Abstract
BACKGROUND
The risk of type 2 diabetes mellitus is increased in people who have low birth weights and who subsequently become obese as adults. Whether their obesity originates in childhood and, if so, at what age are unknown. Understanding the origin of obesity may be especially important in developing countries, where type 2 diabetes is rapidly increasing yet public health messages still focus on reducing childhood “undernutrition.”
METHODS
We evaluated glucose tolerance and plasma insulin concentrations in 1492 men and women 26 to 32 years of age who had been measured at birth and at intervals of three to six months throughout infancy, childhood, and adolescence in a prospective, population-based study.
RESULTS
The prevalence of impaired glucose tolerance was 10.8 percent, and that of diabetes was 4.4 percent. Subjects with impaired glucose tolerance or diabetes typically had a low body-mass index up to the age of two years, followed by an early adiposity rebound (the age after infancy when body mass starts to rise) and an accelerated increase in body-mass index until adulthood. However, despite an increase in body-mass index between the ages of 2 and 12 years, none of these subjects were obese at the age of 12 years. The odds ratio for disease associated with an increase in the body-mass index of 1 SD from 2 to 12 years of age was 1.36 (95 percent confidence interval, 1.18 to 1.57; P<0.001).
CONCLUSIONS
There is an association between thinness in infancy and the presence of impaired glucose tolerance or diabetes in young adulthood. Crossing into higher categories of body-mass index after the age of two years is also associated with these disorders.
The prevalence of type 2 diabetes mellitus is increasing rapidly in developing countries such as India.1-3 This epidemic has been attributed to what has been called “nutritional transition” (increased availability of food, reduced physical activity, and increases in obesity) — changes that are most marked in urban populations. Type 2 diabetes may originate from events initiated during fetal development and in patterns of childhood weight gain. Studies have consistently shown high rates of this disease in people who were born small but became overweight as adults.4-8 It is unclear whether the crucial phase of postnatal weight gain is in infancy, childhood, adolescence, or adulthood. Thus, longitudinal studies are required to determine the age at which preventive interventions should be initiated. We examined the incidence of impaired glucose tolerance and type 2 diabetes in a population study of young adults raised in the era of nutritional transition in India whose growth has been recorded prospectively since birth.9
METHODS
STUDY COHORT AND FOLLOW-UP
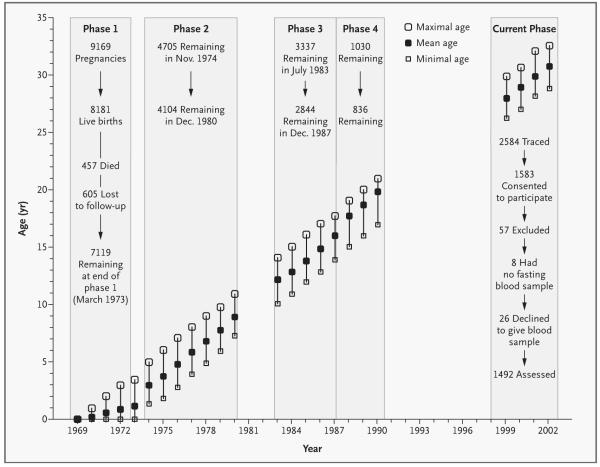
This study of pregnancy outcomes and childhood growth was begun in 1969 in a defined area of 12 km2 in South Delhi, India.10 All families living there between December 1, 1969, and November 30, 1972, were identified. Among a population of 119,799, there were 20,755 married women of reproductive age who were assessed every other month (±3 days) in order to record menstrual dates. Women who became pregnant were seen by a health visitor every 2 months (±3 days) initially and on alternate days from the 37th week of gestation. There were 9169 pregnancies, resulting in 8181 live births (8030 singletons and 151 twins), 202 stillbirths, and 867 abortions. Trained personnel recorded the weight and the length or height of the babies within 72 hours after birth; at the ages of 3, 6, 9, and 12 months (±7 days); and at 6-month intervals (±15 days) thereafter. There were several phases in this cohort study (Fig. 1). More than 30 percent of the cohort (2414 subjects) was lost to follow-up between the end of phase 1 and the beginning of phase 2, a time when unauthorized housing was demolished in South Delhi.
Figure 1. Summary of the Various Phases of the Cohort Study in Relation to the Age of Subjects and the Number Available for Study.
In Phase 4, recruitment was restricted to the subjects who would have reached 20 years of age during this period. The mean, minimal, and maximal ages of the subjects at each year of follow-up are shown by the diagonal rows of symbols. Numbers of subjects remaining in follow-up at the beginning and end of phases 1 through 4 are shown.
