Abstract
Nature abounds with a rich variety of altruistic strategies, including public resource enhancement, resource provisioning, communal foraging, alarm calling, and nest defense. Yet, despite their vastly different ecological roles, current theory typically treats diverse altruistic traits as being favored under the same general conditions. Here we introduce greater ecological realism into social evolution theory and find evidence of at least four distinct modes of altruism. Contrary to existing theory, we find that altruistic traits contributing to “resource-enhancement” (e.g., siderophore production, provisioning, agriculture) and “resource-efficiency” (e.g., pack hunting, communication) are most strongly favored when there is strong local competition. These resource-based modes of helping are “K-strategies” that increase a social group’s growth yield, and should characterize species with scarce resources and/or high local crowding caused by low mortality, high fecundity, and/or mortality occurring late in the process of resource-acquisition. The opposite conditions, namely weak local competition (abundant resource, low crowding), favor survival (e.g., nest defense) and fecundity (e.g., nurse workers) altruism, which are “r-strategies” that increase a social group’s growth rate. We find that survival altruism is uniquely favored by a novel evolutionary force that we call “sunk cost selection”. Sunk cost selection favors helping that prevents resources from being wasted on individuals destined to die before reproduction. Our results contribute to explaining the observed natural diversity of altruistic strategies, reveal the necessary connection between the evolution and the ecology of sociality, and correct the widespread but inaccurate view that local competition uniformly impedes the evolution of altruism.
INTRODUCTION
Altruism is widespread in nature, with organisms ranging in complexity from microbes to insects and mammals expressing a vast diversity of specific strategies for helping others at a personal cost (Buss 1987; Dugatkin 1997; Crespi 2001). Organisms help by warning others of the presence of predators, and by assisting in the construction of defensive nests. Organisms help by feeding reproductive queens, and by caring for her offspring. They risk misfortune by foraging outside of the protective confines of the nest to retrieve food for nestmates, they help in the planting and harvesting of crops, and they expend personal energy to synthesize biomolecules that they release for use by neighbors. They help in the hunt, and by communicating the location of food. These traits provide benefits in different currencies (survival, fecundity, resources, efficiency), and appear to serve different ecological functions. Some appear to increase the growth rate of individuals and groups, others their growth yield. Some appear to increase resource competition, others to ameliorate it. Yet, despite this observed diversity, and despite the fact that evolutionary theory is principally concerned with explaining diversity, social evolution theory, in its attempt to identify general principles, has been largely silent when it has come to describing and explaining the origin and/or maintenance of altruism diversity.
Explaining altruism diversity requires identifying the ecological roles played by alternative altruistic strategies, and determining how these roles influence selection. To this end, we introduce here a simple consumer-resource model of fitness where reproductive success is the outcome of an explicitly ecological process of resource-acquisition, metabolism, growth and reproduction in patches of conspecifics competing over a shared resource pool. Our formulation allows results to be tuned among the three canonical functional responses of ecology (Type’s I, II, and III) (Holling 1959; Tilman 1982; Murdoch et al. 2003), which determine how resource supply maps onto reproductive output. Our model identifies the existence of at least four distinct types of altruism, and shows that these altruism types differ in their ecological roles, and, as a consequence, in the selection pressures they experience.
Most investigations into the evolutionary ecology of altruism have revolved around the concept of “local competition”. Local competition is universally thought to impede altruism evolution by pitting social partners against one-another in a zero-sum game wherein helping one individual necessarily harms others (Boyd 1982; Grafen 1984; Wade 1985; Taylor 1992; Wilson et al. 1992; Queller 1994; Frank 1998; Lehmann and Rousset 2010; Van Dyken 2010). In these models, local competition is imposed by constraining the reproductive output of groups via density regulation. With strictly local density regulation, all social groups are regulated by external (non-heritable) factors to a constant, uniform size at some stage in the life cycle (these populations are said to be “inelastic”). At the other extreme is strictly global density regulation, where groups vary in size in proportion to their (heritable) productivity (these populations are “elastic”). Three approaches for modeling social evolution with local competition currently dominate. The “free-parameter approach” introduces a scale of competition parameter that is tuned between 0 and 1 to modulate results between strict local or global density regulation (Grafen 1984; Frank 1998; Whitlock 2002). Alternatively, the “structural approach” assumes a specific pattern of dispersal among groups and then imposes an assumption about the scale of density regulation, typically either strictly local or strictly global regulation (e.g., (Wade 1985; Frank 1986; Taylor 1992; Rousset 2004; Lehmann et al. 2006; Lehmann and Rousset 2010). Models using a hybrid of these two approaches have also been proposed (Gardner and West 2006b; Van Dyken 2010). Finally, “graph-based approaches” use individual-based simulations and corresponding analytical theory (such as spatial moment equations (van Baalen and Rand 1998)) wherein individuals compete for occupancy of vacant sites on a spatial array (Nowak and May 1992; Wilson et al. 1992; Nakamaru et al. 1997; van Baalen and Rand 1998; Mitteldorf and Wilson 2000; Ohtsuki et al. 2006; Alizon and Taylor 2008; Lion and Gandon 2009, 2010). Models on graphs have the advantage that individuals explicitly compete over a shared limiting resource (space), but since the number of available sites is fixed by the initial structure of the graph, populations are necessarily inelastic and, in existing formulations, evolvable traits cannot increase local carrying capacity.
Methods that impose competition via density regulation assumptions face two shortcomings. First, density regulation is a consequence of competition, not its cause. Such models, then, impose an outcome by assumption. A preferable approach would be to create a causal model of competition and allow the consequences to emerge as a result, rather than by assumption. Secondly, density regulation assumptions force competition to be a function of the same parameters that determine genetic relatedness, namely group size, dispersal rate and dispersal pattern (Kelly 1992; Taylor 1992; Kelly 1994a; Queller 1994; Van Dyken 2010), rather than the parameters that actually control competition, namely resource availability relative to physiological requirements (Tilman 1982). We suggest that this feature of models creates a correlation between relatedness and local competition that needn’t reflect biological reality. Yet, many biological conclusions have been drawn from such models, including the conclusion that population viscosity (low dispersal rates from natal groups) does not promote altruism despite increasing genetic relatedness (Taylor 1992; Wilson et al 1992), and the universally accepted conclusion that local competition impedes altruism evolution.
Here, we decouple the causes of genetic relatedness from those governing competition, allowing each to be controlled by different, independent causal forces (competition by resource availability relative to physiological need, relatedness by population structure and dispersal). We find that some altruistic traits intrinsically create population elasticity, which causes them to be beneficial when competition is most intense. Methods that assume a fixed scale of density regulation have artificially prevented such a result. We show that there are also altruism types that cannot increase elasticity on their own, and so require extrinsic sources of population elasticity (abundant resources, low crowding). In general, we provide a simple mechanistic model for the interaction between sociality and ecology that resolves some previous dilemmas, provides a set of novel empirical predictions, and reveals a number of new phenomena.
MODEL
Life Cycle
We assume a metapopulation composed of a large (technically, an infinite) number of discrete social groups (e.g., families, demes, habitat patches, nests) linked by dispersal. Generations are discrete and non-overlapping. We further assume a monoecious, haploid population for simplicity of presentation, but our methods are readily extended to include other breeding and genetic systems, such as diploidy and haplo-diploidy (Agrawal 2010; Van Dyken 2010; Van Dyken et al. 2011). The life cycle begins with the formation of groups, followed by reproduction. A group produces a total of offspring, where is the mean fecundity of adults founding group i and Ni is the number of these adults (all symbols are defined in Table 1.). Following reproduction, offspring compete locally for access to a spatially localized, limited resource essential for growth and reproduction. We assume that offspring consume resources but that adults do not, although adults may still contribute parental care, for example in the form of resource provisioning or nest defense. Thus, adults may stay alive after reproduction and even contribute parental care, but they do not compete for resources. After the resource acquisition stage of the life cycle, adults die and offspring disperse to form new groups, completing the life cycle. Note that these life cycle assumptions (non-overlapping generations, competition and altruism in the same life stage, and spatially localized resources) are the most restrictive for the evolution of altruism according to previous models (Van Dyken 2010).
Table 1.
Symbols and definitions.
| Symbol | Definition |
|---|---|
| a | Intensity of local competition |
| e | Intensity of sunk cost selection |
| r | Coefficient of relatedness |
| pij, pi, p | Frequency of altruism allele (in individual ij, in group i, and in global metapopulation, respectively) |
| b | Maximum benefit of altruism |
| c | Direct effect of altruism |
| Wij, Wi, W | Fitness (of individual ij, mean of group i, and mean of global metapopulation, respectively) |
| fij, fi, f | Fecundity, i.e., the gross number of pre-regulation offspring produced (individual, mean group, and in absence of altruists, respectively) |
| f (t −1) | Mean fecundity of parents |
| vij, vi, v | Viability, i.e., resource-independent probability of survival to reproductive stage (individual, mean group, and in absence of altruists, respectively) |
| Si, S | Resource supply, i.e., per-consumer amount of resources available to a group (mean group, and in absence of altruists, respectively) |
| TD | Sunk cost parameter, i.e., the average proportion of total resources consumed by an individual before pre-reproductive death |
| kS,i, kS | Half-saturation constant, i.e., per-capita resource supply at which fitness is half its asymptotic value (mean group, and in absence of altruists, respectively) |
| ωi, ω | Coefficient of local crowding (in group i and in a group without altruists, respectively) |
| Ni, N | Number of founders of a deme (in group i and in a group without altruists, respectively) |
| x | Dummy variable used to modulate among Type I, II and III functional responses |
| y | As directly above, but can also be interpreted as the “handling time” of prey |
We assume that the population dynamics of the resource species is weakly coupled to the focal (consumer) species (Murdoch et al. 2002; Murdoch et al. 2003), such that evolution of the focal species, or activities such as differential consumption or enhancement of resources, does not alter the amount of resource available at the beginning of each generation. For examples of evolutionary models that relax this assumption, see (Gurney and Lawton 1996; Laland et al. 1996; Odling-Smee et al. 1996; Murdoch et al. 2003; Odling-Smee et al. 2003; Hauert et al. 2006; Lehmann 2007, 2008; Wakano et al. 2009).
