, 1997) ranged from 0.05 to 0.07.
There were reductions in both cell numbers and brain region volumes in the NPC−/− animals in most of the brain regions examined, but the extent of the reduction was not the same (Table 1). The greatest cell number reduction occurred in the cerebellum where there were 96% fewer Purkinje neurons in the NPC−/− animals vs. NPC+/+ (t = 9.64, P < 0.001). Figure 2 illustrates Purkinje cells in tissue immunostained with a Purkinje cell-specific antibody, calbindin-D28k. There was a dramatic reduction in the number of Purkinje cells, and there were numerous swellings along the Purkinje cell axons. There was also a significant reduction in the volume of the cerebellar hemisphere estimated by using the Cavalieri volume estimator (29%; t = 6.16, P < 0.001). In the thalamus there was a 20% reduction in the number of neurons (t = 4.25, P = 0.005) and a 10% reduction in whole thalamus volume (t = 3.94, P = 0.008) in the NPC−/− mice. There was a significant reduction in the number of neurons in the prefrontal cortex (28%; t = 5.64, P < 0.001) and a significant reduction in the volume of the prefrontal cortex (19%; t = 4.01, P = 0.007). There was also a significant reduction in the number of glial cells in the corpus callosum of the NPC−/− mice (63%; t = 8.23, P < 0.001) and a 45% reduction in the volume of the corpus callosum (t = 4.58, P = 0.004).
TABLE 1.
Cell Numbers and Brain Regional Volumes in the NPC Mouse1
| NPC+/+ |
NPC−/− |
|||
|---|---|---|---|---|
| Brain region | Cell no. | Volume (mm3) | Cell no | Volume (mm3) |
| Prefrontal cortex | 546,750 ± 20,314 | 5.65 ± 0.18 | 391,875 ± 18,497* | 4.56 ± 0.20* |
| Thalamus | 267,907 ± 5,921 | 3.02 ± 0.03 | 214,447 ± 11,108* | 2.69 ± 0.06* |
| Cerebellum2 | 71,959 ± 2,176 | 11.44 ± 0.22 | 2,697 ± 312* | 8.15 ± 0.31* |
| Corpus callosum3 | 225,600 ± 22,650 | 0.60 ± 0.01 | 83,178 ± 3,183* | 0.33 ± 0.01* |
In each brain region there were n = 4 mice/group, and numbers represent mean ± SEM. Cell counts were determined using stereological methods described in the text.
In the cerebellum, Purkinje cells were counted.
In the corpus callosum, glial cell somata were counted.
Student’s t-test, P < 0,01 NPC+/+ vs. NPC−/− for respective brain regions.
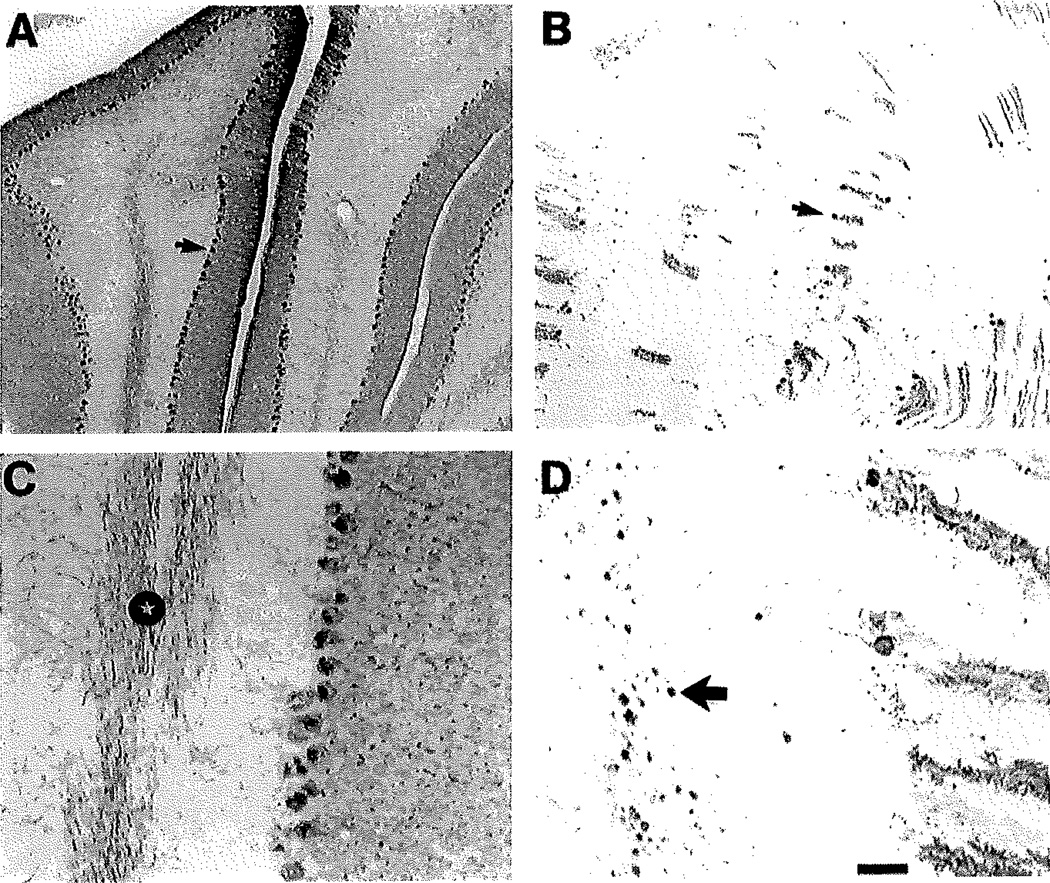
Fig. 2.
