Abstract
BACKGROUND AND OBJECTIVE:
Optimal treatment decisions in children require sufficient evidence on the safety and efficacy of pharmaceuticals in pediatric patients. However, there is concern that not enough trials are conducted in children and that pediatric trials differ from those performed in adults. Our objective was to measure the prevalence of pediatric studies among clinical drug trials and compare trial characteristics and quality indicators between pediatric and adult drug trials.
METHODS:
For conditions representing a high burden of pediatric disease, we identified all drug trials registered in ClinicalTrials.gov with start dates between 2006 and 2011 and tracked the resulting publications. We measured the proportion of pediatric trials and subjects for each condition and compared pediatric and adult trial characteristics and quality indicators.
RESULTS:
For the conditions selected, 59.9% of the disease burden was attributable to children, but only 12.0% (292/2440) of trials were pediatric (P < .001). Among pediatric trials, 58.6% were conducted without industry funding compared with 35.0% of adult trials (P < .001).
Fewer pediatric compared with adult randomized trials examined safety outcomes (10.1% vs 16.9%, P = .008). Pediatric randomized trials were slightly more likely to be appropriately registered before study start (46.9% vs 39.3%, P = .04) and had a modestly higher probability of publication in the examined time frame (32.8% vs 23.2%, P = .04).
CONCLUSIONS:
There is substantial discrepancy between pediatric burden of disease and the amount of clinical trial research devoted to pediatric populations. This may be related in part to trial funding, with pediatric trials relying primarily on government and nonprofit organizations.
KEY WORDS: clinical trials, evidence-based medicine, pediatrics, medication use, research subjects
What’s Known on This Subject:
Many drugs are not approved for use in pediatric patients and there is limited evidence on their safety and efficacy in children. Furthermore, there is concern that the quality of pediatric trials is inferior compared with adult trials.
What This Study Adds:
For conditions with a high disease burden in children, only a small proportion of clinical drug trials study pediatric patients. Most pediatric trials are not funded by industry, and the deficiency of evidence is largest in developing countries.
As many as one-third of outpatient medication prescriptions given to children are unapproved by regulatory authorities or “off-label.”1 Among children who are hospitalized, as many as 79% are treated with off-label drugs.2 The paucity of pediatric clinical drug trials and prescribing information is attributable to ethical challenges of exposing children to risk, to the low prevalence of many childhood diseases, and to considerations by drug manufacturers about market size and profitability.3–5 When treating children, physicians therefore frequently extrapolate findings from adult studies and prescribe medications to children on a trial-and-error basis without age-specific research on dosing, safety, or efficacy.6,7
However, the safety and efficacy profiles of drugs may differ for pediatric and adult patients because of developmental pathophysiology and age-related changes in the absorption and metabolism of drugs.5,8 As a result, children given untested medications may experience harm or may forgo potentially effective treatments if a clinician is unwilling to use a medication for an unlicensed indication. Well-documented pediatric adverse events for drugs used off-label include chloramphenicol-induced gray-baby syndrome and propofol sedation associated with fatal metabolic acidosis and cardiac failure.9,10
There is also concern that the quality of pediatric trials needs to be strengthened and that there are fewer pediatric compared with adult randomized trials.11–14 Studies examining pediatric trial reports have found high risks of bias in trial design and insufficient assessment of drug safety and toxicity.11,14,15 Drug trials conducted in children in developing countries may be particularly prone to these deficiencies.16,17
Without trials addressing relevant drug interventions and conducted with methodological rigor, the practice of pediatric evidence-based medicine is severely compromised. To identify areas in pediatric drug research that deserve intensified focus in the current clinical research agenda, we determined the prevalence of pediatric studies among clinical trials conducted in the past 5 years and compared trial characteristics and quality indicators between pediatric and adult trials. We focused on conditions that are treated with medications and that have high pediatric disease burden. We examined the respective clinical trial agenda in both high-income and developing countries.
Methods
Pediatric Global Burden of Disease Data
Conditions causing the greatest pediatric burden of disease were selected based on the World Health Organization’s (WHO) 2004 Global Burden of Disease study which uses national and WHO program information to create comprehensive morbidity and mortality estimates for specific diseases in all regions of the world.18 Estimates include a composite measure, disability-adjusted life-years (DALYs), which accounts for years lost both because of disability and because of premature mortality. One DALY represents the loss of 1 year of healthy life because of disease. As such, it allows us to account for not only how many children are affected by a given disease, but also the severity of the disease, which is particularly apt for comparing against the amount of research activity pertaining to a condition.19,20 Data are classified according to gross national income per capita into high-income (US$10 066 or more), middle-income (US$826–$10 065), and low-income (US$825 or less) countries. The high-income group comprises 53 countries (1.0 billion persons), the middle-income group comprises 93 countries (3.0 billion persons), and the low-income group comprises 59 countries (2.4 billion persons).
