India has one of the highest burdens of cardiovascular disease (CVD) worldwide. The annual number of deaths from CVD in India is projected to rise from 2.26 million (1990) to 4.77 million (2020) (1). Coronary heart disease prevalence rates in India have been estimated over the past several decades and have ranged from 1.6% to 7.4% in rural populations and from 1% to 13.2% in urban populations (2).
The INTERHEART study showed that CVD risk factors such as abdominal obesity, hypertension, and diabetes are higher among Indians, even at young ages, than among other ethnic groups (3). The prevalence rates of CVD risk factors have been rapidly rising within India over the past 25 years, particularly within urban communities (4). The reasons for this high burden of risk factors are speculative and have been poorly investigated. In this regard, cohort studies provide unbiased estimates of the relationship of exposure to outcomes, which would increase understanding of the determinants of CVD.
The New Delhi Birth Cohort (5) provides a unique opportunity to evaluate the incidence of CVD risk factors in a young, urban Indian population. We report here the incidence of CVD risk factors in 1,100 members of the cohort across a 7-year period from a mean age of 29 to 36 years and factors at baseline that were associated with increased risk.
Methods
Study cohort
Details of the New Delhi Birth Cohort have been published elsewhere (5). Briefly, 20,755 married women of reproductive age in South Delhi participated in a study of pregnancy outcomes and childhood growth from 1969 to 1972. Over that period, trained personnel recorded the weight and length of the 8,181 newborns within 72 h after birth. The infants were followed at ages 3, 6, 9, and 12 months and thereafter at 6-month intervals until 1973 (end of phase 1). In the first 4 phases, which were conducted during 1969 to 1990, only anthropometric and survey data were collected. In phase 5 (1998 to 2002), data on CVD risk factors were collected to assess their relationship to birth and early life anthropometry (5,6).
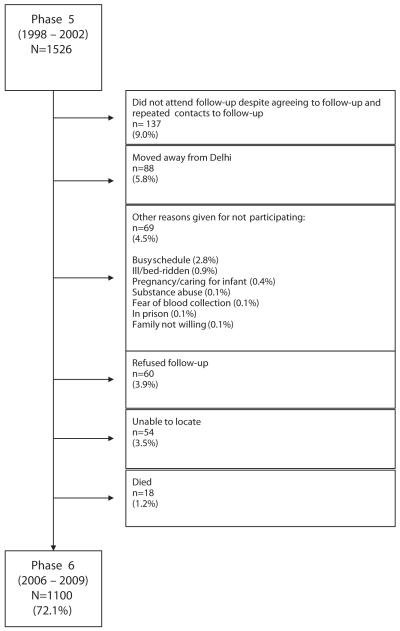
By the fifth phase, the number of participants had decreased to 1,526 owing to attrition, loss to follow-up, and municipal closure of unauthorized housing at the beginning of phase 2 (which began in 1974 and concluded in 1980). Loss to follow-up between phases 5 and 6 was minimized through the following algorithm until all nonresponders were accounted for: telephone calls/e-mails, house visits, meeting with neighbors, and contact with local post offices. Failure to locate a participant occurred for 3.5% of phase 5 participants. Reasons for nonparticipation included not attending follow-up despite agreeing to follow-up (9.0% of phase 5 participants), moving away from New Delhi (5.8%), refusal (3.9%), “busy schedule” (2.8%), and death (1.2%). Other reasons that occurred in fewer than 1% of participants were ill/bedridden (0.9%), pregnancy/caring for infant (0.4%), substance abuse (0.1%), fear of blood collection (0.1%), in prison (0.1%), and family unwilling (0.1%) (Fig. 1).
Figure 1. New Delhi Birth Cohort Flow Diagram.
Flow diagram of reasons for loss to follow-up between phases 5 and 6.
The initial cohort had a literacy rate of 85.1% (national mean 33.7%). Hindus comprised the majority (84.3%), with Sikhs, Christians, Muslims, and Jains contributing to the remainder of the sample (11.6%, 2.1%, 1.1%, and 0.7%, respectively). Although the families had a higher income than the national mean, many lived in 1-room tenements (43%).
The ethics committee from the All India Institute of Medical Sciences approved the study. Informed consent was obtained from each participant.
