Abstract
Interactions of leptin and leptin receptors play crucial roles during animal development and regulation of appetite and energy balance. In this study we analyzed expression pattern of a zebrafish leptin receptor gene in both developing and adult zebrafish using in situ hybridization and Q-PCR methods. Zebrafish leptin receptor message (lepr) was detected in all embryonic and larval stages examined, and in adult zebrafish. In embryonic zebrafish, lepr was mainly expressed in the notochord. As development proceeded, lepr expression in the notochord decreased, while its expression in several other tissues, including the trunk muscles and gut, became evident. In both larval and adult brains, large lepr expressing cells were detected in similar regions of the hindbrain. In adult zebrafish, lepr expression was also observed in several other brain regions including the hypothalamic lateral tuberal nucleus, the fish homolog of the arcuate nucleus. Q-PCR experiments confirmed lepr expression in the adult fish brain, and also showed lepr expression in several adult tissues including liver, muscle and gonads. Our results showed that lepr expression was both spatially and temporally regulated.
Keywords: obese gene, brain, notochord, muscle, leptin, zebrafish, leptin receptor, in situ hybridization
1. Introduction
Leptin is a small (16 kDa) protein hormone, whose discovery in 1994 by Jeff Friedman’s lab (Zhang et al., 1994) led to intense research into its properties and physiological action (> 25,000 reports to date; Friedman, 2009). Although initially characterized exclusively as a product of adipose tissue, more recent investigations document leptin expression in stomach (Bado et al., 1998), placenta (Sagawa et al., 2002), brain, and pituitary (Wilkinson et al., 2007). Similarly, the initial emphasis on leptin’s physiological effects focused upon its influence on metabolic rate and mobilization of fat stores. We now know that leptin (in mammals) is pleiotropic, exerting effects on reproduction, immune function, capillary growth, and bone remodeling (see Friedman, 2009 for review). Ahima and Flier recognized this pleiotropy relatively early in leptin’s history, and called for an evolutionary approach to unraveling leptin’s many functions/effects (Ahima and Flier, 2000). They advanced the hypothesis that leptin signaling evolved as a sensitive indicator of starvation; leptin signaling mediates decreased activity of energetically demanding pathways (e.g. reproduction and immunity) and thus increases the chance of surviving the starvation event.
This compelling idea was extended by comparative endocrinologists investigating leptin signaling in lower vertebrates; such a signaling system would have obvious selective benefits. The search for non-mammalian leptin homologues was difficult, however, with the first accepted non- mammal leptin sequence published 11 years after leptin was cloned in mice (Kurokawa et al., 2005). Kurokawa’s group identified pufferfish leptin via gene synteny, which revealed that leptin sequence is poorly conserved among vertebrates (11–30% amino acid conservation between non-mammals and mammals; Londraville and Niewiarowski, 2010) although with apparently strong conservation of tertiary structure. In contrast, the leptin receptor was more tractable, with the first non-mammalian receptor (chicken) published in 2000 (Dunn et al., 2000; Horev et al., 2000), although no chicken leptin sequence has been identified in the (largely solved) chicken genome (Sharp et al., 2008). To date there are several verified leptin sequences from non-mammals, including amphibians (Xenopus (Crespi and Denver, 2006), and several species of fish (Fugu; Kurokawa et al., 2005, carp; Huising et al., 2006, Medaka; Kurokawa and Murashita, 2009, and rainbow trout; Murashita et al., 2008). For the leptin receptor, several sequences have been cloned in birds (Horev et al., 2000), amphibians (Crespi and Denver, 2006), and fishes (Kurokawa et al., 2008; Kurokawa and Murashita, 2009). A notable gap in our knowledge of leptin evolution is that there are no reptilian sequences for either leptin or its receptor, although reptiles do respond to injections of mammalian leptin (Niewiarowski et al., 2000) and anti-mammal leptin antibodies recognize ‘leptins’ in reptiles (Paolucci et al., 2001; Spanovich et al., 2006).
Although there has been considerable progress on the molecular evolution of leptin and the leptin receptor, little physiological data exist on the expression or function of native leptins in non- mammals. Wong et al. (2007) characterized expression of leptin receptor in adult marine medaka (Oryzias melastigma) and its response to hypoxia, but their study did not include developmental studies. Most physiologically-oriented leptin studies in fish document response to a mammalian leptin (Londraville and Duvall, 2002; Volkoff et al., 2003), with only one study (to date) that investigates the effects of native leptin in fish (Murashita et al., 2008). The only study that addresses leptin’s effects on developing and adult lower vertebrates is Crespi and Denver’s seminal work on Xenopus (2006). It is our long-term goal to characterize the physiology of both leptin and its receptor in zebrafish (Danio rerio), to elucidate its roles in both development and adults. In this study, we used Q-PCR and in situ hybridization to describe tissue level-expression of zebrafish leptin receptor. The expression pattern is strikingly similar to that described for mammalian leptin receptors and for medaka, which both supports our contention that this is a long-form leptin receptor homologue, and that the zebrafish model system is ideally suited for the study of how leptin function evolved.
2. Materials and Methods
Animals
Zebrafish embryos were obtained from in house breeding, and maintained as described in the Zebrafish Book (Westerfield, 2005). Adult zebrafish were raised from embryos obtained from in house breeding. Embryos for whole mount in situ hybridization were raised in PTU (1-phenyl-2- thiourea, 0.003%) treated fish tank water, while embryos for in situ hybridization on tissue sections were raised in regular fish tank water, both at 28.5°C. Ages of the embryos or larvae are given as hours post fertilization (hpf) or days post fertilization. All animal-related procedures were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Akron Committee on Use and Care of Animals.
Tissue preparation
Animals were anesthetized in 0.02% MS-222. Adult fish were killed by cervical transection. The brain of adult fish or whole embryos and larvae were fixed for 2 hours at room temperature or overnight at 4°C, in phosphate-buffered 4% paraformaldehyde (pH 7.4). Embryos for whole mount in situ hybridization were washed in 0.1 M phosphate-buffered saline (PBS, pH 7.4), placed in increasing concentrations of methanol and stored in 100% methanol at −20°C till use. Whole larval fish or adult brains were rinsed in PBS and prepared for cryosections (15 µm) as described previously (Barthel and Raymond, 1990).
Probe synthesis and in situ hybridization
To obtain a zebrafish lepr cDNA fragment as a template for synthesizing in situ hybridization cRNA probes, RT-PCR was performed using zebrafish lepr specific primers (forward primer 1, 5’- GGTCTCACTGCCTGTCCATT-3’; reverse primer 1, 5’-AGATGGTGCTGCTCCACT-3’) and total RNA from zebrafish 20–50 hpf embryos. The resulting DNA fragment, corresponding to nucleotides 2565–3303 of the published zebrafish lepr sequence (GenBank accession number: NM_001113376), was cloned into the pCRII-TOPO vector (Invitrogen), and was verified by restriction enzymes digestion, a PCR experiment using a pair of zebrafish lepr specific primers that were internal to the last set of primers (forward primer 2, 5’-GACGAAGGCAACTTCTCTGC-3’; reverse primer 2, 5’-TTCTTTCTCCTCTCCGGTCA-3’), and sequencing.
Detailed procedures for digoxigenin-labeled cRNA probe synthesis, whole mount in situ hybridization, and in situ hybridization on tissue sections were described previously (Liu et al., 1999). To verify the specificity of the above lepr cRNA probe, we also performed in situ hybridization using a shorter cRNA probe (transcribed from a lepr cDNA fragment corresponding to nucleotides 2565–3168 of the published zebrafish lepr sequence). Staining patterns in both developing and adult tissues were identical. Moreover, there was no staining in zebrafish embryos from 21–72 hpf using sense lepr probes (data not shown).
