Summary
Neurotrophin signaling is crucial for neuron growth. While “signaling endosomes” hypothesis is one of accepted models, the molecular machinery that drives retrograde axonal transport of TrkB signaling endosomes is largely unknown. In particular, mechanisms recruiting dynein to TrkB signaling endosomes have not been elucidated. Here, using snapin deficient mice and gene rescue experiments combined with compartmentalized cultures of live cortical neurons, we reveal that Snapin, as a dynein adaptor, mediates retrograde axonal transport of TrkB signaling endosomes. Such a role is essential for dendritic growth of cortical neurons. Deleting snapin or disrupting Snapin-dynein interaction abolishes TrkB retrograde transport, impairs BDNF-induced retrograde signaling from axonal terminals to the nucleus, and decreases dendritic growth. Such defects were rescued by reintroducing snapin gene. Our study indicates that Snapin-dynein coupling is one of the primary mechanisms driving BDNF-TrkB retrograde transport, thus providing new mechanistic insights into the regulation of neuronal growth and survival.
Keywords: BDNF, compartmentalized cultures, cortical neurons, dendritic growth, dynein motor, dynein-cargo linkage, late endosome, motor adaptor, retrograde signaling, retrograde transport, signaling endosome, Snapin, TrkB
INTRODUCTION
During neuronal development, the density of dendritic arborization determine the number of synaptic inputs, and are thus critical for assembling the central nervous system (CNS) (see review by Jan and Jan, 2010). Many lines of evidence indicate that the neurotrophic signaling pathway from axonal terminals to cell bodies is crucial for dendrite growth and neuron survival (see reviews by Cosker et al., 2008; Ginty and Segal, 2002; Howe and Mobley, 2005). The neurotrophin family, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin 3 or 4/5 (NT-3, NT-4/5), are mainly produced in post-synaptic cells and initiate signals at axonal terminals by binding to their receptor tyrosine kinases (Trks); NGF binds to TrkA, BDNF and NT4/5 to TrkB, and NT3 to TrkA-C (see reviews by Chao, 2003; Huang and Reichardt, 2001; Segal, 2003). Ligand binding induces Trk dimerization and phosphorylation, triggers the internalization of ligand-receptor complexes into signaling endosomes, and activates signal transduction cascades, ultimately leading to retrograde signaling and gene transcription in the nucleus (Lemmon and Schlessinger, 2010; Sorkin and von Zastrow, 2009; Zweifel et al., 2005).
BDNF is one of the well-studied neurotrophic factors regulating dendrite outgrowth and branching (Cheung et al., 2007; Dijkhuizen and Ghosh, 2005; Horch et al., 1999; McAllister et al., 1997). A previous study in dorsal root ganglion (DRG) neurons showed that TrkB-GFP is expressed in “signaling endosomes” undergoing axonal retrograde transport and this relocation is required for nuclear signaling responses (Heerssen et al., 2004). However, it remains unclear whether retrograde axonal transport of TrkB signaling endosomes has a direct impact on dendritic growth in cultured CNS neurons. Cytoplasmic dynein motors are responsible for retrograde transport in axons and dynein-cargo linkage via an adaptor complex may be a general mechanism in neurons (Hirokawa et al., 2010). A long-standing question is how activated BDNF-TrkB signaling complexes are delivered from axonal terminals to cell bodies. Thus, a major challenge has been to determine how dynein motors are recruited to TrkB signaling endosomes. In the current study, we reveal that Snapin acts as a dynein adaptor by binding to dynein intermediate chain (DIC) and mediates retrograde axonal transport of TrkB signaling endosomes. Deleting snapin or disrupting the Snapin-DIC interaction abolishes TrkB retrograde transport, impairs BDNF-induced signaling pathway from axonal terminals to the nucleus, and reduces dendritic growth in cortical neurons. Such defects were effectively rescued by reintroducing the snapin gene into mutant neurons. Thus, our study reveals the motor-adaptor machinery that is essential for retrograde transport of TrkB signaling endosomes.
RESULTS
Retrograde Axonal TrkB Transport Impacts Dendritic Growth of Cortical Neurons
We first examined whether dendritic growth is correlated with the mobility of axonal TrkB signaling endosomes in the same cortical neurons co-expressing GFP and TrkB-RFP at DIV7. Mobility data was acquired by time-lapse imaging and converted to kymographs. Cortical neurons with enriched primary dendrites display predominant retrograde movement of TrkB endosomes along axonal processes (Figure S1A). However, cortical neurons with relatively few primary dendrites show less mobility of TrkB endosomes where the majority of them remain stationary (Figure S1B). Pearson correlation analysis revealed a significant positive correlation between the retrograde mobility of TrkB signaling endosomes and the total dendritic length (p<0.001) or tip number (p<0.019) of the same neurons (Figure S1C). Conversely, there is a significant negative correlation between the number of stationary TrkB endosomes and total dendritic length (p<0.002) (Figure S1D). No correlation was found between dendritic length and anterograde transport (p=0.902) or retrograde mean velocity (p=0.178) of TrkB signaling endosomes along the same axons (Figure S1D). As a control, dendritic growth was not correlated with axonal mitochondrial mobility (Figure S1E), thus excluding the possibility for general axonal transport defects in neurons with less dendritic branches. These results provide cellular evidence that axonal TrkB retrograde trafficking may play a critical role in supporting dendritic growth of CNS neurons.