Condition-specific DALYs were examined for children 0 to 17 years of age in high-income and middle- and low-income countries, combined. Because data are presented in predefined age groups, we combined DALYs for persons 0 to 4 years, 5 to 14 years, and three-fifths of 15 to 19 years to approximate estimates of DALYs for children 0 to 17 years. The 5 conditions with the highest DALYs for children 0 to 17 years were selected separately for the high-income countries and for middle- and low-income countries combined. We excluded conditions specific to pediatric or adult age groups as well as conditions that are generally not conducive to drug therapy (eg, refractive errors and injuries).
Selection of Clinical Drug Trials
Clinical trials pertaining to the selected high-burden diseases were identified in ClincialTrials.gov, a Web-based registry of clinical studies that provides a publicly available source of information on clinical studies conducted in the United States and internationally.21 ClinicalTrials.gov is the largest and most widely used trial registry, and it is estimated that at least 86% of registered trials are recorded in this registry.22,23 By using a registry search function, we selected clinical trials addressing the conditions of interest. All data were downloaded on February 8, 2011. Trials were individually reviewed, and those selected had started January 1, 2006 and later and studied a drug intervention. We excluded trials that were suspended or terminated before subject enrollment (n = 124) or that were missing information on minimum age eligibility (n = 4).
Trial Data Extraction and Definitions
Data elements extracted from ClinicalTrials.gov included registration date, study start and completion dates, funding source, trial phase, estimated enrollment number, subject age eligibility, outcome measures, number and location of study centers, comparator type, allocation strategy, and masking. Double-data extraction was performed independently by 2 investigators for 10% of trials, and a κ-coefficient was calculated to assess interrater reliability for the variables extracted. Agreement was excellent (κ ranging from 0.89 to 1.0).
Outcome measures were categorized as efficacy, safety, or pharmacokinetic/dynamic. The primary funding sources were categorized as government, industry, or nonprofit by use of the funding sponsors listed in the record.24 We also examined secondary funding sources and further categorized nonprofit-funded trials as with or without industry contributions. Pediatric trials were defined as those enrolling only patients <18 years as well as those that also included participants ≥18 years, but in which the minimum age was <18 and the midpoint of the age range for enrolled participants was <18 years.
Study Publication and Results Data
For trials with a start date between January 1, 2006 and June 30, 2008, we sought to identify publications describing trial results. We limited our search to trials with these start dates to allow at least 3 years between study start and publication. We systematically searched PubMed by using names of principal investigators, drug names, conditions studied, design characteristics, and study location. All searches were conducted between June 28 and August 25, 2011.
Results for the primary outcome in each publication were reviewed and classified as “positive” if they were nominally statistically significant for the experimental drug based on P values <.05 or 95% confidence intervals (CIs) excluding the null. Noninferiority trials that demonstrated noninferiority of the test drug were also classified as positive, as were safety trials without formal statistical testing where the authors concluded that the drug was “safe and well-tolerated.” Publications without a hypothesis (eg, head-to-head comparative trials) and publications reporting both “negative” and “positive” results were classified as “neither.” The outcome classification was performed independently by 2 authors (F.B. and S.M.), and disagreements were resolved by consensus. Interrater agreement for assigning study outcomes had κ = 0.75 (95% CI, 0.66–0.84).
Trial Characteristics and Quality Indicators
Information on trial characteristics and quality indicators for comparison of pediatric and adult trials was collected from ClinicalTrials.gov and from published trial reports. The selected quality indicators were based on previous work examining study quality and strengths in the pediatric literature.11,13–15,25 The evaluation focused on randomized controlled trials. We identified comparator types (active versus placebo), type of masking, scope of patient recruitment (single center versus multicenter), sample size, and whether the primary outcome included safety measures. The types of comparators were examined because of recent efforts to increase comparative effectiveness research which requires the comparison of active therapies.26 We also determined whether a trial was registered before study start, because prospective trial registration has become the accepted standard.27 Based on trial publications, we examined the probability of publication over time and whether the anticipated study sample was attained.28,29
Statistical Analysis
We calculated the proportion of pediatric trials and pediatric participants for each disease. In calculating the number of pediatric participants, we summed the enrollment figures for trials with all participants ≤18 years as well as the proportion of pediatric participants in trials enrolling both pediatric and adult participants, with the proportion based on the proportion of the age range that was ≤18 years. We compared the proportions of pediatric trials and participants with the proportion of pediatric disease burden with use of the binomial test. Characteristics of pediatric and adult trials were compared by using χ2 and Kruskal-Wallis tests. Time to publication was compared by using Kaplan-Meier curves and log-rank tests. All data were analyzed with SAS software version 9.2 (SAS Institute, Inc, Cary, NC).