At the time of recruitment, 59.9 percent of families had an income above 50 rupees per month (national average, 28.4). Only 14.9 percent of parents were illiterate (national average, 66.3). Nevertheless, 43.0 percent of families lived in only one room. Hindus were the majority religious group (84.3 percent), followed by Sikhs (11.6 percent), Christians (2.1 percent), Muslims (1.1 percent), and Jains (0.7 percent).
CURRENT PHASE OF STUDY
From August 1998 to August 2002, we located 2584 (31.6 percent) of the initial cohort. A social worker performed home visits and recorded each subject’s occupation and level of education, physical activity, and alcohol and tobacco consumption. The subjects were asked to attend a clinic after an overnight fast for further investigations. Of the 1583 subjects (61.3 percent) who agreed to participate, 57 were excluded (24 were pregnant, 2 withdrew, and 31 were unreliably linked to earlier data), leaving 1526. In comparison with the original cohort, this cohort had 7 percent more male subjects, the rate of maternal literacy was 6 percent higher, the mean birth weight was 32 g higher, and the mean birth length was 2 mm longer. The height, weight, and body-mass index (the weight in kilograms divided by the square of the height in meters) in childhood and adolescence were approximately 0.1 SD lower than in the original cohort.
The subjects’ blood pressure, weight, height, waist and hip circumferences, and skinfold thicknesses (triceps and subscapular) were measured according to standardized techniques. Subjects were categorized as obese if their body-mass index was 30 or more.11 Two definitions of overweight were used, the standard World Health Organization11 cutoff value of a body-mass index of 25 and that recommended for Asians12 of 23.
A standard glucose-tolerance test with an oral 75-g anhydrous glucose load was administered.13 Plasma glucose concentrations in samples obtained after an overnight fast and 30 and 120 minutes after the ingestion of glucose (fasting, 30-minute, and 120-minute values) were analyzed by means of a glucose oxidase method (GOD-PAP, Randox) with a Beckman autoanalyzer. Aliquots of plasma were stored at −70°C for up to eight months, and insulin concentrations were measured by radioimmunoassay (Coat-a-Count insulin kit, Diagnostic Products). The intraassay and interassay coefficients of variation were less than 5 percent and less than 7.5 percent, respectively. Insulin resistance was calculated according to the homeostasis-model assessment.14
The 30-minute increment in insulin — calculated as the (30-minute insulin concentration–the fasting insulin concentration)÷the 30-minute glucose concentration — was used as a measure of first-phase insulin secretion.15 Impaired glucose tolerance was defined as a fasting plasma glucose concentration of less than 126 mg per deciliter (7.0 mmol per liter) and a 120-minute value of at least 141 mg per deciliter (7.8 mmol per liter); diabetes was defined as a fasting glucose concentration of at least 126 mg per deciliter or a 120-minute concentration of at least 200 mg per deciliter (11.1 mmol per liter).13
The All India Institute of Medical Sciences approved the study. Informed consent was obtained from each subject.
STATISTICAL ANALYSIS
Using all recorded data, not just those for subjects recruited for the current studies, we generated height, weight, and body-mass index standards so as to derive internal sex-specific SD scores (the SD score is the number of standard deviations by which an observation differs from the mean for the cohort). Recruited subjects had an average (±SD) of 23±5.5 observations between birth and the age of 21 years. We modeled the progress of the median, spread, and skewness of the measurements as age increased. For each subject we interpolated values linearly between successive SD scores to estimate SD scores at 6 months and at birthdays from 1 to 21 years of age.16 The interpolated values were used if a measurement had been made within 6 months (up to 1 year), 1 year (age of 2 years), 1.5 years (age of 3 years), and 2 years (all ages after 3 years). Back transformation provided estimates of the measurements at all these ages. The ponderal index at birth was calculated as 1000 times the weight in grams divided by the cube of the height (or the crown–heel length) in centimeters.
We calculated the age at the time of adiposity rebound — the age after infancy at which the bodymass index starts to rise — as the age in years between two and nine years at which the lowest body-mass index occurred; the analysis was restricted to 1311 subjects whose body-mass index was estimated at every birthday. Variables with skewed distributions were log-transformed. Data were analyzed with the use of multiple linear and logistic regression.
RESULTS
Table 1 shows the characteristics of the 886 men and 640 women in the current sample. Most were married, college graduates (with a bachelor’s degree or above), and not in manual employment. Few women drank alcohol or smoked tobacco. Almost half the subjects were overweight according to the conventional definition,11 and nearly two thirds were overweight when the Asian cutoff value was used.12
Table 1.