Snapin and DIC Associate with TrkB Signaling Endosomes
To determine whether TrkB is expressed in Rab7-labeled endosomes undergoing retrograde transport, we co-expressed TrkB-RFP with the endosomal marker YFP-Rab7 in cortical neurons followed by time-lapse imaging at DIV7. In axons, the majority of TrkB-RFP signals co-localizes with Rab7-YFP as vesicular structures and co-migrates from the distal axon toward the soma (Figure 1A and Movie S1). Thus, TrkB-containing vesicles in axons are specialized late endocytic organelles predominantly undergoing retrograde transport. In contrast, a significant number of dendritic TrkB is not co-localized with Rab7 and instead, undergoes anterograde transport from the soma to dendrites (Movie S2).
Figure 1. Snapin and DIC Associate with Rab7-labeled TrkB Signaling Endosomes.
(A) Co-localization and co-retrograde transport of Rab7 and TrkB along axonal processes. Cortical neurons were co-transfected with YFP-Rab7 and TrkB-RFP at DIV5, followed by time-lapse imaging at DIV7. The axon image was taken ~100 μm away from the cell body. Note that YFP-Rab7 (Green) and TrkB-RFP (Red) co-localize as vesicular structures (white arrows) and co-migrate towards the soma. Mobile vesicles are labeled as “1” or “2” during the 110 second recording period. (See also Movie S1 and S2).
(B–D) Both Snapin and DIC associate with Rab7- and TrkB-labeled signaling endosomes. Membranous organelles were immunoisolated with Dynal magnetic beads coated with an antibody against Trk (C-14) (B), Snapin (C), or DIC (D), followed by sequential immunoblotting on the same membrane after stripping between each antibody application. Note that TrkB, Snapin, and DIC all associate with Rab7-labeled endocytic organelles, while any association with the Golgi marker P115 is undetectable. pErk1/2 also associates with membrane organelles containing Snapin and DIC.
(E) Reduced recruitment of the dynein DIC to the TrkB-signaling endosomes in snapin−/− brains. Representative blots of three repeats showing immunoisolation of dynein-associated membranous organelles (left) and Trk signaling endosomes (right) from mouse brain light membrane fractions. The same membranes (in black boxes) were sequentially immunoblotted with antibodies as indicated. Note that deleting snapin results in a reduced attachment of DIC with the activated signaling endosomes containing pan-Trk, TrkB, pErk1/2, and pCREB.
Next, we examined whether dynein motors associate with TrkB signaling endosomes. One of the approaches in literature in identifying Trk signaling endosomes was achieved by using subcellular fractionation, in which Trk-containing membranous compartments are separated into various density fractions (Yano and Chao, 2004). Instead, we combined density fractionation and immunoisolation of TrkB membrane organelles from mouse brain light membrane fractions using magnetic beads coated with an anti-Trk antibody. Immunoblot analysis of purified membrane organelles showed that dynein DIC was detected with TrkB and pErk1/2, a Trk-activated signaling kinase, along with endosomal markers Rab7 and EEA1 (Figure 1B). Interestingly, Snapin was also detected in the purified Trk membrane organelles. To confirm this observation, we alternatively immunoisolated Snapin or DIC-associated membrane organelles. Consistently, both Snapin and DIC associate with the signaling organelles containing TrkB, Rab7, and pErk1/2 (Figures 1C and 1D).
Our previous study revealed that Snapin recruits the dynein motor to the late endocytic membrane via the Snapin–DIC linkage and contributes to retrograde transport of late endocytic organelles in neurons (Cai et al., 2010). Given that signaling endosomes are specialized late endosomes undergoing retrograde transport, we asked whether Snapin is also required to recruit dynein to TrkB signaling endosomes. To test this hypothesis, we immunoisolated dynein-associated membranous organelles and Trk signaling endosomes from snapin+/+ and snapin−/− mouse brain with Dynal magnetic beads coated with an antibody against dynein DIC or pan-Trk. Deleting snapin results in a reduced attachment of DIC with the signaling endosomes containing pan-Trk, TrkB, pErk1/2, and pCREB, the phospho-cyclic AMP-response element binding protein (Figure 1E), suggesting a critical role of Snapin in coupling dynein motors with activated TrkB signaling endosomes.
