Abstract
Environmental variation in signalling conditions affects animal communication traits, with possible consequences for sexual selection and reproductive isolation. Using spectrophotometry, we studied how male coloration within and between populations of two closely related Lake Victoria cichlid species (Pundamilia pundamilia and P. nyererei) covaries with water transparency. Focusing on coloration patches implicated in sexual selection, we predicted that in clear waters, with broad-spectrum light, (1) colours should become more saturated and (2) shift in hue away from the dominant ambient wavelengths, compared to more turbid waters. We found support for these predictions for the red and yellow coloration of P. nyererei but not the blue coloration of P. pundamilia. This may be explained by the species difference in depth distribution, which generates a steeper gradient in visual conditions for P. nyererei compared to P. pundamilia. Alternatively, the importance of male coloration in intraspecific sexual selection may differ between the species. We also found that anal fin spots, that is, the orange spots on male haplochromine anal fins that presumably mimic eggs, covaried with water transparency in a similar way for both species. This is in contrast to the other body regions studied and suggests that, while indeed functioning as signals, these spots may not play a role in species differentiation.
1. Introduction
Heterogeneous signaling conditions exert divergent selection on animal communication traits, leading to the divergence of sexual signals between environments [1–3]. For example, bird song characteristics may covary with the sound transmission properties of the vegetation (e.g., [4]) and fish coloration may covary with underwater light conditions (e.g., [5]). These adaptations could contribute to reproductive isolation between populations and possibly promote speciation [6–9]. In addition, signalling conditions may influence the opportunity for sexual selection, by compromising signal perception or by increasing the costs of mate searching [10–12].
The haplochromine cichlids of East Africa constitute a species-rich assemblage with extensive variation in male coloration. Several lines of evidence suggest that variation in underwater light conditions influences the evolution of these colour patterns. In Lake Victoria, for example, male colours tend to become more distinctive in locations with relatively high water transparency [13, 14] and some colour morphs are completely absent in turbid waters [15].
Haplochromine coloration mediates both intraspecific sexual selection [16, 17] and interspecific behavioural isolation [18–20]. Thus, environment-dependent adaptation in male colours may contribute to reproductive isolation. Indeed, there is a relationship between species diversity and colour diversity along water transparency gradients in Lake Victoria, indicating that constraints on visual communication may explain variation in species richness [13].
Here, we focus on the species pair P. pundamilia and P. nyererei. These two closely related species are morphologically similar, and the cryptically coloured females of both species are difficult to distinguish. Males however differ markedly in coloration: male P. pundamilia are blue-grey while male P. nyererei are bright red and yellow (Figure 1). The species co-occur at various locations in Lake Victoria that differ in water transparency. In the present study, we investigate how this variation in signalling conditions may affect male coloration in both species. Previous work indicated that, within P. nyererei, populations inhabiting turbid waters exhibit less red coloration in males [13, 14] and weaker colour preferences in females [14] compared to clear-water populations.
Figure 1.
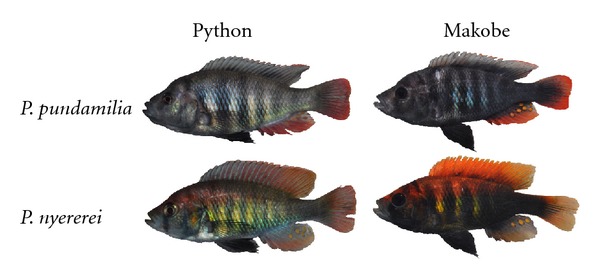
Examples illustrating the colour variation between species and populations.
We address the following predictions. First, we predict that colours are less saturated (i.e., less chromatic) in turbid waters. Since less-saturated colours can reflect a broader range of wavelengths, we expect these to be favoured (i.e., reflect more light and thus be more conspicuous) in turbid water. Second, we assume that colour conspicuousness is constrained by the ambient light intensity at the wavelengths of reflectance. As a result, colours outside the dominant wavelengths of the ambient spectrum will be favoured only in clear waters where their absolute intensities are high enough for receivers to detect. We therefore predict that, in clear water, reflectance should shift towards either shorter (blue) or longer (red) wavelengths, away from the dominant wavelengths (green) in the ambient light.
Finally, as a result of the above changes, we predict that colour differentiation between P. pundamilia and P. nyererei will be more pronounced in clear waters.
2. Methods
2.1. Study Species and Sampling Locations
Pundamilia pundamilia and P. nyererei are two closely related species of haplochromine rock-dwelling cichlids that co-occur throughout a gradient of light environments in Lake Victoria. Both species are morphologically very similar. Females of both species show a yellowish cryptic coloration and are difficult to tell apart. P. pundamilia males are blue-grey while males of P. nyererei are red dorsally with yellow flanks (Figure 1). Females of both species exert species-assortative colour preferences [18]. In P. nyererei, male yellow and red coloration is subject to directional sexual selection as well [14, 17]. Due to its shallow depth, Lake Victoria has relatively turbid waters. Light scattering and absorption are mostly due to nonphytoplankton particles, derived from soil erosion and resuspended sediment [21]. In the present study, we focus on five islands in the south of the lake (Figure 2; Table 1). Two of these islands (Makobe and Ruti) are located offshore and have relatively clear waters. Here, P. pundamilia inhabits the top shallower waters while P. nyererei dwells in deeper waters. In turbid waters (Kissenda and Python), both species inhabit the same shallow depth layers [22]. At even more turbid locations (i.e., Luanso island), the two species are replaced by a single panmictic population with variable coloration, referred to as P. sp. “Luanso” [22]. Pundamilia sp. breed year-round, with no marked seasonality in breeding activity. All data were collected during May-June 2010.
Figure 2.
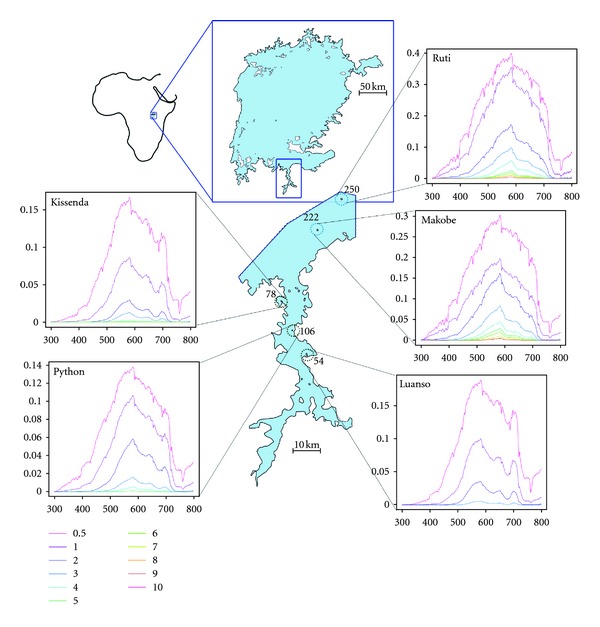
Sampling locations and their underwater light environments. In each panel, curves show underwater ambient light spectra at different depths (m). Numbers shown next to the islands are the mean Secchi disk measurements (cm).