Competition in our model emerges as a consequence of resource availability. Our model differs from those proposed previously in that the parameters determining local competition are not the same as those determining relatedness, so open-form solutions conceal no hidden relationships between model parameters (given our specific life cycle assumptions). We thus report all our solutions (Table 2) in open form, which highlights their generality with respect to population structures. Also note that our weak selection assumption, which we make throughout, obviates the need to assume constant Ni, as Ni changes in size by a factor of (b − c) over the course of evolution, which has a negligible impact on results when b, c ≪1.
Table 2. Conditions for positive selection of altruism types.
These conditions arise from first-order “inclusive fitness effects” as derived in the main text. b is the maximum altruistic benefit, c the cost of acting altruistically, r is the genetic relatedness of social partners, a is the intensity of local resource competition (Equation 3) and e accounts for “sunk-cost selection” (Equation 4). Results apply for Types I, II, and III functional responses.
| Benefit | Cost | Condition For Spread (“Hamilton’s Rule”) | Examples |
|---|---|---|---|
| Survival | Survival Cost | (1 − a + e)br − c(1 − ar + re)> 0 | Nest defense Alarm calling Nest climate control Communal rearing |
| Fecundity Cost | (1 − a + e)br − c(1 − ar) > 0 | ||
| Fecundity | Survival Cost | (1 − a)br − c(1 − ar + re)> 0 | Worker care of queens (“nurse workers”) Allocation of survival gains to reproductive effort |
| Fecundity Cost | (1 − a)br − c(1 − ar) > 0 | ||
| Resource-Enhancement | Survival Cost | abr − c(1 − ar + re)> 0 | Provisioning Agriculture Livestock rearing Exoenzymes Siderophores |
| Fecundity Cost | abr − c(1 − ar) > 0 | ||
| Resource-Efficiency | Survival Cost | abr − c(1 − ar + re)> 0 | Pack hunting Communal foraging Communication |
| Fecundity Cost | abr − c(1 − ar) > 0 |
Consumer-Resource Fitness Model
Fitness is the outcome of the following process: organisms consume resources acquired from the environment, which they then convert into biomass (growth, offspring) and chemical and mechanical work (metabolism, behavior). The mapping of resource availability onto fitness requires the specification of a “functional response”, which comes in three canonical forms (Holling 1959; Tilman 1982; Murdoch et al. 2003). A Type I (linear) functional response assumes that reproductive output is linearly proportional to resource availability, such that individuals produce an infinite number of offspring in an unlimited environment. This is clearly unrealistic. Necessary biological factors such as respiration, energetic costs of foraging, search and handling times, and satiation prevent a 1:1 correspondence between resource supply and growth (Holling 1959; Tilman 1982; Yodzis and Innes 1992). In the strictest sense, Type I functional responses violate the Second Law of Thermodynamics. More common are Type II (concave, e.g., Monod or Michaelis-Menten kinetics) and Type III (sigmoidal) functional responses, which make reproductive output a saturating function of resource abundance, so that a finite maximum (asymptotic) number of offspring is produced when resources are infinite. This accords with most laboratory experiments and empirical observations (Gause 1934; Monod 1949; Tilman 1982), with Type II functional responses having the most widespread empirical support. We employ a general model that allows us to modulate between Type I, II and III functional responses (Yodzis and Innes 1992; Otto and Day 2007). Allowing for a Type II or III functional response is one feature that distinguishes our model from most existing niche-construction models (Odling-Smee et al. 1996; Kerr et al. 1999; Odling-Smee et al. 2003; Lehmann 2007, 2008) and most other explicit resource models of social evolution (although see Whitlock et al (2007)), which typically model fitness as a Type I (linear, non-saturating) functional response.
In Appendix A we derive the following equation for the absolute fitness (total number of offspring produced) by the jth individual in the ith social group (see also Agrawal (2010)):
| (1) |
vijfij is individual ij’s asymptotic fitness; that is, its resource-independent probability of survival to maturity (viability, vij) times its maximum fecundity (fij). The parameters x ∈ {1,2} and y ∈ {0,1} modulate Equation 1 between Type I (x = 1, y = 0), Type II (x = 1, y = 1) and Type III (x = 2, y = 1) functional responses (Otto and Day 2007). Note that y may be defined as a continuous variable ( y ∈ [0,1] ) representing the “handling time” required to subdue, consume and digest a resource item (Holling 1959). The quantity, Si = ST,i/Ni, is the per-capita resource supply in the ith group at the beginning of a generation (before births or stochastic deaths), which we assume is constant (i.e., held in a steady quasi-equilibrium). kS,i is the mean “half-saturation constant” (Monod 1949; Healey 1980; Tilman 1982) of individuals in the ith group, which is the value of the resource concentration, S, at which fitness is half its maximum.
ωi measures crowding. Specifically, it is a function of the mean fecundity of group founders ( ), the group mean resource-independent survival probability (vi), and the mean rate at which resources within the group are consumed by individuals that die before reproduction, TD,i. We refer to TD as the “sunk cost” parameter because it quantifies the unrecoverable loss in resources due to deaths (note that “cost” here is in units of resources, not necessarily fitness). When TD = 0, deaths occur before any resources are consumed, while TD = 1 indicates that deaths occur after individuals have consumed as much of the local resource as those which survive to reproduction (Agrawal 2010). We make the simplifying assumption that TD and v are uncorrelated, which may often be violated (A. Agrawal, pers. comm.). Here, we write crowding as , or, approximately,
| (2) |
ω corresponds to the degree of “habitat saturation” (Lion and Gandon 2010), modeled as “empty sites” in graph-based spatial models (Alizon and Taylor 2008; Lion and Gandon 2010). Notably, here ω includes cases where “sites” have been vacated by deaths, but the dead individuals have already consumed resources. Unlike in graph theoretic models, such deaths do not relax local resource competition.
The following parameter appears in our results after rearrangement, and we define it as a measure of the intensity of local competition,
| (3) |
Importantly, this parameter is a model outcome, not an input parameter. (Throughout, unsubscripted parameters (f, v, kS, ω and S) indicate values in the absence of altruists). Eqn. 3 shows that local competition is most intense with high crowding, scarce resources, and high resource demand. When costs and/or benefits affect survival, the following “sunk-cost selection” term arises:
| (4) |
This term accounts for additional fitness consequences resulting from deaths that waste resources but do not contribute to group productivity. Note that e ∈ [0, a], where e = 0 when TD = 0, and e = a when TD = 1.
Results in Table 2 apply for all three functional responses (Type I, II, and III), with the appropriate values of x and y substituted throughout. Importantly, a Type I functional response creates a condition where a = 1 for all values of S and ω (see Equation 3). This is not true of Types II and III functional responses, where local competition is strictly determined by resource availability and crowding, and approaches zero as S → ∞. To understand this effect of a Type I functional response on local competition, we can substitute x = 1 and y = 0 into Equation 1, giving: Wij = Sivijfij/(kS,ivjfi) for TD = 0, and Wij = Sivijfij/(kS,ifi) for TD = 1. This shows that a linear functional response makes the fitness consequences of survival and fecundity effects (when TD = 0) or just fecundity effects (when TD = 1) strictly relative to the performance of others in the group. This dependence of absolute fitness on the fitness of social partners is, by definition, local competition (Frank 1998). Most previous models have tacitly or implicitly assumed a Type I functional response. Allowing for the more realistic Type II and III functional responses is responsible for many of the novel results we identify here.
Allele Frequency Recursions
The evolutionary change in global frequency of an altruism allele is given by the Robertson-Price equation (Robertson 1966; Price 1970),
| (5) |
where cov() is covariance, pij is the frequency of the altruism allele in the jth individual in the ith group, and Wij/W̄ is the global relative fitness of individual ij. In our haploid model, for carriers of the altruism allele, pij = 1, and for carriers of the non-altruistic allele, pij = 0 (Wade 1985). Eqn. 5 is quite simple to use. First, one defines a fitness function, Wij (we will use Equation 1 throughout), which then gives W̄ by taking expectations. Once the relative fitness (Wij/W̄) is found, one simply applies the rules of covariance’s and expectations (e.g. Lynch and Walsh(1998)) to find the solution. The solution will contain genetic covariances that can be written as a function of global allele frequency and the degree of population genetic structure (Wright 1931; Wright 1951; Wright 1965; Whitlock 2002; Rousset 2004). In our present model, we need only the following haploid genetic covariances:
| (6a) |
| (6b) |
Here r is the kin selection coefficient of genetic relatedness, which is equal to the proportion of total genetic variance among groups (Crow and Kimura 1970; Michod and Hamilton 1980; Frank 1998), and which, in our haploid model, is equivalent to Wright’s FST, the probability that two alleles chosen at random from within a group are identical-by-descent (Wright 1965).
We assume linear social effects throughout, such that the effect of two altruists is twice that of one, and so on. Thus, while the fitness surface is nonlinear due to the functional response (eq. 1), we assume that it behaves approximately linearly throughout the course of invasion and fixation of a single mutant allele. This is reasonable provided phenotypic effects are small, i.e., b and c ≪ 1. Our aim here is not to investigate how non-linear fitness interactions influence the statics and dynamics of altruism evolution, which has been investigated elsewhere (Smith et al. 2010; Archetti and Scheuring 2011), but instead to demonstrate the existence of multiple, diverse ecological modes of altruism and to investigate their interaction with the factors influencing local competition. Our weak selection assumption can be relaxed using previous methods (Smith et al. 2010). In addition, we derive results in terms of “whole-group” fitness (Pepper 2000), where altruists receive their own benefit. This is by no means a necessary assumption, and is only made to simplify the presentation and to provide results in a familiar form. A simple change of variables suffices to obtain the “others-only” analogues (e.g., Van Dyken (2010)).
RESULTS
Altruism is typically modeled as increasing a recipients’ survival or fecundity. However, our model (Eqn. 1) provides four different parameters that control fitness, each of which can be modified by altruism, as we now show. Analytical results are summarized in Table 2.
