Abstract
Oxyntomodulin (OXM) is a peptide secreted postprandially from the L-cells of the gut that has a weak affinity for both the glucagon-like peptide-1 receptor (GLP1R) and the glucagon receptor (GCGR). Peripheral administration of OXM in humans and rodents causes weight loss reducing food intake and increasing energy expenditure. It has been suggested that OXM modulates energy intake solely through GLP1R agonism. Because glucagon decreases food intake in rodents and humans, we examined whether activation of the GCGR is involved in the body weight-lowering effects of OXM. We identified an equipotent GLP1R-selective peptide agonist that differs from OXM by only one residue (Q3→E, OXMQ3E), but has no significant GCGR agonist activity in vitro and ~100-fold reduced ability to stimulate liver glycogenolysis. Chronic treatment of obese mice with OXM and OXMQ3E demonstrated that OXM exhibits superior weight loss and lipid-lowering efficacy, and antihyperglycemic activity that is comparable to the corresponding GLP1R-selective agonist. Studies in Glp1r−/− mice and coadministration of OXM and a GCGR antagonist revealed that the antiobesity effect of OXM requires activation of both GLP1R and GCGR. Our data provide new insight into the mechanism of action of OXM and suggest that activation of GCGR is involved in the body weight-lowering action of OXM.
Repeated subcutaneous administration of oxyntomodulin (OXM) causes weight loss in obese patients (1) and rodents (2,3), where OXM reduces body weight via suppression of food intake and increases in energy expenditure (3,4). OXM, a longer isoform of glucagon that is co-secreted together with GLP-1 from L-cells in the gut, is a dual glucagon-like peptide-1 receptor (GLP1R)/glucagon receptor (GCGR) agonist in vitro (5,6,7). It has been proposed that OXM modulates energy homeostasis solely by GLP1R agonism, since its acute anorectic effects in rodents are abolished by coadministration of the GLP1R antagonist exendin (9–39) and are not observed in Glp1r−/− mice (3,8,9). However, the weak affinity of OXM for GLP1R compared to the cognate ligand GLP-1 (2,6) does not explain the effect of OXM on body weight. Other effects of OXM including its improvement of β-cell function and stimulation of heart rate and energy expenditure appear to be independent of GLP1R signaling (3,8,10), suggesting the involvement of other signaling pathways. That glucagon decreases food intake and increases energy expenditure is well established (11,12,13,14,15,16) suggesting a potential role for GCGR signaling in the action of OXM. OXM activates the GLP1R in vitro with ~100-fold reduced agonist potency compared to GLP-1 (6). OXM and GLP-1 have different half-lifes in vivo (T1/2 ~12 and 2–3 min, respectively) (17,18). These differences in receptor potencies and pharmacokinetics limit any mechanistic insight into the relative contributions of GLP1R activation and GCGR signaling to the effect of OXM that may be gained by direct comparison of OXM to GLP-1 in vivo. As such, we identified an OXM analog, OXMQ3E, that differs from OXM by only one residue (Q3→E). This peptide retains comparable GLP1R agonism and pharmacokinetics to OXM but has no significant GCGR agonist activity in vitro and has ~100-fold reduced ability to stimulate ex-vivo glycogenolysis in perfused mouse liver. The objective of this study was to leverage the matched GLP1R agonist potencies and pharmacokinetics of OXM and OXMQ3E to investigate the therapeutic potential of OXM and the contribution of each receptor to the body weight-lowering effect of OXM in a mouse model of obesity.
Methods and Procedures
Animals
Experiments were performed in diet-induced obese (DIO) wild-type or human GCGR (hGCGR) (19) C57BL/6 mice and in weight- and sex-matched Glp1r−/− (20) or lean C57BL/6 control mice. Mice were housed in Tecniplast cages (obese mice single-housed, Glp1r−/− mice housed 4 per cage) in a conventional Specific (SPF) pathogen-free facility. All mice were obtained from Taconic Farms (Germantown, NY) and were maintained on either regular chow (Harlan Teklad 7012; Harlan Teklad, Madison, WI) or a high-fat diet (D12492: 60% kcal from fat; Research Diets, New Brunswick, NJ or S3282: 58% kcal from fat; Bio-Serv, Frenchtown, NJ) in a 12-h light/12-h dark cycle. Animal protocols used in these studies were approved by the Merck Research Laboratories Institutional Animal Care and Use Committee in Rahway, NJ.