Snapin-DIC Interaction Mediates TrkB Retrograde Axonal Transport
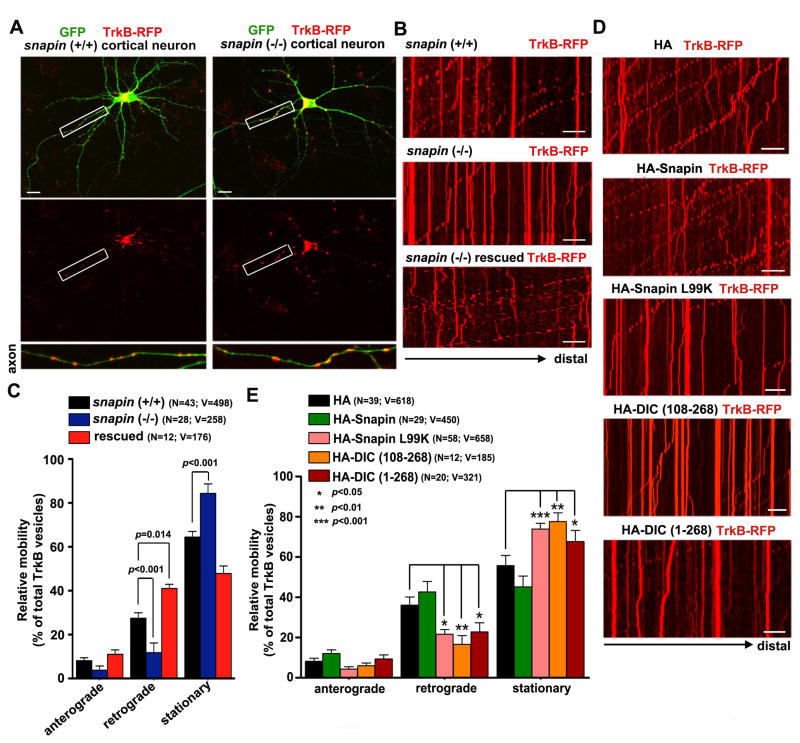
We next asked whether Snapin, as a dynein adaptor, could mediate retrograde axonal transport of TrkB signaling endosomes in cortical neurons. Snapin−/− mice provide us with a genetic tool to address this question. We examined the distribution and transport of TrkB signaling endosomes in both snapin+/+ and snapin−/− neurons expressing GFP and TrkB-RFP at DIV7. In snapin+/+ neurons, TrkB appeared as small, fine vesicular structures evenly distributed along processes. In contrast, it was clustered as large vesicular structures along snapin−/− processes (Figure 2A). We then performed time-lapse imaging to assess the relative mobility of TrkB signaling endosomes along axons. As we previously reported (Cai et al., 2010; Kang et al., 2008), axons were distinguished from dendrites based upon known morphological characteristics: greater length, thin and uniform diameter, and sparse branching. This was further confirmed by immunostaining with an anti-MAP2 antibody (Figure S2). In wild-type neurons, mobile TrkB endosomes undergo predominantly retrograde transport towards the soma (of the total 35%mobile, 8.08 ± 0.67% for anterograde and 27.43 ± 1.26% for retrograde, n = 43, mean ± SEM) (Figures 2B and 2C, Movie S3). In contrast, snapin deletion reduced retrograde transport to 11.93 ± 1.97% (n = 28, p<0.001) and thus relatively increased their ratio in the stationary phase (Movie S4). Re-introducing the snapin transgene into snapin−/− neurons not only rescued the defective retrograde transport of TrkB, but also further enhanced their retrograde transport ratio relative to snapin+/+ neurons (anterograde: 11.08 ± 1.00%, retrograde: 41.06 ± 0.85%, n = 12, p=0.014) (Figures 2B and 2C, Movie S5). Thus, elevated Snapin expression recruits more stationary TrkB endosomes into the retrograde mobile pool.
Figure 2. Snapin-DIC Interaction Mediates TrkB Retrograde Axonal Transport.
(A) Representative images showing distribution patterns of TrkB signaling endosomes in snapin+/+ and snapin−/− cortical neurons expressing with GFP and TrkB-RFP at DIV7. Lower panels are close-up views of the boxed axonal processes. TrkB signaling endosomes in snapin−/− neurons appear in large clusters along processes.
(B, C) Impaired retrograde transport of TrkB signaling endosomes in snapin−/− cortical neurons. Kymographs (B) and quantitative analysis (C) showing relative mobility of TrkB signaling endosomes during 6-min recordings of axons of snapin+/+, snapin−/−, and rescued neurons. In kymographs, vertical lines represent stationary organelles; oblique lines or curves to the left represent retrograde transport. Note that reintroducing snapin into snapin−/− neurons (rescued) effectively recruits stationary organelles into the retrograde mobile pool. (See also Movies S3–S5)
(D, E) Disrupting the Snapin-DIC interaction inhibits axonal retrograde transport of TrkB signaling endosomes. Kymographs (D) and relative mobility (E) of TrkB signaling endosomes during 6-min recordings in axons of wild-type neurons co-expressing TrkB-RFP with Snapin, or with Snapin mutant (L99K), or with DIC-truncated mutants (DIC108–268 or DIC 1–268). Cortical neurons were co-transfected at DIV5 and time-lapse images were taken at DIV7. Relative mobility is expressed as a percentage of total TrkB vesicles and compared with data from neurons expressing an HA vehicle vector. (See also Movies S6–S9).
Data were quantified from the total number of TrkB signaling endosomes (V) from the total number of neurons examined (N) in > 3 experiments as indicated in parentheses. Scale bars: 10 μm. Error bars: SEM. Student’s t test.