Results
Burden of Disease and Trial Participation Among Children
The 5 conditions with the highest disease burden among children in high-income countries were depression (1 233 415 DALYs), asthma (947 259 DALYs), migraine headaches (558 052 DALYs), schizophrenia (479 009 DALYs), and bipolar disorder (306 687 DALYs). In middle- and low-income countries, the top 5 conditions were lower respiratory tract infections (74 455 917 DALYs), diarrheal disease (65 370 104 DALYs), malaria (32 646 855 DALYs), HIV/AIDS (11 527 459 DALYs), and depression (9 609 990 DALYs). Table 1 shows proportions of total disease burden attributable to children compared with adults for each of these conditions.
TABLE 1.
Comparison of Disease-Specific Pediatric Burden and Pediatric Research Activity in Terms of Pediatric Trials and Subjects
| Conditiona | Disease Burden That Is Pediatric, % | Trials Tha Are Pediatricb, % | Binomial Test, P | Pediatric Subjectsc, % | Binomial Test, P |
|---|---|---|---|---|---|
| Overall | 59.9 | 12.0 (292/2440) | <.001 | 19.9 (121 082/608 438) | <.001 |
| High-income countries | 21.4 | 9.8 (159/1618) | <.001 | 10.0 (35 150/352 497) | <.001 |
| Asthma | 49.4 | 17.9 (90/503) | <.001 | 18.2 (19 632/107 910) | <.001 |
| Migraine | 38.8 | 9.8 (13/133) | <.001 | 16.4 (6281/38 201) | <.001 |
| Schizophrenia | 30.8 | 4.6 (20/430) | <.001 | 5.0 (3889/77 637) | <.001 |
| Bipolar disorder | 19.9 | 13.5 (26/193) | .03 | 12.8 (3729/29 061) | <.001 |
| Depression | 12.3 | 3.4 (14/417) | <.001 | 2.3 (2504/106 843) | <.001 |
| Middle- and low-income countries | 61.9 | 11.9 (148/1241) | <.001 | 24.4 (88 545/363 044) | <.001 |
| Malaria | 96.2 | 38.8 (59/152) | <.001 | 50.4 (51 610/102 485) | <.001 |
| Diarrheal illness | 90.4 | 59.1 (13/22) | <.001 | 82.3 (13 566/16 467) | <.001 |
| Lower respiratory tract infection | 79.8 | 38.9 (28/72) | <.001 | 54.1 (16 281/30 110) | <.001 |
| Depression | 17.3 | 3.4 (14/417) | <.001 | 2.3 (2504/106 843) | <.001 |
| HIV/AIDS | 19.9 | 6.0 (35/583) | <.001 | 5.4 (5872/109 451) | <.001 |
Top 5 conditions based on pediatric burden of disease (DALYs) for high-income and middle- and low-income countries.
Defined as trials with maximum age criteria of 17 y as well as trials with a maximum age criteria of ≥18 y but where the midpoint of the age range is <18 y.
Based on sum of enrollment figures in trials with subjects ≤18 y and proportion of pediatric subjects in trial enrolling pediatric and adult subjects with the proportion based on the proportion of the age range that is ≤18 y.
A total of 2440 registered clinical drug trials pertaining to these 9 conditions were eligible for analysis. Overall, 59.9% of the disease burden for the selected conditions was attributable to children, but only 12.0% of trials were pediatric (P < .001). In high-income countries, children contributed 21.4% of the disease burden with 9.8% of trials representing pediatric trials (P < .001). Among middle- and low-income countries, 61.9% of the disease burden was borne by children and 11.9% of trials were pediatric (P < .001).
The proportion of pediatric clinical drug trials was substantially lower than that of pediatric disease burden for each of the conditions examined (P < .001 for all conditions except bipolar disorder with P = .03) (Table 1). Similarly, the proportion of pediatric subjects enrolled in drug trials was smaller than the corresponding pediatric burden of disease for each of the conditions (P < .001 for all conditions).
In comparison of disease burdens for high-income and middle- and low-income countries, 98.2% (193.6 million/197.1 million DALYs) of the total pediatric disease burden for the conditions studied derived from middle- and low-income countries. By contrast, only 48.2% (134/278) of pediatric trials (P < .001) and 72.6% (86 041/118 578) of pediatric subjects (P < .001) addressed conditions unique to middle- and low-income countries. Among pediatric trials addressing conditions pertinent to middle- and low-income countries, 77.0% included at least 1 study location in a middle- or low-income country.
