Abstract
Exposure to mercury is normally assessed by measuring its accumulation in hair, blood or urine. Currently, the biomarkers of effect that have been proposed for mercurials, such as coproporphyrines or oxidative stress markers, are not sensitive enough and lack specificity. Selenium and selenoproteins are important targets for mercury and thioredoxin reductase (TrxR) in particular was shown to be very sensitive to mercury compounds both in vitro and in vivo. In this study we looked into the relation between the inhibition of thioredoxin reductase (TrxR) activity and histopathological changes caused by exposure to mercurials. Juvenile zeabra-seabreams were exposed to Hg2+ or MeHg for 28 days and histopathological changes were analyzed in the liver and kidney as well as TrxR activity. Both mercurials caused histopathological changes in liver and kidney, albeit Hg2+ caused more extensive and severe lesions. Likewise, both mercurials decreased TrxR activity, being Hg2+ a stronger inhibitor. Co-exposure to Hg2+ and Se fully prevented TrxR inhibition in the liver and reduced the severity of lesions in the organ. These results show that upon exposure to mercurials, histopathological alterations correlate with the level of TrxR activity and point to the potential use of this enzyme as a biomarker of mercury toxicity.
1. Introduction
Adverse health effects of mercury include neurotoxicity, nephrotoxicity, cardiotoxicity, teratogenicity and immunotoxicity. However, the molecular mechanisms underlying mercury toxicity remain unclear with detrimental consequences on the development and validation of appropriate biomarkers of predictive toxic effects. Neurotoxic symptoms are the most visible aspect of mercury poisoning [1]. Nevertheless, the liver and kidney also accumulate high amounts of mercurials [2–4] that may impair their regular functioning. As happens with most xenobiotics, mercury compounds are mainly metabolized in the liver, where demethylathion [5, 6] and conjugation with Se [7, 8] or glutathione can occur [1]. In the liver of animals exposed to high levels of mercurials, hepatocytes are frequently hypertrophied with large-size vacuoles and widespread areas of necrosis can often be observed [3, 9]. The proximal tubule is the kidney structure most affected by mercurials; the cellular changes include swelling of the mitochondrial matrix and endoplasmatic reticulum, loss of membrane integrity and eventual cellular necrosis [10]. Nephrotoxicity caused by to Hg2+ accumulation, is well recognized but may also arise from MeHg exposure [10, 11].
In risk assessment, biomarkers are important tools to assess the exposure, effect or susceptibility of individuals to a given xenobiotic. In the case of mercury compounds, only the use of biomarkers of exposure, such as the determination of mercury levels in hair [12, 13], blood [14, 15] and urine [16, 17] is generalized. However, the correlation between symptoms and Hg levels in hair, blood or urine is not always evident due to inter-individual variability in susceptibility to mercury [17] and to the delayed onset of effects that characterizes mercury poisoning [1]. Mercury toxic effects are frequently evaluated in humans and animals by conducting psychological and motor tests to assess the degree of neurological damage [17–19] although, changes in mental and motor skills might signify that mercury impairment of biological functions is already established. Therefore, the imposing challenge is still to identify a biomarker predictive of effect for mercury. Coproporphyrines levels and their excretion pattern in urine were proposed to evaluate early effects of mercurials [20, 21], but they have not proven to be a sensitive and useful indicator of mercury toxicity [19] and its use has been quite limited.
The affinity of mercury to thiol groups (–SH) makes peptides and proteins vulnerable to its presence, especially when sulfhydryl groups are in the active site of enzymes. Changes in the activity of several enzymes involved in antioxidant action, such as glutathione reductase (GR) [22], superoxide dismutase (SOD) [23], and catalase (CAT) [24] are indicative of mercury induced oxidative stress. Glutathione depletion [25, 26], resulting from complexation with mercurials is also a good but unspecific indicator of mercury effects. Metallothionine induction [27] is a biochemical change that has been previously related to mercury exposure. Nevertheless, these changes are not specific and do not allow to distinguish mercury related effects in a multi-contaminant context.
Selenols (–SeH) have a lower pKa than thiols (5.3 versus 8.5) and under physiological conditions are fully ionized to selenolates (–Se−) and thus are more reactive and can easily interact with mercury [28]. Selenoenzymes such as glutathione peroxidases (GPxs) are good targets for mercury [29–32] but, recently [28], the involvement of the thioredoxin system-comprising thioredoxin (Trx), the selenoenzyme thioredoxin reductase (TrxR) and NADPH-on the molecular mechanism of mercury toxicity was proven. The inhibitory effects of mercurials on the thioredoxin system have been shown both in vitro [28, 33] and in vivo [4, 34, 35]. Thioredoxin reductase is particularly sensitive to mercurials which results from its highly nucleophylic structure. Reduced TrxR has two active sites in each homodimer that include a dithiol in the FAD domain and a selenolthiol in the interface domain [34, 36]. By contrast, the homologous enzyme GR, which differs from TrxR by lacking Se in the C-terminal active site, is not inhibited in the presence of mercury compounds [4, 28]. Given the importance of the TrxR and the thioredoxin system to several cellular functions such as protein repair and regulation of the cellular cycle [36], we hypothesize that TrxR inhibition might be a key mechanism by which mercury toxicity develops. Thus, this work investigates the incidence of histopathological changes in the liver and kidney of zeabra-seabreams and its correlation with the decrease in TrxR activity caused by MeHg or Hg2+ exposure. The influence of Se co-exposure and post-exposure treatment on enzyme activity and on the alterations observed is also discussed.