Table 1.
Study site characteristics and numbers of individuals collected.
| Luanso | Kissenda | Python | Makobe | Ruti | |
|---|---|---|---|---|---|
| Maximum depth of the rock-sand interface (m)1 | 5-6 | 7-8 | 7-8 | 8–12 | >13 |
| Mean Secchi transparency (cm, mean ± se)2 |
54 ± 4 (n = 9) | 78 ± 8 (n = 8) | 106 ± 7 (n = 11) | 222 ± 7 (n = 88) | 250 ± 23 (n = 7) |
| Spectral width (and range, nm) of the light spectrum at 2 m depth and 0.002 W/m2 light intensity | 195 (497–692) | 247 (477–724) | 264 (455–719) | 366 (362–728) | 390 (343–733) |
| Sampling dates for irradiance spectrophotometry (2010) | 29/5, 7/6 | 17/5, 1/6, 9/6 | 20/5, 26/5, 4/6, 5/6 | 22/5, 27/5, 3/6, 10/6 | 24/5, 31/5, 12/6 |
| Sample size P. pundamilia | 103 | 8 | 10 | 11 | 9 |
| Sample size P. nyererei | 6 | 16 | 19 | 17 |
1Data from [23] and pers. obs.
2Data collected between 2000 and 2010. Water transparency varies seasonally, but differences between sampling locations are highly consistent (for Secchi readings collected during 2000–2010 at our four sampling sites: anova controlling for sampling date: F 3,107 = 25.41, P ≪ 0.0001).
3 P. sp. “Luanso” replaces both species at this locality.
2.2. Underwater Light Environments
At each island, water transparency was measured using a white Secchi disk (Table 1). We measured downwelling irradiance at each island using a BLK-C-100 spectrophotometer and an F-600-UV-VIS-SR optical fiber with CR2 cosine receptor (StellarNet, FL). Measurements were collected in 0.5 m depth increments down to 5 m depth and subsequent 1 m increments down to 12 m depth. At turbid locations, light intensities were too low to obtain reliable measures over this entire depth range (Luanso: measurements down to 4 m; Kissenda and Python: down to 7 m). During each measurement series, we took a minimum of two irradiance spectra at each depth and used the average for further analysis (for repeatability estimates see Supplementary Table S1 in Supplementary Material available online at doi:10.1155/2012/161306). We collected 2 independent measurement series for Luanso island, 3 series each for Kissenda and Ruti islands and 4 series each for Python and Makobe islands (Table 1).
To characterise variation in light environments between locations and depth ranges we calculated the orange ratio for each spectrum [24, 25]: the light intensity in the 550–700 nm range (yellow, orange, red) divided by the intensity in the 400–550 nm range (blue, green). This ratio reflects the spectral composition of the ambient light and tends to increase with depth and with increasing turbidity, as short wavelengths are selectively scattered and absorbed [22, 26]. We subsequently fitted island-specific exponential curves to obtain estimated orange ratios at each depth. Using the species-specific depth ranges (obtained from [22] and assuming equal distributions at Makobe and Ruti) we subsequently identified the range of orange ratios that each species experiences in its natural habitat.
2.3. Reflectance Spectrophotometry
Adult males of the three Pundamilia species were collected by gillnetting and angling (sample sizes are given in Table 1). Immediately after collection, reflectance spectra at different areas of the body (Figure 3) were taken using the above-mentioned spectrophotometer, an SL4-DT (Deuterium/Tungsten) light source and an R600-8-UV-VIS reflectance probe (StellarNet, FL). We focused on body parts that are potentially subject to (divergent) sexual selection. In P. nyererei, sexually selected coloration (red and yellow; [14, 17]) is mostly present on the flank, dorsum, and dorsal fin. In P. pundamilia, intraspecific sexual selection has not been explored and we therefore analysed the same body areas, that are grey-blue in this species. However, red coloration is present also in P. pundamilia, namely, on the edges (“lappets”) of the unpaired fins. In order to capture potentially important variation in this trait, we included “dorsal fin lappets” as an additional body area for both species. Finally, for both species we also measured the spectra of the anal fin spots (“egg dummies”) as these brightly coloured spots have been implicated in sexual communication [27–30]. For correlations between body areas, see Supplementary Tables S4 and S5.
Figure 3.
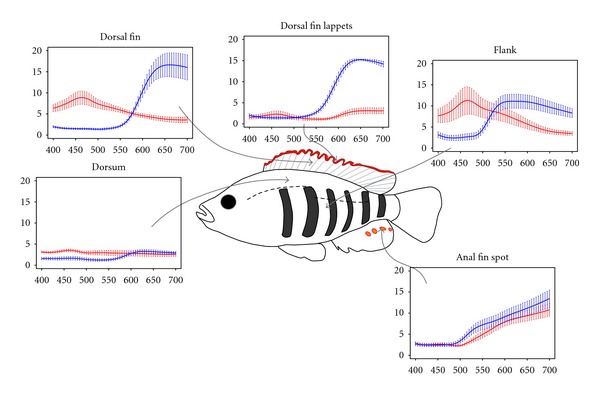
Body areas measured and reflectance spectra for each species (average with standard error; P. pundamilia in blue, P. nyererei in red; both from Makobe island).
About halfway through the field work, the light source stopped working and subsequent measurements had to be taken using the sun as a light source (see below for statistical incorporation of this variation).
2.4. Calculation of Colour Metrics
A minimum of two reflectance spectra were measured for each body region for each fish, and the mean of these was used for calculations (unless after visual inspection, one of the spectra was outside expected limits and was discarded, less than 10% of all spectra; repeatability estimates for included spectra are given in Supplementary Tables S2 and S3). We then extracted two colour metrics (see Table 2), excluding the UV part of the spectrum (300–400 nm) because UV-sensitive pigments have not been detected in Lake Victoria cichlids including Pundamilia species [31, 32]. (1) Chroma (or saturation): a measure of the purity of a colour, indicating how much of the reflectance is concentrated in a particular segment of the spectrum. It ranges from 0 (e.g., grey or white) to 1 (a pure colour). (2) Hue: related to the wavelength at the maximum absolute slope in the reflectance spectrum, and the property that in common language we understand as colour (e.g., red, blue, green, etc.). As a measure of hue, we calculated λP 50, the wavelength at which 50% of the total reflectance between 400–700 occurs [33, 34].