Calbindin-immunostained sections illustrating the loss of cerebellar Purkinje cells in the 11-week-old NPC−/− mouse. Calbindin is a selective marker for Purkinje cells in the cerebellum, their dendrites and axons. A: In the 11-week-old NPC+/+ mouse there are numerous Purkinje cell closely spaced together (arrow points to the cell body of one cell), with dendrites radiating into the molecular layer. B: In the NPC−/− mouse there is a marked loss of Purkinje cells. Arrow points to one of the few remaining Purkinje cells. C: Illustration of calbindin-containing Purkinje cell axons in the white matter of the cerebellum in the NPC+/+ brain. Note the many immunostained Purkinje cell axons (star). D: Illustration of calbindin-containing Purkinje cell axons with marked swellings (large arrow points to one of many swellings) in the white matter of the NPC−/− brain. Scale bar in D = 160 µm for A,B; 30 µm for C,D.
Glial cells
In the NPC+/+ brain, GFAP-positive astrocytes were only observed in white matter regions like the corpus callosum. In contrast, the NPC−/− animals had high densities of GFAP-reactive astrocytes in many gray matter areas. As an example of the enhanced GFAP staining in the NPC−/− mice, Figure 3 shows one folia of the cerebellum in NPC+/+ and NPC−/− animals. Note the lack of GFAP immunostaining in the normal animal and the high density of immunostaining in the NPC−/− mouse. The astrocyte somata are located near the Purkinje cell layer, and the GFAP-positive processes radiate toward the molecular layer of the cerebellar cortex.
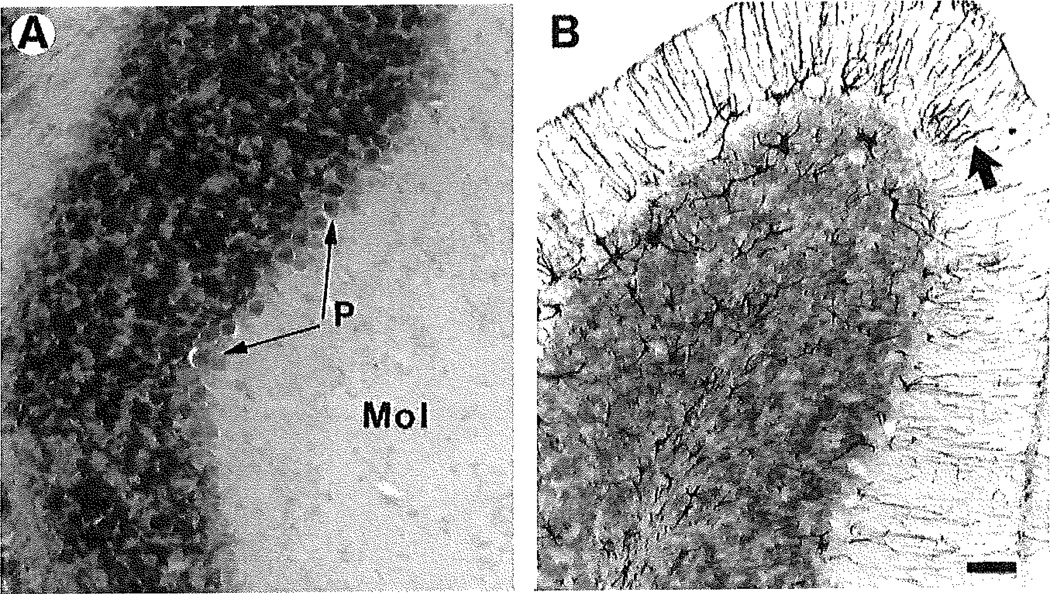
Fig. 3.
Astrocytes in the NPC mouse cerebellum as revealed by immunostaining for GFAP. Tissues were immunostanined for GFAP (black) and counterstained with creayl violet. A: In the NPC−/− mouse, there is no GFAP immunostaining. B: In the NPC−/− mouse, GFAP immunostaining is intense within the astrocytic fibers radiating from the Purkinjo cell layer up to the surface of the molecular layer (arrow points to one of many such glial fibers). Note the few astrocyte somata in the Purkinje cell layer. Abbreviations: Mol, molecular layer; P, Purkinje cell layer. Scale bar = 30 µm.
Cholinergic neurons in the NPC mouse
Cells that were immunoatained with an antibody against ChAT were examined in both the striatum and basal forebrain of NPC−/− and NPC+/+ mice. The basal forebrain neurons were examined in a sagittal plane that included cholinergic cells in the nucleus accumbens (rostrally), substantia innominata, and nucleus basalis magnocellularis (caudally, in the region of the globus pallidus). The basal forebrain cholinergic neurons were studied to determine whether the NPC mice exhibit neurodegeneration that resembles that found in Alzheimer’s disease patients (Whitehouse et al., 1982; Nagai et al., 1983). The striatal cholinergic neurons were counted because they have been implicated in schizophrenia (Holt et al., 1999), and a schizophrenic-like disorder is observed in NPC patients. In both cholinergic brain regions sampled, however, there was no significant difference in the number of neurons/section in the NPC−/− vs. NPC+/+ mice (Welch’s t-values < 1.0) (Table 2).
TABLE 2.