Q-PCR experiments
Total RNA was isolated from adult tissues or developing embryos at 0, 5–6, 12, 24, 36, 48, 72 hpf intervals using a column based extraction (EZRNA, Omega Biotech). Approximately 20–50 embryos were pooled and frozen at −80°C for each stage; adult tissues were pooled from 2 individuals (fresh tissue, immediately extracted). Samples were homogenized in a bead mill to avoid cross-contamination. RNA samples were digested with DNAse during extraction to reduce possible genomic DNA contamination. cDNA was synthesized from total RNA using a high efficiency reverse transciptase (Applied Biosystems) primed with random hexamers. cDNA was quantified with a NanoDrop ND-1000 spectrophotometer (Nanodrop Technologies). Duplicate reactions without reverse transcriptase were performed for negative control-templates for quantitative PCR. Q-PCR analysis of lepr temporal and tissue expression profiles was performed using lepr primers (forward primer 5’-CTCCAGTGACGAAGGCAACTT-3’; reverse primer 5’- GGGAAGGAGCCGGAAATGT-3’), and primers for zebrafish ribosomal protein L13A (60s) as a reference gene (forward primer 5’-TCTGGAGGACTGTAAGAGGTATGC-3’; reverse primer 5’- AGACGCACAATCTTGAGAGCAG-3’) as in Tang et al. (2007). L13A is a validated control gene for zebrafish, showing no significant change in expression among tissues or during early development (Tang et al., 2007). cDNAs (100 ng/reaction) were amplified and quantified with SYBR green master mix (Sigma) on an Applied Biosystems 7300 (ABI).
3. Results
Alignment of several leptin receptor sequences indicate that the zebrafish receptor is relatively divergent from other vertebrate receptors (~20% primary sequence identity), and is most similar to other fish (32%; Fig. 1 alignment and identity table). Regions of the sequence contain blocks of sequence that are highly conserved, including several blocks within the putative leptin binding region identified by Kurokawa et al., 2008 and Kurokawa and Murashita, 2009 (residues 387–592 on the Takifugu sequence).
Figure 1.
Amino acid alignment of long-form leptin receptors (LEPR). Danio (zebrafish, Acc# NP_001106841.1), Takifugu (puffer,NM_001130869), Xenopus (African clawed frog, NP_001037866.1), Gallus (chicken, NP_989654.1), Mus (mouse, NP_666258.1), Homo (human, NP_002294.2). Sequences were aligned with CLUSTALW(Larkin et al., 2007); shaded residues indicate conservation. Table indicates percentage primary sequence identity between taxa (computed with BioEdit v.7; Hall, 1999).
Q-PCR analysis of lepr expression in embryonic and adult zebrafish
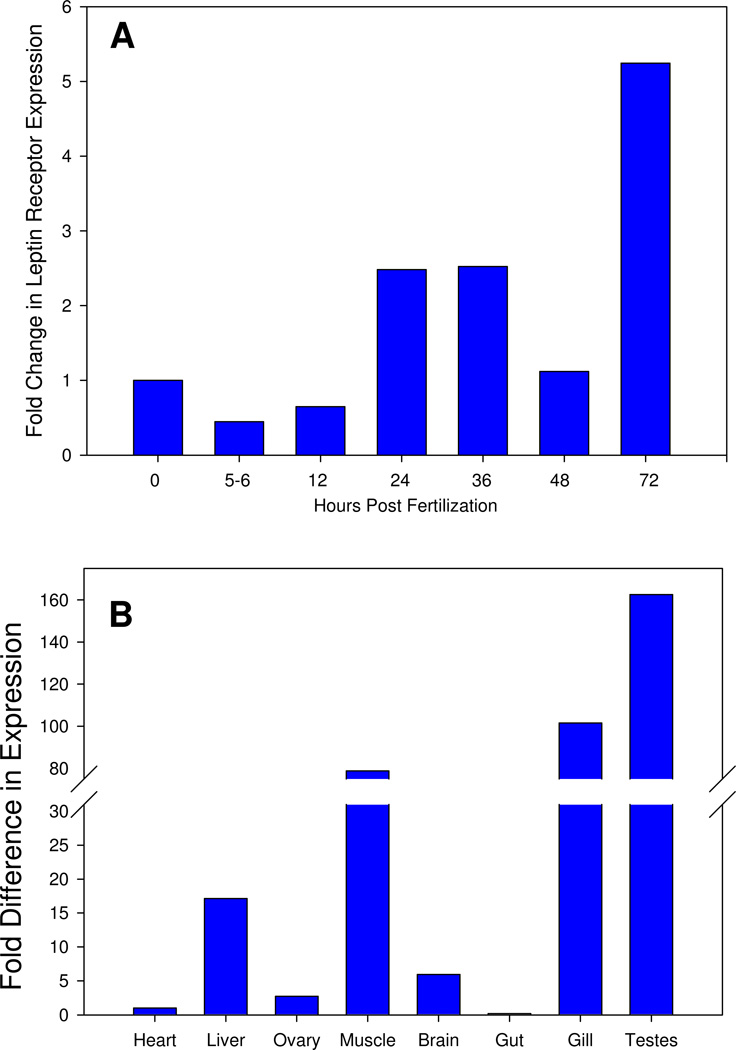
We measured relative lepr expression in embryonic and adult zebrafish using quantitative PCR. Lepr transcripts were detected in all the stages examined, but their expression levels varied during development and among adult tissues. Lepr transcripts were weakly expressed by young embryos (0–12 hours post fertilization hpf), increased expression in 24–36 hpf embryos, followed by a decrease in expression at 48 hpf and an increase at 72hpf (Fig. 2A. In adult zebrafish, lepr transcripts were detected in all the tissues examined, with strongest expression in liver, muscle, gill, and testes (Fig. 2B).
Figure 2.
Q-PCR analysis of lepr expression in whole embryos (panel A) and adult tissues (panel B). Fold change was calculated as 2−ΔΔCt with zebrafish ribosomal protein L13A (60s) as a reference gene as detailed in Tang et al., 2007. Bars represent mean value from triplicate samples.
In situ hybridization analysis of lepr expression
To determine spatial and temporal expression pattern of lepr in embryonic and larval zebrafish, and distribution of lepr in adult zebrafish brain, we performed in situ hybridization experiments on whole mount embryos, and tissue sections of larval zebrafish and adult brain.