Characteristics of the Study Cohort.*
| Characteristic | Men (N=886) | Women (N=640) | P Value† | ||
|---|---|---|---|---|---|
| No. | Value | No. | Value | ||
| At birth | |||||
| Weight — g | 803 | 2891±436 | 561 | 2791±383 | <0.001 |
| Length — cm | 779 | 48.8±2.1 | 558 | 48.3±1.9 | <0.001 |
| Ponderal index | 779 | 24.8±2.6 | 558 | 24.7±2.5 | 0.65 |
| Head circumference — cm | 775 | 33.8±1.3 | 555 | 33.3±1.1 | <0.001 |
| Gestation — wk | 791 | 38.7±2.6 | 588 | 39.1±2.5 | 0.003 |
| During childhood | |||||
| 2 Yr | |||||
| Weight — kg | 834 | 10.3±1.3 | 609 | 9.8±1.2 | <0.001 |
| Height — cm | 840 | 81.1±3.6 | 609 | 79.6±3.6 | <0.001 |
| Body-mass index | 833 | 15.8±1.2 | 604 | 15.4±1.2 | <0.001 |
| 12 Yr | |||||
| Weight — kg | 867 | 30.9±5.9 | 625 | 32.2±6.7 | <0.001 |
| Height — cm | 864 | 140.3±6.7 | 623 | 141.4±7.6 | 0.002 |
| Body-mass index | 864 | 15.6±2.0 | 623 | 16.0±2.1 | 0.002 |
| Current assessment‡ | |||||
| Age — yr | 886 | 29.2±1.3 | 640 | 29.2±1.4 | 0.78 |
| Married — % | 886 | 69.8 | 640 | 81.1 | <0.001 |
| Educational level — % | 886 | 640 | <0.001 | ||
| Middle school or less | 15.6 | 7.7 | |||
| High school or less | 32.5 | 28.3 | |||
| College graduate | 40.0 | 48.8 | |||
| Postgraduate or professional | 12.0 | 15.3 | |||
| Employment status — % | 886 | 639 | <0.001 | ||
| Housewife | — | 61.0 | |||
| Unemployed | 2.1 | 7.5 | |||
| Unskilled or semiskilled manual labor | 10.9 | 1.1 | |||
| Skilled manual labor | 24.2 | 2.5 | |||
| Nonmanual labor, business or professional | 62.8 | 27.9 | |||
| Current alcohol use — % | 886 | 56.2 | 640 | 1.4 | <0.001 |
| Tobacco use (smoking or oral) — % | 886 | 640 | <0.001 | ||
| Former | 5.1 | 0.2 | |||
| Current | 29.8 | 0.2 | |||
| Weight — kg | 886 | 71.8±14.0 | 640 | 59.2±13.4 | <0.001 |
| Height — m | 886 | 1.70±0.06 | 638 | 1.55±0.06 | <0.001 |
| Body-mass index | 886 | 24.9±4.3 | 638 | 24.6±5.1 | 0.31 |
| Waist:hip ratio | 886 | 0.92±0.06 | 639 | 0.82±0.07 | <0.001 |
| Skinfold thickness — mm | |||||
| Subscapular | 883 | 23.6±9.6 | 637 | 25.5±10.4 | <0.001 |
| Triceps | 885 | 16.7±7.4 | 638 | 25.5±9.5 | <0.001 |
| Subscapular:triceps ratio — % | 883 | 635 | <0.001 | ||
| Median | 1.42 | 1.00 | |||
| Interquartile range | 1.21–1.71 | 0.85–1.14 | |||
| Overweight — % | |||||
| Body-mass index ≥25 | 886 | 47.4 | 638 | 45.5 | 0.47 |
| Body-mass index ≥23 | 886 | 66.0 | 638 | 61.8 | 0.09 |
| Obese (body-mass index ≥30) — % | 886 | 9.5 | 638 | 13.0 | 0.03 |
| Central obesity — %§ | 886 | 65.5 | 639 | 31.0 | <0.001 |
| Plasma glucose — mmol/liter | |||||
| Fasting | 869 | 623 | 0.09 | ||
| Median | 5.4 | 5.3 | |||
| Interquartile range | 4.9–5.9 | 4.8–5.8 | |||
| 30 Min | 864 | 613 | <0.001 | ||
| Median | 8.2 | 7.6 | |||
| Interquartile range | 7.2–9.4 | 6.6–8.6 | |||
| 120 Min | 848 | 591 | 0.02 | ||
| Median | 5.9 | 6.1 | |||
| Interquartile range | 5.1–7.1 | 5.3–7.1 | |||
| Plasma insulin — pmol/liter | |||||
| Fasting | 868 | 623 | <0.001 | ||
| Median | 38.4 | 32.4 | |||
| Interquartile range | 18–66 | 14.4–60 | |||
| 30 Min | 864 | 612 | <0.001 | ||
| Median | 360 | 294 | |||
| Interquartile range | 216–576 | 192–460.5 | |||
| 120 Min | 847 | 591 | 0.68 | ||
| Median | 186 | 186 | |||
| Interquartile range | 102–348 | 108–300 | |||
| Diabetes — no. (%) | 849 | 41 (4.8) | 593 | 22 (3.7) | 0.36 |
| Impaired glucose tolerance — no. (%) | 849 | 95 (11.2) | 593 | 61 (10.3) | 0.61 |
| Diabetes or impaired glucose tolerance — no. (%) | 849 | 136 (16.0) | 593 | 83 (14.0) | 0.30 |
Plus–minus values are means ±SD. To convert glucose values to milligrams per deciliter, divide by 0.05551. To convert insulin values to milliunits per liter, divide by 6.0. The ponderal index is equal to 1000 times the weight in grams divided by the cube of the height (or the crown–heel length) in centimeters.