Cholinergic Cell Characteristics in the NPC Mouse1
| NPC+/+ |
NPC−/− |
|||
|---|---|---|---|---|
| Brain region | Cell no. | Cell size | Cell no. | Cell size |
| Basal forebrain | 70.7 ± 8.2 | — | 77.7 ± 2.2 | — |
| Striatum | 77.5 ± 9.2 | 15.11 ± 0.36 | 69.0 ± 13 | 16.51 ± 0.24* |
Numbers for basal forebrain and strinatal neurons represent the mean ± SEM number of ChAT-immunostained neurons/20-µm-thick section. In each brain region there were n = 4 mice/group. Cells were counted in sections that were closely matched across brains to represent the same neuroantomical portion of each nucleus. The basal forebrain cells were located within several nuclei including the nucleus accumbens, substantia innominata, and nucleus basalis magnocellularis. Cell size represents the long axis, in µm, of only ChAT-immunostained somata with an unstained nucleus (n = 3/group).
Welch’s t = 3.23 (P = 0.038), NPC+/+ vs. NPC−/− cell size.
There were differences in the morphology of the cholinergic neurons in the NPC mouse. The cholinergic neurons were slightly larger in the NPC−/− animals vs. the NPC+/+ mice (9%; Welch’s t = 3.23, P = 0.038; n = 3/group). Qualitative examination of cholinergic somata in the cerebral cortex indicated that the somata were present in both mouse groups. The ChAT-stained axonal varicosities in the cerebral cortex, however, were often swollen in the NPC−/− mice (n = 4/group) (Fig. 4), as was observed for the Purkinje cell axons.
Fig. 4.
Cholinergic neurons are present in the cerebral cortex, basal forebrain, and striatum of the NPC+/+ and NPC−/− mouse. Cholinergic neurons, immunostained with an antibody against ChAT appear black and are illustrated in the NPC+/+ mouse (A,C,E) and NPC−/− mouse (B,D,F). The distribution of cholinergic neurons is similar in the two mouse types. A,B: Cholinergic nerve terminals in the cerebral cortex are found in both mouse types, but they are often swollen in size in the NPC−/− animals (small arrows). There are also small cholinergic somata in the cortox of both animal types (curved arrows). C,D: Large cholinergic somata are present in the basal fore-brain in both animal types (curved arrows point to one of several such cells). E,F:Largo cholinergic somata are present in the striatum in both animal types (curved arrows point to one of several such cells). The size of the striatal cholinergic neurons is 9% larger in the NPC−/− animal vs. NPC+/+ animal (see Table 2). Scale bar = 20 µm.
Neurofibrillary tangles
Light microscopic study
Histochemical staining was used to determine whether the NPC mice, like NPC humans, exhibit NFTs within the brain. Control tissue from the hippocampus of an Alzheimer’s disease case, a 79-year-old man, was run in parallel with the mouse tissues as a positive control, Thioflavin-S histochemistry (data not shown) revealed numerous plaques and tangles in the Alzheimer’s disease hippocampus but no histochemical staining of NFTs in the mouse (n = 3).
EM studies
The cerebellum from three 5-week-old NPC−/− mice was examined to determine whether the Purkinje cells contained cytoskeletal filamentous abnormalities that are characteristic of NFTs within neurons. In the Purkinje cell soma there was no evidence of NFTs, although the cells contained numerous inclusion bodies (Fig. 5). There were no classical NFTs observed within neurons or glial cells in the cerebellum of the mutant NPC mice. The EM studies revealed only inclusion bodies and membrane swirls within neurons, as has been observed in both NPC human (e.g., Love et al., 1995) and NPC mouse (Tanaka et al., 1988).
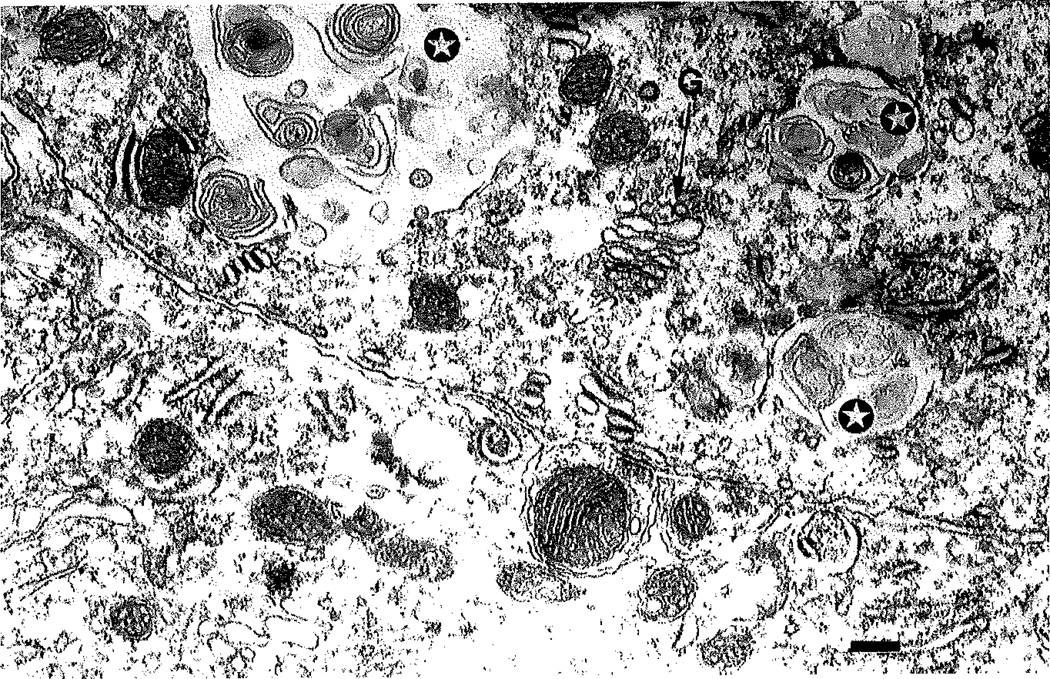
Fig. 5.