To determine whether Snapin-mediated TrkB transport occurs through its binding to dynein DIC, we expressed Snapin and DIC mutants in wild-type neurons including Snapin-L99K, a DIC-binding defective mutant by replacing a single hydrophobic residue with lysine, and DIC (108–268) and DIC (1–268), truncated DIC mutants containing the Snapin-binding domain. These transgenes act as dominant-negative mutants by effectively disrupting the endogenous Snapin-DIC interaction and impairing retrograde transport of late endosomes in cortical neurons (Cai et al., 2010). Consistently, expressing these transgenes in wild-type neurons reduced TrkB retrograde transport by recruiting more signaling endosomes into the stationary pool (Figures 2D and 2E, Movies S6–S9). These phenotypes mimic what were found in snapin−/− neurons (Figures 2B and 2C), suggesting a dominant-negative effect by disrupting the Snapin-DIC interaction. Altogether, the live imaging analysis provides compelling evidence that the Snapin-DIC interaction is necessary for dynein-driven retrograde transport of TrkB signaling endosomes.
Deleting snapin Impairs BDNF-Induced TrkB Retrograde Signaling
The phosphorylation at Ser133 of CREB is critical for controlling gene activation (Bito et al., 1996; Li et al., 2009), and pCREB is relocated to the nucleus following brief BDNF stimulation at distal axons of DRG neurons (Watson et al., 2001). pCREB-dependent transcription is necessary for regulation of dendritic morphology and development (Redmond et al., 2002; Wayman et al., 2006). A previous report revealed an anterograde pathway that conveys BDNF signals from the dendrite to the nucleus for synaptic plasticity although proposed signaling endosomes were not identified (Cohen et al., 2011). This BDNF anterograde dendrite-to-nucleus signaling pathway induces the expression of the immediate early genes Arc and c-Fos, which is not found in the BDNF retrograde axon-to-nucleus pathway. To address whether Snapin is required for BDNF-induced retrograde signaling from axonal terminals to the nucleus, we examined the relative intensity of nuclear pCREB in a microfluidic chamber system with a longer micro-channels (450 μm). This chamber system allows better spatial isolation of distal axons from somas/dendrites, thus eliminating the dendrite-to-nucleus signaling. Snapin+/+ and snapin−/− cortical neurons were plated in the soma chamber; somas and dendrites stained with MAP2 are mainly restricted in the soma chamber while axons labeled by Tau grow into the axonal terminal chamber through the micro-channels (Figure 3A). Neurons were treated with 25 ng/ml BSA as control or 25 ng/ml BDNF at the terminal chamber for 5 min followed by fixation and co-staining with MAP2 and phospho-specific pCREB. Under BSA treatment, both snapin+/+ and snapin−/− cortical neurons showed low basal levels of nuclear pCREB signals. Brief treatment of snapin+/+ axon terminals with BDNF robustly increases the mean intensity of nuclear pCREB signals. Deleting snapin abolishes enhancement of nuclear pCREB staining following BDNF stimulation at axon terminals (Figures 3B and 3C). The relative frequency distribution of nuclear pCREB signal intensity in snapin+/+ cortical neurons was shifted toward the right following the brief BDNF induction (Figure 3D, left). However, a similar shift was not observed in snapin−/− cortical neurons upon the same treatment (Figure 3D, right). Normalized mean intensity of nuclear pCREB signals was substantially increased in snapin+/+ neurons (1.691 ± 0.027, n=499, p<0.001) relative to that in snapin−/− somas (1.209 ± 0.029, n=425)(Figure 3E), suggesting impaired BDNF-induced retrograde signaling from axon terminals to the nucleus in snapin deficient cortical neurons.
Figure 3. Microfluidic Analysis Showing Impaired Axon Terminal BDNF-Induced Retrograde Signaling in snapin−/− Cortical Neurons.
(A) Representative images showing the microfluidic chamber system for the spatial isolation of axonal terminals from somas/dendrites in cultures. Mouse cortical neurons are plated in the soma chamber. Dendrites labeled by MAP2 are restricted in the soma chamber or proximal micro-channels (white arrows) while axons labeled by Tau (red) grow into the axon terminal chamber through the micro-channels.
(B, C) Deleting snapin reduces the BDNF-induced nuclear targeting of pCREB. Representative images of snapin+/+ (B) or snapin−/− (C) cortical neurons grown in the microfluidic chambers at DIV5. Neurons were treated with 25 ng/ml BSA or 25 ng/ml BDNF at the terminal chamber for 5 min followed by fixation and co-staining for MAP2 and pCREB. Yellow lines separate the chambers of somas/dendrites from micro-channels (axonal terminal chambers are not shown). Note that brief treatment of axonal terminals with BDNF substantially increases the intensity of nuclear pCREB signals in snapin+/+ somas, but only slightly increases in snapin−/− somas. Only those cultures where MAP2 did not pass through the micro-channels were selected for quantitative analysis.