P values were derived with the use of two-sample t-tests for continuous variables, chi-square tests for categorical variables, and chi-square tests for trend in cases in which there were more than two categories (education and employment status and tobacco smoking).
The current age of subjects was 26 to 32 years.
Central obesity was defined as a waist:hip ratio greater than 0.90 for men and 0.85 for women.13
Eight of the 1526 subjects who attended the clinic had not fasted, and a further 26 declined to provide a blood sample. Fifty subjects did not complete the glucose-tolerance test and therefore could not be classified as having normal or impaired glucose tolerance or diabetes. Of the remaining 1442 subjects, 156 (10.8 percent) had impaired glucose tolerance and 63 (4.4 percent) had diabetes.
Fasting and 120-minute plasma glucose concentrations were correlated positively with age (P<0.001 for both), body-mass index (P<0.001 for both), and waist:hip ratio (P=0.001 for both). Mean 120-minute glucose values were 101 mg per deciliter (5.61 mmol per liter) in subjects who were 26 or 27 years of age, as compared with 118 mg per deciliter (6.57 mmol per liter) in those who were 31 or 32 years of age; the corresponding figures for the combined prevalence of impaired glucose tolerance and diabetes were 8.4 percent and 17.7 percent. These trends remained significantly associated with age (P<0.001) after adjustment for body-mass index and the waist:hip ratio.
As compared with subjects with normal glucose tolerance, those with impaired glucose tolerance or diabetes had higher mean values for body-mass index, waist:hip ratio, fasting and 120-minute plasma insulin concentrations, and insulin resistance (Table 2). The 30-minute insulin-increment values, however, were significantly lower in the subjects with diabetes or impaired glucose tolerance than in the subjects with normal glucose tolerance and were lowest in those with diabetes. The presence of impaired glucose tolerance and diabetes was unrelated to a subject’s level of education or employment status, alcohol consumption, smoking status, or level of physical activity. A history of diabetes in a first-degree relative (present in 36.7 percent of subjects) was associated with an increased risk of impaired glucose tolerance or diabetes (P=0.004), but this relation was no longer statistically significant (P=0.08) after adjustment for adult body-mass index and the waist:hip ratio. In further analyses of the predictors of impaired glucose tolerance and diabetes, we adjusted for age, sex, adult body-mass index, and the waist:hip ratio unless otherwise stated.
Table 2.
Mean Plasma Glucose and Insulin Concentrations, Values for Body-Mass Index, and Waist:Hip Ratios According to Glucose-Tolerance Status.*
| Variable | Normal Glucose Tolerance (N=1223) |
Impaired Glucose Tolerance (N=156) |
Diabetes (N=63) |
P Value† | All Subjects (N=1442) median (interquartile range) |
|---|---|---|---|---|---|
| Body-mass index | 24.6 | 26.2 | 26.2 | <0.001 | 24.7 (21.8–27.6) |
| Waist:hip ratio | |||||
| Men | 0.92 | 0.95 | 0.95 | <0.001 | 0.92 (0.88–0.96) |
| Women | 0.82 | 0.83 | 0.85 | 0.006 | 0.81 (0.77–0.86) |
| Plasma glucose (mmol/liter) | |||||
| Fasting | 5.2 | 5.6 | 7.8 | <0.001 | 5.4 (4.9–5.9) |
| 30 Min | 7.7 | 9.1 | 10.9 | <0.001 | 8.0 (7.0–9.0) |
| 120 Min | 5.6 | 8.6 | 9.4 | <0.001 | 6.0 (5.1–7.0) |
| Glucose increment‡ | 2.6 | 3.6 | 3.5 | <0.001 | 2.6 (1.8–3.6) |
| Plasma insulin (pmol/liter) | |||||
| Fasting | 31 | 40 | 48 | <0.001 | 36 (16–63) |
| 30 Min | 314 | 353 | 248 | 0.4 | 333 (207–517) |
| 120 Min | 159 | 365 | 219 | <0.001 | 185 (107–328) |
| 30-Min insulin increment§ | 42 | 39 | 23 | <0.001 | 37 (22–57) |
| Insulin resistance¶ | 1.18 | 1.68 | 2.79 | <0.001 | 1.41 (0.62–2.58) |
All variables have been adjusted for age and sex. To convert glucose values to milligrams per deciliter, divide by 0.05551. To convert insulin values to milliunits per liter, divide by 6.0.