Table 2.
Coloration metrics.
| Name/description | Formula | Reference |
|---|---|---|
| LM = B R − B G | ||
| MS = B Y − B B | ||
| where: | ||
| Chroma | [35] | |
| A measure of the “purity” or saturation of a colour; a function of how rapidly intensity changes with wavelength | ||
| , | ||
|
| ||
| λP 50 | ||
| Wavelength that divides the spectrum in two parts with equal spectral energy (i.e., the median of the cumulative distribution between 400–700 nm) | [33, 34] | |
Brightness, that is, the total intensity of light reflected, is another potentially important component of coloration. However, due to the failure of the light source we did not obtain reliable brightness estimates (see below) and therefore excluded this property from the analyses.
2.5. Data Analysis
We built linear models allowing for random effects as well as differences in variances among the explanatory variables, using Linear Mixed Effect models (LME) [36]. We fitted models for each coloration property, each body area, and each species separately. We chose this approach (as opposed to collapsing metrics and body areas into, for example, Principal Components) because it allows evaluation of specific predictions and exposes potential differences between body areas. All analyses incorporated four populations of each species (Luanso was excluded from the analyses but included in the figures as a reference). Because water transparency was bimodal rather than continuous (i.e., the waters at Kissenda and Python islands were similarly turbid, and Makobe and Ruti similarly clear, Table 1; Figure 4), water clarity was modelled as a categorical variable (i.e., turbid versus clear). A factor for population was included as a random effect in all models. In addition to water clarity, the effect of using either the lamp or the sun as a light source was included as explanatory variable. To address colour differentiation between species, species identity was added as a third explanatory variable and the interaction with water clarity evaluated.
Figure 4.
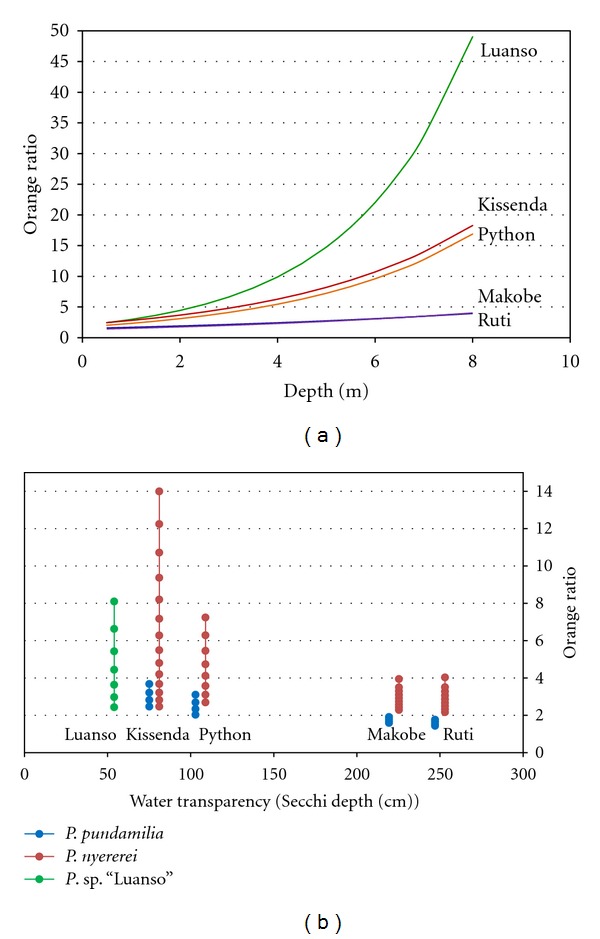
Variation in underwater light environments between sampling locations and species-specific depth ranges. For each location, plotted values derive from fitting an exponential function to all measured orange ratios at that location. (a) The increase in orange ratio with depth for the five sampling locations. Ruti and Makobe show virtually identical curves. (b) The orange ratios at the species- and island-specific depth ranges. Each symbol represents the orange ratio at a specific water depth (in 0.5 m increments) where the species occur.
For model selection, we explored all possible variance structures (variance components were functions that included the actual Secchi depths (Table 1) and a factor for light source) and selected the most parsimonious model using restricted maximum likelihood ratio and Akaike's information criterion, corrected for small sample size (AICc) [37]. After remaining with the best variance structure, we used maximum likelihood to reduce the complexity of the models and AICc to select the covariates that remain in the model. We then used ANOVA to test whether a model including the clarity covariate (or the interaction between species: clarity, when applicable) was significantly better than one that did not, and we report likelihood ratio and P values for this comparison.
All statistical analyses were conducted in R 2.12 [38], applying packages nlme and MuMIn. To adjust for multiple testing of the same prediction in multiple body areas, we used corrected P values (i.e., we multiplied the actual P values with the number of body areas, 5).
Our estimates of chroma and hue were not strongly influenced by the light source used (lamp or sun, see Supplementary Table S6) but there were major effects on brightness, showing significant interactions between water clarity and light source for all models. Therefore, we had to discard this metric.
3. Results
3.1. Light Environments
At all study sites, the proportion of longer wavelengths in the light spectrum (i.e., wavelengths >550 nm) increased towards deeper waters (Figure 4(a)). The increase was steepest at Luanso, intermediate at Kissenda and Python islands, and very gentle at Makobe and Ruti islands. Incorporating species-specific depth ranges at each location, we estimated the range of orange ratios that the two species experience in their natural habitats. Both species are exposed to higher orange ratios in the turbid waters of Kissenda and Python, compared to Makobe and Ruti (Figure 4(b)). P. nyererei in particular experiences a large difference in light environment between turbid and clear locations, although the decrease in orange ratio was not significantly different between the species (ANOVA, interaction effect between Secchi reading and species on orange ratio: F2,4 = 4.49, P = 0.10).
3.2. Chroma
In P. pundamilia (Figure 5(a)), we did not observe a significant increase in chroma in any of the measured body areas. There was a trend for anal fin spots (L = 5.66, P = 0.087), but a significant decrease in chroma for dorsal fin (L = 6.81, P = 0.045). There were no changes in the chroma of the dorsum, flank, or dorsal fin lappets. The changes in P. nyererei were more consistent (Figure 5(b)), with significantly increased chroma in clearwater populations for dorsum (L = 9.16, P = 0.013) and dorsal fin (L = 12.53, P < 0.001) and a trend in the same direction for flank (L = 5.99, P = 0.072). No significant changes were observed in anal fin spots and dorsal fin lappets.
Figure 5.
