SUMMARY
The phosphatidylinositol 3-kinase (PI3K)/Akt pathway is important for tissue proliferation. Previously, we found that tissue regeneration after partial pancreatic resection was markedly attenuated in aged mice as compared to young mice and that this attenuation was due to an age-dependent reduction of PI3K/Akt signaling in the pancreatic acini; however, the mechanisms for the age-associated decline of pancreatic PI3K/Akt signaling remained unknown. To better delineate the mechanisms for the decreased PI3K/Akt activation with aging, age-associated changes in cell proliferation and PI3K/Akt signaling were investigated in the present study using in vitro primary pancreatic acinar cell cultures derived from young and aged mice. In response to treatment with insulin-like growth factor 1 (IGF-1), acinar cells from young but not aged mice showed increased activation of PI3K/Akt signaling and cell proliferation, indicating that intrinsic cellular mechanisms cause the age-associated changes in pancreatic acinar cells. We also found that the expression of PI3K p85α subunit, but not IGF-1 receptor or other PI3K subunits, was significantly reduced in pancreatic acinar cells from aged mice; this age-associated reduction of p85α was confirmed in both mouse and human pancreatic tissues. Finally, siRNA-mediated knockdown of p85α expression in acinar cells from young mice resulted in markedly attenuated activation of PI3K/Akt downstream signaling in response to IGF-1. From these results, we conclude that exocrine pancreatic expression of PI3K p85α subunit is attenuated by aging, which is likely responsible for the age-associated decrease in activation of pancreatic PI3K signaling and acinar cell proliferation in response to growth promoting stimuli.
Keywords: Aging, Cell proliferation, Pancreatic acinar cells, PI3K
INTRODUCTION
Phosphatidylinositol 3-kinase (PI3K) comprises a large and complex family that includes 3 classes with multiple subunits and isoforms (Fruman et al. 1998; Vanhaesebroeck & Waterfield 1999). The Class I PI3Ks are composed of an 85-kDa regulatory subunit (p85) and a 110-kDa catalytic subunit (p110) (Cantley 2002). PI3K catalyzes the production of phosphatidylinositol-3, 4, 5-triphosphate (PIP3). PIP3 recruits a subset of signaling proteins, such as the protein serine-threonine kinase Akt (also known as protein kinase B [PKB]), to the membrane where they are activated by phosphorylation. Phosphorylated Akt (p-Akt) in turn promotes phosphorylation of downstream proteins (such as glycogen synthase kinase 3β [GSK3β], mammalian target of rapamycin [mTOR], and p70S6 kinase [p70S6K]) that affect cell growth, cell cycle distribution, apoptosis, and survival (Vanhaesebroeck et al. 2001; Cantley 2002). Previously, we showed that the PI3K/Akt pathway plays a critical role in the regulation of intestinal cell proliferation and colon cancer cell differentiation (Wang et al. 2001; Sheng et al. 2003; Shao et al. 2004).
Insulin-like growth factor 1 (IGF-1) is a potent stimulator of the PI3K/Akt pathway (Sanchez-Margalet et al. 1995; Ludwig et al. 1999). IGF-1 binds to the type 1 IGF-1 receptor (IGF-1R) (Sanchez-Margalet et al. 1995; Baserga et al. 1997; Unger & Betz 1998) and induces its intrinsic tyrosine kinase activity that, in turn, phosphorylates members of the insulin receptor substrate (IRS) family and leads to PI3K-dependent downstream activation (Pollak et al. 2004). Both protein and mRNA levels of IGF-1 increase in the proliferating remnant pancreas shortly after partial pancreatectomy (Px), suggesting an important role for IGF-1 in pancreatic regeneration (Smith et al. 1991; Hayakawa et al. 1996; Calvo et al. 1997). Indeed, we previously demonstrated that stimulation with IGF-1 induced cell proliferation and Akt phosphorylation in cultured pancreatic acinar cells from young adult mice (Watanabe et al. 2005). We also showed that Akt phosphorylation was significantly increased in the remnant pancreas of young adult mice after partial Px. Treatment of mice after partial Px with the PI3K inhibitor wortmannin or small interfering RNA (siRNA) directed to the PI3K p85α subunit completely blocked both Akt phosphorylation and tissue regeneration of the remnant pancreas, suggesting that Akt activation is essential for pancreatic tissue growth (Watanabe et al. 2005).