Electron micrograph of a Purkinje cell in the NPC−/− mouse. In the cell body of the 5-week-old mouse, there were no NFTs. The soma did, however, contain the characteristic ultrastructural features of NPC disease; inclusion bodies with “myelin figures” inside (stars), Abbreviation: G, Golgi apparatus. Scale bar = 0.27 µm.
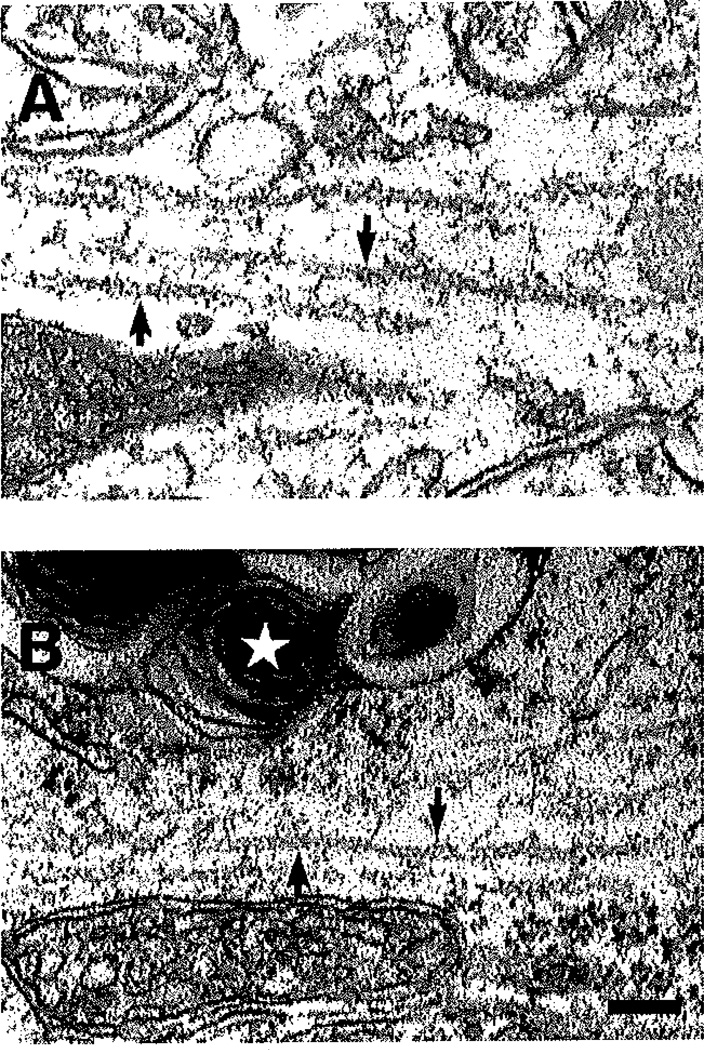
In the prefrontal cortex of three 11-week-old NPC −/− mice, there was no evidence of NFTs. Over 20 neurons were examined in the NPC−/− brain via EM, and no cytoskeletal filamentous abnormalities characteristic of NFTs were found. In both mutant and wild-type control mice, the neurofilaments found within neurons looked similar in density and morphology (Fig. 6).
Fig. 6.
Electron micrographs illustrating neurofilaments in neurons in the prefrontal cortex of the NPC+/+ and NPC−/− mouse. In both NPC+/+ (A) and NPC−/− (B) animals, the neurofilaments (arrows) have a similar appearance. Note the inclusion bodies in the NPC−/− tissue (star indicates one inclusion body with myelin swirl), In no case was an accumulation of filamentous material observed in neurons or glial cells in the NPC−/− brain. Scale bar = 0.12 µm.
DISCUSSION
The present data indicate that both neurons and glial cells are decreased in number in the 11-week-old NPC−/− brain. The 11-week age group represents an advanced age for these mice because shortly thereafter the animals die. The neuronal reductions appear to represent neurodegeneration in the NPC−/− animals and are not the result of a developmental abnormality. Degeneration appears to be taking place because 1) there is an age-related decrease in the number of Purkinje cells; until about 3 weeks of age there is a normal number of Purkinje cells in the NPC−/− mouse, but thereafter the cell numbers decrease (Tanaka et al., 1988; Quintero et al., 2000); 2) there is an age-related reduction in the number of prefrontal cortex and thalamic neurons in the NPC−/− mouse, with normal numbers at 3 weeks of age (Quintero et al., 2000); and 3) apoptosis has been observed within several brain regions of the NPC−/− mouse (Wu et al., 1999).
All neurons do not exhibit equal vulnerability to degeneration in the NPC mouse. By 11 weeks of age, there are low levels of neuron loss in the thalamus and prefrontal cortex. On the other hand, there is a 96% loss of Purkinje cells in the cerebellum. Others have also reported a profound loss of Purkinje cells in the NPC mouse (Morris et al., 1982; Tanaka et al., 1988; Higashi et al., 1993). Tanaka et al. (1988) have performed the only quantitative study of cell loss in the NPC mouse, and they found almost 100% loss of Purkinje cells by 10 weeks of age. It is unclear why Purkinje cells are the most vulnerable neurons to degeneration in the NPC mouse. Purkinje cells are a target for degeneration in several mutant mouse strains; in the case of lurcher, the degeneration has been related to a mutation in. the delta2 glutamate receptor gene (Zuo et al., 1997). This represents the first study to demonstrate degeneration of neurons outside of the cerebellum. Thus, the NPC disease phenotype is like other neurodegenerative diseases, including Parkinson’s and Alzheimer’s disease, in that specific neurons are the target of the disease whereas other neurons are less vulnerable to degeneration.