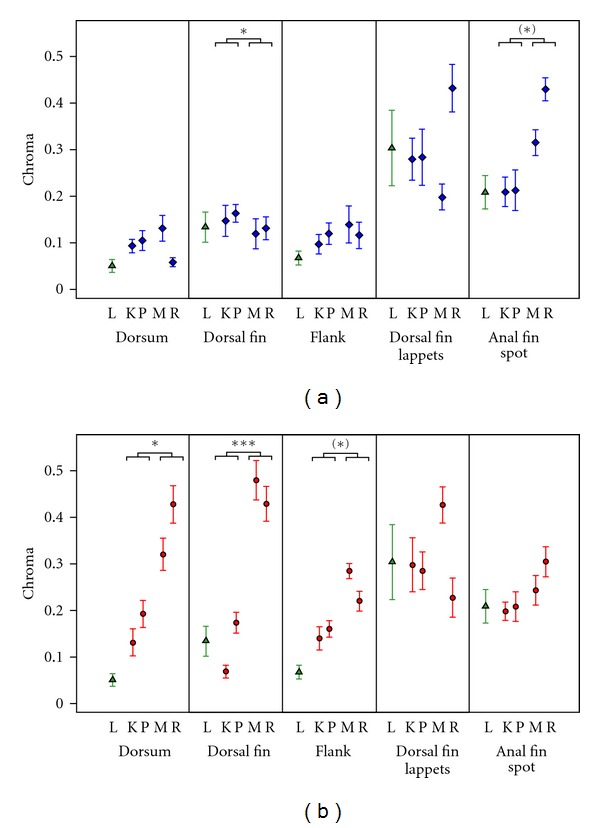
Chroma of different body parts at four sampling locations for (a) P. pundamilia (blue diamonds) and (b) P. nyererei (red circles). In both panels, P. sp. “Luanso” (green triangles) is included as a reference. Symbols indicate means with standard errors. Statistically significant differences between clear and turbid locations are indicated with asterisks (after correction for multiple testing; (*) P < 0.10; *P < 0.05; ***P < 0.001). L: Luanso, K: Kissenda, P: Python, M: Makobe, R: Ruti.
3.3. Changes in Hue
λP 50 (the wavelength that halves the total reflectance) was expected to shift towards more extreme wavelengths in clear waters. For the blue coloration elements in P. pundamilia, results were inconsistent with this prediction (Figure 6(a)). We found small and nonsignificant changes towards longer rather than shorter wavelengths for dorsum (L = 6.36, P = 0.059) and dorsal fin (L = 5.76, P = 0.082). There was no significant change in the hue of flank coloration. The red dorsal fin lappets also did not increase in λP 50. Only the yellow anal fin spots tended to follow the prediction, but the increase towards longer wavelengths in clear water was not statistically significant (L = 6.17, P = 0.065).
Figure 6.
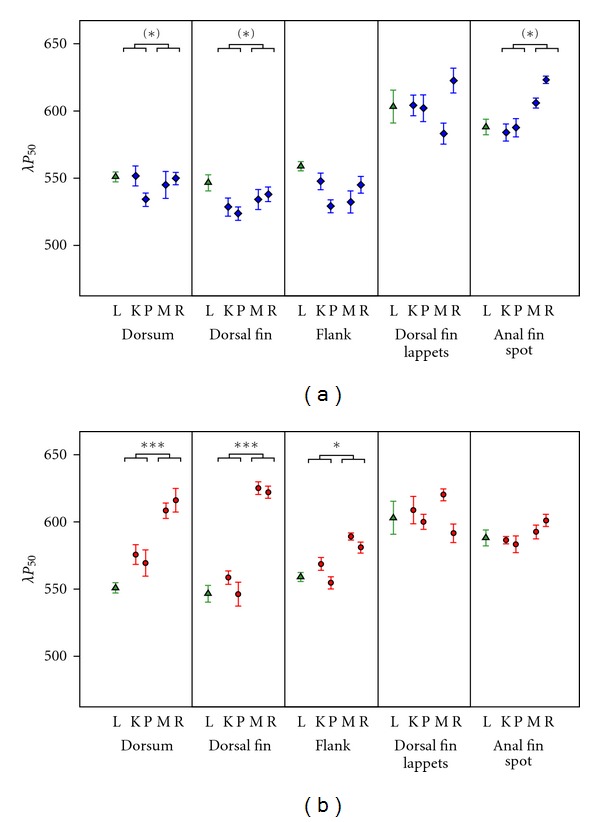
Hue (λP 50) of different body parts at four sampling locations for (a) P. pundamilia and (b) P. nyererei. Symbols and labels as in Figure 5.
In P. nyererei (Figure 6(b)), we observed a highly significant shift towards longer wavelength reflectance for the dorsum (L = 11.51, P < 0.001), dorsal fin (L = 15.69, P < 0.001) and flank (L = 9.28, P = 0.012). Anal fin spots and dorsal fin lappets did not show significant changes.
3.4. Colour Differentiation between Species
λP 50 was also used to test for the extent of differentiation between the two species' coloration (Figure 7). We found increased differentiation in clear waters for dorsal fin (L = 27.29, P < 0.001), and flank (L = 8.77, P = 0.016) and a trend in the same direction for dorsum (L = 5.52, P = 0.094). In contrast, coloration of anal fin spots and dorsal fin lappets did not show increased differentiation with water clarity.
Figure 7.
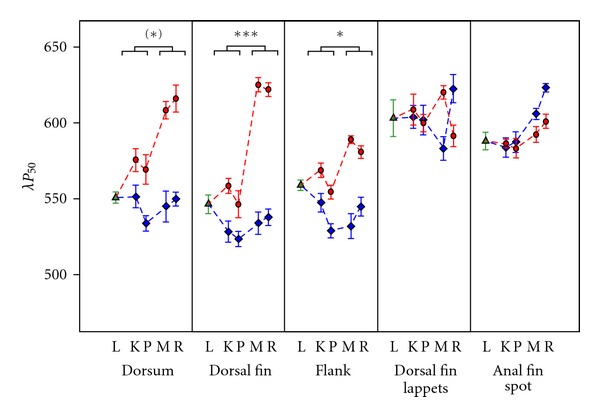
Differentiation in coloration hue (λP 50) of different body regions between P. pundamilia and P. nyererei. Symbols and labels as in Figure 5.
4. Discussion
We examined patterns of colour variation within and between two cichlid species that inhabit different signalling environments. We specifically tested whether fish coloration becomes more saturated and increasingly exploits wavelength ranges outside the dominant ambient light spectrum, in populations inhabiting clearer waters. We found support for these predictions in P. nyererei, but inconsistent results for P. pundamilia. For those body areas that are differently coloured between the species, we observed increasing species differentiation in coloration towards clear waters.