(D, E) Frequency distribution of pCREB signal intensity in somas of snapin+/+ (D, left) or snapin−/− (D, right) cortical neurons and normalized pCREB mean intensity (E) following incubation of axonal terminals with BSA or BDNF. The total number of neurons examined in > 3 experiments as indicated in parentheses (D) or in bar graphs (E). The intensity of nuclear pCREB was analyzed using Image J. Scale bars in B and C: 50μm. Error bars: SEM (Student’s t test).
Deleting Snapin or Disrupting Snapin-DIC Coupling Reduces Dendritic Growth
BDNF regulates the dendritic growth and morphology in cortical neurons (McAllister et al., 1997). Given the snapin deficient phenotypes, we asked whether snapin deficiency has any influence on the growth of cortical neurons from snapin+/+ and snapin−/− mice (Figure 4A). Dendritic and axonal morphology at DIV7 was visualized by expressing GFP and confirmed by MAP2 staining. Deleting snapin substantially reduces total dendritic length, tip number, and total axonal length (Figure 4B), and slows the time course of dendritic growth from DIV1 to DIV7 (Figure 4C). Reintroducing the snapin gene into snapin−/− cortical neurons rescues dendritic growth defects (Figure 4D). The reduced axonal length in snapin−/− cortical neurons (Figure 4B) also suggests an important role of the retrograde signaling for axonal growth, which is consistent with previous studies showing that activated signaling effectors, including pCREB, contribute to axonal extension (Lonze et al., 2002).
Figure 4. Deleting Snapin or Disrupting Snapin-DIC Coupling in Cortical Neurons Impairs Dendritic Growth.
(A) Representative images showing growth of dendrites and axons at DIV7 in snapin+/+ (left), snapin−/− (middle), or rescued (right) snapin−/− cortical neurons. Neuron and dendritic morphology was visualized by expressing GFP and confirmed by MAP2 staining.
(B–E) Quantitative analysis of total dendritic length, tip number, and axonal length (B), the time course of dendritic growth in both snapin+/+ and snapin−/− cortical neurons from DIV1 to DIV7 (C), total dendritic length of snapin−/− cortical neurons rescued by expressing HA-Snapin (D) and following BDNF treatment (E). For measuring axonal length, cortical neurons were fixed at DIV2 and stained by Tau-1. Total dendritic length, tip number, and axonal length are normalized to the data from wild-type neurons. For rescue experiments, cortical neurons were transfected with HA or HA-Snapin at DIV4, followed by MAP2 staining at DIV7.
(F–H) Snapin-DIC interaction is required for proper dendrite growth in cortical neurons. Representative images (F) and quantitative analysis showing dendrite growth in wild-type cortical neurons expressing various Snapin and DIC dominant-negative mutants without or with brief BDNF stimulation. Cortical neurons at DIV4 were co-transfected with GFP and HA, HA-Snapin, or Snapin-L99K, Snapin-C66A, or HA-DIC (108–268), followed by MAP2 staining at DIV7. The dendrite growth is expressed as total dendritic length and dendritic tip number normalized to that from neurons expressing the HA control. Quantification is from the number of neurons (indicated in parentheses within bars) from >3 independent experiments. Scale bars: 100 μm. Error bars: SEM. Student’s t test.
Next, we examined dendritic growth in both snapin+/+ and snapin−/− cortical neurons before and after BDNF treatment. Although applying BDNF could slightly increase dendritic growth in snapin−/− neurons (normalized total dendrite length: 1.161 ± 0.070), it failed to fully rescue the mutant neurons to the levels observed in snapin+/+ neurons following BDNF induction (2.035 ± 0.124, p<0.001). In addition, we disrupted the Snapin-DIC interaction in wild-type neurons by expressing the binding defective mutant Snapin-L99K or DIC (108–268) or Snapin-C66A, a mutation that has normal binding to DIC but defective binding to SNAP25, thus impairing synaptic vesicle release. Cortical neurons expressing Snapin-L99K (p=0.037 and p=0.021) or DIC (108–268) (p=0.001 and p=0.019), but not Snapin or its C66A mutant, display reduced dendritic length and tip numbers compared to neurons expressing the vehicle vector HA (Figures 4F and 4G). Furthermore, we treated the transfected neurons with BDNF. Altered neuronal morphology in those neurons cannot be rescued by applying BDNF in cultures (p<0.01) (Figure 4H), suggesting an impaired BDNF retrograde signaling pathway. Thus, using different Snapin loss-of-function mutants combined with BDNF application suggests that snapin deficiency reduces cortical neuron growth by impairing BDNF retrograde signaling, rather than being attributed to other defects in synaptic vesicle release (Pan et al., 2009) and lysosomal maturation (Cai et al., 2010) observed in snapin−/− neurons.