Survival Altruism
“Survival altruism” includes common altruistic traits such as defense against predators or parasites, alarm calling to warn of danger (e.g., in ground squirrels, meerkats, monkeys, ungulates), nest climate control (e.g., in naked mole rats, termites, ants, bees) and collective thermoregulation (e.g., bees, mice). The defensive sting of ants, bees and wasps (Starr 1985) is a salient example of survival altruism, as stinging deters predators but often causes death of the defender. Construction and defense of nests, i.e., “fortress defense”, is a more general example of survival altruism (Queller and Strassmann 1998; Wilson 2008). Targets of selection include stingers, mandibulate mouthparts used in nest construction (Holldobler and Wilson 1990), and stereotyped behaviors contributing to nest construction and agonistic response to invaders.
As is typical in kin selection models, we assume that the survival of an individual, vij, is increased by an amount bk for every altruist in its group. Let b = bkN be the “maximum benefit” received in a hypothetical group fixed for altruism. In the case of a survival trade-off, which we derive for the sake of illustration, altruists suffer reduced personal survival by an amount, c. Let v and f be the survival and fecundity rates of individuals in the absence of altruism. Thus, assuming small phenotypic effects, we can write,
| (7a) |
| (7b) |
Substituting these values into Eqn. 1 and using Eqn’s 2–4, we find to first order,
| (8) |
Again, the parameters a and e (eqns. 3 and 4, respectively) emerge after some algebra. Substituting Eqn. 8 into Eqn. 5 and using the identities in 6a,b gives,
| (9) |
Altruism spreads when Δp > 0 (Table 2).
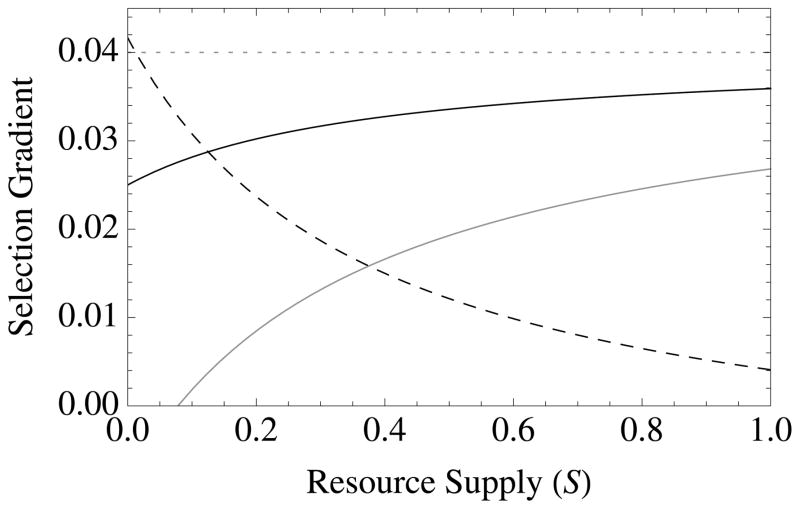
It is clear from eq. [9] that local competition (high crowding, scarce resources) impedes survival altruism. When resources are infinite (and thus not limiting), such that a = e = 0, we recover the familiar form of Hamilton’s rule (Hamilton 1964), with resource-independent linear benefits, b, and costs, c (Table 2). When TD = 0 (deaths occur before resources are consumed) such that e = 0 (no wasted resources), we recover as a special case previous theoretical results for altruism under local competition (Boyd 1982; Grafen 1984; Frank 1998; Van Dyken 2010), namely, (1 − a)br − c(1 − ar)> 0, except that the abstract “scale of competition” (Frank 1998) parameter used previously is replaced by eq. [3], which is an explicit, causal function of environmental and physiological variables.
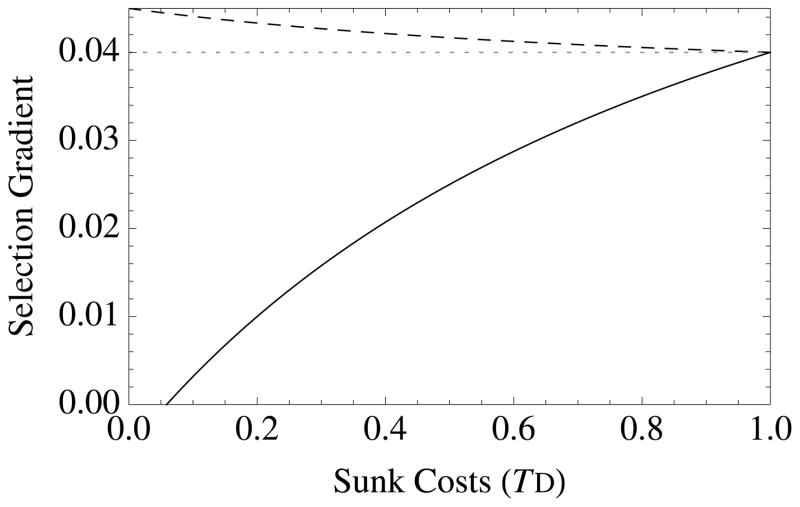
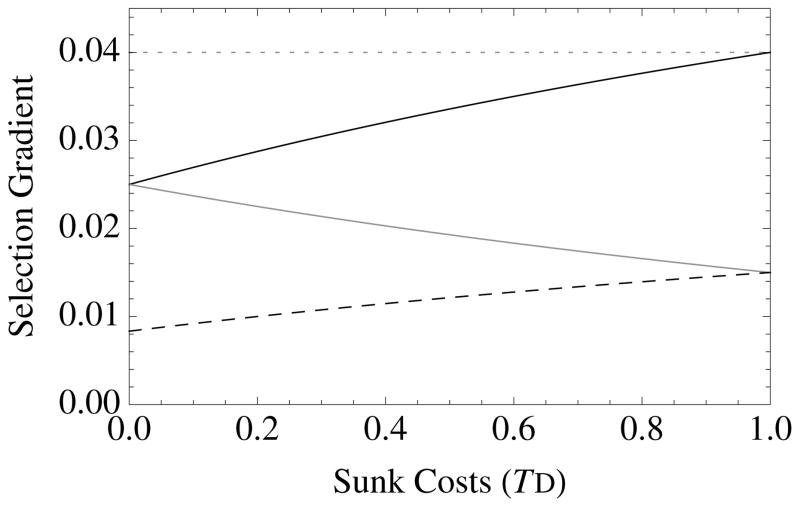
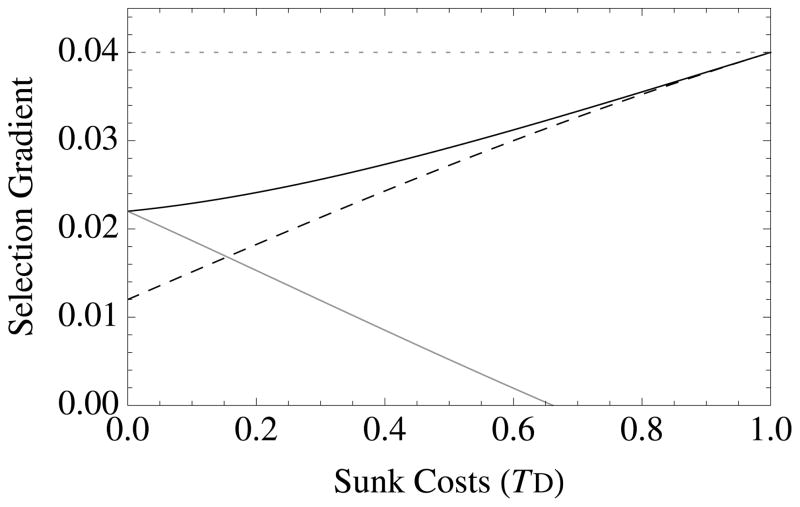
Novel results appear when TD > 0, which corresponds to the existence of “sunk costs” or “wasted resources” (Agrawal 2010), wherein deaths remove resources from the group without contributing to group productivity. Sunk cost selection, which is controlled by the parameter e, favors traits that increase survival when sunk costs are high (TD > 0) (Figure 1). This selection acts to reduce resource waste. To demonstrate this effect, note the following: When TD = 1, then e = a, so that survival altruism evolves when br − c > 0, which is independent of resources or crowding. This occurs because individuals that have already consumed resources are no longer competitors: helping them does not increase local competition. Another way to look at this effect is to note that reducing wasted resources creates elasticity: groups that eliminate waste are more efficient with their collective resource use, which increases their relative productivity. This sunk-cost effect distinguishes survival altruism from fecundity altruism (see next section), not only quantitatively but qualitatively (Figure 1). Survival altruism is always more strongly favored than fecundity altruism when TD > 0. This is because survival altruism, but not fecundity altruism, reduces resource waste due to premature death.