For the red and yellow coloration elements in P. nyererei, we found that colours are more saturated and shifted towards longer wavelengths (i.e., redder) in clearer waters. For the blue coloration of P. pundamilia however, we did not observe any statistically significant shift towards greater chroma or shorter wavelengths (i.e., more blue). One reason for this incongruence may lie in the different depth distributions of the two species. The change in the environmental light spectrum from turbid to clear waters is more pronounced in the deeper waters where P. nyererei is most abundant (Figure 4), possibly generating stronger divergent selection between allopatric populations for this species. It is also possible that the importance of male coloration for intraspecific female choice differs between the species. Sexual selection on red and yellow colour elements is well established in P. nyererei [14, 17], but intraspecific sexual selection remains to be studied in P. pundamilia. Just like P. nyererei, P. pundamilia females use colour cues during interspecific mate choice [18]. However, they might use other characteristics, such as body size, behaviour or chemical cues in their choice among conspecific males. Recent work in these and other haplochromines indicates that chemical cues could play a role in mate choice in some species [39–41]. Methodological constraints may also contribute to the difference between species, as the blue-grey coloration of P. pundamilia may be more difficult to capture with spectrophotometry [42]. This is consistent with the observation that the yellow anal fin spots did tend to change in the predicted direction for both hue and chroma.
Although not statistically significant, we observed similar variation in anal fin spot coloration in both species. This is consistent with earlier suggestions of adaptation of these spots to environmental light: Goldschmidt [29] found that species inhabiting darker habitats had relatively large anal fin spots. Anal fin spots have been suggested to mimic eggs and contribute to fertilisation success (e.g., [43] but see [44]). This functional context raises the question whether the observed variation in spot coloration influences the resemblance to eggs. Pundamilia sp. eggs are orange, but no data exist regarding egg colour variation between species or populations. Anal fin spots have also been suggested to play a role in speciation (e.g., [30, 45–48]. Here, we do not find evidence for species-specific effects in spot coloration and a role in species recognition is thus unlikely.
We found no consistent changes in the coloration of the red dorsal fin lappets in either species. Interestingly, this trait is shared not only between our study species, that are very closely related, but also occurs in many other haplochromines [49]. This may indicate that there is little genetic variation in this trait, preventing adaptive divergence between populations and species.
We propose that the differences in coloration that we observed across the four studied populations are adaptations to different underwater light environments. Fish coloration can be phenotypically plastic [50, 51] and in haplochromines, colour expression varies with diet, territorial status [52, 53], and stress ([54]; pers. obs.). However, given the maintenance of colour differences in the laboratory, and significant genetic differentiation between populations [22], evolutionary adaptation is both feasible and likely. We hypothesise that the observed patterns are driven by selection for signal conspicuousness, which requires that signals have sufficient intensity as well as provide contrast against the sensory background [2].
Colour signals that rely on reflection of incident light (as opposed to luminescence or iridescence) will maximise signal intensity by reflecting most strongly in the wavelength range of the incident light (e.g., [55, 56]). However, maximising colour contrast requires reflectance of wavelengths that are underrepresented in the background (e.g., [57]). When the illuminating and background spectra are similar, signal evolution will likely reflect a tradeoff between signal intensity and contrast. This situation occurs in many aquatic systems, where signals are viewed against the water column [58]. In some fish species, conspicuousness is achieved by reflectance of colours that contrast against the prevalent ambient light (e.g., [59, 60]). In other species, colour variation is positively correlated with the prevalence of the reflected wavelengths in the environmental light spectrum [5, 50, 61]. The patterns we observe in Pundamilia may reflect a compromise between these two strategies. The blue P. pundamilia are restricted to shallow waters where short wavelengths are still present, whereas the red and yellow P. nyererei inhabit deeper waters with red-shifted ambient light. At the same time, colour contrast against the background can be maintained by exploiting the shoulders rather than the peak of the ambient spectrum and by reflecting in a relatively narrow wavelength range. We hypothesise that this explains the shift in hue and chroma in the clearwater populations of P. nyererei, that experience a broader and more intense illumination spectrum than their counterparts in turbid waters. The failure of our light source precluded analysis of brightness variation in the present dataset. As a consequence, we are unable to test whether the conspicuousness of male coloration is optimised for local viewing conditions. Moreover, recent studies suggest that there is variation in visual systems between sympatric species and allopatric populations of Pundamilia [22, 31], and ongoing work is aimed at identifying the visual pigments and expression levels in the populations studied here. This information will subsequently be incorporated into quantitative visual models.
Different patterns of variation may also result from other factors than intraspecific perceptual processes. For example, colour production may be subject to physiological constraints [62, 63]. The red and yellow coloration in Pundamilia is carotenoid based [14] and the availability of dietary carotenoids may covary with underwater light intensity [64, 65]. Thus, redder coloration in clearer waters could be due to greater availability of carotenoids. Observations that colour variation between populations is maintained in the laboratory indicate a heritable component, but this does not rule out that carotenoid limitation selectively favours different levels of colour expression [66–68]. Testing this hypothesis requires evaluating whether haplochromines are carotenoid limited in their natural habitat. Second, sexually selected traits are often subject to increased predation (e.g., in fish: [69–72]). In Lake Victoria, however, piscivorous birds and fish tend to be more numerous in clearwater locations [13]; pers. obs), possibly because turbidity hampers visual predation [12, 73]. This would favour less chromatic and less contrasting colours in clearwater, which is not what we observe in Pundamilia. Finally, male colour evolution will likely reflect variation in female preferences among populations. Relaxed sexual selection on visual signals in turbid water has been documented in several fish species [74–76]. In addition to immediate effects of reduced signal perception, variation in water turbidity may lead to heritable changes in female preference behaviour. This seems to be the case in P. nyererei. Females from turbid waters are less selective with respect to male coloration, even when tested under broad-spectrum illumination in the laboratory [14]. The observed colour variation across populations might therefore be driven by heterogeneous sexual selection regimes, rather than selection for optimal local conspicuousness. To resolve this question, we need more detailed analyses of variation in female preference and choosiness to establish sexual selection strength for the different aspects of male coloration (hue, chroma), as well as quantitative estimates of visual conspicuousness in relation to these aspects. Such studies should also help to identify the mechanisms underlying preference variation. Beside sensory biases for conspicuous signals, haplochromine female preferences are likely influenced by selection for heritable benefits (e.g., parasite resistance [52]). Thus, if signal conspicuousness in turbid waters is maximised by lower carotenoid deposition, for example, carotenoid-dependent aspects of male coloration may become less informative and therefore less important in mate selection (e.g., [77–79]). We suggest that the interactions between sensory processes and signal content in shaping haplochromine colours constitute an important and rewarding avenue for further study.