We and others have shown that aging alters physiological function, secretion and motility of the gastrointestinal tract and the pancreas (Evers et al. 1994; Majumdar et al. 1997). Both endocrine and exocrine pancreatic secretions decrease with aging (Khalil et al. 1985; Elahi et al. 2002). Pancreatic growth is also attenuated by aging; the trophic response to the cholecystokinin (CCK) analogue caerulein in aged rats is decreased compared to young rats (Greenberg et al. 1988). We previously demonstrated that aging is associated with significantly decreased pancreatic regeneration after partial Px (Watanabe et al. 2005). In the same study, phosphorylation of Akt, which was increased in acinar cells of the remnant pancreas of young mice after partial Px, was not observed in aged mice, suggesting that this age-dependent absence of Akt phosphorylation may explain, in part, the loss of tissue regeneration with age (Watanabe et al. 2005). However, the mechanisms for this age-dependent suppression of Akt phosphorylation in the remnant pancreas remain unclear.
In the present study, we sought to determine whether the suppression of Akt phosphorylation in the pancreas of aged mice is caused by an age-dependent loss of responsiveness to growth factor(s). We demonstrate that, unlike pancreatic acinar cells from young mice, acinar cells from aged mice do not exhibit Akt activation or increased cell proliferation in response to IGF-1 treatment in vitro. We further demonstrate significant age-associated reduction of the PI3K regulatory subunit, p85α, in pancreatic acinar cells of both mouse and humans, which appears to be causally linked to suppression of PI3K/Akt signaling associated with old age.
RESULTS
IGF-1-Induced Pancreatic Acinar Cell Proliferation Is Lost with Aging
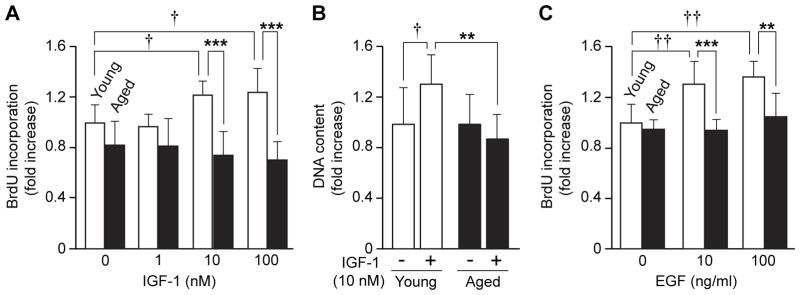
To determine the effect of aging on pancreatic acinar cell proliferation, acinar cells were isolated from young and aged mice, cultured in vitro, and cell proliferation was assessed after stimulation with various concentrations of IGF-1 (1–100 nM). BrdU incorporation in acinar cells from young mice was increased an average of 22% by 10 nM (p=0.023) and 25% by 100 nM IGF-1 (p=0.012) (Fig. 1A). In marked contrast, BrdU incorporation in cells from aged mice was not increased by IGF-1 treatment. Upon stimulation with IGF-1 (10 nM), total DNA content was increased 32% in cells from young mice as compared to PBS-treated controls (p=0.013); however, IGF-1 treatment did not increase DNA content of acinar cells from aged mice (Fig. 1B). To confirm the age-associated decrease in cell proliferation, acinar cells from young and aged mice were stimulated with EGF (10 and 100 ng/mL), another PI3K/Akt stimulator, and BrdU incorporation was compared. BrdU incorporation in acinar cells from young mice was significantly increased 31% with 10 ng/ml (p=0.05) and 36% with 100 ng/ml (p=0.001) of EGF as compared with untreated controls; BrdU incorporation in cells from aged mice was not increased after EGF treatment (Fig. 1C). These results demonstrate that pancreatic acinar cell proliferation in response to growth factors is lost with aging.
Figure 1. Age-associated loss of pancreatic acinar cell proliferation in response to IGF-1 and EGF.
(A) BrdU incorporation in pancreatic acinar cells after IGF-1 treatment was compared. Isolated pancreatic acinar cells were treated with various doses of IGF-1 for 16 h. and BrdU incorporation was determined by ELISA. (B) Effects of IGF-1 treatment (10 nM, 16 h) on DNA content in acinar cells from young and aged mice were compared. (C) EGF-induced BrdU incorporations in pancreatic acinar cells from young and aged mice were compared. BrdU incorporation values were normalized by total cellular DNA content of cells at 0 h (for (A) and (C)). Values are mean ± standard deviation (SD); n=4. **p<0.01 and ***p<0.001 comparing aged with young mice. †p<0.05 and ††p<0.01 comparing IGF-1 with control PBS treatment.