Peptides
GLP-1 and glucagon were purchased from Anaspec (Fremont, CA). OXM and OXMQ3E were synthesized by solid-phase synthesis and purified by reverse-phase high-performance liquid chromatography using water/acetonitrile (0.1% trifluoroacetic acid) gradients. Purified peptides were characterized by electrospray MS on a Micromass LCZ platform spectrometer.
Determination of mouse GLP1R and GCGR potency
In vitro agonist potencies of peptides were determined in Chinese hamster ovary cells stably expressing murine GLP1R or murine GCGR using the Cisbio cAMP Dynamic 2 assay. Peptides were diluted in assay buffer and incubated with cells. The assay was terminated with the addition of the Cisbio detection reagents as per the manufacturer's instructions. cAMP was quantified by measuring the decrease in time-resolved fluorescence energy transfer using an EnVision platereader (PerkinElmer, Waltham, MA).
Ex-vivo liver glycogenolysis assays
The ability of OXM or OXMQ3E to stimulate glycogen breakdown was evaluated ex vivo in perfused livers harvested from C57BL/6 mice using a 13C- nuclear magnetic resonance-based assay as previously described (6).
Measurement of plasma peptide exposures
Mice were anesthetized with isofluorane, and blood was collected by cardiocentesis into EDTA-coated microtainer tubes containing DPP-4 inhibitor (Linco/Millipore, Billerica, MA) and aprotinin (Sigma, St Louis, MO). The in vitro cell-based cAMP bioassay for determining GLP1R agonist potency was used with Chinese hamster ovary cells stably transfected with GLP1R to determine peptide concentrations as previously described (6).
Chronic studies
Mice were anesthetized with isofluorane and a micro-osmotic pump (Alzet; Durect, Cupertino, CA) designed to deliver 0.5 µl/h was implanted subcutaneously in the intrascapular space.
Study 1—Chronic food intake and body composition study in DIO mice. Thirty-seven 23-week-old male DIO mice (body weight ~ 44 g, diet D12492) were infused subcutaneous with OXM (1.1 µmol·kg–1·day–1), OXMQ3E (1.1 µmol·kg–1·day–1), or vehicle (buffer for glucagon formulation; Bell Medical Services, Marlboro, NJ) for 14 days. Body composition was determined using Quantitative Magnetic Resonance (EchoMRI; Echo Medical Systems, Houston, TX).
Study 2—OXM and OXMQ3E in Glp1r−/− mice. Weight-matched (~30 g) wild-type (n = 16) and Glp1r−/− (n = 16) mice were infused with vehicle, OXM (1.1 µmol·kg–1·day–1), or OXMQ3E (1.1 µmol·kg–1·day–1) for 12 days.
Study 3—OXM and glucagon receptor antagonist in hGCGR mice. Forty-eight 27-week-old male hGCGR DIO mice (body weight ~ 49 g, diet S3282) were infused with vehicle, OXM (1.1 µmol·kg–1·day–1) or OXMQ3E (1.1 µmol·kg–1·day–1) in the presence or absence of Cpd A (21) (10 mg/kg) in feed for 14 days.
Biochemical analyses
Insulin and leptin were measured by ELISA (Linco/Millipore). Free-fatty acids and ketone bodies were measured using commercial enzyme-coupled spectrophotometric assays (Wako Chemicals, Richmond, VA). Plasma triglyceride and total cholesterol were determined using an Olympus AU400e Bioanalyzer (Mishima Olympus, Shizooka-Ken, Japan). Adiponectin was measured with a mouse adiponectin RIA kit (Linco/Millipore). Blood glucose levels were measured using a OneTouch glucometer (LifeScan, Milpitas, CA).
Statistical analysis
All data are presented as mean ± SE. Comparisons among groups were made using ANOVA or unpaired Student's t-test, as appropriate. P < 0.05 was regarded as statistically significant.