2. Materials and Methods
2.1. In Vivo Assays
Zeabra-seabreams (Diplodus cervinus), were used as a model. This species is easy to handle in captivity and, since the organs of fishes are similar to those of mammals, they constitute a good alternative to rodents as a model [37]. A total of 63 fishes were divided into 6 experimental groups: control (C; n = 12); selenium (Se, provided as sodium selenite; n = 9); exposure to Hg2+ (HgII; n = 12); exposure to MeHg (MeHg; n = 12); co-exposure to Hg2+ and Se (HgSe; n = 12); co-exposure to MeHg and Se (MeHgSe; n = 12). Juvenile zeabra-seabreams were kept in tanks at a density of 2.5 g of fish per liter. Oxygen saturation, in water was kept close to 100% and ammonia and pH were kept within normal limits. Exposure concentrations were set at 2 μg L−1 for both Hg2+ and MeHg and at 10 μg L−1 for Se. Exposure lasted 28 days and was followed by 14 days of depuration. During the depuration, fishes from HgII and MeHg groups were divided into two sub-groups, one kept in clean water and the other one kept in water supplemented with Se (HgRSe and MeHgRSe). The experiment was described in detail in Branco et al. [34].
2.2. Organ Collection
Sampling of fish took place at days 14, 28 and 42 (hereafter referred as d14, d28 and d42). Three fishes were taken from each group at each sampling day. The liver and kidney were collected and rinsed with 0.9% NaCl. Sub-samples of these organs (were immediately fixed in 10% neutral buffered formalin (diluted in 10% salt water) for histopathological observation and the remaining organs frozen at −80°C until analysis.
2.3. Histopathology Analysis
Following fixation in 10% neutral buffered formalin, samples were washed with distilled water and dehydrated in a progressive series of ethanol, embedded in paraffin and then cut into 3 μm thick sections further stained with Harris haematoxylin (Merck) and counterstained with Eosin (Merck) according to the standard method described by Lillie and Fullmer [39]. Histopathological observations were carried out by using a Olympus BX 51light microscope linked to a digital camera (Olympus DP-20).
The Organ Damage Index (ODI) was calculated for each exposure group at d28 and d42 according to the formula:
| (1) |
where Pi is the pathological importance factor following the proposal of Bernet et al. [38] for a given organ lesion (OL) observed in the number of fishes (N) tested at each time-point.
2.4. Total Protein Determination
Organ samples were homogenized with a glass mortar and Teflon pestle in TE buffer (pH 7.5) containing a protease inhibitor cocktail (Roche), followed by centrifugation for 7 min at 12,000 rpm and 4°C. The pellet was discarded and supernatants used for enzyme activity assays. The total amount of protein in samples was determined in the supernatant fraction by measuring absorption at 595 nm in a microplate reader, according to Bradford [40], using Coomassie Brilliant Blue G-250 dye (Bio-Rad). Concentration of protein was quantified by using a calibration curve prepared by sequential dilution of a BSA standard solution.
2.5. Thioredoxin Reductase Activity
The activity of TrxR was determined using the insulin reduction endpoint assay proposed by Arnér and Holmgren [41]. Samples were incubated with TE buffer, fully reduced human Trx (3 μM; IMCO Corp. Sweden), insulin (0.3 mM), NADPH (2.5 mM), EDTA (2.5 mM) and HEPES (85 mM; pH 7.6) for 20 min at room temperature. Control wells containing the same mixture but without added Trx were simultaneously prepared. After incubation, the reaction was stopped by adding 250 μL of a 1 mM DTNB solution in 6 M guanidine·HCl and absorbance at 412 nm was measured in a microplate reader.
2.6. Glutathione Reductase Activity
For GR activity, supernatants were incubated in 96-well plates with phosphate buffer (100 mM; pH 7.0), NADPH (1 mM), and GSSG (200 μM). The reaction was monitored for 5 min at 30°C in a microplate reader and the decrease in absorption at 340 nm, resulting from NADPH oxidation was registered [42].
2.7. Statistical Analysis
Differences between groups were assessed by computing the Mann-Whitney test for independent samples. Differences were considered significant at a P value below 0.05 [43].
3. Results and Discussion
3.1. Liver Histopathology
Fish dissection showed that the liver of fishes from MeHg and HgII groups had softer consistency, when compared with the C group. No lesions or alterations were observed in the liver of controls (Figure 1(a)). The liver of fishes exposed for 28 days to MeHg and Hg2+ showed signs of hepatocyte alterations, namely, degenerative cytoplasm alterations, architectural pattern changes, loss of typical polygonal cell shape and undefined cell limits. Additionally, vacuolar degeneration with lateral migration of the nuclei, hydropic degeneration (Figure 1(b)), vacuolization within the hepatocytes with lipid-type vacuoles, which can be infiltrated fats, and appearance of some typical globular bodies may result from an increase in the lipid, water and/or glycogen content [3]. Hypertrophied hepatocytes were also disseminated at the parenchyma in fish from both HgII and MeHg groups, which is in good agreement with alterations described in the literature [3, 44, 45]. Liver extensive focal necrosis associated to congestion and pigments deposition was observed in two fishes belonging to the HgII group (Figure 1(c)). The MeHg group displayed three cases of little focal liver necrosis. During exposure to both mercurials the type of lesions was the same, but the necrotic lesions observed in the liver of fishes exposed to Hg2+ were more predominant and severe, leading to higher ODI values (Table 1). Despite the fact that, the accumulation of Hg2+ was lower than MeHg (Table 2), the ODI was higher. This result contrasts with the observations by Ribeiro et al. [45] where Hg2+ failed to cause any significant liver change in the artic charr, in comparison to MeHg. However, it should be stressed that Ribeiro et al. [45], used oral administration of mercurials with food and in those circumstances Hg2+, is much less absorbed in the GI tract than MeHg [1]. Although fishes from HgSe and MeHgSe groups (Figure 1(d)) showed at d28 the same kind of lesions observed in fish exposed only to mercurials (i.e, focal cellular vacuolization, megalocytic hepatocytes focal necrosis and congestion of the hepatic parenchyma), these lesions were observed side by side with normal cells.