Funding Sources for Pediatric and Adult Drug Trials
Pediatric and adult trials differed in the distribution of reported funding sources (Table 2). Half (n = 146) of pediatric trials were funded by nonprofit organizations without industry contributions, and an additional 8.6% (n = 25) were funded by government sources. Only 36.6% (n = 107) of all pediatric drug trials were sponsored by industry. Among adult trials, 51.5% (n = 1106) were industry funded and an additional 13.2% (n = 284) were funded by nonprofit sources in conjunction with industry. Overall, 58.6% (n = 171) of pediatric drug trials and 35.0% (n = 752) of adult drug trials were conducted entirely without any reported industry funding (P < .001). Among pediatric trials for middle- and low-income country conditions, 70.3% (n = 104) received no industry support compared with 43.4% (n = 69) among trials for high-income country conditions (P < .001).
TABLE 2.
Funding Source for Pediatric and Adult Drug Trials
| Primary Funding Source | All Conditions (N = 2440), n (%)a | Conditions in High-Income Countries (N = 1618), n (%)a | Conditions in Middle- and Low-Income Countries (N = 1241), n (%)a | |||
|---|---|---|---|---|---|---|
| Pediatric Trials (N = 292) | Adult Trials (N = 2148) | Pediatric trials (N = 159) | Adult Trials (N = 1459) | Pediatric trials (N = 148) | Adult Trials (N = 1093) | |
| Industry | 107 (36.6) | 1106 (51.5) | 80 (50.3) | 854 (58.5) | 38 (25.7) | 461 (42.2) |
| Governmentb | 25 (8.6) | 108 (5.0) | 12 (7.6) | 60 (4.1) | 14 (9.5) | 72 (6.6) |
| Nonprofit with industry contributionc | 14 (4.8) | 284 (13.2) | 10 (6.3) | 158 (10.8) | 6 (4.0) | 176 (16.1) |
| Nonprofit without industry contributionc | 146 (50.0) | 650 (30.3) | 57 (35.8) | 387 (26.5) | 90 (60.8) | 384 (35.1) |
χ2 P < .001 for funding source among all conditions, P = .005 for funding source among high-income country conditions, and P < .001 for funding source among middle- and low-income country conditions.
Among government-funded trials, none of the pediatric trials and 6 of the adult trials also received secondary industry funding.
Industry contributions determined based on secondary funding sources.
Trial Characteristics and Quality Indicators for Pediatric and Adult Drug Trials
The sample included 2004 (82.1%) safety/efficacy trials, 314 (12.9%) pharmacokinetic/dynamic trials, 55 (2.2%) bioequivalence/availability trials, and 67 (2.8%) dose-finding trials (Table 3). There were no differences between pediatric and adult trials in the proportions that were randomized (P = .82). The outcome measures differed between pediatric and adult trials, with a greater proportion of pediatric trials examining efficacy outcomes (92.1% [269/292] vs 83.0% [1783/2148], P < .001) and fewer incorporating pharmacokinetic/dynamic end points (16.1% [47/292] vs 22.2% [476/2148], P = .02).
TABLE 3.
Trial Type
| Trial Type | All Trials (N = 2440) | Pediatric Trials (N = 292) | Adult Trials (N = 2148) | χ2, P |
|---|---|---|---|---|
| Safety/efficacy trial, n (%) | 2004 (82.1) | 252 (86.3) | 1752 (81.6) | .06 |
| Randomized trial | 1636 (81.7) | 207 (82.5) | 1429 (81.6) | |
| Nonrandomized comparative trial | 23 (1.2) | 1 (0.4) | 22 (1.3) | |
| Nonrandomized noncomparative trial | 345 (17.2) | 44 (17.5) | 301 (17.2) | |
| Pharmacokinetic/dynamic trials, n (%) | 314 (12.9) | 29 (9.9) | 285 (13.3) | .11 |
| Bioequivalence/availability trials, n (%) | 55 (2.2) | 4 (1.4) | 51 (2.4) | .28 |
| Dose-finding trial, n (%) | 67 (2.8) | 7 (2.4) | 60 (2.8) | .70 |
For the subset of randomized controlled trials, trial characteristics and quality indicators were examined in further detail (n = 1636) (Table 4). Pediatric trials differed from adult trials in terms of funding source, with pediatric trials less likely to report funding by industry (29.5% vs 52.6%, P < .001). A smaller proportion of pediatric trials were in early phases (20.3% vs 39.9%, P < .001), fewer included a primary safety outcome (10.1% vs 16.9%, P = .008), and fewer were multicenter (49.3% vs 58.5%, P = .01). However, registered pediatric trials involved larger anticipated sample sizes (median 220 vs 124, P < .001).
TABLE 4.