There is a marked reduction in the number of glial cells in the corpus callosum of the NPC mouse. There are 63% fewer glial cells in the 11-week-old NPC−/− mice compared with the age-matched normal littermates. At 1 week of age there are numerous glial cells in the corpus callosum, and the size of the corpus callosum appears normal in the NPC−/− brain (unpublished observation). Thus, the reduction in glial cell number at 11 weeks of age appears to represent a degenerative process and not a developmental abnormality. The specific type of glial cell that is lost is unclear. However, it is likely that some of the loss is due to a reduction in the number of oligodendrocytes, as this would explain the profound demyelination that occurs with the NPC1 mutation (Weintraub et al., 1987). Astrocytes reacted strongly for GFAP in the NPC−/− animals and appeared in many gray matter areas in addition to the normal localization in white matter areas. There may also be a loss of astroeytes in the corpus callosum. High levels of expression of NPC1 have been reported in astrocytes in the monkey, and astrocytes have been suggested to play a crucial role in the neurodegenerative process (Patel et al., 1999). Because at 3 weeks of age there are 48% fewer glial cells in the corpus callosum in the NPC−/− mouse but only 13% fewer Purkinje cells, the glial cell appears to be the most vulnerable cell type to degeneration in these mice (Quintero et al., 2000).
There was no clear loss of cholinergic neurons in the basal forebrain or striatum in the NPC mouse. In Alzheimer’s disease, there is a loss of basal forebrain cholinergic neurons (Whitehouse et al., 1982) and a reduction in the density of cholinergic nerve terminals in the cortex (Mesulam and Geula, 1988). Although NPC patients also exhibit dementia (e.g., Love et al., 1995), NPC mice do not model the dementia of Alzheimer’s disease because they do not exhibit a reduction in the number of basal forebrain cholinergic neurons. Although schizophrenic brains exhibit reductions in the density of ChAT-immunostained striatal neurons (Holt et al., 1999), the NPC mouse does not model this aspect of schizophrenia as they have a normal density of these neurons. It is interesting, however, that schizophrenic brains have reductions in the number of neurons in specific nuclei of the thalamus (Young et al., 2000; Popken et al., 2000), and the NPC mouse has significant loss of neurons in the thalamus. Additional study is needed to determine whether the thalamic neuron loss in the NPC mouse results in similar circuit alterations as observed in schizophrenia.
NFTs are thought to play a role in neurodegeneration and are present in several degenerative diseases. Diseases that have neurodegeneration and NFTs include NPC disease (Horoupian and Yang, 1978; Love et al., 1995), progressive supranuclear palsy (Steele et al., 1964; Tellez-Nagel and Wisniewski, 1973), postencephalitic parkinsonism (Hallervorden, 1933,1935), myotonic dystrophy (Kiuchi et al., 1991), dementia pugilistica (Corsellis et al., 1973), frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17; Wilhelmsen et al., 1994), and related syndromes (e.g., pallidopontonigral degeneration [Wijker et al, 1996], disinhibition-dementia-parkinsonism-amyotrophy complex [Lynch et al., 1994], and multiple system tauopathy with presenile dementia [Spillantini et al., 1997]). The latter syndromes involve mutations in the tau protein, arguing that tau protein dysfunction by itself may be a cause of neurodegeneration (Hong et al., 2000). Data from the present NPC mouse study, however, indicate that NFTs are not specifically involved in neurodegeneration because there is marked neurodegeneration but no evidence of NFTs.
NFTs have not been found in mouse models of neurodegenerative diseases. In a mouse model of Alzheimer’s disease, for example, in which mutant amyloid precursor protein (APP717) was over expressed, there were β-amyloid-containing neuritic plaques in the cortex and hippocampus, but no NFTs (Games et al., 1995), In humans that express the APP717 mutation, there are both neuritic plaques and NFTs. In other Alzheimer’s disease mouse models that have been examined, there are also neuritic plaques but no NFTs (e.g., Hsiao et al., 1996). It has been suggested that differences between human and mouse tau are such that hyperphosphorylation of tau in the mouse does not produce a tangle (Vickers et al., 1994). Perhaps the mouse cannot make a tangle. In the present study in which EM was used, no evidence of NFTs was found. Thus, the NPC mouse model is like the Alzheimer’s disease mouse models in that some of the neuropathology resembles that found in the human disease but NFTs are not present in either mouse model. When the human tau mutation that is most common in FTDP-17 (P301L) is expressed in transgenic mice, NFTs do occur (Lewis et al., 2000).
Does abnormal cholesterol metabolism play a role in the neurodegenerative process in the NPC mouse? The gene mutation in NPC1 blocks the utilization of cholesterol that comes into the cell via the clathrin-coated pit pathway. In organs like the liver and spleen in both human NPC (Brady, 1983) and NPC mouse (Xie et al., 1999a), there are marked increases in the concentrations of cholesterol. In neurons from NPC mice (Wu et al., 1999) and fibroblasts from NPC humans (Sokol et al., 1988), there are increased concentrations of cholesterol stored in lysosomal-like structures, as indicated in studies by using filipin fluorescence. Whether the increased cellular accumulation of unesterified cholesterol plays a role in neurodegeneration is unclear. There is evidence that increased accumulation of lysosomes causes an increase in lysosomal hydrolases (e.g., cathepsin-D), which can have toxic effects on cells when released from damaged/leaky lysosomal membranes (Nixon and Cataldo, 1995; Deiss et al., 1996; Isahara et al., 1999). In the NPC mouse there are age-related elevations in cathepsin-D in both neurons and microglial cells, with a 10-fold elevation in the number of microglial cells in the molecular/Purkinje cell layer by 11 weeks of age (German et al., 2000). This age-related increase in the number of cathepsin-D-containing microglial cells corresponds to the age-related decrease in the number of Purkinje cells. It has been proposed that microglial activation plays an important role in the neurodegenerative process in another lysosomal storage disease, Sandhoff disease (Wada et al., 2000). Further studies are needed to determine the mechanisms responsible for degeneration caused by the mutation in NPC1.