Figure 1.
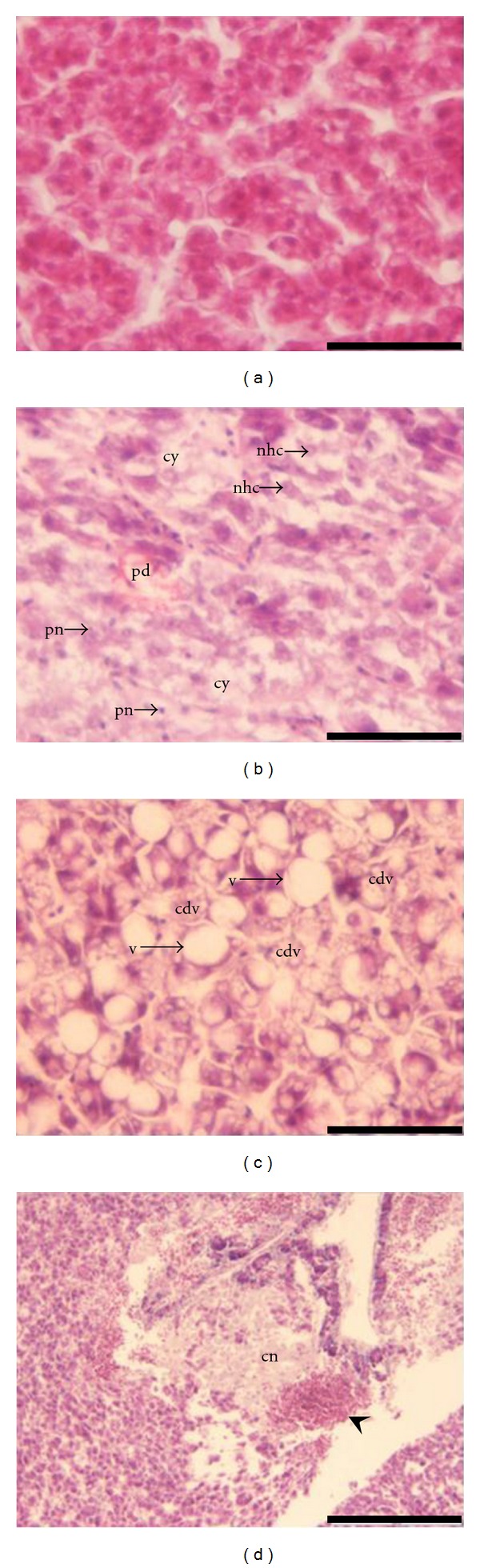
Histopathological observations in the liver of Diplodus cervinus after 28 days of exposure. (a) control group: section of polygonal hepatocytes cords (bar = 50 μm); (b) exposure to Hg2+: extensive necrosis with congestion of sinusoids and pigment deposition (pd), nuclear hypercromatose (nhc), picnotic nucleus (pn) and cytolysis (cy) (bar = 50 μm); (c) exposure to MeHg: extensive cytoplasmic degenerative vacuolization (cdv) with large, smooth-edged vacuoles (v) (bar = 50 μm); (d) co-exposure to MeHg and Se: focal coagulative necrosis (cn) associated to blood congestion (arrowhead) (bar = 200 μm).
Table 1.
Values of the organ damage index∗ (ODI) calculated for the liver and kidney of zeabra-seabreams exposed to the different treatments. No lesions were observed in control fishes. MeHg: exposure to Methylmercury; HgII: exposure to Hg2+; MeHgSe: coexposure to MeHg and Se; HgSe: coexposure to Hg2+ and Se; MeHgRSe: exposure to MeHg followed by exposure to Se during depuration; HgRSe: exposure to Hg2+ followed by exposure to Se during depuration; Se: exposure to selenium.
| Organ lesion (OL) | Pi | MeHg | HgII | MeHgSe | HgSe | MeHgRSe | HgRSe | Se | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d28 | d42 | d28 | d42 | d28 | d42 | d28 | d42 | d42 | d42 | d28 | d42 | |||
| N OL | N OL | N OL | N OL | N OL | N OL | N OL | N OL | N OL | N OL | N OL | N OL | |||
| Liver | Architectural pattern lost | 1 | 4 | 3 | 4 | 4 | 3 | 2 | 4 | 0 | 3 | 3 | 2 | 0 |
| Vacuolar degeneration | 1 | 3 | 1 | 4 | 1 | 2 | 0 | 1 | 2 | 3 | 3 | 2 | 0 | |
| Hydropic degeneration | 1 | 4 | 2 | 3 | 1 | 1 | 2 | 1 | 1 | 0 | 3 | 0 | 0 | |
| Hypertrophy of hepatocytes | 2 | 2 | 3 | 1 | 4 | 4 | 0 | 4 | 1 | 0 | 0 | 0 | 0 | |
| Extensive focal necrosis | 3 | 0 | 3 | 2 | 4 | 0 | 2 | 1 | 3 | 0 | 2 | 0 | 0 | |
| Focal necrosis | 2 | 3 | 0 | 0 | 0 | 1 | 2 | 2 | 0 | 3 | 1 | 0 | 0 | |
| Parenchymal congestion | 1 | 0 | 1 | 2 | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | |
| Pigment deposition | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
|
| ||||||||||||||
| ODI | 21 | 22 | 23 | 26 | 17 | 14 | 24 | 14 | 12 | 17 | 4 | 0 | ||
|
| ||||||||||||||
| Kidney | Vacuolar degeneration | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 1 | 2 | 0 | 0 |
| Hydropic degeneration | 1 | 3 | 2 | 1 | 0 | 1 | 3 | 0 | 3 | 0 | 2 | 0 | 0 | |
| Pigment deposition | 1 | 3 | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | |
| Parenchymal necrosis | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
|
| ||||||||||||||
| ODI | 7 | 3 | 3 | 12 | 2 | 6 | 3 | 5 | 2 | 4 | 0 | 0 | ||
Pi: Pathological index of each lesion according to their importance to organ function [38].