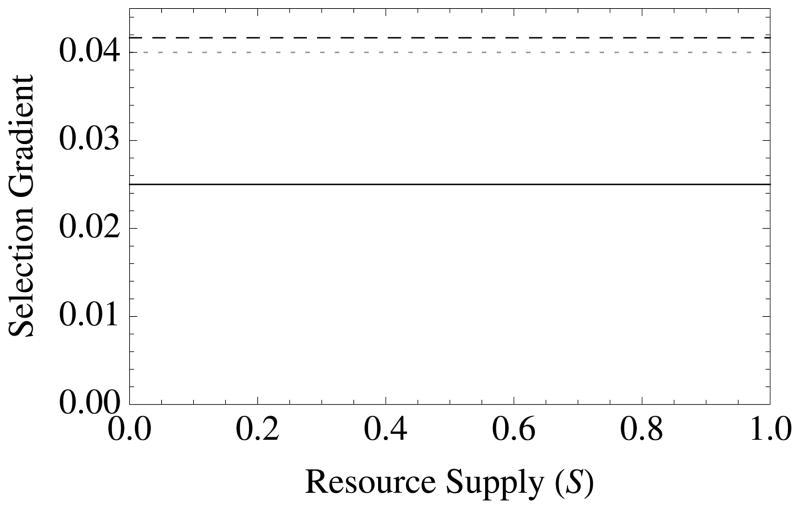
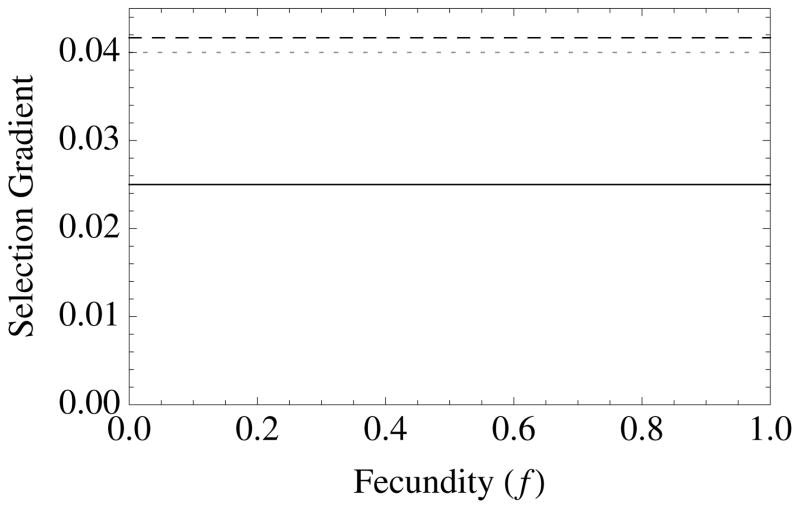
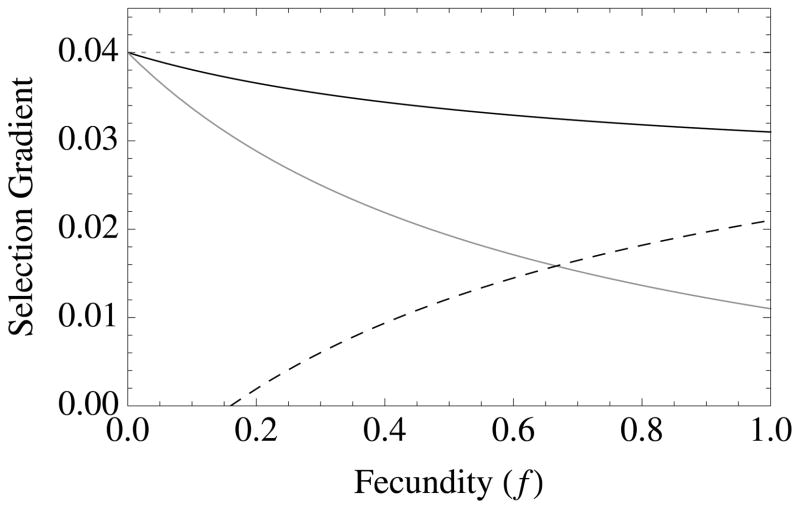
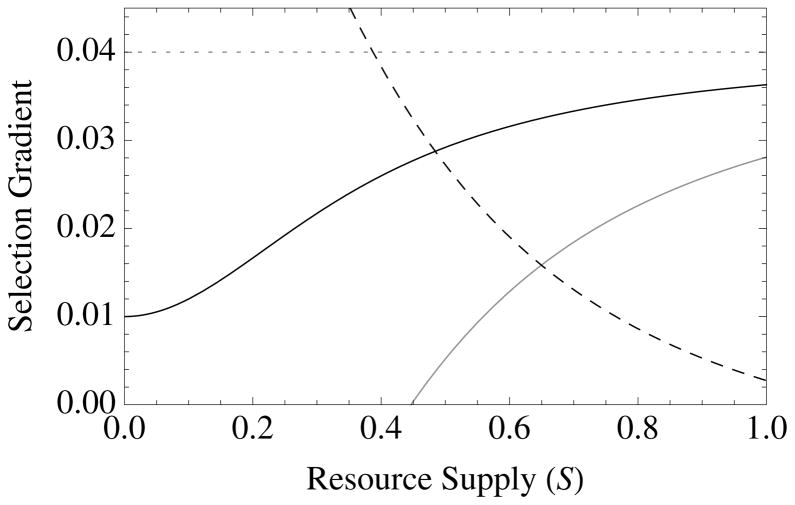
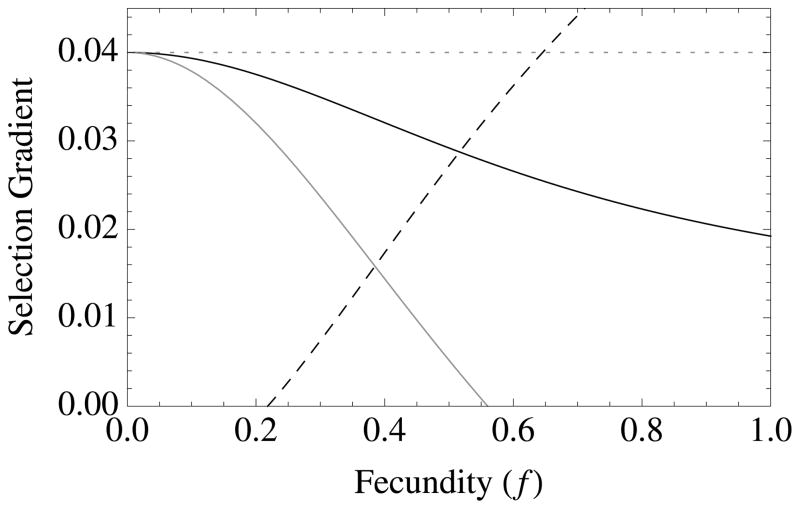
Figure 1. The diversity of altruistic strategies and their evolutionary response to model parameters.
Survival altruism (solid black line), fecundity altruism (solid gray line), resource-enhancement altruism (dashed black line), and resource-efficiency altruism (dashed black line), with survival trade-offs (fecundity trade-offs not shown). The horizontal dotted line at Selection Gradient = 0.04 is the value for (br − c), which is “Hamilton’s rule” for survival or fecundity altruism in the absence of resource competition. Note that resource-enhancement and resource-efficiency altruism have identical first order effects on fitness, which is why the plots show three instead of four curves. Column 1 gives results for Type I functional responses (x = 1, y = 0), Column 2 for Type II responses (x = 1, y = 1), and Column 3 for Type III responses (x = 2, y = 1). Note also that fecundity altruism cannot evolve under these parameter values with a Type I response, so that a gray line does not appear in Column 1. Parameter values for all curves are b = 0.1, c = 0.01, and r = 0.5. Unless used as the independent variable for a given graph, the remaining parameter values are S = v = f = TD = 0.5.
Fecundity altruism
“Fecundity altruism” occurs when help donated by an actor causes recipients to increase their investment in reproductive effort, thereby increasing the gross number of offspring produced by a maximum amount b. Fecundity-altruism is best exemplified by the eusocial Hymenoptera and termites, where care by sterile “nurse workers” allows queens to lay hundreds to millions of eggs (Holldobler and Wilson 2009). The greatly distended, egg-filled abdomen of termite queens (Shellman-Reeve 1997; Holldobler and Wilson 2009) is a clear morphological example. One must be careful to distinguish between survival and fecundity benefits, as increasing the survival of an individual may be mistakenly counted as increasing the fecundity of that individual’s parents. We avoid double-counting costs and benefits by simply restricting the effects of a behavior to within a single generation (Wolf and Wade 2001).
The fecundity of an individual, fij, is increased by a maximum amount b by associating with altruists. We present again the case of a survival trade-off, where altruists suffer reduced personal survival by an amount, c (results for fecundity trade-offs are found in Table 2). Thus, assuming small phenotypic effects, we have,
| (10a) |
| (10b) |
Once again assuming weak selection and following the previous derivation, we find that the change in allele frequency is, to first order,
| (11) |
Like survival altruism, fecundity altruism is favored in elastic environments (low crowding and/or abundant resources) (Table 2; Figure 1). However, as discussed above, fecundity altruism is more heavily favored when deaths occur early in life (TD is small) (Figure 1c), because wasted resources increase local competition.
Another fact to note is that, whenever altruistic costs reduce personal survival or fecundity, the negative impact of this cost on fitness is diminished with increasing local competition and relatedness (that is, the cost is ameliorated by a factor (1 − ar)). This effect is a consequence of “byproduct altruism,” wherein the premature death or reduced reproductive output of the altruist frees up resources for group members. This effect on fitness depends on the intensity of local competition, a, as freed-up resources are a greater boon when competition is fierce, and on the proportion of those that benefit from this incidental resource-enhancement who are related, by r, to the prematurely deceased altruist.
Resource-enhancement Altruism
“Resource-enhancement” helping occurs when altruists act to increase local resource concentration, S. Such traits are widespread in nature, and include provisioning behaviors (Hawkes et al. 1997; Marlowe 2003; Costa 2006; Page et al. 2006; Wilson 2008), agriculture (Diamond 1997; Mueller et al. 2005) rearing of livestock (Diamond 1997) (e.g., aphids milked by ants for their “honeydew” (Holldobler and Wilson 2009)) and many types of niche construction (Jones et al. 1994; Odling-Smee et al. 1996, 2003). Microbial resource-supply altruism typically involves costly production of publicly available biomolecules (Platt and Bever 2009), such as secretion of “siderophores” that increase the local supply of usable iron (Buckling et al. 2007; Kummerli et al. 2009b), (Chao and Levin 1981; Brown et al. 2009), proteolytic exo-enzymes used to subdue and digest prey (Velicer and Mendes-Soares 2009), or exo-enzymes involved in carbohydrate digestion, such as invertase in yeast (Greig and Travisano 2004; Gore et al. 2009).
Because these traits modify the resource component of fitness, they need to be modeled accordingly. Allowing the per-capita resource concentration in a group to be increased by a maximum amount b by the altruists within it, and again assuming small phenotypic effects, gives,
| (12) |
This feature formally makes this a niche-construction model (Gurney and Lawton 1996; Laland et al. 1996; Odling-Smee et al. 1996, 2003), wherein organisms influence fitness by modifying their environment. Viability and fecundity trade-offs can be modeled as above, and the results in Table 2 are found by substituting eq. [12] into eq. [1] and following the derivations above.