Taken together, we found that different body regions and different species show different responses to environmental heterogeneity in visual conditions: divergence at the level of allopatric populations as well as sympatric species (flank, dorsum, dorsal fin), divergence between populations but not species (anal fin spots), or no consistent pattern of change (dorsal fin lappets). Importantly, our findings confirm earlier suggestions that divergent sexual selection is involved in haplochromine species divergence [13, 80], as we found significantly stronger species differentiation towards clear waters for the same body areas that were previously shown to be subject to intraspecific sexual selection in P. nyererei [17]. As such, our study implicates species- and habitat-specific selective pressures as well as potential genetic or functional constraints to adaptive divergence and thereby contributes to identifying the traits involved in the buildup of reproductive isolation.
Supplementary Material
Table S1: Repeatability for irradiance data: orange ratio.
Table S2: Repeatability for reflectance data: chroma.
Table S3: Repeatability for reflectance data: λP50.
Table S4: Spearman correlations between body areas for chroma.
Table S5: Spearman correlations between body areas for λP50.
Table S6: Descriptive statistics for the effect of using different light sources.
Acknowledgments
The authors thank the Tanzanian Commission for Science and Technology for research permission and the Tanzanian Fisheries Research Institute (Y. L. Budeba, B. P. Ngatunga, E. F. B. Katunzi, and H. D. J. Mrosso) for hospitality and facilities. M. Kayeba and M. Haluna provided expert assistance in the field. For discussion and suggestions on earlier versions of this paper, the authors thank R. K. Butlin, K. E. Knott, F. J. Weissing, and two anonymous reviewers. I. R. Pen and L. M. Enqvist provided statistical advice. For general discussion and feedback, they thank the members of the Marie Curie Initial Training Network on Speciation and of the Department of Fish Ecology & Evolution at Eawag. This study was supported by the Swiss National Science Foundation (SNSF), the Netherlands Foundation for Scientific Research (NWO-ALW), and the European Union (EU-Marie Curie ITN).
References
- 1.Marchetti K. Dark habitats and bright birds illustrate the role of the environment in species divergence. Nature. 1993;362(6416):149–152. [Google Scholar]
- 2.Endler JA. Signals, signal conditions, and the direction of evolution. The American Naturalist. 1992;139:S125–S153. [Google Scholar]
- 3.Badyaev AV, Leaf ES. Habitat associations of song characteristics in Phylloscopus and Hippolais warblers. Auk. 1997;114(1):40–46. [Google Scholar]
- 4.Tobias JA, Aben J, Brumfield RT, et al. Song divergence by sensory drive in amazonian birds. Evolution. 2010;64(10):2820–2839. doi: 10.1111/j.1558-5646.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 5.Morrongiello JR, Bond NR, Crook DA, Wong BBM. Nuptial coloration varies with ambient light environment in a freshwater fish. Journal of Evolutionary Biology. 2010;23(12):2718–2725. doi: 10.1111/j.1420-9101.2010.02149.x. [DOI] [PubMed] [Google Scholar]
- 6.Slabbekoorn H, Smith TB. Bird song, ecology and speciation. Philosophical Transactions of the Royal Society B. 2002;357(1420):493–503. doi: 10.1098/rstb.2001.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray SM, McKinnon JS. Linking color polymorphism maintenance and speciation. Trends in Ecology & Evolution. 2007;22(2):71–79. doi: 10.1016/j.tree.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Boughman JW. How sensory drive can promote speciation. Trends in Ecology & Evolution. 2002;17(12):571–577. [Google Scholar]
- 9.Maan ME, Seehausen O. Ecology, sexual selection and speciation. Ecology Letters. 2011;14(6):591–602. doi: 10.1111/j.1461-0248.2011.01606.x. [DOI] [PubMed] [Google Scholar]
- 10.Richardson C, Lengagne T. Multiple signals and male spacing affect female preference at cocktail parties in treefrogs. Proceedings of the Royal Society B. 2010;277(1685):1247–1252. doi: 10.1098/rspb.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones AG, Ratterman NL. Mate choice and sexual selection: what have we learned since Darwin? Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10001–10008. doi: 10.1073/pnas.0901129106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Sluijs I, Gray SM, Amorim MCP, et al. Communication in troubled waters: responses of fish communication systems to changing environments. Evolutionary Ecology. 2011;25(3):623–640. [Google Scholar]
- 13.Seehausen O, van Alphen JJM, Witte F. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science. 1997;277(5333):1808–1811. [Google Scholar]
- 14.Maan ME, Seehausen O, van Alphen JJM. Female mating preferences and male coloration covary with water transparency in a Lake Victoria cichlid fish. Biological Journal of the Linnean Society. 2010;99(2):398–406. [Google Scholar]
- 15.Lande R, Seehausen O, van Alphen JJM. Mechanisms of rapid sympatric speciation by sex reversal and sexual selection in cichlid fish. Genetica. 2001;112-113:435–443. [PubMed] [Google Scholar]
- 16.Pauers MJ, McKinnon JS, Ehlinger TJ. Directional sexual selection on chroma and within-pattern colour contrast in Labeotropheus fuelleborni. Proceedings of the Royal Society B. 2004;271(6):S444–S447. doi: 10.1098/rsbl.2004.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maan ME, Seehausen O, Söderberg L, et al. Intraspecific sexual selection on a speciation trait, male coloration, in the Lake Victoria cichlid Pundamilia nyererei. Proceedings of the Royal Society B. 2004;271(1556):2445–2452. doi: 10.1098/rspb.2004.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seehausen O, van Alphen JJM. The effect of male coloration on female mate choice in closely related Lake Victoria cichlids (Haplochromis nyererei complex) Behavioral Ecology and Sociobiology. 1998;42(1):1–8. [Google Scholar]
- 19.Knight ME, Turner GF. Laboratory mating trials indicate incipient speciation by sexual selection among populations of the cichlid fish Pseudotropheus zebra from Lake Malawi. Proceedings of the Royal Society B. 2004;271(1540):675–680. doi: 10.1098/rspb.2003.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger B, Mattersdorfer K, Sefc KM. Variable discrimination and asymmetric preferences in laboratory tests of reproductive isolation between cichlid colour morphs. Journal of Evolutionary Biology. 2010;23(2):433–439. doi: 10.1111/j.1420-9101.2009.01906.x. [DOI] [PubMed] [Google Scholar]