lepr expression in the notochord
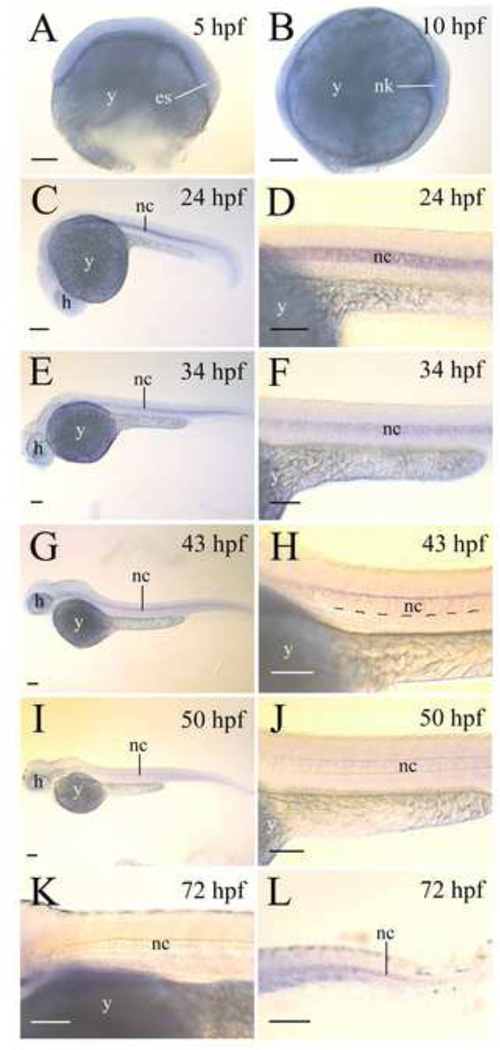
There was no lepr expression observed in 5 hpf and 10 hpf whole mount embryos (Fig. 3B and C), but lepr was strongly expressed by the notochord of 21–24 hpf embryos (Fig. 3D). In older embryos (34–50 hpf), lepr continued to be confined to the notochord, but its expression in the notochord became more restricted (Fig. 3). In 34 hpf embryos, lepr expression levels, judged by staining intensities, in the notochord were reduced, more evident in the ventral regions of the notochord, compared to 21–24 hpf embryos (Fig. 3B and F). By 43 hpf, lepr expression in the notochord was limited to the most dorsal region (Fig. 3C and G), and in 50 and 72 hpf embryos, lepr expression in the notochord became undetectable except in the tail region (Fig. 3D, H, J and K). No lepr expression was detected in the notochord of 4- or 6-day old zebrafish larvae (data not shown).
Figure 3.
lepr expression in zebrafish embryos. All panels, except for panels A and B, show lateral views of whole mount embryos (anterior to the left and dorsal up). Panels D, F, H, J and L are higher magnifications of the mid-trunk region of their respective embryos shown on the left side. Panels K and L show 72 hpf larvae mid-trunk and tail regions, respectively. Abbreviations: es, embryonic shield; y, h, head; nc, notochord; nk, neural keel; yolk. Scale bar = 100 µm.
lepr expression in developing and adult zebrafish brain
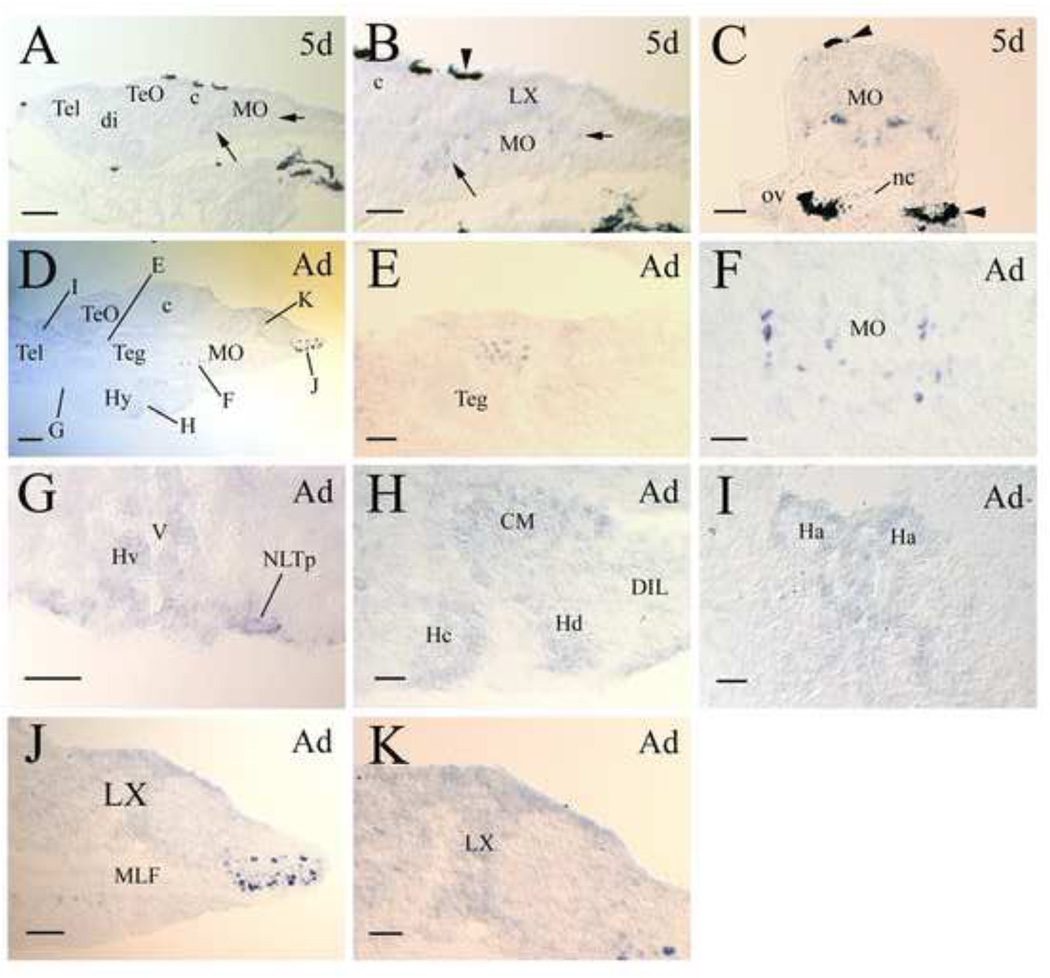
Expression of lepr in the larval brain was first detected in the hindbrain (medulla oblongata) of 5-day old zebrafish (Fig. 4A-C). There were two groups of large lepr expressing cells (10–15 µm diameter) in the hindbrain. The first group, containing more cells, was situated posteroventral to the cerebellum and anteroventral to the vagal lobe. The second group was located posteroventral to the vagal lobe (Fig. 4B). Examination of cross sections showed that these lepr expressing cells were symmetrically located adjacent to the midline (Fig. 4C). In addition to these large cells, smaller cells with weaker staining were observed in the vagal lobe and dorsal regions of the hindbrain (Fig. 4B and C). Similar to the larval zebrafish hindbrain, there were two groups of large lepr expressing cells (15–20 µm diameter) in the adult medulla oblongata (MO). They also occupied similar positions in the adult MO as in the larval brain. According to the atlas of the adult zebrafish brain (Wullimann et al., 1996), the first and second groups of large lepr expressing cells were located adjacent to the medial longitudinal fascicle in the intermediate reticular formation of the ventral MO and the inferior reticular formation of the caudal MO, respectively (Fig. 4D, F and I). The first group of large lepr expressing cells were more scattered than the second group of cells. In addition to these two groups of large lepr expressing cells in the MO, two groups of large lepr positive cells were detected in other brain regions. One group was found in the medial region of the anterodorsal tegmentum (Fig. 4E). The other was found in the hypothalamic lateral tuberal nucleus in the anteroventral region of the ventral zone of the periventricular hypothalamus (Fig. 4G). Compared to the hindbrain large lepr expressing cell groups, these two groups contained far fewer cells.