Current phase of study
In the latest phase (phase 6), from 2006 to 2009, 1,100 (72%) of the phase 5 participants consented to participate. Home visits were performed by trained personnel to obtain informed consent and to record information about the participants’ medical history, family medical history, medication use, material possessions, tobacco use, and alcohol consumption. Study personnel measured the participants’ blood pressure (Omron 7070, Bannockburn, Illinois), weight, height, and waist and hip circumferences according to standardized techniques (6).
Standard glucose tolerance tests (World Health Organization [WHO] protocol) were administered using a 75-g anhydrous glucose load. Plasma glucose concentrations were measured 120 min after the glucose challenge. Fasting plasma glucose, cholesterol, and triglyceride levels were analyzed by enzymatic methods (GOD-PAP, Randox Laboratories Ltd., Crumlin, United Kingdom) with a Beckman auto analyzer; high-density lipoprotein (HDL) cholesterol was measured in the same manner after phosphotungstate precipitation.
Definitions
Education was recorded as one of 7 categories from “no schooling” (category 1) through “professional degree” (e.g., Master’s degree, PhD, medical qualification).
Intake of salt (added to cooked food), fruit, raw vegetables, and cooked vegetables was recorded using a food frequency questionnaire and was divided into 8 frequency categories (never, sometimes, once per month, twice per month, once per week, ≥3 times per week, once daily, and twice daily).
Overweight was defined by using both the WHO criterion (body mass index [BMI] ≥25 kg/m2) and the International Obesity Task Force criterion for Asians (BMI ≥23 kg/m2) (7). Obesity was defined as BMI ≥30 kg/m2, as recommended by WHO (8). Central obesity was defined as a waist circumference ≥90 cm for men and ≥80 cm for women, as recommended for South Asians by the International Diabetes Federation (9).
Pre-hypertension was defined as a systolic blood pressure of 120 to 139 mm Hg or diastolic blood pressure of 80 to 89 mm Hg. Hypertension was defined as a systolic blood pressure of ≥140 mm Hg, a diastolic pressure of ≥90 mm Hg, or being on drug treatment for hypertension.
Metabolic syndrome was defined by the presence of central obesity and at least 2 of the following: 1) triglycerides ≥150 mg/dl (or drug therapy for lipids); 2) HDL cholesterol <40 mg/dl for men and <50 mg/dl for women; 3) blood pressure ≥130/85 mm Hg (or drug therapy for hypertension); and 4) fasting plasma glucose ≥100 mg/dl, per the International Diabetes Federation (9). Diabetes mellitus was defined as a fasting plasma glucose concentration ≥126 mg/dl (7.0 mmol/l) or 120-min concentration of ≥200 mg/dl (11.1 mmol/l). Impaired glucose tolerance (IGT) was defined as a fasting glucose ≥110 mg/dl (6.1 mmol/l) or 120-min concentration of ≥140 mg/dl (7.8 mmol/l) in participants without diabetes (10).
Alcohol consumption was recorded as the frequency of intake and quantity of spirits, beer, and wine per week. These data were converted into units of alcohol (1 U = 25 ml of spirits, 282 ml of beer, or 125 ml of wine) and categorized as none, <7 U, 7 to 14 U, and >14 U/week (6). Tobacco (smoking and nonsmoking) use was defined as never, past, and current users.
Information on material possessions was recorded; participants were given a score of 1 or 0 for each of 17 household items (electricity, fan, cycle, radio, motorized 2-wheeler, gas stove, television, cable television, electric mixer, electric grinder, electric air cooler, washing machine, air conditioner, home computer, TV dish antenna, telephone) and car. This information was used to create a score derived as the first principal component from a correlation matrix of the 17 binary variables (11).
Statistical analysis
Continuous variables are reported as mean ± SD, or if skewed in distribution, as medians with interquartile ranges. Categoric variables are reported as proportions (%). We compared phase 5 measurements between participants and nonparticipants of phase 6 for sensitivity analysis of nonparticipants. We compared continuous variables using paired t tests and categoric variables using McNemar tests for participants seen in both phases 5 and 6. We used multiple logistic regression to assess the determinants of changes in incident binary outcomes between phases 5 and 6. The principal component analysis score was transformed into a Fisher-Yates normal score with a mean of 0 and SD of 1 because it was skewed in distribution.