IGF-1-Induced PI3K/Akt Signaling Pathway in Pancreatic Acinar Cells Is Suppressed by Aging
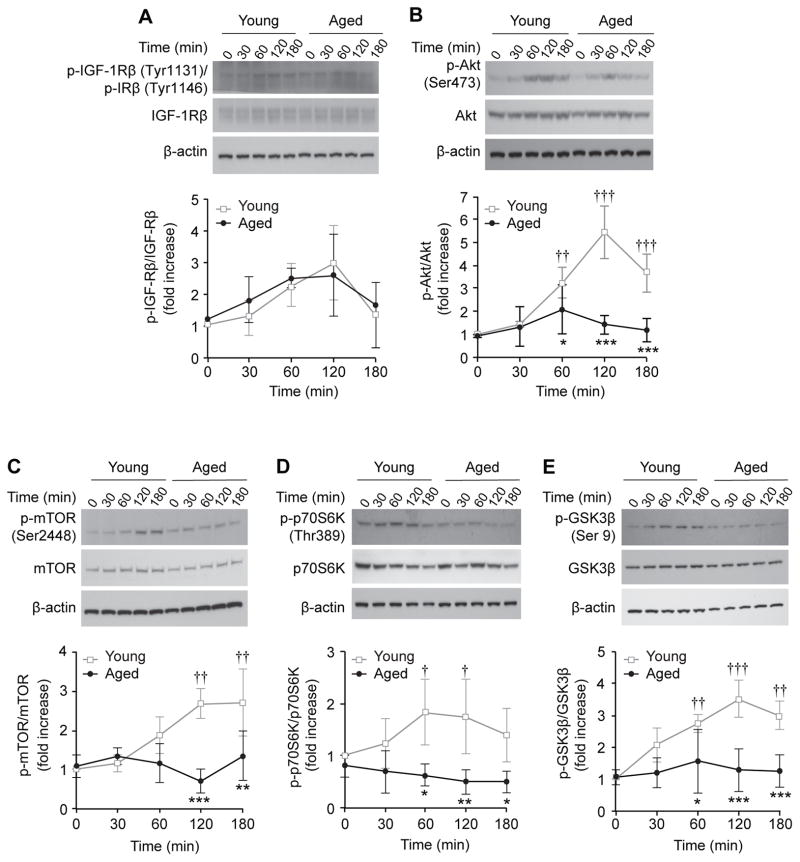
We next examined the effects of aging on activation of the IGF-1/PI3K/Akt pathway in acinar cells. For this study, we assessed IGF-1-induced phosphorylation of IGF-1Rβ, Akt, and downstream signaling factors mTOR, p70S6K, and GSK3β, in acinar cells from young and aged mice. Since IGF-1Rβ and insulin receptor β (IRβ) have significant homology around their tyrosine residues, antibodies used for our Western blot analysis recognized phosphorylation of both receptors (p-IGF-1Rβ and p- IRβ). Phosphorylation of IGF-1Rβ/IRβ appeared to be modestly increased in acinar cells from both young and aged mice 30–120 min after IGF-1 treatment although statistical significance was not obtained; there was no significant age-associated difference in the levels of phosphorylated IGF-1Rβ/IRβ in the acinar cells. Total protein levels of IGF-1Rβ did not show changes by aging or IGF-1 treatment (Fig. 2A). In addition, total protein levels of IRβ also showed no changes by aging or IGF-1 treatment (Fig. S1). By 2 h after IGF-1 treatment, phosphorylated Akt (p-Akt) was increased 5.4-fold in acinar cells derived from young mice (p<0.001); however, p-Akt was not induced in acinar cells from aged mice. The age-associated difference was statistically significant at 60, 120 and 180 min after IGF-1 treatment (p<0.05, Fig. 2B). In addition, phosphorylation of mTOR, p70S6K and GSK3β was significantly increased by 120 min after IGF-1 treatment in acinar cells from young mice. In contrast, the IGF-1 mediated phosphorylation of these proteins was not observed on cells from aged mice; there was a significant age-associated difference in phosphorylation of these proteins at 120 to 180 min after IGF-1 treatment (Fig. 2C–E). These findings demonstrate that aging suppresses IGF-1-induced activation of Akt and its downstream factors in pancreatic acinar cells without affecting receptor activation.