P values refer to the trend across the three glucose-tolerance groups with the use of linear regression.
The glucose increment is derived by subtracting the fasting value from the 30-minute value.
The 30-minute insulin increment is derived by subtracting the fasting value from the 30-minute value and dividing the result by the 30-minute glucose value.
Insulin resistance was calculated according to the homeostasis-model assessment.14
SIZE AT BIRTH AND DURING INFANCY
Across the range of birth weights, 120-minute plasma glucose concentrations in the young adult subjects fell from 113 mg per deciliter (6.28 mmol per liter) in subjects with a birth weight of 2.25 kg or less to 105 mg per deciliter (5.85 mmol per liter) in those with a birth weight of more than 3.5 kg (P=0.02). This relationship was not changed by further adjustment for the length of gestation. There were similar trends in fasting insulin concentrations (P=0.02), 120-minute insulin concentrations (P=0.008), and insulin resistance (P=0.009). These variables were also inversely related to the ponderal index at birth (P=0.04, P=0.01, and P=0.03, respectively). Although the development of impaired glucose tolerance and diabetes was not related to birthweight, in combination the prevalence of these conditions was inversely related to weight and body-mass index at one year of age (P=0.04 and P=0.03, respectively; odds ratio, 1.6; 95 percent confidence interval, 1.0 to 2.5) for those in the lowest quartile of body-mass index as compared with those in the highest quartile of body-mass index at one year.
CHILDHOOD GROWTH AND OBESITY
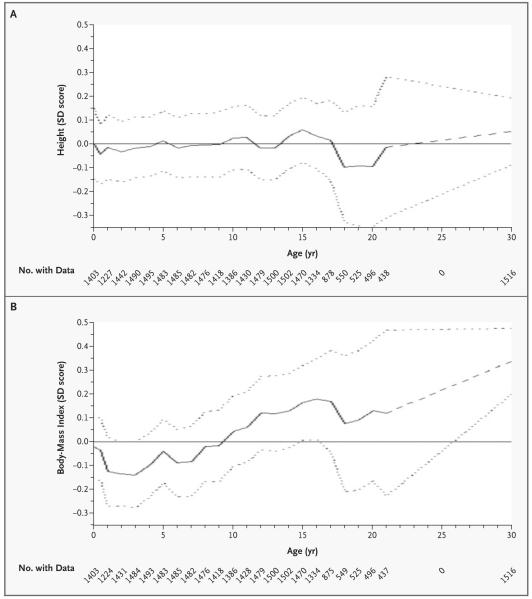
Figure 2 shows the growth of boys and girls in whom impaired glucose tolerance or diabetes subsequently developed. The SD score for the cohort is set at zero. A child maintaining a steady position as large or small in relation to other children would follow a horizontal path on the figure. The SD scores for body-mass index fell between birth and two years of age among children in whom impaired glucose tolerance or diabetes later developed, although this decrease was not statistically significant (P=0.29). From two years of age onward they had an accelerated increase in body-mass index, while SD scores for height remained relatively constant.
Figure 2. Mean Sex-Specific Unadjusted SD Scores for Height (Panel A) and Body-Mass Index (Panel B), According to Age, for Subjects in Whom Impaired Glucose Tolerance or Diabetes Developed.
The mean SD scores (solid lines) are obtained by linear interpolation of yearly means, with one additional observation at six months. The dotted lines represent 95 percent confidence intervals. The dashed portions of lines indicate years in which there was no follow-up. The SD score for the cohort is set at zero (solid horizontal lines).
As shown in Table 3, the highest prevalence of impaired glucose tolerance and diabetes was among subjects who were in the lowest third of the group with respect to body-mass index at the age of 2 years and the highest at the age of 12 years. In a simultaneous regression, the opposing effects of body-mass index at 2 years and at 12 years were both statistically significant (P=0.002 for body-mass index at 2 years and P<0.001 for body-mass index at 12 years, adjusted for age and sex). An increase of 1 SD in body-mass index between the ages of 2 and 12 years was associated with an odds ratio of impaired glucose tolerance or diabetes of 1.36 (95 percent confidence interval, 1.18 to 1.57; P<0.001). This finding was similar after further adjustment for current body-mass index and the waist:hip ratio (odds ratio, 1.26; 95 percent confidence interval, 1.08 to 1.48; P=0.004). An increase of 1 SD in body-mass index between 2 years of age and adulthood was associated with only a slightly higher odds ratio than that for such an increase between 2 and 12 years of age (odds ratio, 1.46; 95 percent confidence interval, 1.28 to 1.66; P<0.001, adjusted for age and sex).