Trial Characteristics and Quality Indicators Among Randomized Controlled Pediatric and Adult Drug Trials
| Domain | Measure | All Trials (N = 1636) | Pediatric Trials (N = 207) | Adult Trials (N = 1429) | Pa |
|---|---|---|---|---|---|
| Trial design | Primary funding source, n (%) | <.001 | |||
| Industry | 812 (49.6) | 61 (29.5) | 751 (52.6) | ||
| Government | 100 (6.1) | 16 (7.7) | 84 (5.9) | ||
| Nonprofit | 724 (44.2) | 130 (62.8) | 593 (41.5) | ||
| Study phase, n (%) | <.001 | ||||
| Phases 1, 2, 2/3 | 608 (37.2) | 41 (19.8) | 567 (39.7) | ||
| Phases 3, 4 | 866 (52.9) | 128 (61.8) | 738 (51.6) | ||
| Unknown phase | 162 (9.9) | 38 (18.4) | 124 (8.7) | ||
| Comparator type, n (%) | .41 | ||||
| Active agent | 770 (47.1) | 103 (49.8) | 666 (46.6) | ||
| Placebo | 866 (52.9) | 104 (50.2) | 768 (53.4) | ||
| Masking, n (%) | .18 | ||||
| Double-blind | 1217 (74.4) | 144 (69.6) | 1073 (75.1) | ||
| Single blind | 72 (4.4) | 9 (4.4) | 62 (4.3) | ||
| No blinding | 347 (21.2) | 54 (26.1) | 293 (20.5) | ||
| Inclusion of primary safety outcome, n (%) | 261 (16.0) | 20 (9.7) | 241 (16.9) | .008 | |
| Scope, n (%) | .007 | ||||
| Multicenter | 938 (57.3) | 102 (49.3) | 835 (58.5) | ||
| Single center | 600 (36.7) | 96 (46.4) | 504 (35.3) | ||
| Unknown | 98 (6.0) | 9 (4.4) | 89 (6.2) | ||
| Anticipated sample size, median (IQR)b | 142 (50–390) | 220 (90–450) | 124 (50–380) | <.001 | |
| Length of trial conduct, y, median (IQR)c | 1.7 (1.0–2.7) | 1.8 (1.0–2.7) | 1.7 (1.0–2.7) | .53 | |
| Trial conduct | Registration before study start, N (%) | 659 (40.3) | 97 (46.9) | 562 (39.3) | .04 |
| Registration before study completion, n (%)c | 1359 (95.4) | 170 (92.9) | 1189 (95.7) | .09 | |
| Attainment of ≥75% anticipated study sample, n (%)d | 180 (90.0) | 34 (89.5) | 146 (90.1) | .90 | |
| Provision of study results, n (%) | 120 (7.3) | 16 (7.7) | 104 (7.3) | .82 | |
| Results reporting | Outcomed,e | .62 | |||
| Positive | 121 (67.2) | 22 (71.0) | 99 (66.4) | ||
| Negative | 59 (32.8) | 9 (29.0) | 50 (33.6) |
IQR, interquartile range.
P value based on χ2 for categorical variables, Student t test for mean values, and Kruskal-Wallis test for median values.
Based on 1622 trials with enrollment data.
Based on 1425 trials with available completion dates.
Based on 200 trials with publications.
Five publications included results pooled from 2 studies; 15 trials designated as neither positive or negative.
In terms of trial conduct, a slightly greater proportion of pediatric trials were appropriately registered before study start (46.9% vs 39.3%, P = .04). There were no differences between pediatric and adult trials in the provision of study results or the enrollment of at least 75% of the anticipated study sample among published trials.
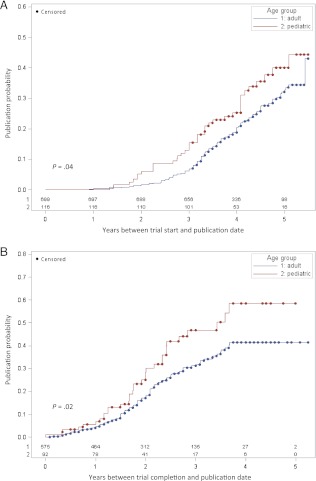
We identified 200 publications corresponding to the randomized controlled trials. The probability of publication was greater for pediatric compared with adult trials overall (32.8% vs 23.2%, log-rank P = .04 for time from study start to publication) and also when limited to completed trials (35.9% vs 23.8%, log-rank P = .02 for time from completion to publication) (Fig 1). There were no differences in the proportion of positive or negative trial outcome reports between pediatric and adult trials.
FIGURE 1.
Kaplan-Meier curves for the cumulative probability of publication for randomized controlled trials. A, Probability of publication for the period beginning with the study start date. The time at which 25% of trials were published was 3.9 years (95% CI: 3.2–4.2) for pediatric trials and 4.4 years (95% CI: 4.1–4.7) for adult trials. B, Probability of publication for the period beginning with the study completion date. The time at which 25% of trials were published was 2.0 years (95% CI: 1.7–2.3) for pediatric trials and 2.4 years (95% CI: 2.1–2.7) for adult trials.