Figure 4.
lepr expression in developing and adult zebrafish brains. All panels show tissue sections, with anterior to the left and dorsal up for all sagittal sections, and dorsal up for all cross sections. Panel A shows a sagittal brain section from a 5-day old larva. Panel B is a higher magnification of the medulla oblongata (MO) shown in panel A. The short and long arrows in these two panels indicate the same respective regions with lepr expressing cells. The arrowhead in panel B points to a piece of pigmented epithelium. Panel C is a cross section of the MO at the otic vesicle (ov) level. Arrowheads indicate pigmented tissues. Panel D is a sagittal section showing the posterior telencephalon (Tel) and the rest of the brain. Letters in panel D refer to the area of this section shown in higher magnification in subsequent panels (E-I). Panels E, F, H, J and K are from sagittal sections, while panels G and I are from cross sections. Other abbreviations: c, cerebellum; CM, corpus mamillare; di, diencephalon; DIL, diffuse nucleus of the inferior lobe; Ha, habenula; Hc, caudal zone of periventricular hypothalamus; Hd, dorsal zone of periventricular hypothalamus; Hv, ventral zone of periventricular hypothalamus; Hy, hypothalamus; LX, vagal lobe; MLF, medial longitudinal fascicle; nc, notochord; NLTp, posterior part of the lateral tuberal nucleus; Teg, tegmentum; TeO, tectum opticum; V, ventricle. Scale bar = 100 µm for panels A and J, 200 µm for panel D, and 50 µm for the remaining panels.
Smaller cells with weaker staining were observed in several other brain regions including areas along the ventricle and dorsal to the lateral tuberal nucleus in the ventral zone of the periventricular hypothalamus (Fig. 4G), the corpus mamillare in the hypothalamus, caudal zone and dorsal zone of periventricular hypothalamus (Fig. 4H), habenula nucleus (Fig. 4I), and the vagal lobe in the MO (Fig. 4K).
lepr expression in larval muscles and internal organs
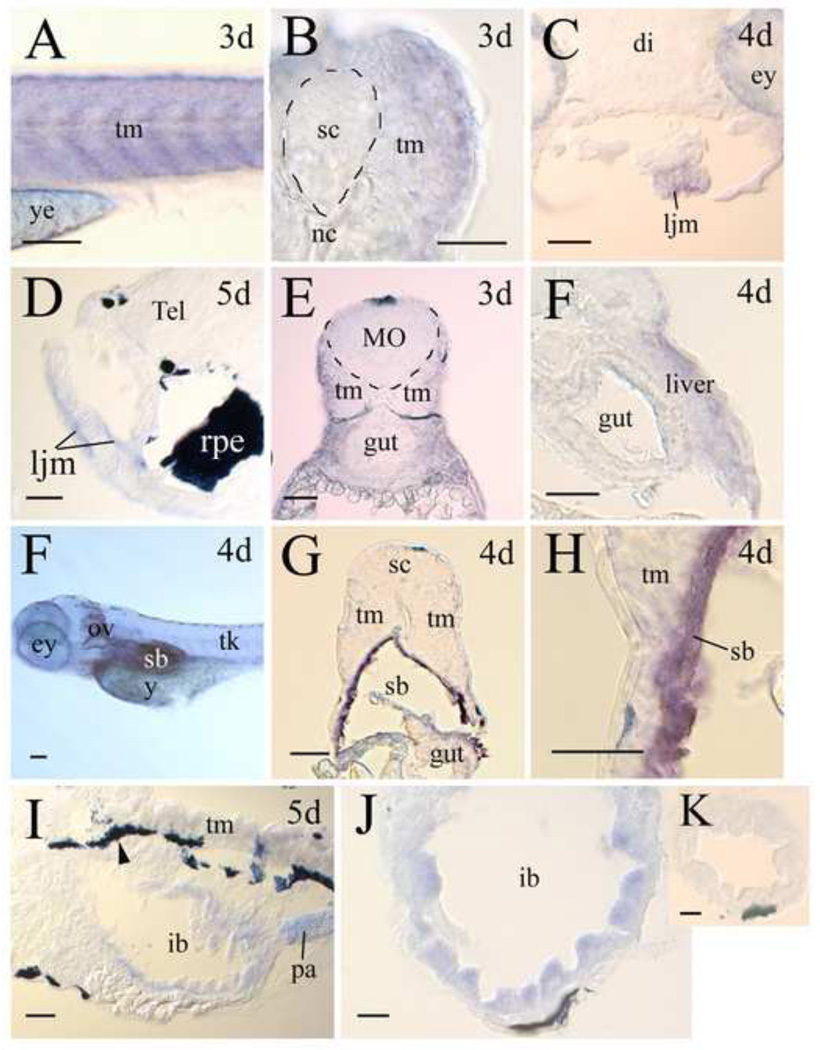
In zebrafish larvae (3–5 days post fertilization), lepr expression was detected in the muscular (Fig. 5) tissues and several internal organs. In the trunk muscles, lepr expression was strongest, judged by staining intensity, in 3-day old larvae (Fig. 5A and B), and became reduced in 4- and 5- day old larvae (Fig. 5G and I). The jaw muscles also contained lepr (Fig. 5C and D). Unlike the trunk muscles, lepr expression in the jaw muscles was similar between 3- (Fig. 5C) and 5- day (Fig. 5D) old larvae. lepr expression was also detected in the developing liver (Fig. 5F), swim bladder (Fig. 5F-H), pancreas (Fig. 5I) and gut (Fig. 5I and J). There was little or no lepr expression in 3- and 4-day old fish gut. lepr expression became obvious in 5-day old fish gut, but lepr expression in the gut was confined mainly to the epithelial layer of the enlarged portion, the intestine bulb, of the gut (Fig. 5I-K).
Figure 5.
lepr expression in muscles and internal organs. The anterior is to the left and dorsal is up for lateral views or sagittal sections, while the dorsal is up for cross sections. Panel A is a lateral view of the mid-trunk region of a 3-day (3d) larva focuses on the skeletal trunk muscles (tm) staining. Panel B is a high magnification of a cross section of the mid-trunk region showing trunk muscle staining. Panels C (cross section) and D (sagittal section) show lepr expression in lower jaw muscles (ljm). Panels E and F (both cross section) show that there is no staining in the gut, but the liver contains lepr. Panel G is a cross section from the whole mount larva in panel F showing swim bladder (sb) labeling. Panel H is a high magnification of the left lower portion of the image shown in panel G. Panels I (sagittal section) and J (cross section) show lepr expression in the intestinal bulb, but not in other intestine regions (panel K, cross section). Other abbreviations: ey, eye; pa, pancreas; rpe, retinal pigmented epithelium; sc, spinal cord; tk, trunk; ye, yolk sac. The remaining abbreviations are the same as in Figures 3 and 4. Scale bars = 100 µm for panels A and F, 25 µm for panels B, H, J and K, 50 µm for the remaining panels.