Results
The mean follow-up period between phases 5 and 6 was 6.9 ± 1.0 years (range 4.0 to 10.2 years). Participants had a mean age of 29 ± 1 years in phase 5 and 36 ± 1 years in phase 6 (Table 1). Ownership of 2-wheeler (81.3%), television (99.6%), and gas stove (98.6%), largely reflecting middle-class status, was common in phase 6 participants. Other material possessions, such as car (45.0%) and home computer (17.5%), were owned by fewer than one-half of respondents.
Table 1.
Continuous Anthropometric and CVD Risk Factor Variables at Phases 5 and 6 of the New Delhi Birth Cohort
| Men |
Women |
|||
|---|---|---|---|---|
| Phase 5 (n = 652) | Phase 6 (n = 652) | Phase 5 (n = 448) | Phase 6 (n = 448) | |
| Age, yrs | 29.1 ± 1.3 | 36.1 ± 1.1 | 29.2 ± 1.3 | 36.1 ± 1.0 |
| BMI, kg/m2 | 24.9 ± 4.2 | 26.7 ± 4.6 | 24.8 ± 5.0 | 27.3 ± 5.2 |
| Waist circumference, cm | 90.3 ± 11.8 | 96.1 ± 11.7 | 80.4 ± 12.1 | 86.8 ± 12.0 |
| Hip circumference, cm | 97.7 ± 8.2 | 100.2 ± 8.2 | 97.4 ± 10.1 | 101.4 ± 10.2 |
| Waist/hip ratio | 0.92 ± 0.06 | 0.96 ± 0.06 | 0.82 ± 0.07 | 0.85 ± 0.07 |
| SBP, mm Hg | 118.8 ± 11.1 | 130.4 ± 14.7 | 106.9 ± 11.1 | 118.7 ± 13.3 |
| DBP, mm Hg | 77.8 ± 10.0 | 82.9 ± 10.7 | 73.7 ± 9.1 | 77.0 ± 9.8 |
| Fasting plasma glucose, mg/dl | 98.8 ± 20.6 | 102.2 ± 25.9 | 96.3 ± 15.2 | 96.3 ± 17.2= |
| 120-min glucose, mg/dl | 107.5 (91.4, 127.1) | 111.5 (96.4, 129.6)* | 111.5 (96.4, 129.6) | 114.0 (99.0, 132.0)* |
| Total cholesterol, mg/dl | 198.4 ± 43.6 | 203.5 ± 40.3 | 185.2 ± 37.2 | 190.8 ± 36.0 |
| Calculated LDL cholesterol, mg/dl | 122.1 ± 35.7 | 122.7 ± 41.2* | 115.8 ± 33.8 | 116.8 ± 32.7* |
| HDL cholesterol, mg/dl | 44.4 ± 11.0 | 46.4 ± 8.6 | 49.0 ± 11.6 | 51.6 ± 10.4 |
| Triglycerides, mg/dl | 131.0 (95.0, 198.0) | 141.5 (105.0, 199.0) | 94.0 (71.0, 122.0) | 99.0 (79.0, 136.0) |
Data are presented as mean ± SD or median values (25th, 75th percentile). All differences for within-sex comparisons between participants seen in phases 5 and 6 are statistically significant, unless noted (*).
BMI = body mass index; CVD = cardiovascular disease; DBP = diastolic blood pressure; HDL = high-density lipoprotein; LDL = low-density lipoprotein; SBP = systolic blood pressure.
Sensitivity analysis
There were no significant differences between phase 6 participants and nonparticipants for the following phase 5 variables: sex, BMI, hip circumference, systolic blood pressure, diastolic blood pressure, fasting and post-load glucose, and low-density lipoprotein (LDL) cholesterol or in the proportions of overweight, obesity, hypertension, IGT, and diabetes. However, we observed certain differences: as compared with phase 6 male nonparticipants, phase 5 age was 0.3 years younger (p = 0.005) and HDL cholesterol (p = 0.027) was 2.0 mg/dl lower in participants; as compared with phase 6 female nonparticipants, phase 5 waist circumference, waist-to-hip ratio (WHR), and triglycerides were 2.7 cm (p = 0.014), 0.16 (p = 0.015), and 6.3 mg/dl (p = 0.04) lower in participants.