N OL: number of fishes displaying one type of organ lesion (OL).
∗ODI: sum of lesions observed for each organ taking into account their relative severity and the number of fishes (NOL).
Table 2.
Quantification of total mercury (HgT) and selenium (Se) values (μg g−1) in liver and kidney of zeabra-seabreams exposed to different treatments at days 28 and 42. Results were previously reported in Branco et al. [34]. MeHg: exposure to Methylmercury; HgII: exposure to Hg2+; MeHgSe: co-exposure to MeHg and Se; HgSe: co-exposure to Hg2+ and Se; MeHgRSe: exposure to MeHg followed by exposure to Se during depuration; HgRSe: exposure to Hg2+ followed by exposure to Se during depuration; Se: exposure to selenium; C: control group.
| MeHg | HgII | MeHgSe | HgSe | MeHgRSe | HgRSe | Se | C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d28 | d42 | d28 | d42 | d28 | d42 | d28 | d42 | d42 | d42 | d28 | d42 | d28 | d42 | ||
| Liver | HgT (μg g−1) | 10.2 ± 4.8 | 6.2 ± 1.4 | 1.4 ± 0.2 | 2.8 ± 0.4 | 5.8 ± 1.7 | 4.8 ± 1.3 | 1.5 ± 0.1 | 2.3 ± 0.5 | 5.8 ± 1.5 | 2.4 ± 0.5 | 0.07 ± 0.02 | 0.09 ± 0.02 | 0.10 ± 0.06 | 0.07 ± 0.03 |
| Se (μg g−1) | 0.6 ± 0.1 | 0.9 ± 0.2 | 0.5 ± 0.2 | 1.3 ± 0.2 | 0.9 ± 0.02 | 1.3 ± 0.3 | 1.1 ± 0.3 | 1.6 ± 0.3 | 1.3 ± 0.2 | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.0 ± 0.14 | 1.0 ± 0.21 | |
|
| |||||||||||||||
| Kidney | HgT (μg g−1) | 28.7 ± 8.3 | 17.3 ± 6.3 | 5.8 ± 1.9 | 8.3 ± 4.1 | 15.1 ± 1.9 | 11.9 ± 3.9 | 8.6 ± 3.3 | 10.0 ± 3.5 | 23.6 ± 1.1 | 8.8 ± 1.7 | 0.11 ± 0.03 | 0.15 ± 0.02 | 0.16 ± 0.04 | 0.14 ± 0.01 |
| Se (μg g−1) | 1.6 ± 0.6 | 3.3 ± 0.6 | 0.4 ± 0.3 | 1.3 ± 0.2 | 2.7 ± 1.2 | 2.1 ± 1.1 | 1.0 ± 0.3 | 2.8 ± 0.8 | 2.3 ± 0.6 | 4.5 ± 1.2 | 1.7 ± 0.3 | 1.7 ± 0.5 | 1.3 ± 0.6 | 1.0 ± 0.2 | |
After the depuration phase at d42, fish exposed to mercurials still exhibited extensive coagulative necrotic changes. Hypertrophy of the hepatocytes was clear in the parenchyma outside necrotic areas. Fishes depurating in water containing Se presented the same type of necrotic lesion although liver parenchyma beyond the necrotic zones appeared normal, resulting in a lower ODI than the index attained by fishes depurating in clean water (Table 1).
3.2. Kidney Histopathology
Kidney changes observed in fish exposed to both mercurials at days 28 and 42 were vacuolar and hydropic degeneration of tubular epithelium and pigment deposits around the tubules. Comparatively, posterior kidney is quite more susceptible to Hg2+ which was evidenced by larger necrosis areas (Figure 2(b)) and occlusion of the tubular lumen with eosinophilic material (Figure 2(c)). Co-exposure to MeHg and Se seemed to delay the appearance of the more severe lesions in the kidney (Table 1). Co-exposure to Hg2+ and Se decreased the detrimental effects of mercury at the end of the depuration period (Table 1). This decrease in renal toxicity does not reflect a decrease in mercury concentration (Table 2) but instead it might be related to the formation of inorganic inert complexes.
Figure 2.