Results
OXM is a GLP1R/GCGR coagonist and OXMQ3E is an equipotent GLP1R agonist
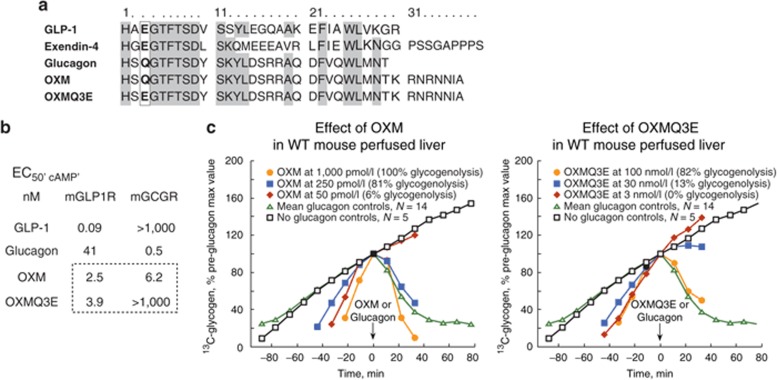
Glucagon and OXM, the only members of the glucagon superfamily that activate GCGR, incorporate a neutral polar residue at position 3 (glutamine, “Q”). In contrast, GLP-1 and the GLP-1 mimetic exendin-4 exhibit no significant GCGR agonist activity and incorporate an acidic residue (glutamate, “E”) at this position (Figure 1a–b). A Q3→E substitution was introduced in native OXM to generate OXMQ3E. The in vitro agonist potencies of these peptides showed that OXM is a full agonist with similar agonist functional potencies at murine GLP1R (mGLP1R, EC50, cAMP = 2.5 nmol/l) and murine GCGR (mGCGR, EC50, cAMP = 6.2 nmol/l). OXMQ3E exhibited comparable agonist potency for mGLP1R (EC50, cAMP = 3.9 nmol/l) but was devoid of significant agonist or antagonist activity at mGCGR at concentrations up to 1 µmol/l (Figure 1b). As an independent measure of mGCGR engagement, we compared the ability of the two peptides to stimulate glycogenolysis in perfused liver obtained from wild-type mice (Figure 1c). Glucagon (EC50, glyco 50 pmol/l) potently induced complete glycogenolysis while comparable glycogenolysis with OXM required higher peptide concentrations, consistent with the mGCGR agonist potency of OXM. The OXM concentration required for half-maximal glycogenolysis (EC50, glyco) was ~0.25 nmol/l. OXMQ3E exhibited >200-fold reduced glycogenolysis activity (EC50, glyco ~50 nmol/l), consistent with its significantly weaker mGCGR agonist potency relative to OXM in vitro.
Figure 1.