Figure 2. Age-associated decreases in IGF-1-mediated PI3K/Akt pathway in pancreatic acinar cells.
IGF-1-mediated activation of IGF-1Rβ and PI3K/Akt pathway components in pancreatic acinar cells from young and aged mice were compared. Phosphorylation of (A) IGF-1Rβ (B) Akt, (C) mTOR, (D) p70S6K, and (E) GSK3β in pancreatic acinar cells from young and aged mice after IGF-1(10 nM) stimulation were assessed by Western blot analysis and densitometric analysis. Each membrane was re-probed for corresponding total protein for normalization. Values are mean ± SD; n=3. *p<0.05, **p<0.01 and ***p<0.001 comparing acinar cells from aged versus young mice. †p<0.05, ††p<0.01 and †††p<0.001 comparing treated with non-treatment control (0 min) in the same age group.
Expression of the PI3K p85α Subunit in Pancreatic Acinar Cells Is Significantly Reduced with Aging
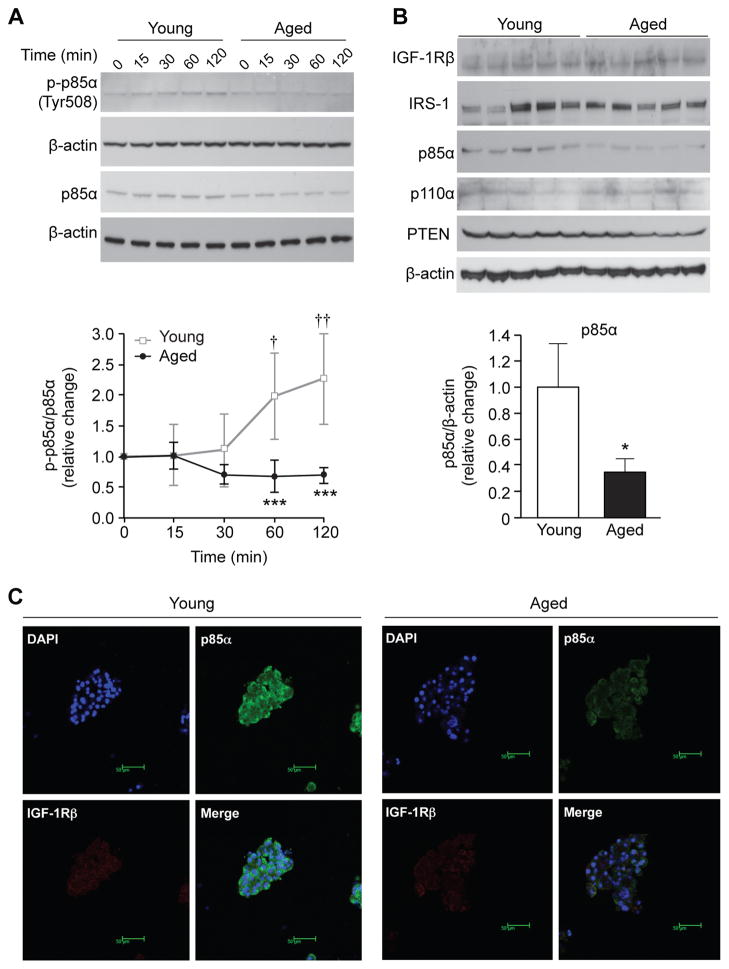
To further investigate the age-associated difference in IGF-1/PI3K/Akt activation, we examined IGF-1-induced phosphorylation of the PI3K p85α subunit in acinar cells. After IGF-1 stimulation, phosphorylation of p85α was significantly increased in acinar cells from young mice (2.0- and 2.3-fold at 60 and 120 min, respectively). In contrast, acinar cells from aged mice did not show IGF-1-induced phosphorylation of p85α; p-p85α levels remained significantly lower as compared to acinar cells from young mice (p<0.001, Fig. 3A). Furthermore, total protein levels of p85α in untreated acinar cells from aged mice were significantly lower (approximately 35%, p=0.019) than that from young mice (Fig. 3B). There was no age-associated difference in the expression of IGF-1Rβ, IRS-1, p110α subunit, and PTEN in acinar cells. Immunofluorescence staining further confirmed age-associated reduction of the p85α subunit in pancreatic acinar cells (Fig. 3C).