In conclusion, the NPC1 mutation in the mouse causes degeneration of both neurons and glial cells, but the magnitude of degeneration differs within specific brain regions. In the 11-week-old NPC−/− mouse, which represents the maximum life span of this mouse type, the greatest magnitude of neuronal loss (96%) occurs within the cerebellum in Purkinje cells. In these same mice, however, there is only 20–28% loss of neurons in the prefrontal cortex and entire thalamus. Furthermore, there is a 63% loss of glial cells in the corpus callosum. However, striatal and basal forebrain cholinergic neuron numbers appear normal. Unlike the human NPC disease, in which there is neurodegeneration along with NFTs, there are no NFTs in the NPC mouse brain. These data provide evidence that neurodegeneration is unrelated to the presence of NFT formation. The NPC1 gene mutation causes a defect in intracellular cholesterol trafficking, accumulation of cholesterol in neurons and glial cells, and future research is needed to determine whether and/or how this defect is related to the selective cellular degenerative process.
ACKNOWLEDGMENTS
The authors thank Dr. Charles White for thioflavin-S staining and Ms. Korri Lane and Mr. Farbod Masrour for technical assistance. Secretarial assistance is acknowledged from Mrs. Ronda Lewis.
Grant sponsor: National Institutes of Health; Grant number: R37 HL09610; Grant sponsor: Tho Carl J. and Hortense M. Thomson Chair in Alzheimer’s Disease Research.
LITERATURE CITED
- Auer IA, Schmidt ML, Lee VM-Y, Curry B, Suzuki K, Shin R-W, Pentchev PG, Carstea ED, Trojanowski JQ. Paired helical filament tau (PHFtau) in Niemann-Pick type C disease is similar to PHFtau in Alzheimer’s disease. Acta Neuropathoil. 1995;90:547–551. doi: 10.1007/BF00318566. [DOI] [PubMed] [Google Scholar]
- Blanchelte-Mackie EJ, Dwyer NK, Amende LM, Kruth HS, Butler JD, Sokol J, Comly ME, Vanier MT, August JT, Brady RO, Pentchev PG. Type-C Niemann-Pick disease; low density lipoprotein uptake is associated with premature cholesterol accumulation in the Golgi complex and excessive cholesterol storage in lysosomes. Proc Natl Acad Sci USA. 1988;85:8022–8026. doi: 10.1073/pnas.85.21.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RO. Sphingomyelin lipidosia: Niemann-Pick disease. In: Stanbury JB, Wyngaarden JB, Fredrickson DS, Goldstein JL, Brown MS, editors. The metabolic basis of inherited disease. New York: McGraw-Hill; 1983. pp. 834–841. [Google Scholar]
- Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang T-Y, Liscum L, Strauss JF, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O’Neill RR, van Diggelen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- Corsellis JAN, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. 1973:270–303. doi: 10.1017/s0033291700049588. [DOI] [PubMed] [Google Scholar]
- Deiss LP, Galinka H, Berissi H, Cohen O, Kimchi A. Cathepsin D protease mediates programmed cell death induced by interferon-γ Fas/APO-1 and TNF-α. Eur Mol Biol Org. 1996;15:3861–3870. [PMC free article] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemes J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieborburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- German DC, Ng MC, Liang C-L, McMahon A, Iacopino AM. Calbindin-D28kin nerve cell nuclei. Neuroscience. 1997;81:735–743. doi: 10.1016/s0306-4522(97)00206-6. [DOI] [PubMed] [Google Scholar]
- German DC, Song T, Dietschy JM, Xie C, Yazdani U, Liang C-L. Cathepsin-D is elevated in neurons and microglia in the Niemann-Pick C mouse. Neurosci Abstr. 2000;26:2059. [Google Scholar]
- Gilbert EF, Callahan J, Viseskul C, Opitz JM. Niemann-Pick disease type C. Pathological, histochemical, ultrastructural and biochemical studies. Eur J Pediat. 1981;136:263–274. doi: 10.1007/BF00442993. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Wilson PD. The coefficient of error of optical fractionator population size estimates: a computer simulation comparing three estimators. J Microsc. 1997;192:163–171. doi: 10.1046/j.1365-2818.1998.00417.x. [DOI] [PubMed] [Google Scholar]
- Goedert M, Crowther RA, Spillantini MG. Tau mutations cause frontotemporal dementias. Neuron. 1998;21:955–958. doi: 10.1016/s0896-6273(00)80615-7. [DOI] [PubMed] [Google Scholar]
- Hallervorden J. Zur Pathogenese des Postencephalitischen parkinsonismus. Klin Wehnschr. 1933;12:692–695. [Google Scholar]
- Hallervorden J. Anatomische untersuchungen zur Pathogenese des Postencephalitischen parkinsonismus. Deutsche Ztschr Nervenh. 1935;136:68–77. [Google Scholar]