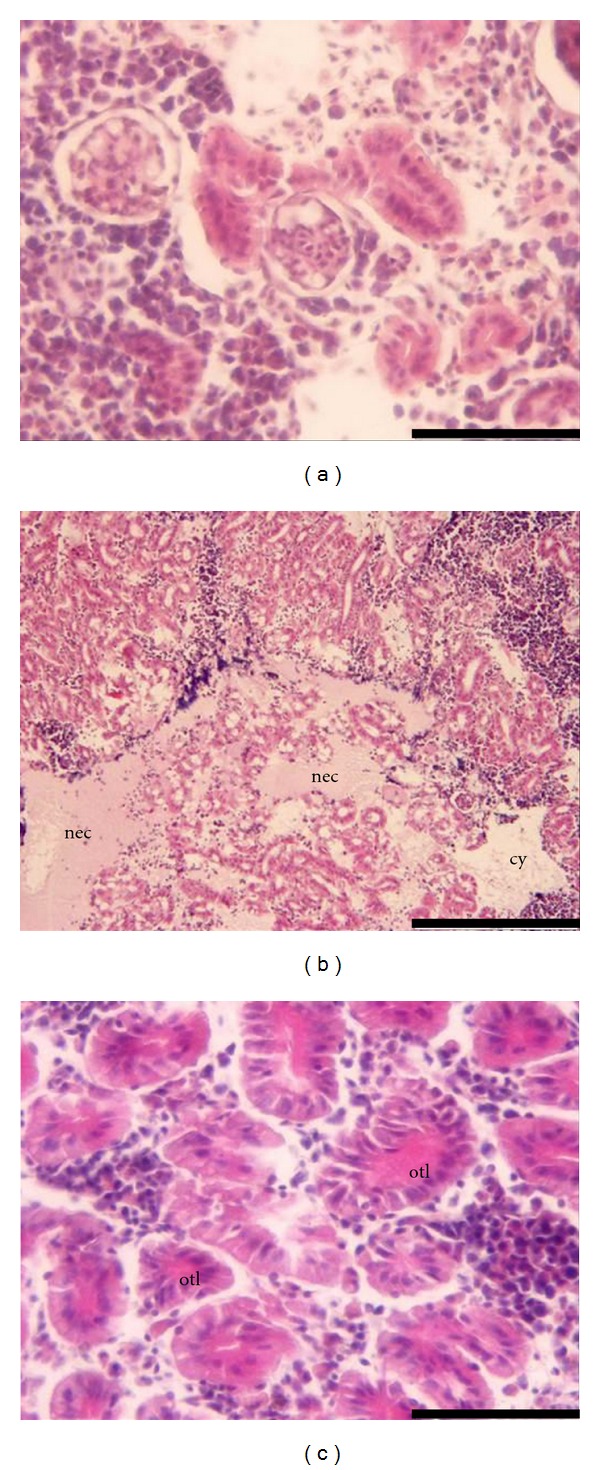
Histopathological observations in the kidney of Diplodus cervinus after 28 days of exposure to Hg2+. (a) control group: posterior kidney section (bar = 50 μm); (b) exposure to Hg2+: posterior kidney section showing a massive necrosis area (nec) and cytolysis (cy) (bar = 200 μm). (c) exposure to Hg2+: occlusion of the tubular lumen (otl) (bar = 50 μm).
3.3. Enzymatic Activities
The activity of TrxR in the liver and kidney of zeabra-seabreams is shown in Figure 3. In the liver, TrxR activity was decreased by 52% at d14 (P < 0.05; Figure 3(a)) during Hg2+ exposure, while in the case of MeHg, the inhibition was only observed by d28 (P < 0.05; Figure 3(b)). As previously shown and discussed [28, 34, 36] Hg2+ is a stronger inhibitor of TrxR than MeHg. Recovery of TrxR activity was complete at the end of depuration and was not influenced by Se supplementation during this period (data not shown). Co-exposure to Se besides reducing the ODI index, clearly prevented the inhibitory effect of Hg2+ over TrxR activity, that is, activity levels do not differ significantly from the control (P > 0.05; Figure 3(a)), but was not effective over MeHg inhibition (Figure 3(b)) whose toxic effects seem to be increased by Se. In vitro experiments also confirmed that the co-exposure to MeHg and Se increased the detrimental effects over the TrxR activity [34]. At the same time it was shown that Se can remove Hg2+ bound to the selenolthiol in the active site of TrxR restoring activity while MeHg showed no displacement from the active site [34]. Further studies to fully elucidate the contradictory role of Se in the presence of different mercury species are being conducted.
Figure 3.
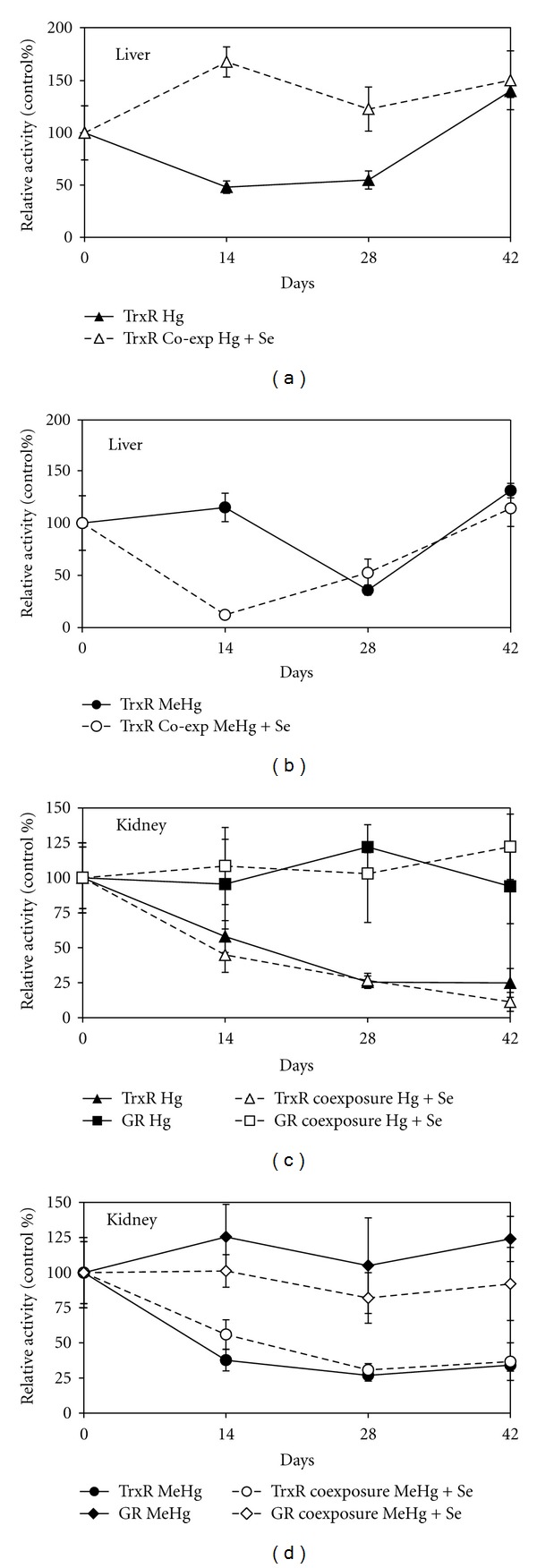
Enzymatic activities in liver and kidney of zeabra-seabreams exposed to mercurials and co-exposed to mercurials and Se (subset of results from a study previously reported in Branco et al. [34]). Exposure lasted 28 days and was followed by 14 days of depuration in clean or Se-supplemented water. (a) TrxR activity in the liver of seabreams exposed to Hg2+ and co-exposed to Hg2+ and Se; (b) TrxR activity in the liver of seabreams exposed to MeHg and co-exposed to MeHg and Se; (c) TrxR and GR activities in the kidney of seabreams exposed to Hg2+ and co-exposed to Hg2+ and Se; (d) TrxR and GR activities in the kidney of seabreams exposed to MeHg and co-exposed to MeHg and Se. GR activity in the liver did not show significant variation and was not represented to improve clarity.