Contrary to previous theory (e.g., (Boyd 1982; Grafen 1984; Wade 1985; Taylor 1992; Queller 1994; Frank 1998; Alizon and Taylor 2008; Platt and Bever 2009; Lehmann and Rousset 2010; Lion and Gandon 2010; Van Dyken 2010), local competition actually enhances selection for altruism in these cases (Table 2; Fig. 1). The fitness benefit of resource-based altruism is equal to, B = ab. Note that this effect is not due to resource altruism overcoming local competition; rather, local competition is a prerequisite for the evolution of resource altruism. This occurs because resource-enhancement increases growth yield, creating elasticity. If the environment is elastic (low crowding and abundant resources), then there is little relative benefits of creating elasticity. Consider for example an altruist that forages away from the nest and brings back food to share with nest mates. This behavior comes with costs, as it exposes the altruist to risks such as predation and causes them to invest in foraging rather than reproductive output. Benefits will outweigh these costs only if food at the nest is sufficiently scarce, but if food is abundant in the nest (a is close to zero), then a marginal increase in provisioning effort will not be worth the accompanying costs.
Resource-efficiency Altruism
“Resource-efficiency” helping enhances the efficiency with which social partners convert resources into fitness. Communication of the location and quality of a food source to colony members using chemical cues (e.g., pheromone trails in ants) or visual cues (e.g., the “waggle dance” in honeybees) increases the efficiency of foraging by preventing wasted time and effort (Holldobler and Wilson 1990, 2009). Other examples include communal foraging (Kelly 1994b) and “pack hunting” (Creel and Creel 1995). Resource-efficiency altruism is possible when the net caloric intake from foraging (calories consumed minus calories expended) is greater when done in groups than when done alone, as has been documented in mammalian (Creel and Creel 1995) and arachnid (Yip et al. 2008) carnivores that cooperatively hunt large prey. For instance, pack hunting increases the success rate of hunting, the size of prey captured, and reduces the probability that prey is stolen by other species (Fanshawe and Fitzgibbon 1993). Altruists increase the hunting efficiency of others but suffer a loss in personal viability or fecundity due to increased investment in cooperation.
Resource-efficiency altruism is modeled by modifying the half-saturation constant, kS, in eq. [1]. Increased efficiency corresponds to decreased kS, as fewer resources are required to produce the same unit of fitness. Thus, altruists increase the efficiency of resource-use by decreasing kS by a total amount, b, such that,
| (13) |
Again, results reported in Table 2 are derived from eq. [8] as shown above. As with resource-enhancement helping, resource-efficiency helping increases growth yield, creating population elasticity. Resource-efficiency helping requires strong local competition in order to be favored by selection.
Constraints on Resource-based Helping
In real populations, the ability of organisms to enhance local resource supply, or to use resources more efficiently, may have an upper maximum determined by global resource limitation or physiology. For example, provisioning by animals enhances local resource supply as they capture resources from outside of the group; as a result, enhancement is constrained by global resource supply (or at least the supply that can be reached by foragers). Microbes are relatively immobile and so typically enhance resources by biochemical conversion of local raw materials into usable substrate (Platt and Bever 2009). In this case, constraints derive from the local availability of these convertible raw materials. Diffusible iron-scavenging siderophore molecules, for example, increase the local supply of usable iron, but they cannot increase this supply above the amount of raw iron provided by the environment. Agriculture may be constrained by technology and crop needs, such as water, fertilizer, or space (Diamond 1997; Mueller et al. 2005).
To formalize these constraints, we define a maximum achievable (given existing strategies) local resource concentration, Smax. Let b̃ denote the asymptotic proportional increase in resource supply due to the actions of altruists, which is the increase in resource supply due to altruism in unlimited populations. It can be thought of as the effort invested in resource enhancement. This is to be distinguished from b, which is the actual proportional increase in resource supply due to altruists, which may vary depending on the environment. Using the logistic equation, we can define the local resource supply under enhancement as,
| (14) |
Equation [14] can be substituted into all our previous fitness models (i.e., eq. [5]) to introduce an enhancement maximum, or, equivalently, the quantity, b̃(1 − S/Smax), can be substituted for b. For example, the condition for invasion of a resource-enhancement allele is,
| (15) |
Note that this maximum enhancement constraint is distinct from a local competition constraint. Local competition constrains (survival/fecundity) altruism through negative feedback caused by increased competition with kin (Grafen 1984; Taylor 1992; Queller 1994). Helping others to survive or reproduce increases crowding, which increases resource competition, which decreases fitness when resources are limiting. On the other hand, resource-based altruism is promoted, not constrained, by local competition (Table 2). The evolutionary constraint imposed by maximum enhancement, then, does not come about via negative feedback owing to increasing local competition, it comes from the inability to acquire more resources despite selection to do so.
Assuming a fixed resource maximum may often be unrealistic. Selection imposed by local competition will favor novel traits that increase the maximum level of resource enhancement (Smax), so that these constraints are themselves evolvable. For example, exhaustion of nearby resources may be overcome by nest relocation (“counteractive relocation” niche-construction (Odling-Smee et al. 2003)), by broadening the resource base through generalism or metabolic novelties (“inceptive perturbation” niche-construction (Odling-Smee et al. 2003)), or by overcoming agricultural limitation with innovation (e.g., better fertilizer, irrigation, crop-rotation, artificial selection (Diamond 1997)). Traits that enhance locomotion, such as adaptations for better flight in bees, expand an individual’s foraging range making available more resources for provisioning. As a consequence, a resource maximum is not a fixed quantity, and may itself serve as a selection pressure favoring specific adaptations. As such, a “fixed” resource maximum may not be as severe a constraint as implied by the static equations above.
DISCUSSION
Theoretical and empirical studies commonly treat diverse modes of altruism as ecologically and evolutionarily equivalent. For instance, when providing real-life examples to motivate theoretical models, workers regularly state that the trait they are modeling can be thought of as, say, either resource sharing or warning of attack by predators, as if these two behaviors were ecologically and evolutionarily equivalent to one another. Empirical studies, whether motivated by theoretical work or not, also conflate diverse altruistic strategies. Studies ranging from investigations of human economic behavior to laboratory experiments with microbes regularly aim to test theory formulated for one altruism type with experiments that probe another (see further below for specific examples). Here we have shown that conflation of altruism types is not only unjustified, it is misleading. Different altruistic strategies face different evolutionary constraints and selection pressures; in fact, resource-based altruism and survival/fecundity altruism are favored under polar opposite environmental conditions. Identifying the specific ecological function of an altruistic trait is not only essential to understanding its evolution, it is required in order to inform experimental design, to avoid false inferences from experimental results, and to accurately predict responses of species to specific medical or management interventions.
Connection to Previous Theoretical Work
Our aim here was introduce a simple, mathematically tractable yet ecologically realistic mechanistic theory of social evolution. Our approach is prefigured by that of Agrawal (2010), although our fitness model is extended to include crowding effects, the half-saturation constant, Type’s I and III functional responses and social interactions. Agrawal (2010) also introduced what we call the “sunk cost” parameter, TD, which figures prominently in our model.
The approach we have taken is similar to that of classic density-dependent selection models, where selection is determined by a logistic growth equation with parameters for growth rate, r, and yield, K, under genetic control (Charlesworth 1971; Roughgarden 1971; Roughgarden 1979). Logistic growth models, in general, have been criticized on the grounds that they abstract away from the causes of competition and include only its consequences (namely density regulation) via the implicit parameters of growth rate and carrying capacity (Stewart and Levin 1973; Tilman 1982). In our consumer-resource model, carrying capacity is caused by resource availability, S, and the physiological requirements of growth, kS, while growth rate is the product of survival and fecundity. We find that survival and fecundity altruism increase the growth rate of social groups, while resource-supply and resource-efficiency altruism increase the growth yield of social groups. These two altruism classes, then, represent r- and K-strategies (MacArthur and Wilson 1967), respectively.
It is straightforward to recover previous results from social evolution theory as special cases of those in Table 2, revealing a number of implicit assumptions in previous work. As noted above, the results for survival and fecundity altruism recover the classic form of Hamilton’s rule when S → ∞, which makes sense because Hamilton did not consider resources in his model. Previous social evolution work also has not considered wasted resources; thus, when TD = 0, our results for survival and fecundity altruism recover previous results for altruism under local competition ((1 − a)br − c(1 − ar) > 0) (Boyd 1982; Grafen 1984; Frank 1998; Van Dyken 2010), although a in our results is an explicit function of resource supply, efficiency of resource use, and crowding. Because we have assumed a life cycle that is least conducive to (survival/fecundity) altruism evolution (i.e., competition for resources is strictly among social partners and occurs in the same life stage as cooperation), we would like to know if we can find the set of assumptions that recovers the previous result that altruism cannot evolve in our life cycle. We find that this previous result for our life cycle is recovered when we assume 1) survival/fecundity benefits, 2) TD = 0, 3) and a linear mapping of resources onto fitness (Type I functional response). While the first assumption is explicit in previous work, the latter two assumptions are entirely implicit, since previous work did not explicitly model resources as a determinant of both fitness and the intensity of local competition. Hence, assuming a Type I functional response, survival/fecundity benefits, and TD = 0, we recover the previous result for the evolutionary dynamics of altruism under our life cycle, Δp = −p(1 − p)c(1 − r) (Rousset 2004; Van Dyken 2010).
Our model identifies several ways that altruism can evolve in this restrictive life cycle. First, with saturating (Type II and III) functional responses, which tend to be the rule in nature, local competition is ameliorated as resource supply increases. Thus, even when competition is spatially localized and directed at kin, survival/fecundity altruism can evolve as long as resources are sufficiently abundant. Second, survival altruism can evolve in this life cycle when TD > 0, even with a Type I functional response, due to sunk-cost selection. The dynamics in this case, with TD = 1, are: Δp= p(1 − p)[br − c(1− r)]. Finally, altruism can evolve in this life cycle when benefits are delivered in the currency of enhanced resource supply or increased efficiency of resource use, even with a linear functional response.