DISCUSSION
Axonal Trk transport was better studied in the peripheral nerve system (PNS). Studies in compartmentalized cultures of sensory and sympathetic neurons provide substantial evidence in support of the ‘signaling endosome model’ for NGF-TrkA retrograde transport (Cosker et al., 2008; Deinhardt et al., 2006; Valdez et al., 2007). Those signaling endosomes undergoing retrograde transport were visualized with quantum dot labeled-NGF (QD-NGF) (Cui et al., 2007), and are essential for the survival of superior cervical ganglia neurons (Ye et al., 2003). Furthermore, signaling endosomes containing NGF, TrkA, and activated signaling complexes including Rap1/Erk1/2, p38MAPK, and PI3K/Akt are found in mature DRG neurons and retrogradely transported in the isolated sciatic nerve (Bhattacharyya et al., 2002; Delcroix et al., 2003). Despite extensive studies in the past decade, there is no general agreement as to how activated BDNF-TrkB complexes are delivered from axonal terminals to the soma for retrograde signaling in the CNS. In particular, the identity of the activated “TrkB endosomal” cargos and adaptor linking the dynein motor to these cargos has not been identified.
Our current study provides mechanistic insights into the motor-adaptor machinery that drives the retrograde transport of TrkB signaling endosomes. Snapin acts as an adaptor recruiting the dynein motor to TrkB signaling endosomes via binding to DIC (Figure S4). Snapin-mediated and dynein-driven retrograde transport is essential to delivery activated BDNF-TrkB signaling complexes from axonal terminals to somas, and thus, is critical for dendritic growth of cortical neurons in vitro. Such a mechanism enables neurons to maintain an efficient response to neurotrophic factors at distal terminals. In snapin-deficient neurons, the recruitment of dynein motors to TrkB signaling endosomes was impaired (Figure 1) resulting in the following three major defects: (1) fewer TrkB endosomes were transported from distal processes toward the soma (Figure 2); (2) the efficacy of BDNF-induced retrograde signaling in the nucleus was reduced (Figure 3); and (3) dendritic growth of cortical neurons was decreased (Figure 4). These phenotypes could be effectively rescued by re-introducing Snapin transgene but not its mutant defective in DIC binding. The proposed Snapin-DIC mechanism was further tested in wild-type neurons expressing dominant-negative Snapin mutant. Disrupting Snapin-DIC interaction immobilizes TrkB signaling endosomes (Figure 2D and 2E) and reduces dendrite growth of cortical neurons in culture (Figure 4G–H). Therefore, Snapin-DIC coupling is one of the primary mechanisms mediating BDNF-TrkB retrograde signaling in cortical neurons. Although alternative models may exist (Ginty and Segal, 2002; Howe and Mobley, 2005), it is widely accepted that the dynein-driven retrograde transport is required for many, if not most, aspects of retrograde signaling mechanisms. Thus, variations in dendritic growth in culture may, at least in part, reflect the relative retrograde transport of TrkB signaling endosomes in those neurons (Figure S1).
Snapin is expressed in mouse hippocampus and cortex during the early developmental stage (E15.5) (PubMed, GENSAT). Snapin-null mice exhibit neonatal death and embryonic snapin−/− mouse brains show reduced cortical plates and intermediate zone cell density (Zhou et al., 2011). In addition, snapin deficiency reduces viability of mature snapin−/− cortical neurons in high-density culture (Cai et al., 2010). Reduced viability, developmental defects, and impaired neurotrophic signaling in snapin-deficient neurons may be attributed to the embryonic lethal phenotype of snapin-null mice. NGF-TrkA signaling pathway is essential for neuronal survival (Heerssen et al., 2004). TrkB, unlike TrkA and TrkC, does not trigger neuronal death (Nikoletopoulou et al., 2010). While BDNF is the most widely expressed neurotrophin in the CNS, elimination of BDNF in mice affects dendrite growth in some specific CNS areas (Rauskolb et al., 2010). Snapin mutant mice display reduced association of dynein motors with endosomes immuno-isolated with an anti-pan-Trk antibody. Thus, we cannot exclude the possibility that Snapin may also recruit dynein motors to TrkA or TrkC signaling endosomes for retrograde transport essential for neuronal survival and development of the CNS.
We also analyzed dendritic arborization in snapin+/− mice using the GolgiStain method. Analysis of node numbers and average dendritic length shows no significant changes in dendritic growth of pyramidal neurons and striatal medium-sized spiny neurons (data not shown). This suggests two possibilities. First, levels of Snapin expression in snapin+/− mouse are sufficient to maintain proper retrograde transport of activated BDNF-TrkB signaling endosomes, which is further supported that the snapin+/− mouse develops normally with no detectable defects. Second, Snapin-DIC coupling is only one of several mechanisms mediating BDNF retrograde signaling in the CNS. Deficiency in this pathway may be complementarily rescued by other retrograde signaling mechanisms. Future studies using snapin conditional KO mice will help clarify this issue.