Anthropometry
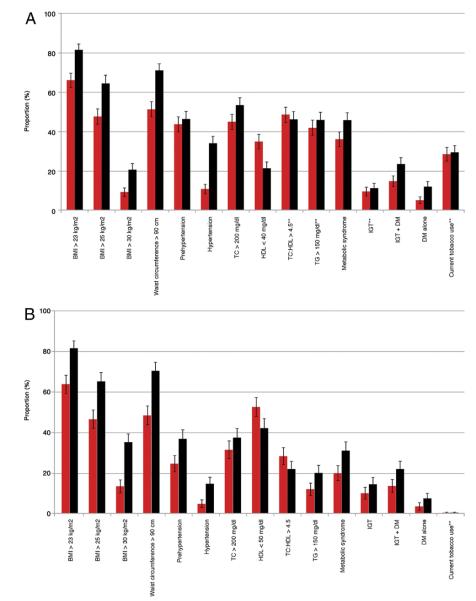
The annual incidence of obesity was 2.0% (95% confidence interval [CI]: 1.6% to 2.4%) for men and 2.2% (95% CI: 1.7% to 2.8%) for women (Table 2). Mean BMI, waist circumference, hip circumference, and WHR (Table 1) and the prevalence of overweight, obesity, and central obesity (Figs. 2A and 2B) all increased significantly in both men and women between phases 5 and 6 (p < 0.05). Mean BMI increased from 25 to 27 kg/m2 in both men and women (Table 1), resulting in an increase in obesity from 9% to 21% in men and from 13% to 25% in women (Figs. 2A and 2B). Waist circumference rose from a mean of 90 to 96 cm in men and from 80 to 87 cm in women (Table 1), resulting in an increase in central obesity from 52% to 71% in men and from 48% to 70% in women (Figs. 2A and 2B).
Table 2.
Incidence Rates of Obesity, Hypertension, and Diabetes Over 6.9 Years of Follow-Up in the New Delhi Birth Cohort
| Incident Cases/ At-Risk Sample |
Annual Incidence (Incidence/6.9 Yrs) |
|
|---|---|---|
| Obesity | ||
| Men | 82 of 588 | 2.0% (95% CI: 1.6%–2.4%) |
| Women | 60 of 386 | 2.2% (95% CI: 1.7%–2.8%) |
| Hypertension | ||
| Men | 168 of 574 | 4.2% (95% CI: 3.7%–4.8%) |
| Women | 52 of 419 | 1.8% (95% CI: 1.3%–2.3%) |
| Diabetes | ||
| Men | 43 of 595 | 1.0% (95% CI: 0.8%–1.4%) |
| Women | 15 of 412 | 0.5% (95% CI: 0.3%–0.9%) |
CI = confidence interval.
Figure 2. New Delhi Birth Cohort CVD Risk Factors.
(A) Categoric anthropometric and cardiovascular disease (CVD) risk factor variable prevalence (with 95% confidence intervals [CIs]) at phases 5 (red bars) and 6 (black bars) in men from the New Delhi Birth Cohort. All differences for within-sex comparisons between participants seen in phases 5 and 6 are statistically significant unless noted (**). (B) Categoric anthropometric and CVD risk factor variable prevalence (with 95% CIs) at phases 5 and 6 in women from the New Delhi Birth Cohort. All differences for within-sex comparisons between participants seen in phases 5 and 6 are statistically significant unless noted (**). BMI = body mass index; DM = diabetes mellitus; HDL = high-density lipoprotein cholesterol; IGT = impaired glucose tolerance; TC = total cholesterol; TG = triglycerides.
After controlling for age and sex, multivariate logistic regression analysis demonstrated significant positive associations between phase 5 BMI, waist circumference, WHR, socioeconomic status (by principal component analysis z-score), total cholesterol, and triglycerides and incident obesity (Table 3). There was a significant inverse association with tobacco use.
Table 3.