Table 3.
Prevalence of and Odds Ratios for Impaired Glucose Tolerance or Diabetes, According to the Body-Mass Index (BMI) at 2 Years and 12 Years of Age and 2 Years of Age and Currently.*
| BMI at 2 yr of Age | Prevalence of Impaired Glucose Tolerance or Diabetes | Odds Ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| BMI <14.7 at Age 12 |
BMI=14.7–16.2 at Age 12 |
BMI >16.2 at Age 12 |
All Subjects |
BMI <14.7 at Age 12 |
BMI=14.7–16.2 at Age 12 |
BMI >16.2 at Age 12 |
|
| percent (number of subjects) | |||||||
| <15.0 | 16.1 (217) | 14.8 (135) | 27.1 (85) | 17.8 (437) | 2.2 (0.8–5.9) | 2.0 (0.7–5.7) | 4.5 (1.6–12.8) |
| 15.0–16.1 | 14.2 (148) | 11.2 (143) | 17.2 (151) | 14.3 (442) | 1.8 (0.6–5.0) | 1.4 (0.5–4.1) | 2.4 (0.9–6.8) |
| >16.1 | 8.2 (61) | 11.8 (169) | 15.1 (219) | 12.9 (449) | 1.0† | 1.5 (0.5–4.3) | 2.1 (0.8–5.6) |
| All subjects | 14.3 (426) | 12.5 (447) | 18.0 (455) | 15.0 (1328) | — | — | — |
| Current BMI <22.7 |
Current BMI=22.7–26.5 |
Current BMI >26.5 |
All Subjects |
Current BMI <22.7 |
Current BMI=22.7–26.5 |
Current BMI >26.5 |
|
| percent (number of subjects) | |||||||
| <15.0 | 10.1 (188) | 21.2 (151) | 28.3 (106) | 18.2 (445) | 1.5 (0.6–3.6) | 3.3 (1.4–7.8) | 4.6 (1.9–11.2) |
| 15.0–16.1 | 9.9 (152) | 14.0 (157) | 18.2 (143) | 13.9 (452) | 1.4 (0.5–3.5) | 2.0 (0.8–4.9) | 2.6 (1.1–6.2) |
| >16.1 | 7.2 (97) | 7.7(168) | 19.9 (196) | 12.8 (461) | 1.0† | 1.0 (0.4–2.6) | 3.1 (1.3–7.2) |
| All subjects | 9.4 (437) | 14.1 (476) | 21.3 (445) | 14.9 (1358) | — | — | — |
Values for body-mass index were divided into three groups of equal size. The subjects’ current age was 26 to 32 years. Odds ratios are adjusted for sex and current age. CI denotes confidence interval.
This group served as the reference group.
Using the definitions of the International Obesity Task Force,17 we found that only 3.3 percent of the children in whom impaired glucose tolerance or diabetes subsequently developed were over-weight at the age of 12 years, and none were obese at this age. These figures had increased by the age of 16 years to 11.4 percent and 0.5 percent, respectively. A 1-unit increase in the body-mass index at the age of 12 years was associated with a corresponding increase of 1.4 units (95 percent confidence interval, 1.3 to 1.5) at the age of 30 years (correlation coefficient, 0.61).
ADIPOSITY REBOUND
The mean body-mass index at the time of adiposity rebound was similar in subjects in whom impaired glucose tolerance or diabetes developed and subjects whose glucose tolerance remained normal (13.8 and 13.9, respectively), but the mean age was younger (6.3 years, as compared with 6.7 years; P=0.007). Children with the earliest adiposity rebound (five years of age or younger) had the highest body-mass index in later childhood, and this difference persisted into adulthood (Table 4). However, they had the lowest ponderal index at birth, the lowest body-mass index at two years, and a small increase in body-mass index from birth to two years of age (P=0.004). The prevalence of impaired glucose tolerance and diabetes fell with increasing age at the time of adiposity rebound.
Table 4.
Ponderal Index at Birth; Body-Mass Index at the Age of 2 Years, 12 Years, and Currently; and Prevalence of Impaired Glucose Tolerance or Diabetes, According to the Age at the Time of Adiposity Rebound.*
| Age at Adiposity Rebound |
No. of Subjects |
Ponderal Index at Birth |
Body-Mass Index at 2 Yr |
Body-Mass Index at 12 Yr |
Current Body-Mass Index |
Impaired Glucose Tolerance or Diabetes % |
|---|---|---|---|---|---|---|
| 2–5 Yr | 233 | 24.5 | 15.2 | 16.8 | 26.0 | 21.0 |
| 6 Yr | 252 | 24.9 | 15.7 | 16.1 | 25.7 | 13.9 |
| 7 Yr | 363 | 24.6 | 15.8 | 15.7 | 24.7 | 14.6 |
| 8–9 Yr | 395 | 25.0 | 15.6 | 15.0 | 23.4 | 12.2 |
| All subjects | 1243 | 24.8 | 15.6 | 15.7 | 24.8 | 14.9 |
| P value | – | 0.06 | <0.001 | <0.001 | <0.001 | 0.006†‡ |
Data are included for the 1243 subjects whose glucose-tolerance status was known. The ponderal index is equal to 1000 times the weight in grams divided by the cube of the height (or the crown–heel length) in centimeters.