Discussion
There is a large discrepancy between pediatric burden of disease and the research supporting the evidence base for the medical care provided to children. Among the conditions examined, children accounted for nearly 60% of total disease burden, but only 12% of drug trials focused on pediatric patients. Some of this mismatch is potentially explained by the sources of funding for these studies. Although pharmaceutical companies funded the majority of adult trials, pediatric trials were funded primarily by government and nonprofit organizations where available budgets for clinical trials are currently limited. The pharmaceutical industry may face multiple disincentives to conduct pediatric trials, including difficulties surrounding the recruitment of pediatric patients, ethical issues in undertaking pediatric research, and the small market share and return on investment for pediatric compared with adult drugs.3,5 Furthermore, regulatory agencies have historically not required inclusion of pediatric patients in premarketing clinical trials, which form the basis of the Food and Drug Administration approval process for new drugs.30 Inclusion of children in later phase trials has also not been required; the Food and Drug Administration has sanctioned use of certain adult trial data to guide pharmacotherapy in pediatric populations if the condition and drugs’ effects are deemed comparable in children and adults.31,32
Despite concerns that have been expressed previously regarding the quality of pediatric trials, we did not identify substantial differences between pediatric and adult trials based on the indicators available. Randomized studies comprised approximately two-thirds of the registered trials both for pediatric and adult populations. Other trial characteristics and quality indicators were also comparable, although pediatric trials were somewhat more likely to be appropriately registered before study start and to result in publication. This disparity in publication rate may in part be related to differences in the funding entities sponsoring pediatric and adult trials and the factors motivating their trial research.
In the United States, the Best Pharmaceuticals for Children Act and The Pediatric Research Equity Act of 2007 seek to increase pediatric drug research conducted by drug manufacturers.33,34 Similar legislation in Europe and global WHO initiatives also aim to improve the evidence guiding the use of pharmaceuticals in children.35,36 However, given the current gap in pediatric drug trial participation, additional regulations may be necessary to increase the number of drugs tested in children by pharmaceutical companies both in the preapproval and postmarketing phase of drug development. Furthermore, in light of growing concerns over potential conflicts of interest inherent in the practice of pharmaceutical companies testing and reporting on their own products, additional programs should be established to support pediatric drug testing by noncommercial entities.37,38
Previous studies have documented a disproportionately small number of pediatric trials in developing countries compared with high-income countries.16,17,20,39 For the conditions we examined, 98.8% of the disease burden is borne by children in middle- and low-income countries, whereas slightly less than half of all pediatric drug trials address conditions most pertinent to these countries. Conducting pediatric trials in developing nations presents additional practical and ethical issues and is generally unprofitable for pharmaceutical companies.40,41 We found that 70% of pediatric drug trials addressing the most pertinent diseases for the developing world were conducted entirely without support by the pharmaceutical industry and relied instead on nonprofit and government organizations alone.
There was a relative paucity of registered pharmacokinetic/dynamic and safety assessments in children compared with adults and, similarly, a lower proportion of registered pediatric trials in early phases, which tend to represent trials with a greater focus on pharmacokinetics and safety of drugs. Establishing the proper dose and safety of medications in children is critical given age-specific differences in drug disposition, action, and toxicity.8 It is unknown whether this deficiency of early-phase trials means that such trials are not done, not registered, or a combination of these factors.
A limitation of our work is that ClinicalTrials.gov may not include all trials, although it is the most comprehensive and accessible of any of the registries.22,23 It also does not contain complete information on trial methodology, which would enable a more detailed comparison of the quality of pediatric and adult trials. It has been suggested that full protocols should be included in the future with trial registration records.42,43 We were also not able to verify the accuracy of the data reported by investigators, and there were some missing data. Furthermore, because we chose to examine recent research activity, it is anticipated that more publications will result from these trials in the future. Late-published trials may differ from early-published trials in the proportion of positive results and other aspects.29 Finally, we did not compare the results of pediatric versus adult trials, because only a limited number of trials are published to date.44 However, such comparisons would only be eventually meaningful and informative if sufficient pediatric evidence exists to juxtapose against the more substantial evidence in adults.
Conclusions
Although there are initiatives in the United States and globally to increase the number of trials studying drugs in children, a large gap remains between the disease burden in children and the amount of pediatric drug research. This disparity is most pronounced in developing countries. Future efforts to increase pediatric drug research should involve both the pharmaceutical industry and noncommercial organizations, and emphasis should be placed on including pharmacokinetic and safety assessments to ensure effective and safe prescribing practices in children.