Multivariate Logistic Regression Analyses for Predictors of Obesity, Hypertension, and Diabetes at Phase 6
| Phase 5 Variable (Unit) | Phase 6 Obesity (BMI ≥30 kg/m2) | Phase 6 Hypertension (BP ≥140/90 mm Hg) | Phase 6 Diabetes Mellitus |
|---|---|---|---|
| BMI (1 kg/m2) | 2.07 (1.84–2.34)* | 1.10 (1.06–1.14)* | 1.12 (1.06–1.18)* |
| Waist circumference (1 cm) | 1.18 (1.15–1.22)* | 1.04 (1.03–1.06)* | 1.04 (1.02–1.06)* |
| Waist/hip ratio (0.01) | 1.13 (1.10–1.17)* | 1.05 (1.02–1.07)* | 1.09 (1.04–1.13)* |
| Alcohol† | 0.84 (0.67–1.06) | 1.41 (1.20–1.65)* | 1.00 (0.75–1.33) |
| Tobacco use (never, former, current) | 0.73 (0.54–0.97)* | 1.20 (0.99–1.45) | 1.00 (0.71–1.42) |
| SES (possessions)‡ | 1.23 (1.02–1.48)* | 1.07 (0.92–1.26) | 0.97 (0.74–1.28) |
| SES (education)§ | 1.10 (0.95–1.28) | 0.99 (0.88–1.12) | 0.92 (0.75–1.12) |
| Add salt|| | 0.94 (0.84–1.04) | 1.02 (0.95–1.11) | 1.00 (0.87–1.16) |
| Fresh fruit|| | 1.06 (0.95–1.17) | 1.01 (0.93–1.10) | 0.93 (0.82–1.07) |
| Raw vegetables|| | 1.09 (0.99–1.21) | 1.06 (0.98–1.15) | 0.98 (0.86–1.12) |
| Cooked vegetables|| | 1.10 (0.97–1.23) | 1.00 (0.91–1.10) | 0.93 (0.80–1.09) |
| Total cholesterol (50 mg/dl) | 1.23 (1.00–1.52)* | 1.44 (1.19–1.75)* | 1.40 (1.04–1.89)* |
| LDL cholesterol (10 mg/dl) | 1.04 (0.99–1.09) | 1.05 (1.00–1.09)* | 1.04 (0.96–1.12) |
| HDL cholesterol (10 mg/dl) | 0.97 (0.82–1.15) | 1.02 (0.89–1.18) | 0.89 (0.69–1.14) |
| Triglycerides (log) | 1.61 (1.12–2.32)* | 2.26 (1.64–3.12)* | 2.87 (1.72–4.79)* |
Data are presented as odds ratio (95% confidence interval). Each cell represents a separate regression model. All analyses were adjusted for age at baseline, difference in age between phases 5 and 6, and sex.
p < 0.05.
Lifestyle factors:
alcohol consumption (4 levels from none to heavy based on number of units per week), tobacco use (categorized into never, ex-user, and current user),
socioeconomic status (SES) in adult life derived from possessions (normalized score based on principal components analysis),
SES in adult life based on education (7 levels from no schooling to professional degree),
food intake (8 levels from none to twice daily), and family history of any of high blood pressure, angina, myocardial infarction, stroke, or diabetes in a first-degree relative.
Abbreviations as in Table 1.
Hypertension
The annual incidence of hypertension was 4.2% (95% CI: 3.7% to 4.8%) for men and 1.8% (95% CI: 1.3% to 2.3%) for women (Table 2). Mean systolic blood pressure rose from 119 to 130 mm Hg in men and from 107 to 119 mm Hg in women (Table 1). There were similar increases in diastolic pressure. The prevalence of hypertension rose from 11% to 34% in men and from 5% to 15% in women (Figs. 2A and 2B). All of these changes were statistically significant. Antihypertensive drug therapy was reported in 6% of phase 6 respondents. After controlling for age and sex, multivariate logistic regression analysis demonstrated significant positive associations between phase 5 BMI, waist circumference, WHR, alcohol use, family history of hypertension, total cholesterol, and triglycerides and incident hypertension (Table 3).
IGT and diabetes mellitus
The annual incidence for diabetes was 1.0% (95% CI: 0.8% to 1.4%) for men and 0.5% (95% CI: 0.3% to 0.9%) for women (Table 2). Mean fasting glucose concentration increased significantly in men but not in women (Table 1), whereas 120-min glucose concentrations did not increase in men or women. There was a significant increase in the prevalence of IGT in women between phases 5 and 6, rising from 10% to 14% (Fig. 2B), whereas the prevalence of diabetes doubled (rising from 5% to 12% in men and from 3.5% to 7% in women). Antidiabetic drug therapy was reported in only 1.5% of phase 6 respondents.
After controlling for age and sex, multivariate logistic regression analysis demonstrated significant positive associations between phase 5 BMI, waist circumference, WHR, total cholesterol, and triglycerides and incident diabetes (Table 3).