- 21.Okullo W, Ssenyonga T, Hamre B, et al. Parameterization of the inherent optical properties of Murchison Bay, Lake Victoria. Applied Optics. 2007;46(36):8553–8561. doi: 10.1364/ao.46.008553. [DOI] [PubMed] [Google Scholar]
- 22.Seehausen O, Terai Y, Magalhaes IS, et al. Speciation through sensory drive in cichlid fish. Nature. 2008;455(7213):620–626. doi: 10.1038/nature07285. [DOI] [PubMed] [Google Scholar]
- 23.Bouton N, Seehausen O, van Alphen JJM. Resource partitioning among rock-dwelling haplochromines (Pisces: Cichlidae) from Lake Victoria. Ecology of Freshwater Fish. 1997;6(4):225–240. [Google Scholar]
- 24.Endler JA, Houde AE. Geographic variation in female preferences for male traits in Poecilia reticulata. Evolution. 1995;49(3):456–468. doi: 10.1111/j.1558-5646.1995.tb02278.x. [DOI] [PubMed] [Google Scholar]
- 25.Maan ME, Hofker KD, van Alphen JJM, Seehausen O. Sensory drive in cichlid speciation. The American Naturalist . 2006;167:947–954. doi: 10.1086/503532. [DOI] [PubMed] [Google Scholar]
- 26.Levring T, Fish GR. The penetration of light in some tropical East-African waters. Oikos. 1956;7:98–109. [Google Scholar]
- 27.Egger B, Klaefiger Y, Theis A, Salzburger W. A sensory bias has triggered the evolution of egg-spots in cichlid fishes. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0025601.e25601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salzburger W, Mack T, Verheyen E, Meyer A. Out of Tanganyika: genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes. BMC Evolutionary Biology. 2005;5:p. 17. doi: 10.1186/1471-2148-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldschmidt T. Egg mimics in haplochromine cichlids (pisces, perciformes) from Lake Victoria. Ethology. 1991;88:177–190. [Google Scholar]
- 30.Hert E. The function of egg-spots in an African mouth-brooding cichlid fish. Animal Behaviour. 1989;37(5):726–732. [Google Scholar]
- 31.Carleton KL, Parry JWL, Bowmaker JK, Hunt DM, Seehausen O. Colour vision and speciation in Lake Victoria cichlids of the genus Pundamilia. Molecular Ecology. 2005;14(14):4341–4353. doi: 10.1111/j.1365-294X.2005.02735.x. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann CM, O’Quin KE, Marshall NJ, Cronin TW, Seehausen O, Carleton KL. The eyes have it: regulatory and structural changes both underlie cichlid visual pigment diversity. PLoS Biology. 2009;7(12) doi: 10.1371/journal.pbio.1000266.e1000266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munz FW, McFarland WN. The significance of spectral position in the rhodopsins of tropical marine fishes. Vision Research. 1973;13(10):1829–1874. doi: 10.1016/0042-6989(73)90060-6. [DOI] [PubMed] [Google Scholar]
- 34.McFarland WN, Munz FW. Part II: the photic environment of clear tropical seas during the day. Vision Research. 1975;15(10):1063–1070. doi: 10.1016/0042-6989(75)90002-4. [DOI] [PubMed] [Google Scholar]
- 35.Endler JA. On the measurement and classification of colour in studies of animal colour patterns. Biological Journal of the Linnean Society. 1990;41(4):315–352. [Google Scholar]
- 36.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. Mixed Effect Models and Extensions in Ecology with R. Springer; 2009. [Google Scholar]
- 37.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information—Theoretic Approach. Springer; 2002. [Google Scholar]
- 38.R Development Core Team. R: A language and environment for statistical computing. 2011. [Google Scholar]
- 39.Plenderleith M, van Oosterhout C, Robinson RL, Turner GF. Female preference for conspecific males based on olfactory cues in a Lake Malawi cichlid fish. Biology Letters. 2005;1(4):411–414. doi: 10.1098/rsbl.2005.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verzijden MN, Ten Cate C. Early learning influences species assortative mating preferences in Lake Victoria cichlid fish. Biology Letters. 2007;3(2):134–136. doi: 10.1098/rsbl.2006.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maruska KP, Fernald RD. Contextual chemosensory urine signaling in an African cichlid fish. Journal of Experimental Biology. 2012;215(1):68–74. doi: 10.1242/jeb.062794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osorio D, Ham AD. Spectral reflectance and directional properties of structural coloration in bird plumage. Journal of Experimental Biology. 2002;205(14):2017–2027. doi: 10.1242/jeb.205.14.2017. [DOI] [PubMed] [Google Scholar]
- 43.Wickler W. ‘Egg-dummies’ as natural releasers in mouth-breeding cichlids. Nature. 1962;194(4833):1092–1093. [Google Scholar]
- 44.Theis A, Salzburger W, Egger B. The function of anal fin egg-spots in the cichlid fish Astatotilapia burtoni. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0029878.e29878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hert E. Female choice based on egg-spots in Pseudotropheus aurora Burgess 1976, a rock-dwelling cichlid of Lake Malawi, Africa. Journal of Fish Biology. 1991;38:951–953. [Google Scholar]
- 46.Couldridge VCK. Experimental manipulation of male eggspots demonstrates female preference for one large spot in Pseudotropheus lombardoi. Journal of Fish Biology. 2002;60(3):726–730. [Google Scholar]
- 47.Salzburger W, Mack T, Verheyen E, Meyer A. Out of Tanganyika: genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes. BMC Evolutionary Biology. 2005;5 doi: 10.1186/1471-2148-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldschmidt T, de Visser J. On the possible role of egg mimics in speciation. Acta Biotheoretica. 1990;38(2):125–134. [Google Scholar]
- 49.Seehausen O. Lake Victoria Rock Cichlids: Taxonomy, Ecology, and Distribution. Verduijn; 1996. [Google Scholar]
- 50.Kelley JL, Phillips B, Cummins GH, Shand J. Changes in the visual environment affect colour signal brightness and shoaling behaviour in a freshwater fish. Animal Behaviour. 2012;83(3):783–791. [Google Scholar]
- 51.Lewandowski E, Boughman J. Effects of genetics and light environment on colour expression in threespine sticklebacks. Biological Journal of the Linnean Society. 2008;94(4):663–673. [Google Scholar]
- 52.Maan ME, van der Spoel M, Quesada Jimenez P, van Alphen JJM, Seehausen O. Fitness correlates of male coloration in a Lake Victoria cichlid fish. Behavioral Ecology. 2006;17(5):691–699. [Google Scholar]
- 53.Fernald RD. Quantitative behavioural observations of Haplochromis burtoni under semi-natural conditions. Animal Behaviour. 1977;25(3):643–653. [Google Scholar]