lepr expression in the sensory organs
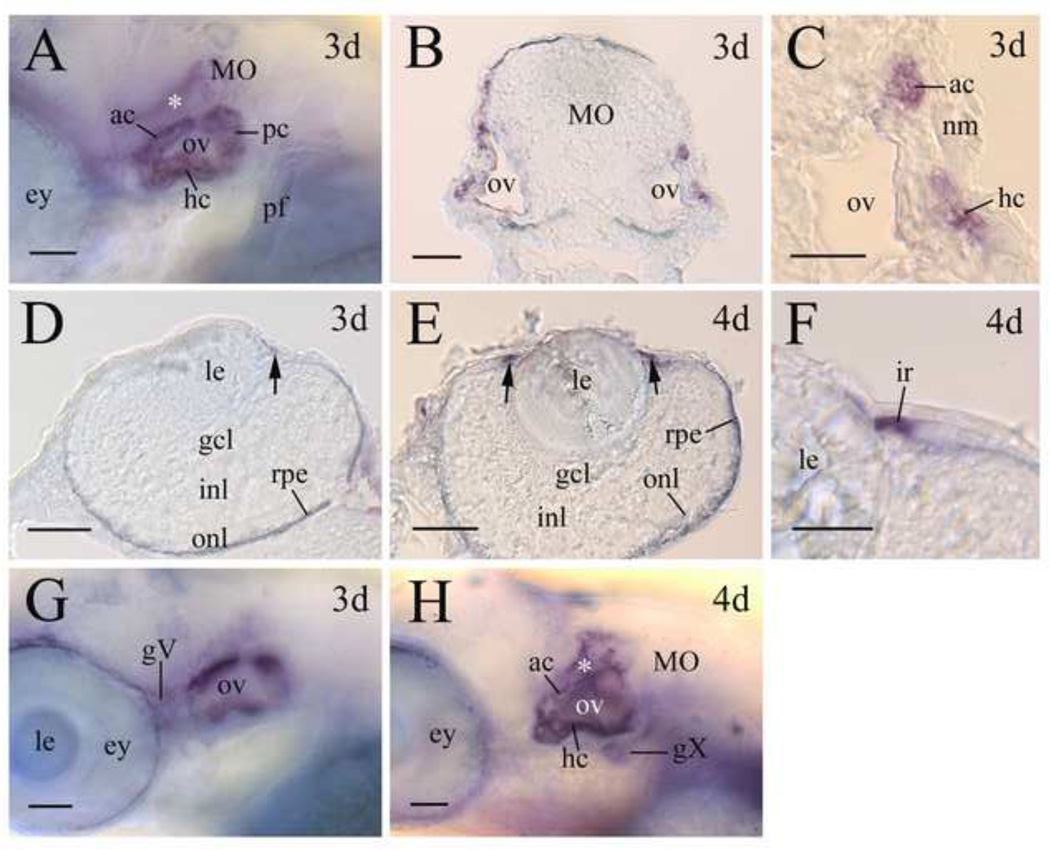
In 72 hpf larval zebrafish, lepr was strongly expressed by the ear (Fig. 6A-C). Examination of both whole mount ears and sections of ears showed that lepr expression was mainly detected in the non-sensory epithelium of the walls of the semicircular canals. lepr expression in the ear was similar in 3–5 days old larval fish. lepr expression in the 3- and 4-day old larval eyes was confined to the iris (Fig. 6D-F). lepr expression in the iris was stronger in 4-day old larvae (Fig. 6E) than 3-day old larvae (Fig. 6D), and the expression became undetectable in 5-day old iris (data not shown). lepr was also detected in two cranial ganglia, the trigeminal ganglion (Fig. 6F) and vagal ganglion (Fig. 6G) in 3–5 days old larvae.
Figure 6.
lepr expression in the sensory organs of larval zebrafish. Panels A, G and H are lateral views (anterior to the left and dorsal up) of whole mount embryos showing the otic vesicle region. Panel B is a cross section (dorsal up) of the medulla oblongata (MO) at the otic vesicle level. Panel C is a higher magnification of the right ov shown in panel B. Panels D and E are cross sections (dorsal to the right) of the eye, while panel F is a higher magnification of the iris (ir) region of a 4- day old larval eye. Arrows in panels D and E indicate lepr expression in the iris. Abbreviations: ac, rostral canal; gcl, retinal ganglion cell layer; hc, horizontal canal; inl, inner nuclear layer; le, lens; nm, neuromast; onl, outer nuclear layer; pc, posterior canal; pf, pectoral fin. Other abbreviations are the same as in Figures 3 and 4. Scale bars = 25 µm for panels C and F, 50 µm for the remaining panels.
Discussion
The leptin receptor gene in mammals produces several alternatively spliced products. Only the long form of the receptor (lepr) codes for the full intracellular domain and allows transmission of the intracellular signal through the JAK-STAT signaling cascade, and thus is responsible for mediating most of the well-characterized effects of leptin (Tartaglia, 1997). Alignment of the zebrafish lepr with established isoforms of lepr from multiple vertebrates reveals that the approximate length of the sequence is conserved (~1100 aa), and several blocks of conserved sequence distributed throughout the sequence, although overall sequence identity is low (Fig. 1). This supports our assertion that we are characterizing the long form of the receptor, and not a splice variant. In addition, the tissue expression pattern, which is consistent with that described for lepr in mammals (below), further supports a long-form leptin receptor in zebrafish.
The Q-PCR results correlated well with those obtained from whole mount in situ hybridization experiments in 18–72 hpf embryos. For example, both methods revealed strong lepr expression in 18–24 hpf embryos, weak lepr expression in 50 hpf embryos. The difference in results from RT-PCR showing weak lepr expression (Fig. 2A) and in situ hybridization showing no lepr expression (Fig. 3) in younger embryos (1.5–5.5 hpf) likely due to differential sensitivities of these two methods, with the Q-PCR being a more sensitive method than in situ hybridization.
The vertebrate notochord is an important source of molecules (e.g. sonic hedgehog) crucial for the development of various tissues and organs including the neural tube and somites (Smith 1993; Bumcrot and McMahon 1995). Formation of the zebrafish notochord begins early (5–6 hpf), and by 12 hpf, a straight rod-shaped notochord has formed (Glickman et al., 2003). lepr is strongly expressed in the notochord in 18–24 hpf embryos, therefore it is unlikely involved in the early formation of the notochord. However, the period of strong lepr expression in the notochord coincided with a critical stage of notochord development during which time the notochord chordamesoderm cells differentiate into mature notochord cells (Hawkins et al., 2008). Moreover, a cell adhesion molecule cadherin-7 is also strongly expressed this time in the zebrafish notochord, and inhibition of cadherin-7 function resulted in severe notochord defects in embryonic zebrafish (Liu et al., 2008). Similar to the zebrafish, developing mouse notochord is one of only a few tissues that express lepr (the splice variant with the long intracellular domain, Chen et al., 2000).
Involvement of the hypothalamus in regulation of feeding has been known for decades. In mammals, many effects of leptin on feeding and energy balance are mediated through a hypothalamic circuitry including a ventral medially located structure immediately above the median eminence called the arcuate nucleus (reviewed by Bouret and Simerly, 2007). The arcuate nucleus contains neurons expressing high levels of leptin receptor and neurons responding directly to leptin (Elmquist et al., 1998; Cowley et al., 2001; Pinto et al., 2004). Similar to mammals, the zebrafish hypothalamic lateral tuberal nucleus, the fish homolog of the mammalian arcuate nucleus (Cerda-Reverter and Peter 2003), contains cells expressing high levels of lepr.
In vertebrates, the caudal brain stem, especially the dorsal vagal complex, contains leptin receptor mRNA and protein (Elmquist et al., 1998; Mercer et al., 1998; Buyse et al., 2001; Grill et al., 2002), and is thought to play crucial roles in integration of leptin signals and meal-related satiety signals (Grill and Kaplan, 2002; Berthoud, 2002; Bouret and Simerly, 2007). Similar to rodents, lepr was detected in similar regions of the zebrafish hindbrain. The hindbrain lepr structures are established early in zefrafish larvae (day 5), about 1 day after the fish begin to feed. Our results suggest that the leptin-sensitive neural pathways are evolutionarily conserved.