In the kidney, TrxR activity was significantly affected (P < 0.05) both in HgII (42% inhibition) and MeHg (62% inhibition) groups at d14 and the inhibitory effect remained throughout the entire experiment (Figures 3(c) and 3(d)). Selenium showed no protective effect during co-exposure or on the recovery of TrxR activity during the depuration phase. As we have previously suggested [36], the protective Se over TrxR is organ specific and might be related to both the Se : Hg ratio and the capacity of selenoenzyme expression within the organ. In the liver, besides higher Se : Hg ratios [36] the level of selenoenzymes expression is assumed to be higher [44]. It should be stressed that, albeit TrxR activity did not recover, the citotoxic effects of MeHg decreased with Se co-administration in part due to the lower MeHg accumulation in both organs, liver and kidney, which reflected in the ODI values (Table 2). Since TrxR presents a high affinity for mercurials, any available mercury will primarily bound the selenolthiol of its active site and therefore it is normal that the inhibition of TrxR is observed in cases where cito-/organ toxicity is largely decreased (Table 1).
For the HgII group possible explanations for the decreased renal toxicity in the presence of Se include the formation of inorganic inert complexes and the participation of Se in antioxidant in cellular pathways. GR activity was not inhibited in the liver (results not shown) or in the kidney (P > 0.05; Figures 3(c) and 3(d)) in any group. On the contrary, the slight increase of GR activity seems to be a compensation mechanism for the loss of antioxidant protection provided by TrxR. Moreover, the fact that GR is homologous to TrxR, but lacks the Sec residue in the active site, reinforces the importance of TrxR active site as a main target for mercurials.
4. Conclusions
Mercury effects have been evaluated using different types of biomarkers. However, these are normally non-specific or are not predictive of toxicity but, instead, correspond to manifestations of toxicity itself. The thioredoxin system is responsible for several key cellular functions that range from anti-oxidant defense to regulation of the cellular cycle [33] and loss of activity will result in apoptosis [46]. Mercury (II) was shown to be a stronger inhibitor of TrxR than MeHg and also produced the most extensive array of organ lesions (Table 1). Also, when zeabra-seabreams were co-exposed to Se and Hg2+ the severity of the lesions in the liver decreased while TrxR activity was kept at normal levels. In the cases of exposure to Hg2+ and MeHg, although we observed full recovery of the activity of TrxR in the liver following depuration, the histopathological lesions reversion was not significant. Possibly, cellular mechanisms downstream of the thioredoxin system were affected to such a degree that recovery of TrxR activity does not reflect in an immediate organ recovery and time will be needed to replace damaged cellular structures. Overall, this work shows that the strong inhibition of TrxR activity is related with the histopathological alterations displayed in the liver and kidney of seabreams indicating the potential use of TrxR as a biomarker of effect of mercury toxicity.
Acknowledgments
The authors would like to thank Rui Silva and Pedro Pousão for assistance with the in vivo experiments. Vasco Branco was financed by a PhD fellowship (SFRH/BD/37388/2007) from the Fundação para a Ciência e Tecnologia (FCT) (http://www.fct.mctes.pt/). This study was financed by the MERTOX-TRX project (PTDC/QUI-BIQ/117281/2010) and by iMed.UL through FCT's strategic project: PEst-OE/SAU/UI4013/2011.