Our findings are consistent with a previous study showing abnormal dendritic growth in neurons with a mutation in dynein DIC (Satoh et al., 2008). Dynein motors are composed of two heavy chains (DHC), several light intermediate chains (DLIC), light chains (DLC), and DIC, While DHC functions as a motor domain, various other chains likely mediate the regulation or association of dynein with transported cargos (Karki and Holzbaur, 1999). TrkA co-localizes with the dynein complex (Bhattacharyya et al., 2002), and TrkB associates with DIC/DILC/DLC in brain lysates (Ha et al., 2008; Yano et al., 2001). However, it is not known whether this association serves as a dynein-recruiting mechanism and whether interrupting this coupling impairs TrkB retrograde transport and BDNF-induced nuclear signaling. In the current study, we failed to detect a native TrkB-DIC complex in brain homogenates under our experimental conditions that consistently allowed us to detect relatively enriched native Snapin-DIC complexes in brain homogenates (Figure S3). Our study consistently demonstrates that Snapin-DIC interaction is required for recruiting dynein motors to TrkB signaling endosomes. In both snapin-null and wild-type neurons expressing Snapin mutants defective in DIC-binding exhibit impaired BDNF retrograde signaling and dendritic growth in cortical neurons. Thus, our study reveals a new cellular pathway in regulating neurotrophic signaling and neuronal growth.
EXPERIMENTAL PROCEDURES
Mouse cortical neuron cultures were prepared from E17–18 mouse embryos as described (Cai et al., 2010). For dendrite growth analysis, neurons were co-transfected with pEGFP and various DNA constructs at DIV5 followed by imaging analysis at DIV7. For immunostaining, cultured neurons were fixed with 4% paraformaldehyde and 4% sucrose followed by incubation with antibodies. Neuron images were acquired on a Zeiss LSM 510 confocal microscope with a 40×Oil immersion objective by sequential-acquisition. For morphological analysis, z-stack (5–8 optical sections) images were acquired by using the same settings at 1-μm intervals at a resolution of 1,024×1,024 pixels (8 bit). All images were acquired below saturation while clearly labeling the dendritic arbor. The morphometric measurements were performed using Neurolucida software. For time-lapse imaging analysis, neurons were plated at 6×104/cm2 upon the astrocyte monolayer. TrkB-RFP and YFP-Rab7 were co-transfected at DIV5 and incubated for an additional 48 hr. Neurons were incubated in a heated-confocal living cell incubator for at least 10 min before imaging with a 40× oil immersion objective lens (N.A 1.3) on an LSM 510 Zeiss confocal microscope. Lightly TrkB-RFP expressing neurons were selected for analysis of signaling endosome transport. Due to distinct microtubule organization in axons and dendrites, only axonal processes were selected for mobility analysis of signaling endosomes. The proximal axonal regions (~100 μm) from isolated soma were selected to determine transport direction. Axonal processes were further confirmed by retrospective MAP2 immunostaining. The microfluidic devices with 450-μm micro-channels (Xona) were used for compartmental cultures.
Supplementary Material
Highlights.
Snapin recruits dynein motors to BDNF-TrkB signaling endosomes
Snapin-dynein coupling drives TrkB retrograde axonal transport
Snapin deficiency impairs terminal BDNF-induced retrograde signaling
Snapin-mediated TrkB retrograde signaling is critical for dendrite growth
Acknowledgments
We thank the members of the Sheng lab for helpful discussions; S. Wang for mouse breeding; S. Yang and M. Davis for manuscript editing; X. Zhuang for YFP-Rab7; M. V. Chao for TrkB-RFP construct; BaoJi Xu for Neurolucida. This work was supported by the Intramural Research Program of NINDS, NIH (Z-H. S.) and an NIH Pathway to Independence Award R00AG033658 (Q.C.).
Abbreviation
- BDNF
brain derived neurotrophic factor
- CNS
the central nervous system
- CREB
cyclic AMP-response element binding protein
- DIC
dynein intermediate chain
- DIV
days in vitro
- DRG
dorsal root ganglion neurons
- MAP2
microtubule-associated protein-2
- NGF
nerve growth factor
- PNS
the peripheral nerve system
- Trk
receptor tyrosine kinase
Footnotes
COMPETING INTERESTS STATMENT
The authors declare no competing financial interests.
Author Contributions
B. Z. conducted most experiments and wrote the manuscript; Q.C performed biochemical analysis; Y.X. measured dendritic arborization in vivo, Z-H. S. is senior author responsible for the project design, data interpretation, and manuscript writing.
Animal care and use in this study was carried out in accordance with NIH guidelines and was approved by the NIH, NINDS/NIDCD Animal Care and Use Committee.