The P value was derived by multiple regression, after adjustment for age and sex.
P=0.05, derived by multiple regression, after adjustment for age, sex, and adult body-mass index.
DISCUSSION
We studied more than 1400 adults who had grown up in the city of Delhi, India, at a time of rapid nutritional transition. Even at the age of 30 years, 15.2 percent had impaired glucose tolerance or diabetes, and 4.4 percent had diabetes. Mean 120-minute plasma glucose concentrations after a standard glucose challenge rose by 17 mg per deciliter (0.96 mmol per liter) between the ages of 26 and 32 years, indicating a sharp deterioration in glucose homeostasis at a relatively young age in adult life. The growth of children in whom impaired glucose tolerance or diabetes later developed was characterized by a low body-mass index between birth and two years of age, a young age at adiposity rebound (as defined by the age after infancy at which the body-mass index starts to rise), and a sustained accelerated gain in body-mass index until adulthood.
The study subjects came from a population of neonates representing all live births within a defined area. Since only 18.7 percent of the original cohort participated in the present study, the subjects may well be unrepresentative of the cohort as a whole. However, the differences in their mean size at birth and in childhood, though statistically significant, were trivial. Our analysis was based on internal comparisons within the study sample and would be biased only if the association between early growth and current glucose or insulin status differed between those who were included in the current study and those who were not. Some of these young adults with diabetes may not have had type 2 diabetes; however, since only one required insulin, the number with type 1 diabetes is likely to be small.
Our study had several strengths. It was population-based; gestational age was assessed prospectively; trained personnel collected anthropometric data at frequent intervals; and the relatively young age of subjects ensured minimal modification as a result of complications of disease or medications. The cohort is unique, in that it represents an urban population of people who grew up during a period of rapid nutritional transition in a developing country and who are now having a rapid loss of glucose homeostasis relatively young in adult life.
The mean insulin concentrations in our cohort would be considered high in whites with similar values for body-mass index in Western countries, but such values have been well described in South Asians.18,19 As in earlier reports,4,8,20 we also found that a small size at birth, defined by a low birth weight or ponderal index, was associated with increased plasma glucose and insulin concentrations and insulin resistance during adulthood. It was not, however, associated with the occurrence of impaired glucose tolerance or diabetes in our study. Given the association between birth weight and both fasting and 120-minute glucose concentrations, the lack of a significant association with disease may be due to a loss of sensitivity resulting in the change from a continuous to a dichotomous variable. Consistent with studies in Hertfordshire, United Kingdom,4 and Helsinki, Finland,21 we found that low weight and thinness at one to two years of age were associated with impaired glucose tolerance and diabetes in adulthood. Children who are thin at two years of age tend to have been thin at birth, though postnatal influences such as infection and feeding practices also contribute.
The children in whom impaired glucose tolerance or diabetes later developed were not over-weight or obese in childhood. They remained below the cohort average for body-mass index until the age of 10 years. At the age of 12 years, only 3.3 percent were overweight according to the current definitions, and none were obese. Instead, they were characterized by their high rate of gain in body mass after the age of two years. We propose that an upward trajectory of body-mass index, starting in early childhood, underlies the current epidemic of diabetes in India.
Our findings are remarkably similar to those in the only Western population with comparable data.21 Among 8760 boys and girls who grew up in Helsinki, Finland, during the Second World War, childhood obesity was uncommon, affecting only 0.4 percent at the age of 12 years, according to International Obesity Task Force definitions.17 The 290 children in that study in whom type 2 diabetes developed in adult life had below-average body size at birth and low weight at one year of age. Thereafter, they had an early adiposity rebound and an accelerated gain in weight and body-mass index, but not height. Their mean body-mass index did not exceed the average for the cohort until around five years of age. Early adiposity rebound was associated with low weight and body-mass index at one year of age. The prevalence of type 2 diabetes fell progressively from 8.6 percent in people whose adiposity rebound occurred before the age of five years to 1.8 percent in those in whom it occurred after seven years.