- Harding AJ, Halliday GM, Cullen K. Practical considerations for the use of the optical disector in estimating neuronal number. J Neurosci Methods. 1994;51:83–89. doi: 10.1016/0165-0270(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M. Genetic dissection of Alzheimer’s disease and related dementias: amyloid and its relationship to tau. Nat Neurosci. 1998;1:355–358. doi: 10.1038/1565. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Murayama S, Pentchev PG, Suzuki K. Cerebellar degeneration in the Niemann-Pick type C mouse. Acta Neuropathol. 1993;85:175–184. doi: 10.1007/BF00227765. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Herman MM, Hyde TM, Kleinmann JE, Sinton CM, German DC, Hersh LB, Graybiel AM, Saper CB. Evidence for a deficit in cholinergic interneurons in the striatum in schizophrenia. Neuroscience. 1999;94:21–31. doi: 10.1016/s0306-4522(99)00279-1. [DOI] [PubMed] [Google Scholar]
- Hong M, Trojanowski JQ, Lee VM-Y. Tau-based neurofibrillary lesions. In: Clark CM, Trojanowski JQ, editors. Neurodegenerative dementias. New York: McGraw-Hill; 2000. pp. 161–175. [Google Scholar]
- Horoupian DS, Yang SS. Paired helical filaments in neurovisceral lipidosis (juvenile dystonic lipidosis) Ann Neurol. 1978;4:404–411. doi: 10.1002/ana.410040504. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, A-beta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Isahara K, Ohsawa Y, Kanamori S, Shibata M, Waguri S, Sato N, Gotow T, Watanabe T, Momoi T, Urase K, Kominami E, Uchiyama Y. Regulation of a novel pathway for cell death by lysosomal aspartic and cysteine proteinases. Neuroscienco. 1999;91:233–249. doi: 10.1016/s0306-4522(98)00566-1. [DOI] [PubMed] [Google Scholar]
- Kiuchi A, Otsuka N, Namba Y, Nakano I, Tomonaga M. Presenile appearance of abundant Alzheimer’s neurofilbrillary tangles without senile plaques in the brain in myotonic dystrophy. Acta Neuropathol (Berl) 1991;82:1–5. doi: 10.1007/BF00310916. [DOI] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegten-horst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Liang C-L, Kozlowski GP, German DC. Leucine5-enkephalin afferents to midbrain dopaminergic neurons: light and electron microscopic examination. J Comp Neurol. 1993;332:269–281. doi: 10.1002/cne.903320302. [DOI] [PubMed] [Google Scholar]
- Loftus SK, Morris JA, Carstea ED, Gu ZL, Cuminings C, Brown A, Ellison J, Ohno K, Rosenfeld MA, Tagle DA, Pentchev PG, Pavan WJ. Murine model of Niemann-Pick C disease: mutation in a cholesterol homcostasis gene. Science. 1997;277:232–235. doi: 10.1126/science.277.5323.232. [DOI] [PubMed] [Google Scholar]
- Love S, Bridges LR, Case CP. Neuroflbrillary tangles in Niemann-Pick disease type C. Brain. 1995;118:119–129. doi: 10.1093/brain/118.1.119. [DOI] [PubMed] [Google Scholar]
- Lynch T, Sano M, Marder KS, Bell KL, Foster NL, Defendini RF, Sima AA, Keohane C, Nygaard TG, Fahn S. Clinical characteristics of a family with chromosome 17-linked disinhibition-dementia-parkinsonism-amyotrophy complex. Neurology. 1994;44:1878–1884. doi: 10.1212/wnl.44.10.1878. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Geula C. Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J Comp Nourol. 1988;275:216–240. doi: 10.1002/cne.902750205. [DOI] [PubMed] [Google Scholar]
- Morris MD, Bhuvaneswaran C, Shio M, Fowler S. Lysosomal lipid storage disorder in NCTR-Balb/C mice 1. Description of the disease and genetics. Am J Pathol. 1982;108:140–149. [PMC free article] [PubMed] [Google Scholar]
- Nagai T, McGeer PL, Peng JH, McGeer EG, Dolman CE. Choline acetyltransferase immunohistochemistry in Alzheimer’s disease patients and controls. Neurosci Lett. 1983;36:195–199. doi: 10.1016/0304-3940(83)90264-1. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Cataldo AM. The endosomal-lysosomal system of neurons: new roles. Trends Neurosci. 1995;18:489–496. doi: 10.1016/0166-2236(95)92772-i. [DOI] [PubMed] [Google Scholar]
- Palmeri S, Battisti C, Federico A, Guzzi GC. Hypoplasia of the corpus callosum in Niemann-Pick type C disease. Neuroradiology. 1994;36:20–22. doi: 10.1007/BF00599187. [DOI] [PubMed] [Google Scholar]
- Patel SC, Suresh S, Kumar U, Hu CY, Cooney A, Blanchette-Mackie EJ, Neufeld EB, Patel RC, Brady RO, Patel YC, Pentchev PG, Ong W-Y. Localization of Niemann-Pick C1 protein in astrocytes: implication for neuronal degeneration in Niemann-Pick type C disease. Proc Natl Acad Sci USA. 1999;96:1657–1662. doi: 10.1073/pnas.96.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed. New York: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Bunney WE, Jr, Potkin SG, Jones EG. Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci USA. 2000;97:9276–9280. doi: 10.1073/pnas.150243397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero EM, Dietschy JM, Xie C, German DC. Neurodegeneration in a cholesterol storage disease: a mouse model of Niemann-Pick C disease. Neuroscience Abstr. 2000;26:2059. [Google Scholar]