Previous work has found that the effect of local competition differs in some cases depending on whether payoffs occur in the currency of survival or fecundity (Ohtsuki et al. 2006; Grafen and Archetti 2008; Lion and Gandon 2010; Taylor 2011). This difference occurs with overlapping generations or births occurring before deaths in graph-based spatial models. Here we have found another factor that causes selection to differ between survival and fecundity altruism, even in a non-spatially explicit model with non-overlapping generations. This factor is sunk-cost selection, which favors traits that prevent resources from being wasted by premature deaths. Graph theoretic models have not included factors analogous to TD, and so have not found such an effect. In addition, graph-based models have not discovered the distinction between survival/fecundity payoffs and resource-based payoffs, as we have here, because resource availability is fixed in these models by the amount of available space. Graphs impose inelasticity, so that even traits that create elasticity in nature, such as resource-based altruism, cannot evolve in these models. Future work should seek to allow resource-based payoffs in graph theoretic models, and to introduce factors that allow resource supply and use to be controlled by traits in the population.
Our model contributes to previous work aimed at introducing greater ecological realism into social evolution theory through explicit resources. Whitlock et al (2007) modeled resource-based reciprocity where fitness was a concave function of resources, similar to our Type II functional response. Most explicit resource models of social evolution assume a Type I functional response, including those applying a niche construction approach (Odling-Smee et al. 2003; Lehmann 2007, 2008, 2010), and dynamical consumer-resource models (Aviles 1999; Hauert et al. 2006; Wakano et al. 2009). As we have shown, the use of a Type I functional response produces results that differ qualitatively from those applying saturating responses. In particular, Type I models have the unrealistic consequence that high resource abundance does not ameliorate local competition, so that survival and fecundity altruism always require special demographic assumptions in order to evolve. On the other hand, increasing resource supply via resource-enhancement benefits is always favored with a Type I response, even when resources are already abundant. While we have avoided these anomalies by introducing saturating functional responses, we have also sacrificed realism for tractability by assuming weak coupling between consumer and resource species, so that we did not have to track the coupled population dynamics of both species (Murdoch et al. 2002; Murdoch et al. 2003). Strong coupling can lead to novel evolutionary outcomes, including instability causing local extinction (Aviles 1999; Hauert et al. 2006; Wakano et al. 2009), so that extending our model in this direction may lead to novel insights.
Non-Linearities
Our model can be generalized further by allowing for non-linear social interactions. These non-linearities will arise in our model when phenotypic effects (b and c) are sufficiently large that the effect of each additional altruist on fitness will be either smaller (negative synergism, diminishing returns) or larger (positive synergism, increasing returns) than adding the previous one (Queller 1985). In the neighborhood of the fitness peak, the sign of fitness may even change as altruists of large effect are added. In this case, altruists can invade a population, but cannot fix, leading to the maintenance of stable polymorphisms of “cheaters” and altruists.
Relaxing the weak selection assumption is straightforward, although often tedious, as it introduces a potentially infinite number of higher order identity coefficients that must be quantified (Smith et al 2010). These higher order identity coefficients are typically difficult to compute, and general equations for computing them are currently lacking for most population structures. Non-linearity is more straightforward to model when assuming panmictic populations (reviewed in (Archetti and Scheuring 2011)), where the complication introduced by higher order identity coefficients is avoided, at the cost of reduced generality. Future work should relax our weak selection assumption in order to provide a fully general model.
Emprical Implications
Ironically, the system most commonly used to experimentally test the prediction that local competition impedes altruism evolution is one involving public resource enhancement. The opportunistically pathogenic bacteria, Pseudomonas aeruginosa, cooperates by secreting “siderophores,” diffusible iron-scavenging molecules that increase the local supply of usable iron (West and Buckling 2003; Buckling et al. 2007). Tests of local competition theory in this system (e.g., (Griffin et al. 2004b; Kummerli et al. 2009a)) have not recognized the distinction between resource-based altruism and survival/fecundity altruism. Instead, results from survival/fecundity altruism models (e.g., (Taylor 1992; Gardner and West 2006a)) were imported to provide the conceptual basis for these experiments. Many of these local competition experiments in P. aeruginosa imposed experimental conditions that directly recreated model assumptions (Griffin et al. 2004a; Kummerli et al. 2009a), rather than actively testing these assumptions. That is, instead of introducing factors that cause local competition, such as iron scarcity, they imposed the consequence of local competition, namely strict local density regulation. This experimental treatment prevents differences in local resource enhancement from contributing to differential group productivity, prohibiting positive selection for public resource enhancement. In contrast, in a local competition experiment in this system where selection was not experimenter-imposed (Kummerli et al. 2009b), siderophore-producing strains evolved more readily in more viscous populations, as predicted by our theory but not previous theory (see also Le Gac and Doebeli (2010), who also found that viscosity promotes altruism in experiments with altruistic bacteriocin-production in Escherichia coli). However, this viscosity effect does not offer unambiguous experimental support of our theory because, as noted by Kummerli et al (2009b) as an explanation for why their results did not conform to predictions, siderophore-production may create direct benefits that prevent it from being truly altruistic. As public goods production in microbes typically involves the secretion of diffusible molecules in diffusion-limited microhabitats, direct benefits may be a universal property of resource-based cooperation in microbes (Gore et al. 2009; Driscoll and Pepper 2010; Smith et al. 2010), frustrating attempts at an unequivocal experimental test of local competition theories in these systems.
Altruistic resource-enhancement in P. aeruginosa highlights the importance of eco-evolutionary theories of sociality. Because P. aeruginosa is a common pathogen, one of the leading causes of infection of patients with cystic fibrosis, our understanding of the factors promoting or impeding the evolution of virulence in this and other species has implications for medicine and public policy. Indeed, previous local competition theory has been used to suggest clinical conditions that could increase or decrease virulence evolution by altering local competition (Griffin et al. 2004a; Buckling et al. 2007). When virulence is caused by an altruistic trait, the ecological mode of altruism must be carefully considered before manipulation of local competition is suggested as a means to control pathogens. More generally, future work on social evolution should be clear about the ecological role played by specific social traits in order that the evolutionary forces impinging on these traits are made clear.
Cheater Diversity
Just as there is a diversity of modes of altruism, so too is there necessarily an associated diversity of modes of social conflict in the form of selfish “cheating” strategies. Cheaters create conflict by reaping the benefits of altruism donated by others but suffering none of the costs of contributing to the public good. The relative abundance of cheaters and their ecological mode of action depend on the strength of selection favoring the altruistic strategies on which they cheat. With linear social interactions, the frequency of cheaters in populations is determined by the balance between their rate of introduction (by mutation, migration, etc.) and their rate of removal by kin selection (the “kin selection-mutation balance” (Van Dyken et al. 2011)). When kin selection is weak, cheaters can prevail. Therefore, we predict that resource-based cheating will be common when resources are abundant, because selection favoring resource-based altruism will be weak. Similarly, survival/fecundity cheating will be common when resources are scarce, because these are the conditions where selection favoring survival/fecundity altruism is weakest. Just as altruism types should be clearly classified based on their ecological mode of action, so too should their associated cheaters.
Acknowledgments
The authors would like to thank A. Agrawal and two anonymous reviewers for their many helpful comments, which greatly improved the content and clarity of the manuscript. This work was supported by NIH R01 GM00279912-03 to MJW, and an NSF Postdoctoral Fellowship to JDVD. Portions of this work were inspired by the diversity of social traits discussed in the 2010 NESCent working group, Evolution of Insect Sociality: An Integrative Modeling Approach (J. Hunt, organizer).
Appendix A
Assuming discrete, non-overlapping generations leads to the fact that fitness, the per capita number of offspring produced, is equivalent to the rate of population growth (Crow and Kimura 1970; Roughgarden 1979; Rice 2004). Mean population fitness, W̄, maps the number of individuals in generation t onto the number at t + 1 (Crow and Kimura 1970; Roughgarden 1979; Rice 2004)
| (A1) |
Subtracting N(t) from both sides gives the total change in population size over a generation, and then dividing by N(t) gives the per capita rate of change,
| (A2) |
This is the birth rate minus the death rate, where the death rate equals one as a consequence of the fact that we are modeling discrete, non-overlapping generations so that the probability of death every generation is one.
We may now substitute any model of population growth for fitness. Previous evolutionary models have used the logistic equation (Charlesworth 1971; Roughgarden 1971; Roughgarden 1979; Frank 2010) and Holling’s disc equation (Type II response curve) (Agrawal 2010). Because we are interested in the relationship between fitness and the underlying causal factors of competition, namely resource abundance, we use an explicit consumer-resource model (Monod 1949; Stewart and Levin 1973; Tilman 1982). Doing so, we can write the per capita rate of change in population size as (Tilman 1982),
| (A3) |
where, again, death rate has been set equal to one and is explicitly density-independent (individuals die by the end of each generation regardless of population density). The parameter λ is the maximal rate of population increase, ST is the total resource concentration (often written as R, but not here in order to avoid confusion with the kin selection coefficient of relatedness), kS,T is the half-saturation constant for the total population, and x ∈ {1,2} and y ∈ {0,1} are parameters that modulates A3 between Type I (y = 0, x = 1), Type II (y = 1, x = 1) and Type III (y = 1, x = 2) functional responses.