References
- 1.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Critical Reviews in Toxicology . 2006;36(8):609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 2.Chang CWJ, Nakamura RM, Brooks CC. Effect of varied dietary levels and forms of mercury on swine. Journal of Animal Science . 1977;45(2):279–285. doi: 10.2527/jas1977.452279x. [DOI] [PubMed] [Google Scholar]
- 3.Liao C, Fu J, Shi J, Zhou Q, Yuan C, Jiang G. Methylmercury accumulation, histopathology effects, and cholinesterase activity alterations in medaka (Oryzias latipes) following sublethal exposure to methylmercury chloride. Environmental Toxicology and Pharmacology . 2006;22(2):225–233. doi: 10.1016/j.etap.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Branco V, Canário J, Holmgren A, Carvalho C. Inhibition of the thioredoxin system in the brain and liver of zebra-seabreams exposed to waterborne methylmercury. Toxicology and Applied Pharmacology . 2011;251(2):95–103. doi: 10.1016/j.taap.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Mottet NK, Vahter ME, Charleston JS, Friberg LT. Metabolism of Methylmercury in the Brain and Its Toxicological Significance . New York, NY, USA: Marcel Dekker; 1997. [PubMed] [Google Scholar]
- 6.Yang D, Yu-Wei C, Gunn JM, Belzile N. Selenium and mercury in organisms: interactions and mechanisms. Environmental Reviews . 2008;16:71–92. [Google Scholar]
- 7.Parizek J, Ostadalova I, Kalouskva J, Babichy A, Benes J. The Detoxifying Effects of Selenium. Interrelation Between Compounds of Selenium and Certain Metals . New York, NY, USA: Marcel Dekker; 1971. [Google Scholar]
- 8.Khan MAK, Wang F. Mercury-selenium compounds and their toxicological significance: toward a molecular understanding of the mercury-selenium antagonism. Environmental Toxicology and Chemistry . 2009;28(8):1567–1577. doi: 10.1897/08-375.1. [DOI] [PubMed] [Google Scholar]
- 9.Wester PW, Canton HH. Histopathological effects in Poecilia reticulata (guppy) exposed to methyl mercury chloride. Toxicologic Pathology . 1992;20(1):81–92. doi: 10.1177/019262339202000110. [DOI] [PubMed] [Google Scholar]
- 10.Zalups RK. Molecular interactions with mercury in the kidney. Pharmacological Reviews . 2000;52(1):113–143. [PubMed] [Google Scholar]
- 11.Lash LH, Hueni SE, Putt DA, Zalups RK. Role of organic anion and amino acid carriers in transport of inorganic mercury in rat renal basolateral membrane vesicles: influence of compensatory renal growth. Toxicological Sciences . 2005;88(2):630–644. doi: 10.1093/toxsci/kfi328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giovanoli-Jakubczak T, Greenwood MR, Smith JC, Clarkson TW. Determination of total and inorganic mercury in hair by flameless atomic absorption, and of methylmercury by gas chromatography. Clinical Chemistry . 1974;20(2):222–229. [PubMed] [Google Scholar]
- 13.Satoh H. Occupational and environmental toxicology of mercury and its compounds. Industrial Health . 2000;38(2):153–164. doi: 10.2486/indhealth.38.153. [DOI] [PubMed] [Google Scholar]
- 14.Gage JC. Distribution and excretion of methyl and phenyl mercury salts. British Journal of Industrial Medicine . 1964;21(3):197–202. doi: 10.1136/oem.21.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berglund M, Lind B, Björnberg KA, Palm B, Einarsson Ö, Vahter M. Inter-individual variations of human mercury exposure biomarkers: a cross-sectional assessment. Environmental Health: A Global Access Science Source . 2005;4, article 20 doi: 10.1186/1476-069X-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gage JC. The distribution and excretion of inhaled mercury vapour. British Journal of Industrial Medicine . 1961;18(4):287–294. doi: 10.1136/oem.18.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bose-O’Reilly S, McCarty KM, Steckling N, Lettmeier B. Mercury exposure and children’s health. Current Problems in Pediatric and Adolescent Health Care . 2010;40(8):186–215. doi: 10.1016/j.cppeds.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwata T, Sakamoto M, Feng X, et al. Effects of mercury vapor exposure on neuromotor function in Chinese miners and smelters. International Archives of Occupational and Environmental Health . 2007;80(5):381–387. doi: 10.1007/s00420-006-0144-1. [DOI] [PubMed] [Google Scholar]
- 19.dos Santos APM, Mateus ML, Carvalho CML, Batoréu MCC. Biomarkers of exposure and effect as indicators of the interference of selenomethionine on methylmercury toxicity. Toxicology Letters . 2007;169(2):121–128. doi: 10.1016/j.toxlet.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Woods JS. Porphyrin Metabolism as Indicator of Metal Exposure and Toxicity . Berlin, Germany: Springer; 1995. [Google Scholar]
- 21.Woods JS, Martin MD, Leroux BG, et al. Urinary porphyrin excretion in children with mercury amalgam treatment: findings from the Casa Pia children’s dental amalgam trial. Journal of Toxicology and Environmental Health A . 2009;72(14):891–896. doi: 10.1080/15287390902959557. [DOI] [PubMed] [Google Scholar]
- 22.Oh S, Lee M. Interaction between inorganic mercury and selenium on tissue sulfhydryl groups and glutathione-linked enzymes in rats. Yonsei Medical Journal . 1981;22(2):122–126. doi: 10.3349/ymj.1981.22.2.122. [DOI] [PubMed] [Google Scholar]
- 23.Livardjani F, Ledig M, Kopp P, Dahlet M, Leroy M, Jaeger A. Lung and blood superoxide dismutase activity in mercury vapor exposed rats: effect of N-acetylcysteine treatment. Toxicology . 1991;66(3):289–295. doi: 10.1016/0300-483x(91)90200-k. [DOI] [PubMed] [Google Scholar]