Mutation of glutamine (Q) to glutamate (E), at position 3 in the native sequence of oxyntomodulin, abolishes glucagon receptor (GCGR) agonist activity. (a) Sequence alignment of oxyntomodulin (OXM) and related peptides. Conserved residues are highlighted. (b) In vitro potencies (EC50, CAMP) against mGLP1R and mGCGR, and (c) effect of OXM and OXMQ3E on ex-vivo glycogenolysis in perfused mouse liver. m, murine.
Chronic effects of OXM and OXMQ3E on food intake and body weight in DIO mice
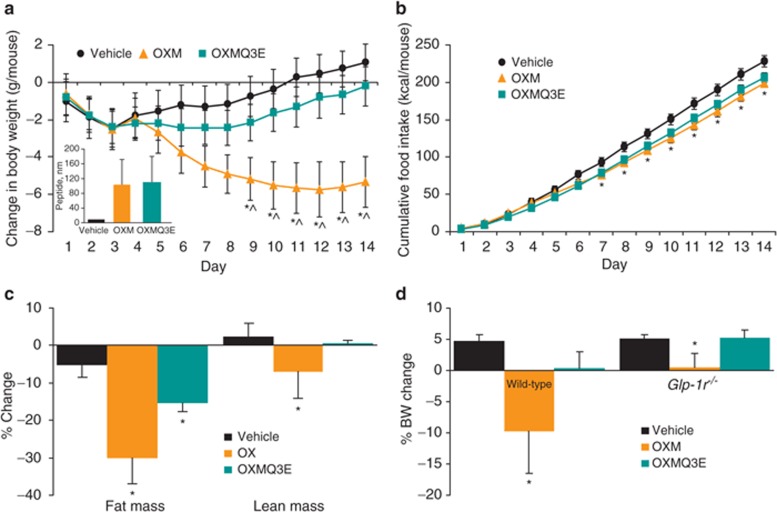
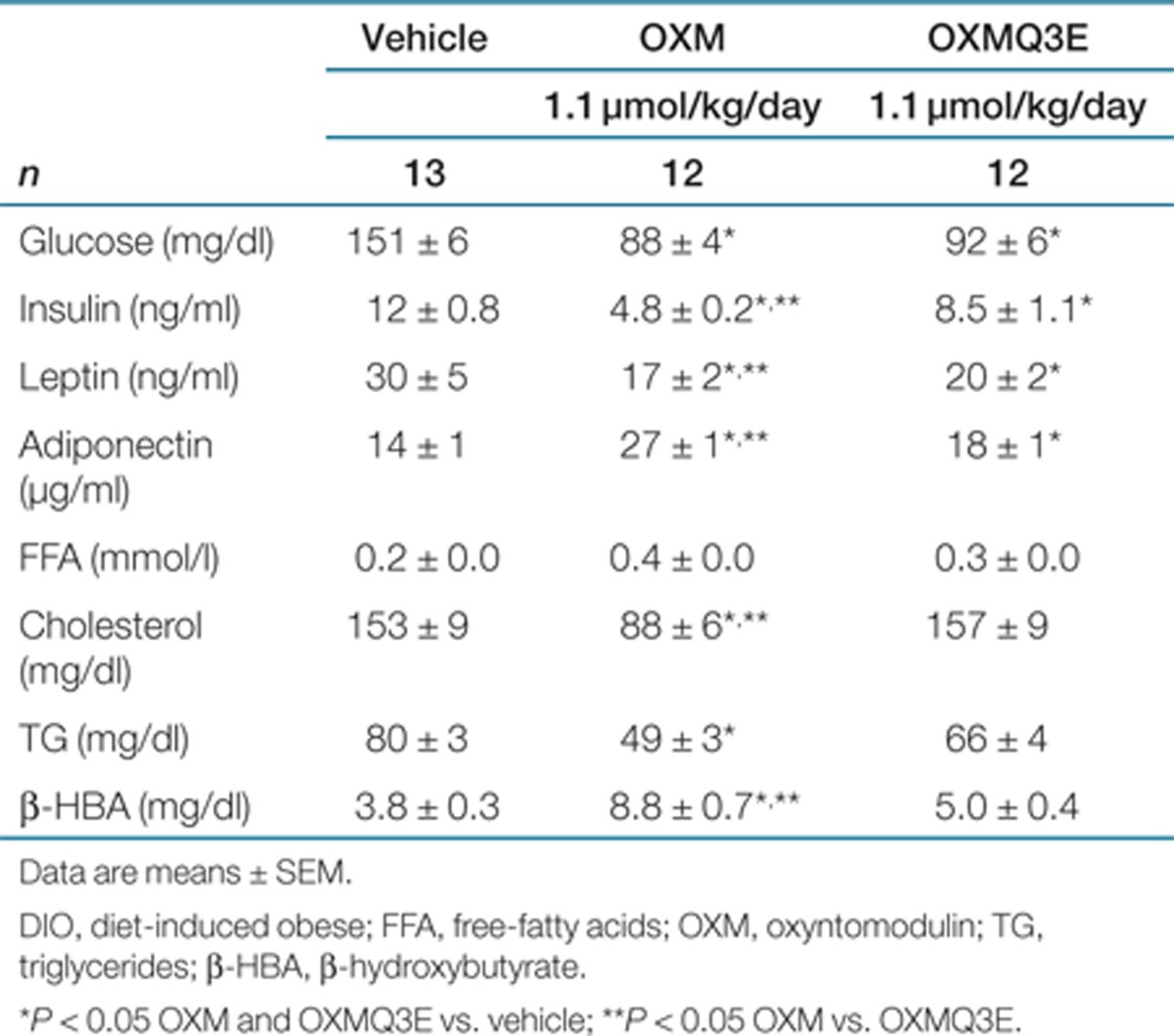
The effect of chronic administration of OXM and OXMQ3E on body weight and food intake was compared in DIO mice. Vehicle or each peptide was infused subcutaneously via osmotic minipumps. As depicted in Figure 2a, OXM exhibited superior weight loss efficacy compared to vehicle (14% reduction at the end of the study, P < 0.05 vs. vehicle) and OXMQ3E (11% reduction, P < 0.05 vs. OXMQ3E) in the presence of similar plasma peptide concentrations (Figure 2a, inset). Chronic treatment with OXMQ3E modestly lowered body weight (3% reduction, P < 0.05 vs. vehicle). OXM and OXMQ3E lowered cumulative food intake to a similar extent (Figure 2b, P < 0.05 OXM and OXMQ3E vs. vehicle). Quantitative magnetic resonance measurements of body composition revealed that the weight loss was driven primarily by decreased fat mass (Figure 2c). At the end of the chronic treatment, ambient glucose was significantly reduced in OXM- and OXMQ3E-treated animals (Table 1). A significant improvement in plasma metabolic parameters was observed in the OXM-treated group, and to a lesser extent, in OXMQ3E-treated animals. Increased plasma adiponectin and decreased leptin and insulin levels correlated well with the observed decrease in body fat and were also associated with an improved lipid profile (Table 1).