In conclusion, the young adults in our study who had impaired glucose tolerance or diabetes were, as a group, overweight. They were not, however, overweight as young children but, rather, became overweight as a result of an accelerated gain in body mass starting in early childhood, having been thin in infancy. The ability of children to have an accelerated increase in body mass may be a recent phenomenon in India, a consequence of nutritional transition. Our data do not allow us to distinguish between the events that lead to increasing body-mass index and the expression of the diabetic phenotype. However, assuming that the change in body-mass index is causal rather than the result of a simple association, we speculate that the primary prevention of the epidemic of diabetes in India may require measures to prevent children from crossing into higher categories of body-mass index after the age of two years. Individual children will need to have serial measurements of body-mass index for such a growth trajectory to be identified.
Acknowledgments
Supported by a grant (RG 98001) from the British Heart Foundation. The original cohort studies were supported by the National Center for Health Statistics and the Indian Council of Medical Research.
We are indebted to the men and women and their families who took part in the study, as well as to the field and laboratory staff for their contribution.
REFERENCES
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Pradeepa R, Mohan V. The changing scenario of the diabetes epidemic: implications for India. Indian J Med Res. 2002;116:121–32. [PubMed] [Google Scholar]
- 3.Ramachandran A, Snehalatha C, Latha E, Vijay V, Viswanathan M. Rising prevalence of NIDDM in an urban population in India. Diabetologia. 1997;40:232–7. doi: 10.1007/s001250050668. [DOI] [PubMed] [Google Scholar]
- 4.Hales CN, Barker DJP, Clark PMS, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–22. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rich-Edwards JW, Colditz GA, Stampfer MJ, et al. Birthweight and the risk of type 2 diabetes mellitus in adult women. Ann Intern Med. 1999;130:278–84. doi: 10.7326/0003-4819-130-4_part_1-199902160-00005. [DOI] [PubMed] [Google Scholar]
- 6.Forsen T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C, Barker D. The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med. 2000;133:176–82. doi: 10.7326/0003-4819-133-3-200008010-00008. [DOI] [PubMed] [Google Scholar]
- 7.Delisle H. Programming of chronic disease by impaired fetal nutrition: evidence and implications for policy and intervention strategies. World Health Organization; Geneva: 2002. (WHO/NHD/02.3, WHO/NPH/02.1.) [Google Scholar]
- 8.Newsome CA, Shiell AW, Fall CHD, Phillips DIW, Shier R, Law CM. Is birth weight related to later glucose and insulin metabolism? A systematic review. Diabet Med. 2003;20:339–48. doi: 10.1046/j.1464-5491.2003.00871.x. [DOI] [PubMed] [Google Scholar]
- 9.Sachdev HPS. Recent transitions in anthropometric profile of Indian children: clinical and public health implications. NFI Bull. 2003;24(2):6–8. [Google Scholar]
- 10.Ghosh S, Bhargava SK, Moriyama IM. Longitudinal study of the survival and outcome of a birth cohort. Department of Paediatrics, Safdarjung Hospital; New Delhi, India: 1979. [Google Scholar]
- 11.Physical status: the use and interpretation of anthropometry: report of a WHO expert committee. World Health Organ Tech Rep Ser. 1995;854:329. [PubMed] [Google Scholar]
- 12.The Asia-Pacific perspective: redefining obesity and its treatment. Health Communications Australia; Sydney, Australia: 2000. p. 18. [Google Scholar]
- 13.Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1. Diagnosis and classification of diabetes mellitus. World Health Organization; Geneva: 1999. (WHO/NCD/NCS/99.2.) [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Wareham NJ, Phillips DIW, Byrne CD, Hales CN. The 30-minute insulin incremental response in an oral glucose tolerance test as a measure of insulin secretion. Diabet Med. 1995;12:931. doi: 10.1111/j.1464-5491.1995.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 16.Royston P. Constructing time-specific reference ranges. Stat Med. 1991;10:675–90. doi: 10.1002/sim.4780100502. [DOI] [PubMed] [Google Scholar]
- 17.Cole TJ, Bellizzi MC, Flegal DM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fall CHD, Stein CE, Kumaran K, et al. Size at birth, maternal weight, and type 2 diabetes in South India. Diabet Med. 1998;15:220–7. doi: 10.1002/(SICI)1096-9136(199803)15:3<220::AID-DIA544>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 19.Yajnik CS. The lifecycle effects of nutrition and body size on adult adiposity, diabetes and cardiovascular disease. Obes Rev. 2002;3:217–24. doi: 10.1046/j.1467-789x.2002.00072.x. [DOI] [PubMed] [Google Scholar]
- 20.Barker DJP, Hales CN, Fall CHD, Osmond C, Phipps K, Clark PMS. Type 2 (noninsulin-dependent) diabetes mellitus, hypertension and hyperlipidemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–7. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJP. Early adiposity rebound in childhood and risk of Type 2 diabetes in adult life. Diabetologia. 2003;46:190–4. doi: 10.1007/s00125-002-1012-5. [DOI] [PubMed] [Google Scholar]