- 24.Perrin-Nadif R, Dusch M, Koch C, Schmitt P, Mur JM. Catalase and superoxide dismutase activities as biomarkers of oxidative stress in workers exposed to mercury vapors. Journal of Toxicology and Environmental Health A . 1996;48(2):107–119. doi: 10.1080/009841096161366. [DOI] [PubMed] [Google Scholar]
- 25.Sarafian T, Verity MA. Oxidative mechanisms underlying methyl mercury neurotoxicity. International Journal of Developmental Neuroscience . 1991;9(2):147–153. doi: 10.1016/0736-5748(91)90005-7. [DOI] [PubMed] [Google Scholar]
- 26.Lund BO, Miller DM, Woods JS. Studies on Hg(II)-induced H2O2 formation and oxidative stress in vivo and in vitro in rat kidney mitochondria. Biochemical Pharmacology . 1993;45(10):2017–2024. doi: 10.1016/0006-2952(93)90012-l. [DOI] [PubMed] [Google Scholar]
- 27.Cherian MG, Clarkson TW. Biochemical changes in rat kidney on exposure to elemental mercury vapor: effect on biosynthesis of metallothionein. Chemico-Biological Interactions . 1976;12(2):109–120. doi: 10.1016/0009-2797(76)90093-4. [DOI] [PubMed] [Google Scholar]
- 28.Carvalho CML, Chew E, Hashemy SI, Lu J, Holmgren A. Inhibition of the human thioredoxin system: a molecular mechanism of mercury toxicity. The Journal of Biological Chemistry . 2008;283(18):11913–11923. doi: 10.1074/jbc.M710133200. [DOI] [PubMed] [Google Scholar]
- 29.Wada O, Yamaguchi N, Ono T, Nagahashi M, Morimura T. Inhibitory effect of mercury on kidney glutathione peroxidase and its prevention by selenium. Environmental Research . 1976;12(1):75–80. [Google Scholar]
- 30.Watanabe C, Yoshida K, Kasanuma Y, Kun Y, Satoh H. In utero methylmercury exposure differentially affects the activities of selenoenzymes in the fetal mouse brain. Environmental Research . 1999;80(3):208–214. doi: 10.1006/enrs.1998.3889. [DOI] [PubMed] [Google Scholar]
- 31.Bulato C, Bosello V, Ursini F, Maiorino M. Effect of mercury on selenium utilization and selenoperoxidase activity in LNCaP cells. Free Radical Biology and Medicine . 2007;42(1):118–123. doi: 10.1016/j.freeradbiomed.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 32.Franco JL, Posser T, Dunkley PR, et al. Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radical Biology and Medicine . 2009;47(4):449–457. doi: 10.1016/j.freeradbiomed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho CML, Lu J, Zhang X, Arnér ESJ, Holmgren A. Effects of selenite and chelating agents on mammalian thioredoxin reductase inhibited by mercury: implications for treatment of mercury poisoning. The FASEB Journal . 2011;25(1):370–381. doi: 10.1096/fj.10-157594. [DOI] [PubMed] [Google Scholar]
- 34.Branco V, Canário J, Lu J, Holmgren A, Carvalho C. Mercury and selenium interaction in vivo: effects on thioredoxin reductase and glutathione peroxidase. Free Radical Biology and Medicine . 2012;52(4):781–793. doi: 10.1016/j.freeradbiomed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Wagner C, Sudati JH, Nogueira CW, Rocha JB. In vivo and in vitro inhibition of mice thioredoxin reductase by methylmercury. BioMetals . 2010;23(6):1171–1177. doi: 10.1007/s10534-010-9367-4. [DOI] [PubMed] [Google Scholar]
- 36.Lillig CH, Holmgren A. Thioredoxin and related molecules—from biology to health and disease. Antioxidants and Redox Signaling . 2007;9(1):25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 37.Wolf JC, Wolfe MJ. A brief overview of nonneoplastic hepatic toxicity in fish. Toxicologic Pathology . 2005;33(1):75–85. doi: 10.1080/01926230590890187. [DOI] [PubMed] [Google Scholar]
- 38.Bernet D, Schmidt H, Meier W, Burkhardt-Holm P, Wahli T. Histopathology in fish: proposal for a protocol to assess aquatic pollution. Journal of Fish Diseases . 1999;22(1):25–34. [Google Scholar]
- 39.Lillie RD, Fullmer HM. Histopathologic Technic and Practical Histochemistry . New York, NY, USA: McGraw- Hill; 1976. [Google Scholar]
- 40.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry . 1976;72(1-2):248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 41.Arnér ESJ, Holmgren A. Measurement of Thioredoxin and Thioredoxin Reductase . New York, NY, USA: John Wiley & Sons; 1999. [Google Scholar]
- 42.Mannervik B. Measurement of Glutathione Reductase Activity . New York, NY, USA: John Wiley & Sons; 1999. [Google Scholar]
- 43.Zar JH. Biostatistical Analysis . New Jersey, NJ, USA: Prentice Hall; 1999. [Google Scholar]
- 44.Mela M, Randi MAF, Ventura DF, Carvalho CEV, Pelletier E, Oliveira CAO. Effects of dietary methylmercury on liver and kidney histology in the neotropical fish Hoplias malabaricus. Ecotoxicology and Environmental Safety . 2007;68(3):426–435. doi: 10.1016/j.ecoenv.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Ribeiro CAO, Belger L, Pelletier E, Rouleau C. Histopathological evidence of inorganic mercury and methyl mercury toxicity in the arctic charr (Salvelinus alpinus) Environmental Research . 2002;90(3):217–225. doi: 10.1016/s0013-9351(02)00025-7. [DOI] [PubMed] [Google Scholar]
- 46.Conrad M. Transgenic mouse models for the vital selenoenzymes cytosolic thioredoxin reductase, mitochondrial thioredoxin reductase and glutathione peroxidase 4. Biochimica et Biophysica Acta . 2009;1790(11):1575–1585. doi: 10.1016/j.bbagen.2009.05.001. [DOI] [PubMed] [Google Scholar]