Figure 2.
Oxyntomodulin reduces body weight to a greater extent than OXMQ3E. (a) Oxyntomodulin (OXM) treatment displays superior body weight-lowering efficacy following chronic subcutaneous (s.c.) infusion in diet-induced obese (DIO) mice. Similar plasma exposures of peptide OXM and OXMQ3E were achieved in the study (inset). (b) Cumulative food intake during the study. (c) Change in body composition. (d) Body weight changes in wild-type or Glp1r−/− mice infused with OXM or OXMQ3E for 14 days. *P < 0.05 vs. vehicle; ^P < 0.05 OXM vs. OXMQ3E.
Table 1. Chronic treatment with OXM and OXMQ3E in DIO mice: plasma determinations at the end of the chronic study.
Chronic infusion of OXM decreases body weight in Glp1r−/− mice
Lean wild-type and weight-matched mice (~30 g) lacking the GLP1R (Glp1r−/−) (20), were infused subcutaneously with OXM, OXMQ3E, or vehicle for 12 days. As demonstrated in DIO mice, chronic OXM infusion in lean mice also resulted in superior weight loss vs. OXMQ3E and vehicle (P < 0.05 OXM vs. vehicle and OXMQ3E, Figure 2d). The efficacy of OXM was partially attenuated in Glp1r−/− mice, where OXM prevented the weight-gain observed in vehicle-treated animals (+0.2 ± 2% vs. +5.4 ± 0.8%, OXM vs. vehicle, P < 0.05, Figure 2d). In contrast, the effects of OXMQ3E were completely ablated in Glp1r−/− mice, confirming its GLP1R-selective nature (Figure 2d).
The body weight-lowering effects of OXM are mediated by GLP-1 and GCG receptors
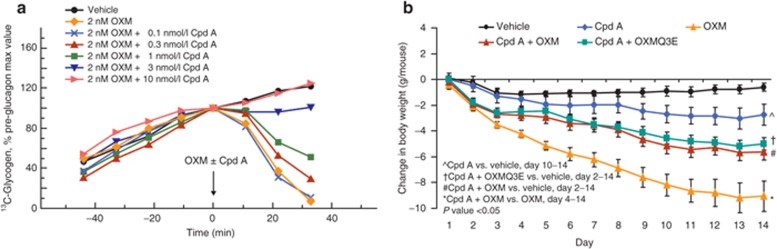
DIO mice expressing the hGCGR (19) were chronically infused with OXM or OXMQ3E in the presence of a small-molecule GCGR antagonist in feed. Compound (Cpd) A (21) inhibits OXM-dependent glycogenolysis in intact perfused liver obtained from hGCGR mice (IC50 = 2 µmol/l, Figure 3a) showing that it has the ability to block OXM-induced hGCGR activation. Chronic infusion with OXM for 14 days resulted in a significant decrease in body weight (–9.1 ± 1.2 g vs. –0.6 ± 0.3 g, OXM vs. vehicle, P < 0.05, Figure 3b). While administration of Cpd A in feed (10 mg/kg) led to a slight but significant reduction in body weight versus vehicle-treated animals (–2.8 ± 0.8 vs. –0.6 ± 0.3 g, Cpd A vs. vehicle, P < 0.05), Cpd A reduced the body weight-lowering effect of OXM to that of OXMQ3E (–5.6 ± 0.6 g vs. –5.0 ± 0.5 g, P = NS, Figure 3b).