Similar to mammals, zebrafish lepr is expressed by a variety of tissues and organs including the eye, gut, heart, liver, muscles, and pancreas (Ahima and Flier, 2000; Margetic et al., 2002). As in mammals, birds, and medaka (Frühbeck et al., 1999; Richards and Poch, 2003; Ramsay and Richards; 2005; Solberg et al., 2005; Guerra et al., 2007, Wong et al. 2007), zebrafish skeletal (trunk) muscles express lepr. Interestingly, the highest lepr expression levels in the trunk muscles, judging from staining intensity, were detected in three day old zebrafish. This temporary increase in lepr expression coincides with two critical events in zebrafish development: 1) transition from exclusively using yolk as nutrient to feeding from external sources; 2) transition from mainly stationary mode of living to free swimming. Fish exploit fatty acids in muscle as a metabolic fuel source (Sidell et al., 1987), and leptin manipulation alters fatty acid metabolism in fish (Londraville and Duvall, 2002). Because fish commonly store neutral lipid as non-membrane bound droplets in muscle (Londraville and Sidell, 1990), leptin signaling may be important for deposition and mobilization of lipid in muscle.
According to Ng et al. (2005), the zebrafish intestinal tract can be divided, as early as the fourth day of development, into three anatomically distinct regions based on epithelial cell morphology and differentiation: the intestinal bulb, mid-intestine and posterior intestine. lepr has been detected in all intestinal regions in several species (Raguso and McCullough, 2000; Aparicio et al., 2005; Hansen et al., 2008), but in the larval zebrafish, only the intestinal bulb contains strong lepr expression in larval zebrafish. Interestingly, the stomach, also located immediately after esophagus, is the only intestinal tissue found to express leptin (Bado et al., 1998). It is possible that other intestinal regions (i.e. the mid- and posterior intestines) may express low levels of lepr that our current in situ hybridization methods failed to detect, or they express lepr in older larvae and adult zebrafish.
Sensory information about specific stimuli such as increases in blood pressure and passage of food through the intestinal tract is sent to the central nervous system via vagal afferent neurons. In mammals, the vagus nerve expresses lepr (Buyse et al., 2001), and vagal mechanoreceptors respond to leptin applications (Gaige et al., 2004). These findings, together with our current results showing that both the zebrafish vagal ganglion and intestine express lepr suggest that the leptin involvement in the parasympathetic control of the gastrointestinal function (reviewed by Guilmeau et al., 2004) is evolutionarily conserved.
lepr expression in the eye was reported in embryonic and adult mice (Chen et al., 2000; Camand et al., 2002). Unlike the mouse where lepr expression was mainly detected in the choroid and sclera of the eye (Camand et al., 2002), lepr expression in zebrafish was confined to the iris of 3 and 4 day larvae. There is no published report, to the best of our knowledge, on lepr expression in the ear. Strong expression of lepr in the semicircular canals during critical stages of their development suggests that leptin is important for the formation of the structure.
Our stated goal is to understand leptin signaling in zebrafish, and the most extensive datasets on leptin function are in mammals. Although we expect and observe that many aspects of lepr will be conserved from fish to mammals, we also expect aspects of leptin signaling to be unique in fishes. Mammalian leptin receptors express a distinct transcript that originates from an alternate start site within the gene, termed the leptin receptor overlapping transcript (LEPROTL or OB-RGRP; Bailleul et al., 1997). Although both lepr and leprotl are coded for by a single gene and under control of a single promoter in mammals (Bailleul et al., 1997), a recent report found lepr and leprotl coded by separate genes in Medaka (Kurokawa and Murashita, 2009). This is potentially important, because leprotl expression was found to influence the number of leptin receptors on a cell’s surface (and thus the cell’s leptin sensitivity) in mice (Couturier et al., 2007). Therefore, control of leptin sensitivity may be markedly different in fish and mammals.
It is clear that the primary structure of leptin itself differs more among vertebrates than its receptor (Kurokawa and Murashita, 2009; Fig. 1). This sequence difference raised doubt about physiological studies in fish (e.g. Londraville and Duvall, 2002; Volkoff et al., 2003) which use mammalian leptin for injection (Kurokawa et al., 2005, Kurokawa et al., 2008; Kurokawa and Murashita, 2009). However, all non-mammal leptins have similar tertiary structures to human leptin (Kurokawa and Murashita, 2009); since interaction with the leptin receptor is likely governed by leptin’s tertiary shape, it is not unreasonable that mammalian leptins could bind to a fish leptin receptor and activate leptin signaling. Indeed, both mouse and Xenopus leptin receptors are activated by both human and Xenopus leptins in vitro (Crespi and Denver, 2006). Further, studies that use anti-mammal leptin antibodies to detect leptins in non-mammals (e.g. Johnson et al., 2000, Kurokawa et al., 2008; Kurokawa and Murashita, 2009) are valid since tertiary structure of the ligand is important for antigen/antibody recognition. One caveat to this argument is that there is only one crystal structure for any leptin, and that is for a human leptin with a modified amino acid (Zhang et al., 1997). All non-mammal leptin structures are based on threading algorithms using the human leptin backbone (Kurokawa and Murashita, 2009). Further studies on non-mammal leptin’s structure, including the nature of leptin/leptin receptor interaction are needed to resolve the issue of whether fish receptors can recognize mammalian leptins.
Given the caution about interpreting leptin injection studies, they do present strong evidence for leptin signaling systems in fish, and that those signaling pathways share some physiological features with mammals. Goldfish reduce food intake with mouse leptin injection, and intracerebroventricular injection was significantly more potent than intraperitoneal injection (Volkoff et al., 2003) consistent with leptin receptor expression in hypothalamus (this study). Murine leptin injection activates pathways of fatty acid metabolism in green sunfish (Londraville and Duvall, 2002). Recombinant human leptin causes follicle stimulating hormone (FSH) and luteinizing hormone (LH) release in male and female rainbow trout, but only when animals are in the reproductive state (Weil et al., 2003). Finally, injection of homologous fish leptin into fish reduces food intake (although during a short-term experiment; Murashita et al.,2008) These studies are all consistent with leptin functions described in mammals (reviewed in Friedman, 2009).
Are there any actions of leptin, therefore, that are unique in fishes? Can we learn anything about the evolution of function for this pleiotropic hormone by studying lower vertebrates? Some recent studies suggest the answer is ‘yes’. Leptin receptor expression is stimulated by hypoxia in marine medaka (Wong et al., 2007), but the leptin response to hypoxia in humans is very complex and unresolved (Ye et al., 2008). The relatively high expression of leptin receptor in gill (Wong et al., 2007, Fig. 2B) indicates a possible role of leptin signaling in sensing environmental hypoxia. Additionally, the high expression of leptin receptor in gonad (Fig. 2B) may indicate a relatively greater role for leptin signaling in reproduction in zebrafish than in mammals. Finally, the intense staining of leptin receptor in ear and eye (Fig 6) indicate that leptin may play a role in sensory development in zebrafish. Together, these data support our contention that leptin signaling exists in fishes, with features that are both reflected in mammals and unique to lower vertebrates.