Total, LDL, and HDL cholesterol and triglycerides
Total cholesterol increased by 5.1 mg/dl in men (p < 0.05) and 5.6 mg/dl in women (p < 0.05). The LDL cholesterol did not change in men or women, and HDL cholesterol increased by 2.0 mg/dl in men and 2.6 mg/dl in women (p < 0.05). Triglycerides increased by 10.5 mg/dl in men and 5.0 mg/dl in women (p < 0.05) (Table 1). However, lipid-lowering therapy was reported in 1.2% of phase 6 respondents.
Discussion
These are among the first cohort data to evaluate the incidence of CVD risk factors in India. Both the incidence and prevalence of risk factors are high at a young age in this urban Indian population that is rapidly transitioning. The incidence rate of obesity was higher in women compared with men in the New Delhi Birth Cohort, but the incidence rates of hypertension and diabetes were higher in men. Central obesity, as measured by waist circumference, appeared to be increasing in both men and women, with a commensurate increase in WHR. Comparing phase 5 and phase 6, the overall prevalence of diabetes is increasing in both men and women.
Participants in the New Delhi Birth Cohort were in the middle to upper range of education and income relative to other people within India. At the time the cohort started, the majority lived in 1 room, which is an indication of the difference in living standards between high-income countries and India at the time. This cohort appeared to remain in the same class from 1969 to 2009 based upon a principal component analysis of material possessions, which estimates household socioeconomic position. However, compared with respondents in the National Family Health Survey in India, the New Delhi Birth Cohort participants represent the 2 highest wealth quintiles in India (4,11,12). The participants live in much more spacious dwellings now, and it is an indication of the transitions that have occurred in India over the last 50 years that participants would be more recognizably “middle class” in terms of their living standards to a Western observer. Although our data may not necessarily be extrapolated to other parts and other ages in India, especially rural India, this rapid increase in CVD risk factors may reflect the huge transitions in wealth and lifestyle occurring in other fast-growing cities in low- and middle-income countries.
These high rates impose a huge economic burden on the country as well as upon individuals. For example, the potential economic burden of increased diabetes prevalence in India is considerable because estimated annual costs for diabetes care (approximately 5,000 rupees in 2005 [13]) are rising and now range from 5% to 34% of personal income in India (14). Lower-income groups spend a greater proportion of their income on diabetes care than higher-income groups, further aggravating disparities that impact the social determinants of health.
Comparison with other Indian populations
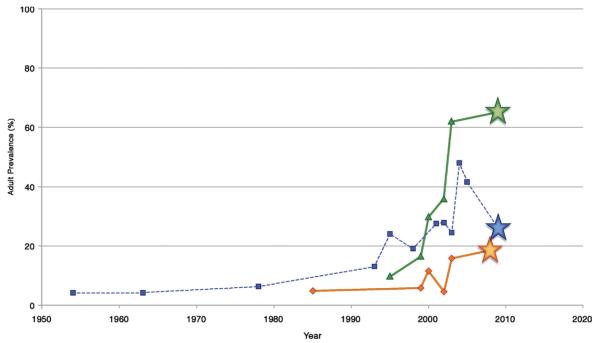
Figure 3 compares the prevalence rates of overweight, hypertension, and diabetes in phase 6 of the New Delhi Birth Cohort with other prevalence studies in urban India for which the participants would be eligible, as described in a WHO India-sponsored systematic review on chronic diseases (4). All 3 curves increased over time, particularly after 2000. Our data represent the leading edge of the CVD risk factor burden in young, urban India.
Figure 3. Risk Factor Trends in India.
Temporal trends of the prevalence of overweight (green line), hypertension (blue line), and diabetes (orange line) in urban India (diagnosis based on laboratory testing). Each star represents the New Delhi Birth Cohort prevalence data during phase 6. Data from World Health Organization (WHO) India (4).
Extrapolation of risk factor trends in urban India also provide context for our data. Secular trends of overweight (BMI >25 kg/m2) and hypertension prevalence from the Jaipur Heart Watch between 1993 and 2005 demonstrated a significant increase in the prevalence of both risk factors (15,16). Overweight prevalence in adults 30 to 39 years increased from 20.7% to 33% in men and from 19.9% to 39.4% in women over 12 years of follow-up, an annual increase in prevalence of 0.9% and 1.5%, respectively. If these increases continued at the same rate from 2005 to 2009 (end of follow-up for phase 6 of the New Delhi Birth Cohort), the obesity prevalence rates in the Jaipur Heart Watch would be 36.6% for men and 43.4% for women (compared with 64.7% and 65.1% in the New Delhi Birth Cohort).