Acknowledgment
We thank Fiona Gore at the World Health Organization, Department of Health Statistics and Information Systems, for her assistance in obtaining age-specific burden-of-disease data.
Glossary
- CI
confidence interval
- DALY
disability-adjusted life-year
- WHO
World Health Organization
Footnotes
Dr Bourgeois contributed to study conception and design, data acquisition, analysis and interpretation of data, drafting and revising the article, and final approval; Dr Murthy contributed to study conception and design, data acquisition, analysis and interpretation of data, revising the article, and final approval; Dr Pinto contributed to study conception and design, data acquisition and analysis, and revision and final approval of the article; Dr Olson contributed to study conception and design, data analysis and interpretation of data, and revision and final approval of the article; and Drs Ioannidis and Mandl contributed to study conception and design, analysis and interpretation of data, and revision and final approval of the article.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Bourgeois was supported by grants from the National Institute of Child Health and Human Development (5T32HD040128 and 1R21HD072382), Dr Mandl by grants from the National Library of Medicine and National Institute of Child Health and Human Development (5G08LM009778 and 1R21HD072382), and Dr Ioannidis by a grant from the National Institute of Child Health and Human Development (1R21HD072382), National Institutes of Health. Funded by the National Institutes of Health (NIH).
References
- 1.Pandolfini C, Bonati M. A literature review on off-label drug use in children. Eur J Pediatr. 2005;164(9):552–558 [DOI] [PubMed] [Google Scholar]
- 2.Shah SS, Hall M, Goodman DM, et al. Off-label drug use in hospitalized children. Arch Pediatr Adolesc Med. 2007;161(3):282–290 [DOI] [PubMed] [Google Scholar]
- 3.Caldwell PH, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet. 2004;364(9436):803–811 [DOI] [PubMed] [Google Scholar]
- 4.Smyth RL, Weindling AM. Research in children: ethical and scientific aspects. Lancet. 1999;354(suppl 2):SII21–SII24 [DOI] [PubMed] [Google Scholar]
- 5.Steinbrook R. Testing medications in children. N Engl J Med. 2002;347(18):1462–1470 [DOI] [PubMed] [Google Scholar]
- 6.Lindkvist J, Airaksinen M, Kaukonen AM, Klaukka T, Hoppu K. Evolution of paediatric off-label use after new significant medicines become available for adults: a study on triptans in Finnish children 1994-2007. Br J Clin Pharmacol. 2011;71(6):929–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waller DG. Off-label and unlicensed prescribing for children: have we made any progress? Br J Clin Pharmacol. 2007;64(1):1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167 [DOI] [PubMed] [Google Scholar]
- 9.Feder HM, Jr, Osier C, Maderazo EG. Chloramphenicol: a review of its use in clinical practice. Rev Infect Dis. 1981;3(3):479–491 [DOI] [PubMed] [Google Scholar]
- 10.Hatch DJ. Propofol-infusion syndrome in children. Lancet. 1999;353(9159):1117–1118 [DOI] [PubMed] [Google Scholar]
- 11.Hamm MP, Hartling L, Milne A, et al. A descriptive analysis of a representative sample of pediatric randomized controlled trials published in 2007. BMC Pediatr. 2010;10:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen E, Goldman RD, Ragone A, et al. Child vs adult randomized controlled trials in specialist journals: a citation analysis of trends, 1985-2005. Arch Pediatr Adolesc Med. 2010;164(3):283–288 [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Castaldi C, Silverstein M, Bauchner H. Child versus adult research: the gap in high-quality study design. Pediatrics. 2008;122(1):52–57 [DOI] [PubMed] [Google Scholar]
- 14.Cohen E, Uleryk E, Jasuja M, Parkin PC. An absence of pediatric randomized controlled trials in general medical journals, 1985-2004. J Clin Epidemiol. 2007;60(2):118–123 [DOI] [PubMed] [Google Scholar]
- 15.Anderson M, Choonara I. A systematic review of safety monitoring and drug toxicity in published randomised controlled trials of antiepileptic drugs in children over a 10-year period. Arch Dis Child. 2010;95(9):731–738 [DOI] [PubMed] [Google Scholar]
- 16.Aripin KN, Choonara I, Sammons HM. A systematic review of paediatric randomised controlled drug trials published in 2007. Arch Dis Child. 2010;95(6):469–473 [DOI] [PubMed] [Google Scholar]
- 17.Nor Aripin KN, Sammons HM, Choonara I. Published pediatric randomized drug trials in developing countries, 1996-2002. Paediatr Drugs. 2010;12(2):99–103 [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. The global burden of disease: 2004 update. Geneva, Switzerland: World Health Organization; 2008. Available at: www.who.int/evidence/bod. Accessed May 5, 2012