Acknowledgements
This work was supported by NIH grants EY13879 and DK079282 to Q. L. and R.L. We thank Dr. Mario Wullimann (Ludwig-Maximilians-University, Germany) and Dr. Tanya Whitfield (University of Sheffield, UK) for helping us identifying lepr expressing structures in larval zebrafish ear and adult zebrafish brain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahima RS, Flier JS. Leptin. Ann. Rev. Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- Aparicio T, Kermorgant S, Darmoul D, Guilmeau S, Hormi K, Mahieu-Caputo D, Lehy T. Leptin and Ob-Rb receptor isoform in the human digestive tract during fetal development. J. Clin. Endocrin. Metab. 2005;90:6177–6184. doi: 10.1210/jc.2005-1498. [DOI] [PubMed] [Google Scholar]
- Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau J, Bortoluzzi M, Moizo L, Lehy T, Guerre-Millos M, Le Marchand- Brustel Y, Lewin M. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- Bailleul B, Akerblom I, Strosberg AD. The leptin receptor promoter controls expression of a second distinct protein. Nuc. Acids Res. 1997;25:2752–2758. doi: 10.1093/nar/25.14.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel LK, Raymond PA. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J. Histochem. Cytochem. 1990;38(9):1383–1388. doi: 10.1177/38.9.2201738. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci. Behav. Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Simerly RB. Development of leptin-sensitive circuits. J. Neuroendocrin. 2007;19:575–582. doi: 10.1111/j.1365-2826.2007.01563.x. [DOI] [PubMed] [Google Scholar]
- Bumcrot DA, McMahon AP. Sonic signals somites. Cur. Biol. 1995;5:612–614. doi: 10.1016/s0960-9822(95)00123-0. [DOI] [PubMed] [Google Scholar]
- Buyse M, Ovesjo ML, Goiot H, Guilmeau S, Peranzi G, Moizo L, Walker F, Lewin MJ, Meister B, Bado A. Expression and regulation of leptin receptor proteins in afferent and efferent neurons of the bagus nerve. Eur.J. Neurosci. 2001;14:64–72. doi: 10.1046/j.0953-816x.2001.01628.x. [DOI] [PubMed] [Google Scholar]
- Camand O, Turban S, Abitbol M, Guerre-Millo M. Embryonic expression of the leptin receptor gene in mesoderm-derived tissues. C.R. Biolog. 2002;325:77–87. doi: 10.1016/s1631-0691(02)01417-8. [DOI] [PubMed] [Google Scholar]
- Cerda-Reverter JM, Peter RE. Endogenous melanocortin antagonist in fish: structure, brain mapping, and regulation by fasting of the goldfish agouti related protein gene. Endocrinol. 2003;144:4552–4561. doi: 10.1210/en.2003-0453. [DOI] [PubMed] [Google Scholar]
- Chen SC, Cunningham JJ, Smeyne RJ. Expression of OB receptor splice variants during prenatal development of the mouse. J. Recep. Sig. Transduc. Res. 2000;20:87–103. doi: 10.3109/10799890009150039. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Couturier C, Sarkis C, Seron K, Belouzard S, Chen P, Lenanin A, Corset L, Dam J, Vauthier V, Dubart A, Mallet J, Froguel P, Rouille Y, Jockers R. Silencing of OB-RGRP in mouse hypothalamic arcuate nucleus increases leptin receptor signaling and prevents diet-induced obesity. P.NA.S. 2007;104(49):19476–19481. doi: 10.1073/pnas.0706671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi EJ, Denver RJ. Leptin (ob gene) of the South African clawed frog Xenopus laevis. P.N.A.S. 2006;103:10092–10097. doi: 10.1073/pnas.0507519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn IC, Boswell T, Friedman-Einat M, Eshdat Y, Burt DW, Paton IR. Mapping of the leptin receptor gene (LEPR) to chicken chromosome 8. Anim. Genet. 2000;31:290. doi: 10.1046/j.1365-2052.2000.00652.x. [DOI] [PubMed] [Google Scholar]
- Elmquist J, Bjorbaek C, Ahima R, Flier J, Saper C. Distribution of leptin receptor mRNA isoforms in the rat brain. J. Comp. Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- Friedman JM. Leptin at 14 y of age: an ongoing story. Am. J.Clin. Nutrit. 2009;89:973S–979S. doi: 10.3945/ajcn.2008.26788B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühbeck G, Gómez-Ambrosi J, Martínez JA. Pre- and postprandial expression of the leptin receptor splice variants OB-Ra and OB-Rb in murine peripheral tissues. Physiol. Res. 1999;48:189–195. [PubMed] [Google Scholar]
- Gaige S, Abou E, Abysique A, Bouvier M. Effects of interactions between interleukin-1 beta and leptin on cat intestinal vagal mechanoreceptors. J. Physiol. 2004;555:297–310. doi: 10.1113/jphysiol.2003.054379. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman NS, Kimmel CB, Jones MA, Adams RJ. Shaping the zebrafish notochord. Develop. 2003;130(5):873–887. doi: 10.1242/dev.00314. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front. Neuroendocrin. 2002;23:2–40. doi: 10.1006/frne.2001.0224. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinol. 2002;143:239–246. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- Guerra B, Santana A, Fuentes T, Delgado-Guerra S, Cabrera-Socorro A, Dorado C, Calbet JA. Leptin receptors in human skeletal muscle. J. Appl. Physiol. 2007;102:1786–1792. doi: 10.1152/japplphysiol.01313.2006. [DOI] [PubMed] [Google Scholar]
- Guilmeau S, Buyse M, Bado A. Gastric leptin: a new manager of gastrointestinal function. Cur. Opin. Pharmacol. 2004;4:561–566. doi: 10.1016/j.coph.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Hall TA. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Hansen GH, Niels-Christiansen LL, Danielsen EM. Leptin and the obesity receptor (OB-R) in the small intestine and colon: a colocalization study. J.Histochem., Cytochem. 2008;56:677–685. doi: 10.1369/jhc.2008.950782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins TA, Cavodeassi F, Erdélyi F, Szabó G, Lele Z. The small molecule Mek1/2 inhibitor U0126 disrupts the chordamesoderm to notochord transition in zebrafish. BMC Developmental Biology. 2008;8:42. doi: 10.1186/1471-213X-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horev G, Einat P, Aharoni T, Eshdat Y, Friedman-Einat M. Molecular cloning and properties of the chicken leptin-receptor (CLEPR) gene. Molec. Cell. Endocrin. 2000;162:95–106. doi: 10.1016/s0303-7207(00)00205-7. [DOI] [PubMed] [Google Scholar]
- Huising MO, Geven EJ, Kruiswijk CP, Nabuurs SB, Stolte EH, Spanings FA, Verburg-van Kemenade BM, Flik G. Increased leptin expression in common carp (Cyprinus carpio) after food intake but not after fasting or feeding to satiation. Endocrinol. 2006;147:5786–5797. doi: 10.1210/en.2006-0824. [DOI] [PubMed] [Google Scholar]