The overall adult prevalence of hypertension in the Jaipur Heart Watch increased from 20.4% to 41.4% in men and from 15.2% to 34.9% in women over 12 years of follow-up, an annual increase in prevalence of 1.8% in men and 1.6% in women (15). If these increases continued at the same rate from 2005 to 2009 (end of follow-up for phase 6 of the New Delhi Birth Cohort), the hypertension prevalence rates in the Jaipur Heart Watch would be 48.6% for men and 41.5% for women (compared with 34% and 15% in the New Delhi Birth Cohort).
In the Chennai Urban Population Study (1997 to 2005), the prevalence rate of diabetes increased from 12% to 18.3% over 8 years (0.8% annual incidence); however, this cohort had a 47% loss to follow-up (17). If this increase continued at the same rate from 2005 to 2009 (end of follow-up for phase 6 of the New Delhi Birth Cohort), the diabetes prevalence rates in the Chennai Urban Population Study would be 20.7% (compared with 8.4% in the New Delhi Birth Cohort). Similar estimates have been reported through secular trends from another study in southern India (18), although our participants were 4 to 5 years younger.
Ramachandran et al. (19) recently reported an overall increase in the prevalence of diabetes in urban India from 5% (1985) to 18.6% (2006). Another report by Chan et al. (20) estimated an increase in diabetes prevalence from 3% (1979) to 7.3% (2005) throughout urban India; however, the 2005 estimates were based upon self-reporting (21).
Reasons for CVD risk factor increases
The causes of these incidence rates of CVD risk factors are complex but likely include lifestyle changes associated with urbanization and the epidemiologic and nutritional transitions that accompany economic development (22). We speculate that increased urban and socioeconomic development in New Delhi over the duration of this cohort was broadly associated with increased caloric intake (particularly of energy-dense foods) and decreased physical activity.
The high rates of metabolic disturbances—central obesity, hypertension, dyslipidemia, IGT, and diabetes mellitus—may also be related to abnormal life-course events, such as maternal undernutrition, low birth weight, and subsequent adiposity rebound (5). Lower weight in infancy, and greater childhood and adolescent BMI or BMI gain have previously been shown to be associated with an increased risk of adult hypertension, IGT, and diabetes in this cohort (23).
Study strengths/limitations
This cohort provides prospective incidence data of key CVD risk factors such as obesity, hypertension, and diabetes in India. Standardized collection of questionnaire, anthropometric, and blood pressure data are strengths of this cohort, as well as the longitudinal follow-up since 1969. The main strengths of the study were the standardized anthropometric and biochemical risk factor measurements and detailed characterization of lifestyle factors, recorded using identical methods at both time points.
However, there are important limitations to these findings. The sample size was fairly small with a relatively narrow age range, which limits the generalizability of our findings. The cohort is composed of participants originally from South Delhi, an upper middle-class urban neighborhood, which cannot represent all the different populations of India, particularly rural populations. There were minor differences in selected phase 5 risk factors between participants and nonparticipants, which may have biased the results. However, these differences may lead to underestimates of risk factor incidence. Finally, these data reflect incidence for CVD risk factors, which may or may not lead to CVD events; however, risk factors such as diabetes confer risk for other competing problems such as blindness and renal failure.
Implications
The high incident rates of obesity, hypertension, and diabetes in this young, urban Indian cohort are likely to lead to a high burden of CVD in this population in the future. The remarkable changes in prevalence rates of these risk factors over such a short span of time in this young, urban cohort could have implications for the use of appropriate risk screening and intervention strategies beginning at younger ages. We believe that a life-course approach should be emphasized, specifically one that includes health promotion during childhood and adolescence, primary prevention for individuals with CVD risk factors, and secondary prevention for those with established coronary heart disease and stroke.
Acknowledgments
The authors thank Ms. Rajeshwari Verma, Mr. Bhaskar Singh, Dr. K. D. Gupta, Dr. Dinesh Mishra, Ms. Arti Mishra, and Mr. Dileep Gupta for their assistance. The authors are also grateful to Professor George Davey-Smith for helpful comments on previous versions of this manuscript.