Figure 3.
Cpd A reduces the body weight lowering effect of OXM. (a) Cpd A dose-dependently inhibited oxyntomodulin (OXM)-mediated glycogenolysis in perfused liver from human glucagon receptor (GCGR) mice. (b) Body weight effect of OXM, OXMQ3E, and Cpd A in hGCGR mice. m, murine; *P < 0.05 vs. vehicle.
Discussion
The anorectic effect of GLP1R agonists in mice requires activation of GLP1R (20). This effect seems to involve direct and indirect regulation of satiety centers, inhibition of gastric emptying, and modulation of aversive signaling pathways (20,22,23,24). Much less is known about OXM. Although OXM inhibits food intake in rodents (2,3) as well as in human subjects (1), the precise receptors and signaling pathways mediating the anorectic action of OXM remain uncertain.
Although OXM has been reported to be a weak activator of the GLP1R and GCGR in vitro (5,7), it has been proposed that the acute effects of OXM on food intake is mediated solely via GLP1R activation because it is abolished by coadministration of the GLP1R antagonist exendin (9–39) and is absent in Glp1r−/− mice (3,10,25). The contribution of GCGR activation or involvement of an unidentified OXM-specific receptor, however, cannot be discounted when considering several aspects of OXM action, including chronic weight loss. Indeed, several reports highlight differences between GLP-1 and OXM. OXM and GLP-1 have been reported to differentially regulate energy expenditure; imaging studies and c-fos labeling have revealed differential neuronal activation in the hypothalamus of treated rodents (3,26,27). Consistent with these observations, Dakin et al. reported that GLP1R antagonism in the arcuate nucleus of the hypothalamus selectively reverses the anorectic effects of OXM but not of GLP-1, which acts mainly through the vagus and brainstem (25). Furthermore, OXM exhibits positive effects on β cells of Glp1r−/− mice (10). These data suggest that OXM has both GLP1R-dependent and independent effects in vivo. Herein we confirm and expand previous observations showing that OXM activates the GCGR in vitro and then show that OXM but not OXMQ3E can stimulate ex-vivo glycogenolysis in DIO mice. We then compared the antiobesity effects of OXM to that of a GLP1R agonist, OXMQ3E, which exhibits similar GLP1R agonist potency and plasma exposures to OXM, during chronic studies. We show that chronic infusion with OXM exerts superior weight loss and lipid lowering and comparable glucose lowering to OXMQ3E in DIO mice. This was associated with reductions in insulin, leptin, and increases in adiponectin, typically more pronounced upon chronic treatment with OXM than with OXMQ3E. We tested OXM in lean Glp1r−/− mice replicating previous findings (4,6) which showed that acute treatment of OXM suppresses body weight in wild type but not in Glp1r−/− mice (data not shown). We demonstrated that chronic infusion of OXM decreases body weight gain in Glp1r−/− mice, although the effect was reduced compared to wild-type mice, suggesting that the body weight-lowering action of OXM is not entirely mediated by activation of the GLP1R. The lack of efficacy observed following a single injection of OXM in Glp1r−/− mice may be explained by the fact that OXM is labile in vivo (14), hence the acute anorectic effect of OXM could be confounded by compensatory mechanisms associated with chronic deletion of the GLP1R (12) and/or, more likely, that the energy expenditure component of GCGR activation may take longer to exert its body weight-lowering effect (11). We then blocked the GCGR during OXM infusion with a selective pharmacological antagonist, demonstrating that the additional body weight lowering observed with OXM vs. OXMQ3E is, at least in part, due to activation of the GCGR. A caveat of this study that may limit the interpretation of each receptor's contribution to the body weight-lowering effect of OXM is the body weight reduction observed in animals treated with Cpd A in feed. The dietary manipulation together with the elevation of endogenous GLP-1 observed during chronic GCGR blockade (28,29) may explain the reduction in body weight. The superior weight loss efficacy of OXM vs. GLP1R agonism, is consistent with previous research on glucagon and energy homeostasis in humans and rodents (6,12,13,14,15,16). A critical role of glucagon in the regulation of body weight is further supported by a recent study performed in T2D patients where a dose-dependent increase in body weight was observed following chronic pharmacological blockade of the GCGR (30).