- Johnson RM, Johnson TM, Londraville RL. Evidence for leptin expression in fishes. J. Exp. Zool. 2000;286:718–724. doi: 10.1002/(sici)1097-010x(20000601)286:7<718::aid-jez6>3.0.co;2-i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling P, Rønnestad I, Stefansson SO, Murashita K, Kurokawa T, Björnsson BT. A homologous salmonid leptin radioimmunoassay indicates elevated plasma leptin levels during fasting of rainbow trout. Gen. Comp. Endocrin. 2009;162:307–312. doi: 10.1016/j.ygcen.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Kurokawa T, Murashita K. Genomic characterization of multiple leptin genes and a leptin receptor gene in the Japanese medaka,Oryzias latipes . Gen. Comp. Endocrinol. 2009;161:229–237. doi: 10.1016/j.ygcen.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Kurokawa T, Murashita K, Suzuki T, Uji S. Genomic characterization and tissue distribution of leptin receptor and leptin receptor overlapping transcript genes in the pufferfish, Takifugu rubripes. Gen. Comp. Endocrinol. 2008;158:108–114. doi: 10.1016/j.ygcen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Kurokawa T, Uji S, Suzuki T. Identification of cDNA coding for a homologue to mammalian leptin from pufferfish, Takifugu rubripes. Pept. 2005;26:745–750. doi: 10.1016/j.peptides.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. ClustalW and ClustalX version 2. Bioinform. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Liu Q, Marrs JA, Londraville RL, Wilson AL. Cadherin-7 function in zebrafish development. Cell & Tissue Research. 2008;334:37–45. doi: 10.1007/s00441-008-0664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Sanborn KL, Cobb N, Raymond PA, Marrs JA. R-cadherin expression in the developing and adult zebrafish visual system. J Comp. Neurol. 1999;410(2):303–319. doi: 10.1002/(sici)1096-9861(19990726)410:2<303::aid-cne11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Londraville DR, Niewiarowski PH. Leptin signaling systems in reptiles and amphibians. In: Paolucci, editor. Leptin in Non-mammalian Vertebrates. Kerala, India: Transworld Research Network; 2010. in press. [Google Scholar]
- Londraville RL, Duvall CS. Murine leptin injections increase intracellular fatty acid-binding protein in green sunfish (Lepomis cyanellus) Gen. Comp. Endocrin. 2002;129:56–62. doi: 10.1016/s0016-6480(02)00510-5. [DOI] [PubMed] [Google Scholar]
- Londraville RL, Sidell BD. Ultrastructure of aerobic muscle in Antarctic fishes may contribute to maintenance of diffusive fluxes. J. Exp. Biol. 1990;150:205–220. [Google Scholar]
- Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int. J. Obes. 2002;26:1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Moar KM, Hoggard N. Localization of leptin receptor (Ob- R) messenger ribonucleic acid in the rodent hindbrain. Endocrinology. 1998;139:29–34. doi: 10.1210/endo.139.1.5685. [DOI] [PubMed] [Google Scholar]
- Murashita K, Uji S, Yamamoto T, Ronnestad I, Kurokawa T. Production of recombinant leptin and its effects on food intake in rainbow trout (Oncorhynchus mykiss) Comp. Biochem.Physiol. B. 2008;150:377–384. doi: 10.1016/j.cbpb.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Ng ANY, de Jong-Curtain TA, Mawdsley DJ, White SJ, Shin J, Appel B, Duc Si Dong P, Stainier DYR, Heath JK. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Develop. Biol. 2005;286:114–135. doi: 10.1016/j.ydbio.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Niewiarowski PH, Balk ML, Londraville RL. Phenotypic effects of leptin in an ectotherm: a new tool to study the evolution of life histories and endothermy? J. Exp. Biol. 2000;203:295–300. doi: 10.1242/jeb.203.2.295. [DOI] [PubMed] [Google Scholar]
- Paolucci M, Rocco M, Varricchio E. Leptin presence in plasma, liver and fat bodies in the lizard Podarcis sicula- Fluctuations throughout the reproductive cycle. Life Sci. 2001;69:2399–2408. doi: 10.1016/s0024-3205(01)01326-1. [DOI] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Raguso C, McCullough AJ. Leptin and the gastrointestinal tract. Cur. Opin. Gastroent. 2000;16:160–165. doi: 10.1097/00001574-200003000-00011. [DOI] [PubMed] [Google Scholar]
- Ramsay TG, Richards MP. Leptin and leptin receptor expression in skeletal muscle and adipose tissue in response to in vivo porcine somatotropin treatment. J. Anim. Sci. 2005;83:2501–2508. doi: 10.2527/2005.83112501x. [DOI] [PubMed] [Google Scholar]
- Richards MP, Poch SM. Molecular cloning and expression of the turkey leptin receptor gene. Comp. Biochem. Physiol. B. 2003;136:833–847. doi: 10.1016/s1096-4959(03)00260-4. [DOI] [PubMed] [Google Scholar]
- Sagawa N, Yura S, Itoh H, Kakui K, Takemura M, Nuamah MA, Ogawa Y, Masuzaki H, Nakao K, Fujii S. Possible role of placental leptin in pregnancy: a review. Endocrine. 2002;19:65–71. doi: 10.1385/ENDO:19:1:65. [DOI] [PubMed] [Google Scholar]
- Sharp PJ, Dunn IC, Waddington D, Boswell T. Chicken leptin. Gen.Comp. Endocrin. 2008;158:2–4. doi: 10.1016/j.ygcen.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Smith JC. Dorso-ventral patterning in the neural tube. Current Biology. 1993;3:582–585. doi: 10.1016/0960-9822(93)90003-7. [DOI] [PubMed] [Google Scholar]
- Sidell BD, Driedzic WR, Stowe DB, Johnston IA. Biochemical correlations of power development and metabolic fuel preferenda in fish hearts. Physiol. Zool. 1987;60:221–232. [Google Scholar]
- Solberg R, Aas V, Thoresen GH, Kase ET, Drevon CA, Rustan AC, Reseland JE. Leptin expression in human primary skeletal muscles cells is reduced during differentiation. J. Cell Biochem. 2005;96:89–96. doi: 10.1002/jcb.20521. [DOI] [PubMed] [Google Scholar]
- Spanovich S, Niewiarowski PH, Londraville RL. Seasonal effects on circulating leptin in the lizard Sceloporus undulatus from two populations. Comp. Biochem. Physiol.B, Biochem. 2006;143:507–513. doi: 10.1016/j.cbpb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Tang R, Dodd A, Lai D, McNabb WC, Love DR. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time PCR normalization. Acta Biochim. Biophys. Sin. 2007;39(5):384–390. doi: 10.1111/j.1745-7270.2007.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia LA. The leptin receptor. J. Biol. Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- Volkoff H, Eykelbosh AJ, Peter RE. Role of leptin in the control of feeding of goldfish Carassius auratus: interactions with cholecystokinin, neuropeptide Y and orexin, A, modulation by fasting. 2003;972:90–109. doi: 10.1016/s0006-8993(03)02507-1. [DOI] [PubMed] [Google Scholar]
- Weil C, Le Bail PY, Sabin N, Le Gac F. In vitro action of leptin on FSH and LH production in rainbow trout (Onchorhychus mykiss) at different stages of the sexual cycle. Gen. Comp. Endocrinol. 2003;130:2–12. doi: 10.1016/s0016-6480(02)00504-x. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. Eugene, OR: University of Oregon Press; 2005. [Google Scholar]
- Wilkinson M, Brown R, Imran SA, Ur E. Adipokine gene expression in brain and pituitary gland. Neuroendocrin. 2007;86:191–209. doi: 10.1159/000108635. [DOI] [PubMed] [Google Scholar]
- Wong MML, Yu RMK, Ng PKS, Law SHW, Tsang AKC, Kong RYC. Characterization of a hypoxia-responsive leptin receptor (omLepRL) cDNA from the marine medaka (Oryzias melastigma) Mar. Pollut. Bull. 2007;54:792–819. doi: 10.1016/j.marpolbul.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Wulliman MP, Rupp B, Reichert H. Neuroanatomy of the Zebrafish Brain: A Topological Atlas. Birkhauser Verlag; 1996. 144 pp. [Google Scholar]
- Ye J, Öztürk L, Xi L, Kukreja RC, Cabrera de Leon A, O'Donnell C, Vats P, Guerre-Millo M, Bigard M. Commentary on viewpoint: Regulation of leptin by hypoxia. J. Appl. Physiol. 2008;105:1687–1690. doi: 10.1152/japplphysiol.zdg-8233-vpcomm.2008a. [DOI] [PubMed] [Google Scholar]
- Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, DiMarchi RD, Furman TC, Hale JE, Hsiung HM, Schoner BE, Smith DP, Zhang XY, Wery JP, Schevitz RW. Crystal structure of the obese protein leptin E-100. Nature. 1997;387(6629):206–209. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and it human homologue. Nature. 1994;372:425–431. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]