- 19.Gross CP, Anderson GF, Powe NR. The relation between funding by the National Institutes of Health and the burden of disease. N Engl J Med. 1999;340(24):1881–1887 [DOI] [PubMed] [Google Scholar]
- 20.Isaakidis P, Swingler GH, Pienaar E, Volmink J, Ioannidis JP. Relation between burden of disease and randomised evidence in sub-Saharan Africa: survey of research. BMJ. 2002;324(7339):702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institutes of Health. ClinicalTrials.gov. Available at: www.clinicaltrials.gov. Accessed May 5, 2012
- 22.Estellat C, Ravaud P. Lack of head-to-head trials and fair control arms: randomized controlled trials of biologic treatment for rheumatoid arthritis. Arch Intern Med. 2012;172(3):237–244 [DOI] [PubMed] [Google Scholar]
- 23.Viergever RF, Ghersi D. The quality of registration of clinical trials. PLoS ONE. 2011;6(2):e14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourgeois FT, Murthy S, Mandl KD. Outcome reporting among drug trials registered in ClinicalTrials.gov. Ann Intern Med. 2010;153(3):158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson D, Hartling L, Cohen E, Vandermeer B, Tjosvold L, Klassen TP. Controlled trials in children: quantity, methodological quality and descriptive characteristics of pediatric controlled trials published 1948-2006. PLoS ONE. 2010;5(9):e13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Institute of Medicine. Learning what works best: the nation's need for evidence on comparative effectiveness in health care. 2007. Available at: www.iom.edu/∼/media/Files/Activity%20Files/Quality/VSRT/ComparativeEffectivenessWhitePaperF.ashx. Accessed May 5, 2012
- 27.Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials.gov results database—update and key issues. N Engl J Med. 2011;364(9):852–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee K, Bacchetti P, Sim I. Publication of clinical trials supporting successful new drug applications: a literature analysis. PLoS Med. 2008;5(9):e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ioannidis JP. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA. 1998;279(4):281–286 [DOI] [PubMed] [Google Scholar]
- 30.Coté CJ, Kauffman RE, Troendle GJ, Lambert GH. Is the “therapeutic orphan” about to be adopted? Pediatrics. 1996;98(1):118–123 [PubMed] [Google Scholar]
- 31.Roberts R, Rodriguez W, Murphy D, Crescenzi T. Pediatric drug labeling: improving the safety and efficacy of pediatric therapies. JAMA. 2003;290(7):905–911 [DOI] [PubMed] [Google Scholar]
- 32. Specific Requirements on Content and Format of Labeling for Human Prescription Drugs; Revision of “Pediatric Use” Subsection in the Labeling; Final Rule. Available at: www.gpo.gov/fdsys/pkg/FR-1994-12-13/html/94-30238.htm. Accessed May 5, 2012. [Google Scholar]
- 33.Best Pharmaceuticals for Children Act. Pub L No. Best Pharmaceuticals for Children Act of 2007. Title V of Pub L No 110-85. 2007 [Google Scholar]
- 34.Pediatric Research Equity Act. Pub L No. Pediatric Research Equity Act of 2007. Title IV of 110-85. 2007
- 35.Choonara I. Regulation of drugs for children in Europe. BMJ. 2007;335(7632):1221–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watts G. WHO launches campaign to make drugs safer for children. BMJ. 2007;335(7632):1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berger E. Ghostwriters, data manipulation and dollar diplomacy: how drug companies pull the strings in clinical research. Ann Emerg Med. 2008;52(2):137–139 [DOI] [PubMed] [Google Scholar]
- 38.Bhandari M, Busse JW, Jackowski D, et al. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. CMAJ. 2004;170(4):477–480 [PMC free article] [PubMed] [Google Scholar]
- 39.Sammons HM, Choonara I. Clinical trials of medication in children, 1996-2002. Eur J Clin Pharmacol. 2005;61(2):165–167 [DOI] [PubMed] [Google Scholar]
- 40.Trouiller P, Olliaro PL. Drug development output: what proportion for tropical diseases? Lancet. 1999;354(9173):164. [DOI] [PubMed] [Google Scholar]
- 41.Wolffers I, Adjei S, van der Drift R. Health research in the tropics. Lancet. 1998;351(9116):1652–1654 [DOI] [PubMed] [Google Scholar]
- 42.Chan AW. Bias, spin, and misreporting: time for full access to trial protocols and results. PLoS Med. 2008;5(11):e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lassere M, Johnson K. The power of the protocol. Lancet. 2002;360(9346):1620–1622 [DOI] [PubMed] [Google Scholar]
- 44.Contopoulos-Ioannidis DG, Baltogianni MS, Ioannidis JP. Comparative effectiveness of medical interventions in adults versus children. J Pediatr. 2010;157(2):322–330.e17 [DOI] [PubMed] [Google Scholar]