It should also be noted that we are uncertain whether the dose used throughout these studies results in physiological levels of circulating OXM at the critical sites involved in the regulation of body weight. Additional data in mice lacking both receptors (31) may help further elucidate the contribution of both receptors and assess whether additional pathways are involved in the effects of OXM on body weight.
In conclusion, our data demonstrate that OXM, a dual GLP1R/GCGR agonist, effects superior weight loss and similar glucose lowering activity compared to an equipotent GLP1R agonist in mice and establishes with pharmacological and genetic approaches that the GCGR pathway is required for the weight-lowering action of exogenous OXM in vivo.
Acknowledgments
The parts of this study were presented in abstract form at the American Diabetes Association 71st Annual Scientific Sessions, San Diego, CA, 24–28 June 2011.
The authors declared no conflict of interest.
See the online ICMJE Conflict of Interest Forms for this article.
REFERENCES
- Wynne K, Park AJ, Small CJ.et al. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial Diabetes 2005542390–2395. [DOI] [PubMed] [Google Scholar]
- Dakin CL, Gunn I, Small CJ.et al. Oxyntomodulin inhibits food intake in the rat Endocrinology 20011424244–4250. [DOI] [PubMed] [Google Scholar]
- Baggio LL, Huang Q, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127:546–558. doi: 10.1053/j.gastro.2004.04.063. [DOI] [PubMed] [Google Scholar]
- Wynne K, Park AJ, Small CJ.et al. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial Int J Obes (Lond) 2006301729–1736. [DOI] [PubMed] [Google Scholar]
- Baldissera FG, Holst JJ, Knuhtsen S, Hilsted L, Nielsen OV. Oxyntomodulin (glicentin-(33-69)): pharmacokinetics, binding to liver cell membranes, effects on isolated perfused pig pancreas, and secretion from isolated perfused lower small intestine of pigs. Regul Pept. 1988;21:151–166. doi: 10.1016/0167-0115(88)90099-7. [DOI] [PubMed] [Google Scholar]
- Pocai A, Carrington PE, Adams JR.et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice Diabetes 2009582258–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros L, Thorens B, Bataille D, Kervran A. Glucagon-like peptide-1-(7-36) amide, oxyntomodulin, and glucagon interact with a common receptor in a somatostatin-secreting cell line. Endocrinology. 1993;133:631–638. doi: 10.1210/endo.133.2.8102095. [DOI] [PubMed] [Google Scholar]
- Sowden GL, Drucker DJ, Weinshenker D, Swoap SJ. Oxyntomodulin increases intrinsic heart rate in mice independent of the glucagon-like peptide-1 receptor. Am J Physiol Regul Integr Comp Physiol. 2007;292:R962–R970. doi: 10.1152/ajpregu.00405.2006. [DOI] [PubMed] [Google Scholar]
- Wynne K, Field BC, Bloom SR. The mechanism of action for oxyntomodulin in the regulation of obesity. Curr Opin Investig Drugs. 2010;11:1151–1157. [PubMed] [Google Scholar]
- Maida A, Lovshin JA, Baggio LL, Drucker DJ. The glucagon-like peptide-1 receptor agonist oxyntomodulin enhances beta-cell function but does not inhibit gastric emptying in mice. Endocrinology. 2008;149:5670–5678. doi: 10.1210/en.2008-0336. [DOI] [PubMed] [Google Scholar]
- Habegger KM, Heppner KM, Geary N.et al. The metabolic actions of glucagon revisited Nat Rev Endocrinol 20106689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter JM. Metabolic effects of glucagon in the Wistar rat. Am J Clin Nutri. 1960;;8:535–539. [Google Scholar]
- Salter JM, Ezrin C, Laidlaw JC, Gornall AG. Metabolic effects of glucagon in human subjects. Metab Clin Exp. 1960;9:753–768. [PubMed] [Google Scholar]
- Langhans W, Zeiger U, Scharrer E, Geary N. Stimulation of feeding in rats by intraperitoneal injection of antibodies to glucagon. Science. 1982;218:894–896. doi: 10.1126/science.7134979. [DOI] [PubMed] [Google Scholar]