- Saper CB, German DC, White CL. Neuronal pathology in the nucleus basalis and associated cell groups in senile dementia of the Alzheimer’s type: possible role in cell loss. Ann Neurol. 1985;35:1089–1095. doi: 10.1212/wnl.35.8.1089. [DOI] [PubMed] [Google Scholar]
- Scheaffer RL, Mendenhall W, Ott L. Elementary survey sampling. 5th ed. Boston: PWS-Kent; 1996. [Google Scholar]
- Schwartz P. Amyloid degeneration and tuberculosis in the aged. Gerontol. 1972;18:321–362. doi: 10.1159/000211943. [DOI] [PubMed] [Google Scholar]
- Sokol J, Blanchette-Mackie EJ, Kruth HS, Dwyer NK, Amende LM, Butler JD, Robinson E, Patel S, Brady RO, Comly ME. Type C Niemann-Pick disease: lysosomal accumulation and defective intracellular mobilization of low density lipoprotein cholesterol. J Biol Chom. 1988;263:3411–3417. [PubMed] [Google Scholar]
- Spillantini MG, Goedert M, Crowther RA, Murrell JR, Farlow MR, Ghetti B. Familial multiple system taupathy with presenile dementia: a disease with abundant neuronal and glial tau filaments. Proc Natl Acad Sci USA. 1997;94:4113–4118. doi: 10.1073/pnas.94.8.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy. Arch Neurol. 1964;10:333–359. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Nakamura H, Miyawaki S. Cerebellar involvement in murine sphingomyelinosis: a new model of Niemann-Pick disease. J Neuropathol Exp Neurol. 1988;47:291–300. doi: 10.1097/00005072-198805000-00008. [DOI] [PubMed] [Google Scholar]
- Tellez-Nagel I, Wisniewski HM. Ultrastructure of neurofibrillary tangles in Steele-Richardson-Olszowski syndrome. Arch Neurol. 1973;29:324–327. doi: 10.1001/archneur.1973.00490290064007. [DOI] [PubMed] [Google Scholar]
- Turpin JC, Masson M, Baumann N. Clinical aspects of Niemann-Pick type C disease in the adult (Review) Dev Neurosci. 1991;13:304–306. doi: 10.1159/000112177. [DOI] [PubMed] [Google Scholar]
- Vickers JC, Morrison JH, Friedrich VL, Elder GA, Perl DP, Katz RN, Lazzarini RA. Age-associated and cell-type-specific neurofibrillary pathology in transgenic mice expressing the human midsized neurofibrillary subunit. J Neurosci. 1994;14:5603–5612. doi: 10.1523/JNEUROSCI.14-09-05603.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada R, Tifft CJ, Proia RL. Microglial activation precedes acute neurodogeneration in Sandhoff disease and is suppressed by bone marrow transplantation. Proc Natl Acad Sci USA. 2000;97:10954–10959. doi: 10.1073/pnas.97.20.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H, Abramovici A, Sandbank U, Booth AD, Pectchev PG, Sela B. Dysmyelination in NCTR-Balb/C mouse mutant with a lysosomal storage disorder. Ada Neuropathol. 1987;74:374–381. doi: 10.1007/BF00687215. [DOI] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle LT, DeLong MR. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Wijker M, Wszolek ZK, Wolters EC, Rooimans MA, Pals G, Pfeiffer RF, Lynch T, Rodnitzky RL, Wilhelmsen KC, Armert F. Localization of the gene for rapidly progressive autosomal dominant parkinsonism and dementia with pallido-ponto-nigral degeneration to chromosome 17q21. Hum Mol Genet. 1996;5:151–154. doi: 10.1093/hmg/5.1.151. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen KC, Lynch T, Pavlou E, Higgins M, Nygaard TG. Localization of disinhibition-dementia-parkinsonism-amytrophy complex to 17q21-22. Am J Hum Genet. 1994;55:1159–1165. [PMC free article] [PubMed] [Google Scholar]
- Wu Y-P, Kubota A, Suzuki K. Neuronal death and reactive glial changes in the brain of the Niemann-Pick disease type C mouse. Neurosci Abstr. 1999;25:1118. [Google Scholar]
- Young KA, Manaye KF, Liang C-L, Hicks PB, German DC. Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biol Psychiatry. 2000;47:944–953. doi: 10.1016/s0006-3223(00)00826-x. [DOI] [PubMed] [Google Scholar]
- Xie C, Turley SD, Pentchev PG, Dietschy JM. Cholesterol balance and metabolism in mice with loss of function of Niemann-Pick C protein. Am J Physiol. 1999a;276:E336–E344. doi: 10.1152/ajpendo.1999.276.2.E336. [DOI] [PubMed] [Google Scholar]
- Xie C, Turley SD, Dietschy JM. Cholesterol accumulation in tissues of the Niemann-Pick type C mouse is determined by the rate of lipoprotein-cholesterol uptake through the coated-pit pathway in each organ. Proc Natl Acad Sci USA. 1999b;96:11992–11997. doi: 10.1073/pnas.96.21.11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Turley SD, Dietschy JM. Centripetal cholesterol flow from the extrahepatic organs through the liver is normal in mice with mutated Niemann-Pick type C protein (NPC1) J Lipid Res. 2000;41:1278–1289. [PubMed] [Google Scholar]
- Zuo J, DeJager PL, Takahashi KA, Jiang W, Linden DJ, Heintz N. Neurodegeneration in Lurcher mice caused by mutation in delta2 glutamate receptor gene. Nature. 1997;388:769–773. doi: 10.1038/42009. [DOI] [PubMed] [Google Scholar]