To get an expression for fitness that is useful for studying evolution in structured populations, we must redefine terms in A3. First, we replace total resource supply, ST, with the per-consumer resource concentration in the ith group, ST,i/ωiNi, where ωi is the crowding factor and Ni is the number of founders of the ith group. As Agrawal (2010) showed, the number of consumers per group depends on the mortality rate and the timing at which mortality occurs with respect to resource consumption. We denote the effect of dead individuals on resources by TD. Following Agrawal (2010), when TD = 0, all deaths occur before the resource consumption phase of the life cycle, while TD = 1 means that all pre-reproductive deaths occur after resource consumption, so that a dead individual has the same effect on resource supply as an individual that survives to reproduction. Thus, we can define the “resource effective number” of group members as ωN, where, ω = v+ (1 − v)TD. We now extend this to allow for life cycles where resource competition occurs among the offspring of group founders before dispersal, and where founders have variable number of offspring. Defining f(t−1) as the fecundity of founders, we now have,
| (A4) |
which we use throughout. In order to be on the proper scale, we replace the total half saturation constant in A3 with the per-capita half-saturation constant, ks,i, where the subscript i indicates that groups may differ in their resource requirements and that the correct value is the mean of the ith group. The competition term is now dimensionless (Healey 1980). Substituting these values into A3, and multiplying through by the crowding term to make it explicit in the function, we have,
| (A5) |
where Si = ST,i/Ni is the per-founder supply of resources at the beginning of a generation.
From Equation A2, we see that fitness corresponds to the first term on the right-hand-side (r.h.s.) of Equation A5. From this, we can now write a function for the fitness of individual j in the ith group:
| (A6) |
Here, W̃ij is the asymptotic fitness of an individual (i.e., fitness when resources are infinite, which is equivalent to the discrete time, per capita intrinsic rate of increase, λ. Asymptotic fitness can be written as a function of individual viability and fecundity, W̃ij = fij vij.
CITATIONS
- Agrawal AF. Ecological determinants of mutation load and inbreeding depression in subdivided populations. The American Naturalist. 2010;176:111–122. doi: 10.1086/653672. [DOI] [PubMed] [Google Scholar]
- Alizon S, Taylor P. Empty sites can promote altruistic behavior. Evolution. 2008;62:1335–1344. doi: 10.1111/j.1558-5646.2008.00369.x. [DOI] [PubMed] [Google Scholar]
- Archetti M, Scheuring I. Review: Game theory of public goods in one-shot social dilemmas without assortment. J Theor Biol. 2011 doi: 10.1016/j.jtbi.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Aviles L. Cooperation and non-linear dynamics: An ecological perspective on the evolution of sociality. Evol Ecol Res. 1999;1:459–477. [Google Scholar]
- Boyd R. Density-dependent mortality and the evolution of social interactions. Animal Behavior. 1982;30:972–982. [Google Scholar]
- Brown SP, Inglis RF, Taddei F. Evolutionary ecology of microbial wars: within-host competition and (incidental) virulence. Evolutionary Applications. 2009;2:32–39. doi: 10.1111/j.1752-4571.2008.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckling A, Harrison F, Vos M, Brockhurst MA, Gardner A, West SA, Griffin A. Siderophore-mediated cooperation and virulence in Pseudomonas aeruginosa. FEMS Microbiol Ecol. 2007;62:135–141. doi: 10.1111/j.1574-6941.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- Buss LW. The Evolution of Individuality. Princeton University Press; Princeton, NJ: 1987. [Google Scholar]
- Chao L, Levin BR. Structured habitats and the evolution of anticompetitor toxins in bacteria. Proc Natl Acad Sci U S A. 1981;78:6324–6328. doi: 10.1073/pnas.78.10.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B. Selection in Density-Regulated Populations. Ecology. 1971;52:469–474. [Google Scholar]
- Costa JT. The Other Insect Societies. Harvard University Press; Cambridge, MA: 2006. [Google Scholar]
- Creel S, Creel NM. Communal hunting and pack size in African wild dogs, Lycaon pictus. Animal Behavior. 1995;50:1325–1339. [Google Scholar]
- Crespi BJ. The evolution of social behavior in microorganisms. Trends Ecol Evol. 2001;16:178–183. doi: 10.1016/s0169-5347(01)02115-2. [DOI] [PubMed] [Google Scholar]
- Crow JF, Kimura M. An Introduction to Population Genetics Theory. Harper & Row; New York: 1970. [Google Scholar]
- Diamond JM. Guns, Germs, and Steel. W.W. Norton & Co; New York: 1997. [Google Scholar]
- Driscoll WW, Pepper JW. Theory for the evolution of diffusible external goods. Evolution. 2010;64:2682–2687. doi: 10.1111/j.1558-5646.2010.01002.x. [DOI] [PubMed] [Google Scholar]
- Dugatkin LA. Cooperation among animals: an evolutionary perspective. Oxford University Press; Oxford: 1997. [Google Scholar]
- Fanshawe JH, Fitzgibbon CD. Factors influencing the hunting success of an African wild dog pack. Anim Behav. 1993;45:479–490. [Google Scholar]
- Frank SA. The genetic value of sons and daughters. Heredity. 1986;56:351–354. doi: 10.1038/hdy.1986.56. [DOI] [PubMed] [Google Scholar]
- Frank SA. Foundations of Social Evolution. Princeton University Press; Princeton, NJ: 1998. [Google Scholar]
- Frank SA. Demography and the tragedy of the commons. J Evol Biol. 2010;23:32–39. doi: 10.1111/j.1420-9101.2009.01893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A, West SA. Demography, altruism, and the benefits of budding. Journal of Evolutionary Biology. 2006a;19:1707–1716. doi: 10.1111/j.1420-9101.2006.01104.x. [DOI] [PubMed] [Google Scholar]
- Gardner A, West SA. Demography, altruism, and the benefits of budding. J Evol Biol. 2006b;19:1707–1716. doi: 10.1111/j.1420-9101.2006.01104.x. [DOI] [PubMed] [Google Scholar]
- Gause GF. The Struggle for Existence. Williams and Wilkins; Baltimore, Md: 1934. [Google Scholar]
- Gore J, Youk H, van Oudenaarden A. Snowdrift game dynamics and facultative cheating in yeast. Nature. 2009;459:253–256. doi: 10.1038/nature07921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafen A. Natural selection, kin selection, and group selection. In: Krebs JR, Davies NB, editors. Behav Ecol. Blackwell Scientific Publications; Oxford: 1984. pp. 62–84. [Google Scholar]
- Grafen A, Archetti M. Natural selection of altruism in inelastic viscous homogeneous populations. Journal of Theoretical Biology. 2008;252:694–710. doi: 10.1016/j.jtbi.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Greig D, Travisano M. The Prisoner’s Dilemma and polymorphism in yeast SUC genes. P Roy Soc Lond B Bio. 2004;271:S25–S26. doi: 10.1098/rsbl.2003.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004a;430:1024–1027. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004b;430:1024–1027. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- Gurney WSC, Lawton JH. The population dynamics of ecosystem engineers. Oikos. 1996;76:273–283. [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour. I Journal of Theoretical Biology. 1964;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Hauert C, Holmes M, Doebeli M. Evolutionary games and population dynamics: maintenance of cooperation in public goods games. Proc Biol Sci. 2006;273:3131–3132. doi: 10.1098/rspb.2006.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes K, O’Connell JF, Blurton Jones NG. Hadza women’s time allocation, offspring provisioning, and the evolution of long postmenopausal life spans. Current Anthropology. 1997;38:551–577. [Google Scholar]
- Healey FP. Slope of the Monod equation as an indicator of advantage in nutrient competition. Microbial Ecology. 1980;5:281–286. doi: 10.1007/BF02020335. [DOI] [PubMed] [Google Scholar]
- Holldobler B, Wilson EO. The Ants. Harvard University Press; Cambridge, MA: 1990. [Google Scholar]
- Holldobler B, Wilson EO. The Superorganism: The Beauty, Elegance, and Strangeness of Insect Societies. W.W. Norton & Co; New York: 2009. [Google Scholar]
- Holling CS. Some characteristics of simple types of predation and parasitism. The Canadian Entomologist. 1959;91:385–398. [Google Scholar]
- Jones CG, Lawton JH, Shachak M. Organisms as ecosystem engineers. Oikos. 1994;69:373–386. [Google Scholar]
- Kelly JK. Restricted Migration and the Evolution of Altruism. Evolution. 1992;46:1492–1495. doi: 10.1111/j.1558-5646.1992.tb01139.x. [DOI] [PubMed] [Google Scholar]
- Kelly JK. The effect of scale dependent processes on kin selection: mating and density regulation. Theor Popul Biol. 1994a;46:32–57. doi: 10.1006/tpbi.1994.1018. [DOI] [PubMed] [Google Scholar]
- Kelly JK. A model for the evolution of communal foraging in hierarchically structured populations. Behav Ecol Sociobiol. 1994b;35:205–212. [Google Scholar]
- Kerr B, Schwilk DW, Bergman A, Feldman MW. Rekindling an old flame: A haploid model for the evolution and impact of flammability in resprouting plants. Evol Ecol Res. 1999;1:807–833. [Google Scholar]
- Kummerli R, Gardner A, West SA, Griffin AS. Limited dispersal, budding dispersal, and cooperation: an experimental study. Evolution. 2009a;63:939–949. doi: 10.1111/j.1558-5646.2008.00548.x. [DOI] [PubMed] [Google Scholar]
- Kummerli R, Griffin AS, West SA, Buckling A, Harrison F. Viscous medium promotes cooperation in the pathogenic bacterium Pseudomonas aeruginosa. Proc Biol Sci. 2009b;276:3531–3538. doi: 10.1098/rspb.2009.0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laland KN, Odling-Smee FJ, Feldman MW. The evolutionary consequences of niche construction: A theoretical investigation using two-locus theory. Journal of Evolutionary Biology. 1996;9:293–316. [Google Scholar]