Supplemental information including Extended Experimental Procedures and four figures and nine movies can be found with this article online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bhattacharyya A, Watson FL, Pomeroy SL, Zhang YZ, Stiles CD, Segal RA. High-resolution imaging demonstrates dynein-based vesicular transport of activated Trk receptors. J Neurobiol. 2002;51:302–312. doi: 10.1002/neu.10062. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Cai Q, Lu L, Tian JH, Zhu YB, Qiao H, Sheng ZH. Snapin-regulated late endosomal transport is critical for efficient autophagy-lysosomal function in neurons. Neuron. 2010;68:73–86. doi: 10.1016/j.neuron.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Cheung ZH, Chin WH, Chen Y, Ng YP, Ip NY. Cdk5 is involved in BDNF-stimulated dendritic growth in hippocampal neurons. PLoS Biol. 2007;5:e63. doi: 10.1371/journal.pbio.0050063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Ortha CB, Kimb HJ, Jeonc NI, Jaffrey SR. Neurotrophin-mediated dendrite-to-nucleus signaling revealed by microfluidic compartmentalization of dendrites. Proc Natl Acad Sci USA. 2011;108:11246–11251. doi: 10.1073/pnas.1012401108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosker KE, Courchesne SL, Segal RA. Action in the axon: generation and transport of signaling endosomes. Current Opinion in Neurobiology. 2008;18:270–275. doi: 10.1016/j.conb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B, Wu C, Chen L, Ramirez A, Bearer EL, Li WP, Mobley WC, Chu S. One at a time, live tracking of NGF axonal transport using quantum dots. Proc Natl Acad Sci USA. 2007;104:13666–13671. doi: 10.1073/pnas.0706192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen PA, Ghosh A. BDNF regulates primary dendrite formation in cortical neurons via the PI3-kinase and MAP kinase signaling pathways. J Neurobiol. 2005;62:278–288. doi: 10.1002/neu.20100. [DOI] [PubMed] [Google Scholar]
- Ginty DD, Segal RA. Retrograde neurotrophin signaling: Trk-ing along the axon. Curr Opin Neurobiol. 2002;12:268–274. doi: 10.1016/s0959-4388(02)00326-4. [DOI] [PubMed] [Google Scholar]
- Ha J, Lo KW, Myers KR, Carr TM, Humsi MK, Rasoul BA, Segal RA, Pfister KK. A neuron-specific cytoplasmic dynein isoform preferentially transports TrkB signaling endosomes. J Cell Biol. 2008;181:1027–1039. doi: 10.1083/jcb.200803150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerssen HM, Pazyra MF, Segal RA. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nat Neurosci. 2004;7:596–604. doi: 10.1038/nn1242. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Horch HW, Krüttgen A, Portbury SD, Katz LC. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23:353–364. doi: 10.1016/s0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- Howe CL, Mobley WC. Long-distance retrograde neurotrophic signaling. Current Opinion in Neurobiol. 2005;15:40–48. doi: 10.1016/j.conb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annual Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Tian JH, Pan PY, Zald P, Li C, Deng C, Sheng ZH. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki S, Holzbaur EL. Cytoplasmic dynein and dynactin in cell division and intracellular transport. Current Opinion in Cell Biology. 1999;11:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhang C, Takemori H, Zhou Y, Xiong ZQ. TORC1 Regulates Activity-Dependent CREB-Target Gene Transcription and Dendritic Growth of Developing Cortical Neurons. J Neurosci. 2009;29:2334–2343. doi: 10.1523/JNEUROSCI.2296-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Riccio A, Cohen S, Ginty DD. Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron. 2002;34:371–385. doi: 10.1016/s0896-6273(02)00686-4. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- Nikoletopoulou V, Lickert H, Frade JM, Rencurel C, Giallonardo P, Zhang L, Bibel M, Barde YA. Neurotrophin receptors TrkA and TrkC cause neuronal death whereas TrkB does not. Nature. 2010;467:59–63. doi: 10.1038/nature09336. [DOI] [PubMed] [Google Scholar]
- Pan PY, Tian JH, Sheng ZH. Snapin facilitates the synchronization of synaptic vesicle fusion. Neuron. 2009;61:412–424. doi: 10.1016/j.neuron.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauskolb, et al. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J Neurosci. 2010;30:1739–1749. doi: 10.1523/JNEUROSCI.5100-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond L, Kashani AH, Ghosh A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron. 2002;34:999–1010. doi: 10.1016/s0896-6273(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Satoh D, Sato D, Tsuyama T, Saito M, Ohkura H, Rolls MM, Ishikawa F, Uemura T. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat Cell Biol. 2008;10:1164–1171. doi: 10.1038/ncb1776. [DOI] [PubMed] [Google Scholar]
- Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annual Rev Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Philippidou P, Rosenbaum J, Akmentin W, Shao Y, Halegoua S. Trk-signaling endosomes are generated by Rac-dependent macroendocytosis. Proc Natl Acad Sci U S A. 2007;104:12270–12275. doi: 10.1073/pnas.0702819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson FL, Heerssen HM, Bhattacharyya A, Klesse L, Lin MZ, Segal RA. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat Neurosci. 2001;4:981–988. doi: 10.1038/nn720. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Yano H, Lee FS, Kong H, Chuang J, Arevalo J, Perez P, Sung C, Chao MV. Association of Trk neurotrophin receptors with components of the cytoplasmic dynein motor. J Neurosci. 2001;21:RC125. doi: 10.1523/JNEUROSCI.21-03-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Kuruvilla R, Zweifel LS, Ginty DD. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron. 2003;39:57–68. doi: 10.1016/s0896-6273(03)00266-6. [DOI] [PubMed] [Google Scholar]
- Zhou B, Zhu YB, Lin L, Cai Q, Sheng ZH. Snapin deficiency is associated with developmental defects of the central nervous system. Biosci Rep. 2011;31:151–158. doi: 10.1042/BSR20100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.