Please note: The original cohort studies were supported by the National Centre for Health Statistics and the Indian Council of Medical Research. The study sponsor did not participate in the study design; collection, analysis, and interpretation of data; writing of the report; nor decision to submit the report for publication. Dr. Huffman is supported by the NIH Fogarty International Clinical Research Fellowship (R24TW007988). Dr. Prabhakaran receives research support from the NIH Fogarty International Center; National Heart, Lung and Blood Institute; UnitedHealth; Wellcome Trust; Canadian Institute of Health Research; Indian Council of Medical Research, Department of Science and Technology (Government of India); and Duke Clinical Research Institute.
Footnotes
The authors have reported that they have no relationships to disclose.
REFERENCES
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R, Joshi P, Mohan V, Reddy KS, Yusuf S. Epidemiology and causation of coronary heart disease and stroke in India. Heart. 2008;94:16–26. doi: 10.1136/hrt.2007.132951. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) India [Accessed April 29, 2010];National cardiovascular disease database. Available at: http://www.whoindia.org/LinkFiles/NMH_Resources_National_CVD_database-Final_Report.pdf.
- 5.Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350:865–75. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sachdev HS, Fall CH, Osmond C, et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–66. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- 7.International Obesity Task Force/WHO . The Asian-Pacific perspective: redefining obesity and its treatment. Health Communications Australia; Sydney, Australia: 2000. [Google Scholar]
- 8.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 9.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 10.WHO . Report of a WHO Consultation. WHO; Geneva: 1999. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. [Google Scholar]
- 11.Rutstein SO, Johnson K. DHS comparative reports 6. ORC Macro; Calverton, MD: 2004. The DHS Wealth Index. [Google Scholar]
- 12.International Institute for Population Sciences and Macro International . National Family Health Survey (NFHS-3), 2005–06. International Institute for Population Sciences; Mumbai, India: [Google Scholar]
- 13.Report of the National Commission on Macroeconomics and Health. Ministry of Health and Family Welfare; New Delhi, India: 2005. [Google Scholar]
- 14.Ramachandran A, Ramachandran S, Snehalatha C, et al. Increasing expenditure on health care incurred by diabetic subjects in a developing country: a study from India. Diabetes Care. 2007;30:252–6. doi: 10.2337/dc06-0144. [DOI] [PubMed] [Google Scholar]
- 15.Gupta R, Gupta VP. Hypertension epidemiology in India: lessons from Jaipur Heart Watch. Curr Sci. 2009;97:349–55. [Google Scholar]
- 16.Gupta R, Misra A, Vikram NK, et al. Younger age of escalation of cardiovascular risk factors in Asian Indian subjects. BMC Cardiovasc Disord. 2009;9:28. doi: 10.1186/1471-2261-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohan V, Deepa M, Anjana RM, Lanthorn H, Deepa R. Incidence of diabetes and pre-diabetes in a selected urban south Indian population (CUPS-19) J Assoc Physicians India. 2008;56:152–7. [PubMed] [Google Scholar]
- 18.Ramachandran A, Mary S, Yamuna A, Murugesan N, Snehalatha C. High prevalence of diabetes and cardiovascular risk factors associated with urbanization in India. Diabetes Care. 2008;31:893–8. doi: 10.2337/dc07-1207. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375:408–18. doi: 10.1016/S0140-6736(09)60937-5. [DOI] [PubMed] [Google Scholar]
- 20.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–40. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 21.Mohan V, Mathur P, Deepa R, et al. Urban rural differences in prevalence of self-reported diabetes in India—the WHO-ICMR Indian NCD risk factor surveillance. Diabetes Res Clin Pract. 2008;80:159–68. doi: 10.1016/j.diabres.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Omran AR. The epidemiologic transition. A theory of the epidemiology of population change. Milbank Mem Fund Q. 1971;49:509–38. [PubMed] [Google Scholar]
- 23.Fall CH, Sachdev HS, Osmond C, et al. Adult metabolic syndrome and impaired glucose tolerance are associated with different patterns of BMI gain during infancy: data from the New Delhi Birth Cohort. Diabetes Care. 2008;31:2349–56. doi: 10.2337/dc08-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]