- PENICK SB, HINKLE LE., Jr Depression of food intake induced in healthy subjects by glucagon. N Engl J Med. 1961;264:893–897. doi: 10.1056/NEJM196105042641801. [DOI] [PubMed] [Google Scholar]
- Schulman JL, Carleton JL, Whitney G, Whitehorn JC. Effect of glucagon on food intake and body weight in man. J Appl Physiol. 1957;11:419–421. doi: 10.1152/jappl.1957.11.3.419. [DOI] [PubMed] [Google Scholar]
- Orskov C, Wettergren A, Holst JJ. Biological effects and metabolic rates of glucagonlike peptide-1 7-36 amide and glucagonlike peptide-1 7-37 in healthy subjects are indistinguishable. Diabetes. 1993;42:658–661. doi: 10.2337/diab.42.5.658. [DOI] [PubMed] [Google Scholar]
- Kervran A, Blache P, Bataille D. Distribution of oxyntomodulin and glucagon in the gastrointestinal tract and the plasma of the rat. Endocrinology. 1987;121:704–713. doi: 10.1210/endo-121-2-704. [DOI] [PubMed] [Google Scholar]
- Dallas-Yang Q, Shen X, Strowski M.et al. Hepatic glucagon receptor binding and glucose-lowering in vivo by peptidyl and non-peptidyl glucagon receptor antagonists Eur J Pharmacol 2004501225–234. [DOI] [PubMed] [Google Scholar]
- Scrocchi LA, Brown TJ, MaClusky N.et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene Nat Med 199621254–1258. [DOI] [PubMed] [Google Scholar]
- Mu J, Jiang G, Brady E.et al. Preclinical efficacy and mechanism of action of a potent small molecule glucagon receptor antagonist Diabetes 201059A1533 [Google Scholar]
- Turton MD, O'Shea D, Gunn I.et al. A role for glucagon-like peptide-1 in the central regulation of feeding Nature 199637969–72. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22:10470–10476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JJ, Gallwitz B, Schmidt WE, Nauck MA. Glucagon-like peptide 1 as a regulator of food intake and body weight: therapeutic perspectives. Eur J Pharmacol. 2002;440:269–279. doi: 10.1016/s0014-2999(02)01434-6. [DOI] [PubMed] [Google Scholar]
- Dakin CL, Small CJ, Batterham RL.et al. Peripheral oxyntomodulin reduces food intake and body weight gain in rats Endocrinology 20041452687–2695. [DOI] [PubMed] [Google Scholar]
- Parkinson JR, Chaudhri OB, Kuo YT.et al. Differential patterns of neuronal activation in the brainstem and hypothalamus following peripheral injection of GLP-1, oxyntomodulin and lithium chloride in mice detected by manganese-enhanced magnetic resonance imaging (MEMRI) Neuroimage 2009441022–1031. [DOI] [PubMed] [Google Scholar]
- Chaudhri OB, Parkinson JR, Kuo YT.et al. Differential hypothalamic neuronal activation following peripheral injection of GLP-1 and oxyntomodulin in mice detected by manganese-enhanced magnetic resonance imaging Biochem Biophys Res Commun 2006350298–306. [DOI] [PubMed] [Google Scholar]
- Gu W, Lloyd DJ, Chinookswong N.et al. Pharmacological targeting of glucagon and glucagon-like peptide 1 receptors has different effects on energy state and glucose homeostasis in diet-induced obese mice J Pharmacol Exp Ther 201133870–81. [DOI] [PubMed] [Google Scholar]
- Mu J, Jiang G, Brady E.et al. Chronic treatment with a glucagon receptor antagonist lowers glucose and moderately raises circulating glucagon and glucagon-like peptide 1 without severe alpha cell hypertrophy in diet-induced obese mice Diabetologia 2011542381–2391. [DOI] [PubMed] [Google Scholar]
- Engel SSXL, Andryuk PJ, Davies MJ, Amatruda J, Kaufman K, Goldstein BJ. Efficacy and tolerability of MK-0893, a glucagon receptor antagonist (GRA), in patients with type 2 diabetes (T2DM. Diabetes. 2011;;Suppl 1:A85.. [Google Scholar]
- Ali S, Lamont BJ, Charron MJ, Drucker DJ. Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis. J Clin Invest. 2011;;121:1917–1929.. doi: 10.1172/JCI43615. [DOI] [PMC free article] [PubMed] [Google Scholar]