- 54.Gray SM, Hart FL, Tremblay MEM, Lisney TJ, Hawryshyn CW. The effects of handling time, ambient light, and anaesthetic method, on the standardized measurement of fish colouration. Canadian Journal of Fisheries and Aquatic Sciences. 2011;68(2):330–342. [Google Scholar]
- 55.Schultz TD, Anderson CN, Symes LB. The conspicuousness of colour cues in male pond damselflies depends on ambient light and visual system. Animal Behaviour. 2008;76(4):1357–1364. [Google Scholar]
- 56.Gomez D, Théry M. Influence of ambient light on the evolution of colour signals: comparative analysis of a Neotropical rainforest bird community. Ecology Letters. 2004;7(4):279–284. [Google Scholar]
- 57.Clark DL, Roberts JA, Rector M, Uetz GW. Spectral reflectance and communication in the wolf spider, Schizocosa ocreata (Hentz): simultaneous crypsis and background contrast in visual signals. Behavioral Ecology and Sociobiology. 2011;65(6):1237–1247. [Google Scholar]
- 58.Cummings ME. Sensory trade-offs predict signal divergence in surfperch. Evolution. 2007;61(3):530–545. doi: 10.1111/j.1558-5646.2007.00047.x. [DOI] [PubMed] [Google Scholar]
- 59.Fuller RC. Lighting environment predicts the relative abundance of male colour morphs in bluefin killifish (Lucania goodei) populations. Proceedings of the Royal Society B. 2002;269(1499):1457–1465. doi: 10.1098/rspb.2002.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gray SM, Dill LM, Tantu FY, Loew ER, Herder F, McKinnon JS. Environment-contingent sexual selection in a colour polymorphic fish. Proceedings of the Royal Society B. 2008;275(1644):1785–1791. doi: 10.1098/rspb.2008.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dugas MB, Franssen NR. Nuptial coloration of red shiners (Cyprinella lutrensis) is more intense in turbid habitats. Naturwissenschaften. 2011;98(3):247–251. doi: 10.1007/s00114-011-0765-4. [DOI] [PubMed] [Google Scholar]
- 62.Stoddard MC, Prum RO. How colorful are birds? Evolution of the avian plumage color gamut. Behavioral Ecology. 2011;22:1042–1052. [Google Scholar]
- 63.Lindstedt C, Morehouse N, Pakkanen H, et al. Characterizing the pigment composition of a variable warning signal of Parasemia plantaginis larvae. Functional Ecology. 2010;24(4):759–766. [Google Scholar]
- 64.Grether GF, Hudon J, Millie DF. Carotenoid limitation of sexual coloration along an environmental gradient in guppies. Proceedings of the Royal Society B. 1999;266(1426):1317–1322. [Google Scholar]
- 65.Kirk JTO. Light and Photosynthesis in Aquatic Ecosystems. Cambridge University Press; 1994. [Google Scholar]
- 66.Svensson PA, Wong BBM. Carotenoid-based signals in behavioural ecology: a review. Behaviour. 2011;148(2):131–189. [Google Scholar]
- 67.Craig JK, Foote CJ. Countergradient variation and secondary sexual color: phenotypic convergence promotes genetic divergence in carotenoid use between sympatric anadromous and nonanadromous morphs of sockeye salmon (Oncorhynchus nerka) Evolution. 2001;55(2):380–391. doi: 10.1111/j.0014-3820.2001.tb01301.x. [DOI] [PubMed] [Google Scholar]
- 68.Evans SR, Sheldon BC. Quantitative genetics of a carotenoid-based color: heritability and persistent natal environmental effects in the great tit. The American Naturalist. 2012;179(1):79–94. doi: 10.1086/663198. [DOI] [PubMed] [Google Scholar]
- 69.Rosenthal GG, Martinez TYF, de León FJG, Ryan MJ. Shared preferences by predators and females for male ornaments in swordtails. The American Naturalist. 2001;158(2):146–154. doi: 10.1086/321309. [DOI] [PubMed] [Google Scholar]
- 70.Maan ME, Eshuis B, Haesler MP, Schneider MV, van Alphen JJM, Seehausen O. Color polymorphism and predation in a Lake Victoria cichlid fish. Copeia. 2008;(3):621–629. [Google Scholar]
- 71.Godin JGJ, McDonough HE. Predator preference for brightly colored males in the guppy: a viability cost for a sexually selected trait. Behavioral Ecology. 2003;14(2):194–200. [Google Scholar]
- 72.Moyaho A, Garcia CM, Manjarrez J. Predation risk is associated with the geographic variation of a sexually selected trait in a viviparous fish (Xenotoca variata) Journal of Zoology. 2004;262(3):265–270. [Google Scholar]
- 73.Meager JJ, Solbakken T, Utne-Palm AC, Oen T. Effects of turbidity on the reactive distance, search time, and foraging success of juvenile Atlantic cod (Gadus morhua) Canadian Journal of Fisheries and Aquatic Sciences. 2005;62(9):1978–1984. [Google Scholar]
- 74.Candolin U, Salesto T, Evers M. Changed environmental conditions weaken sexual selection in sticklebacks. Journal of Evolutionary Biology. 2007;20(1):233–239. doi: 10.1111/j.1420-9101.2006.01207.x. [DOI] [PubMed] [Google Scholar]
- 75.Järvenpää M, Lindström K. Water turbidity by algal blooms causes mating system breakdown in a shallow-water fish, the sand goby Pomatoschistus minutus. Proceedings of the Royal Society B. 2004;271(1555):2361–2365. doi: 10.1098/rspb.2004.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sundin J, Berglund A, Rosenqvist G. Turbidity hampers mate choice in a pipefish. Ethology. 2010;116(8):713–721. [Google Scholar]
- 77.Dunn PO, Whittingham LA, Freeman-Gallant CR, DeCoste J. Geographic variation in the function of ornaments in the common yellowthroat Geothlypis trichas. Journal of Avian Biology. 2008;39(1):66–72. [Google Scholar]
- 78.Grether GF, Kolluru GR, Rodd FH, de la Cerda J, Shimazaki K. Carotenoid availability affects the development of a colour-based mate preference and the sensory bias to which it is genetically linked. Proceedings of the Royal Society B. 2005;272(1577):2181–2188. doi: 10.1098/rspb.2005.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grether GF. Carotenoid limitation and mate preference evolution: a test of the indicator hypothesis in guppies (Poecilia reticulata) Evolution. 2000;54(5):1712–1724. doi: 10.1111/j.0014-3820.2000.tb00715.x. [DOI] [PubMed] [Google Scholar]
- 80.Kocher TD. Adaptive evolution and explosive speciation: the cichlid fish model. Nature Reviews Genetics. 2004;5(4):288–298. doi: 10.1038/nrg1316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Repeatability for irradiance data: orange ratio.
Table S2: Repeatability for reflectance data: chroma.
Table S3: Repeatability for reflectance data: λP50.
Table S4: Spearman correlations between body areas for chroma.
Table S5: Spearman correlations between body areas for λP50.
Table S6: Descriptive statistics for the effect of using different light sources.