- Le Gac M, Doebeli M. Environmental viscosity does not affect the evolution of cooperation during experimental evolution of colicigenic bacteria. Evolution. 2010;64:522–533. doi: 10.1111/j.1558-5646.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- Lehmann L. The evolution of trans-generational altruism: kin selection meets niche construction. Journal of Evolutionary Biology. 2007;20:181–189. doi: 10.1111/j.1420-9101.2006.01202.x. [DOI] [PubMed] [Google Scholar]
- Lehmann L. The adaptive dynamics of niche constructing traits in spatially subdivided populations: Evolving posthumous extended phenotypes. Evolution. 2008;62:549–566. doi: 10.1111/j.1558-5646.2007.00291.x. [DOI] [PubMed] [Google Scholar]
- Lehmann L. Space-Time Relatedness and Hamilton’s Rule for Long-Lasting Behaviors in Viscous Populations. Am Nat. 2010;175:136–143. doi: 10.1086/648554. [DOI] [PubMed] [Google Scholar]
- Lehmann L, Perrin N, Rousset F. Population demography and the evolution of helping behaviors. Evolution. 2006;60:1137–1151. [PubMed] [Google Scholar]
- Lehmann L, Rousset F. How life history and demography promote or inhibit the evolution of helping behaviours. Phil Trans R Soc B. 2010;365:2599–2617. doi: 10.1098/rstb.2010.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lion S, Gandon S. Habitat saturation and the spatial evolutionary ecology of altruism. Journal of Evolutionary Biology. 2009;22:1487–1502. doi: 10.1111/j.1420-9101.2009.01769.x. [DOI] [PubMed] [Google Scholar]
- Lion S, Gandon S. Life history, habitat saturation and the evolution of fecundity and survival altruism. Evolution. 2010;64:1594–1606. doi: 10.1111/j.1558-5646.2009.00933.x. [DOI] [PubMed] [Google Scholar]
- Lynch M, Walsh B. Genetics and Analysis of Quantitative Characters. Sinauer Associates, Inc; Sunderland, MA: 1998. [Google Scholar]
- MacArthur RH, Wilson EO. The theory of island biogeography. Princeton University Press; Princeton, NJ: 1967. [Google Scholar]
- Marlowe FW. A critical period for provisioning by Hadza men: Implications for pair bonding. Evolution and Human Behavior. 2003;24:217–229. [Google Scholar]
- Michod RE, Hamilton WD. Coefficients of relatedness in sociobiology. Nature. 1980;288:694–697. [Google Scholar]
- Mitteldorf J, Wilson DS. Population viscosity and the evolution of altruism. Journal of Theoretical Biology. 2000;204:481–496. doi: 10.1006/jtbi.2000.2007. [DOI] [PubMed] [Google Scholar]
- Monod J. The growth of bacterial populations. Annu Rev Microbiol. 1949;3:371–394. [Google Scholar]
- Mueller UG, Gerardo NM, Aanen DK, Six DL, Schultz TR. The evolution of agriculture in insects. Annual Review of Ecology, Evolution and Systematics. 2005;36:563–595. [Google Scholar]
- Murdoch WW, Briggs CJ, Nisbet RM. Consumer-resource dynamics. Princeton University Press; Princeton, NJ: 2003. [Google Scholar]
- Murdoch WW, Kendall BE, Nisbet RM, Briggs CJ, Mccauley E, Bolser R. Singe-species models for many-species food webs. Nature. 2002;417:541–543. doi: 10.1038/417541a. [DOI] [PubMed] [Google Scholar]
- Nakamaru M, Matsuda H, Iwasa Y. The evolution of cooperation in a lattice-structured population. Journal of Theoretical Biology. 1997;184:65–81. doi: 10.1006/jtbi.1996.0243. [DOI] [PubMed] [Google Scholar]
- Nowak MA, May RM. Evolutionary games and spatial chaos. Nature. 1992;359:826–829. [Google Scholar]
- Odling-Smee FJ, Laland KN, Feldman MW. Niche construction. The American Naturalist. 1996;147:641–648. [Google Scholar]
- Odling-Smee FJ, Laland KN, Feldman MW. Niche Construction: The Neglected Process in Evolution. Princeton University Press; Princeton: 2003. [Google Scholar]
- Ohtsuki H, Hauert C, Lieberman E, Nowak MA. A simple rule for the evolution of cooperation on graphs and social networks. Nature. 2006;441:502–505. doi: 10.1038/nature04605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto SP, Day T. A Biologist’s Guide to Mathematical Modeling in Ecology and Evolution. Princeton University Press; Princeton: 2007. [Google Scholar]
- Page RE, Scheiner R, Erber J, Amdam GV. The development and evolution of division of labor and foraging specialization in a social insect (Apis mellifera L.) Curr Top Dev Biol. 2006;74:253–286. doi: 10.1016/S0070-2153(06)74008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper JW. Relatedness in Trait Group Models of Social Evolution. Journal of Theoretical Biology. 2000;206:355–368. doi: 10.1006/jtbi.2000.2132. [DOI] [PubMed] [Google Scholar]
- Platt TG, Bever JD. Kin competition and the evolution of cooperation. Trends in Ecology & Evolution. 2009;24:370–377. doi: 10.1016/j.tree.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GR. Selection and Covariance. Nature. 1970;227:520–521. doi: 10.1038/227520a0. [DOI] [PubMed] [Google Scholar]
- Queller DC. Kinship, reciprocity and synergism in the evolution of social behaviour. Nature. 1985;318:366–367. [Google Scholar]
- Queller DC. Genetic relatedness in viscous populations. Evolutionary Ecology. 1994;8:70–73. [Google Scholar]
- Queller DC, Strassmann JE. Kin selection and social insects. BioScience. 1998;48:165–175. [Google Scholar]
- Rice SH. Evolutionary theory: mathematical and conceptual foundations. Sinauer; Sunderland, MA: 2004. [Google Scholar]
- Robertson A. A mathematical model of the culling process in dairy cattle. Anim Prod. 1966;8:95–108. [Google Scholar]
- Roughgarden J. Density-Dependent Natural Selection. Ecology. 1971;52:453–468. [Google Scholar]
- Roughgarden J. Theory of population genetics and evolutionary ecology: An introduction. Prentice Hall; Upper Saddle River, NJ: 1979. [Google Scholar]
- Rousset F. Genetic Structure and Selection in Subdivided Populations. Princeton University Press; Princeton, NJ: 2004. [Google Scholar]
- Shellman-Reeve JS, Choe JC, Crespi BJ. The Evolution of Social Behavior in Insects and Arachnids. Cambridge University Press; Cambridge: 1997. The spectrum of eusociality in termites; pp. 52–93. [Google Scholar]
- Smith J, Van Dyken JD, Zee P. A generalization of Hamilton’s rule for the evolution of microbial cooperation. Science. 2010;328:1700–1703. doi: 10.1126/science.1189675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr CK. Enabling mechanisms in the origin of sociality in the Hymenoptera--The sting’s the thing. Annals of the Entomological Society of America. 1985;78:836–840. [Google Scholar]
- Stewart FM, Levin BR. Partitioning of resources and the outcome of interspecific competition: A model and some general considerations. The American Naturalist. 1973;107:171–198. [Google Scholar]
- Taylor P. Altruism in viscous populations — an inclusive fitness model. Evolutionary Ecology. 1992;6:352–356. [Google Scholar]
- Taylor P. Birth-death symmetry in the evolution of a social trait. Journal of Evolutionary Biology. 2011;23:2569–2578. doi: 10.1111/j.1420-9101.2010.02122.x. [DOI] [PubMed] [Google Scholar]
- Tilman D. Resource competition and community structure. Princeton University Press; Princeton, NJ: 1982. [PubMed] [Google Scholar]
- van Baalen M, Rand DA. The unit of selection in viscous populations and the evolution of altruism. Journal of Theoretical Biology. 1998;193:631–648. doi: 10.1006/jtbi.1998.0730. [DOI] [PubMed] [Google Scholar]
- Van Dyken JD. The components of kin competition. Evolution. 2010;64:2840–2854. doi: 10.1111/j.1558-5646.2010.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyken JD, Linksvayer TA, Wade MJ. Kin selection-mutation balance: A model for the origin, maintenance, and consequences of social cheating. The American Naturalist. 2011;177:288–300. doi: 10.1086/658365. [DOI] [PubMed] [Google Scholar]
- Velicer GJ, Mendes-Soares H. Bacterial predators. Curr Biol. 2009;19:R55–56. doi: 10.1016/j.cub.2008.10.043. [DOI] [PubMed] [Google Scholar]
- Wade MJ. Soft Selection, Hard Selection, Kin Selection, and Group Selection. The American Naturalist. 1985;125:61–73. [Google Scholar]
- Wakano JY, Nowak MA, Hauert C. Spatial dynamics of ecological public goods. Proc Natl Acad Sci U S A. 2009;106:7910–7914. doi: 10.1073/pnas.0812644106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West SA, Buckling A. Cooperation, virulence and siderophore production in bacterial parasites. Proc Biol Sci. 2003;270:37–44. doi: 10.1098/rspb.2002.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock MC. Selection, load and inbreeding depression in a large metapopulation. Genetics. 2002;160:1191–1202. doi: 10.1093/genetics/160.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock MC, Davis BH, Yeaman S. The costs and benefits of resource sharing: reciprocity requires resource heterogeneity. J Evol Biol. 2007;20:1772–1782. doi: 10.1111/j.1420-9101.2007.01387.x. [DOI] [PubMed] [Google Scholar]
- Wilson D, Pollock G, Dugatkin L. Can altruism evolve in purely viscous populations? Evolutionary Ecology. 1992;6:331–341. [Google Scholar]
- Wilson EO. One giant leap: How insects achieved altruism and colonial life. BioScience. 2008;58:17–25. [Google Scholar]
- Wolf JB, Wade MJ. On the assignment of fitness to parents and offspring: whose fitness is it and when does it matter? Journal of Evolutionary Biology. 2001;14:347–356. [Google Scholar]
- Wright S. Evolution in Mendelian populations. Genetics. 1931;16:97–159. doi: 10.1093/genetics/16.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. The genetical structure of populations. Annals of Eugenics. 1951;15:323–354. doi: 10.1111/j.1469-1809.1949.tb02451.x. [DOI] [PubMed] [Google Scholar]
- Wright S. The Interpretation of Population Structure by F-Statistics with Special Regard to Systems of Mating. Evolution. 1965;19:395–420. [Google Scholar]
- Yip EC, Powers KS, Aviles L. Cooperative capture of large prey solves scaling challenge faced by spider societies. Proc Natl Acad Sci U S A. 2008;105:11818–11822. doi: 10.1073/pnas.0710603105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yodzis P, Innes S. Body size and consumer-resource dynamics. The American Naturalist. 1992;139:1151–1175. [Google Scholar]