Figure 3. Age-associated decrease in PI3Kp85α subunit in pancreatic acinar cells in vitro.
(A) IGF-1-mediated phosphorylation of PI3Kp85α in pancreatic acinar cells from young and aged mice was compared by Western blot analysis and densitometric analysis. Acinar cells from young and aged mice were treated with IGF-1 (10 nM) and expression of phosphorylated PI3Kp85α (p-p85α) was assessed. Levels of p-p85α were normalized by p85α density. Values are mean ± SD; n=3. ***p<0.001 comparing acinar cells from aged versus young mice. †p<0.05 and ††p<0.01 comparing IGF-1 versus non-treatment control (0 min). (B) The protein levels of IGF-1Rβ, IRS-1, p85α, p110α, and PTEN in untreated isolated pancreatic acinar cells from young and aged mice were assessed by Western blotting and densitometric analysis. Pancreatic acinar cells were isolated from young and aged mice. Each lane represents acinar cells from an individual mouse. Levels of p85α were normalized by β-actin density. Values are mean ± SD, n=5. *p<0.05 comparing aged versus young mice. (C) Expression of p85α in pancreas from young and aged mice was determined by immunofluorescence staining. Cultured pancreatic acinar cells from young and aged mice were immunostained using antibodies against p85α (green) and IGF-1Rβ (red). Each sample was counter-stained with DAPI (blue) (magnification 400×).
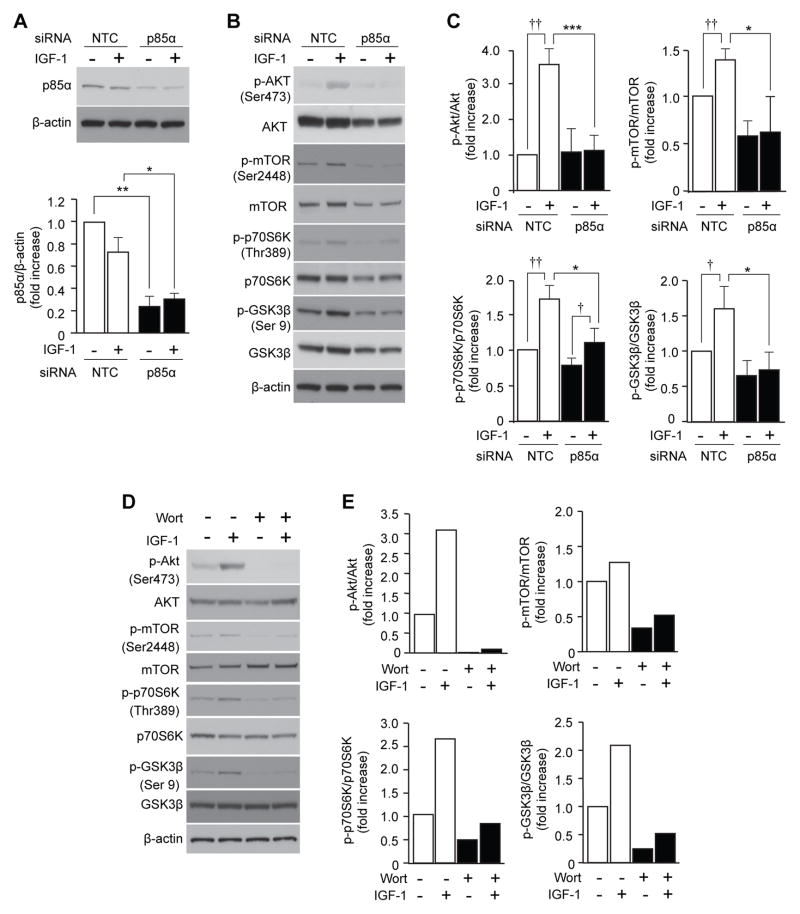
Inhibition of p85α Reduced IGF-1-Induced Activation of PI3K/Akt Downstream Signaling in Pancreatic Acinar Cells
Results from the above analyses indicate that the most upstream age-associated change in IGF-1/PI3K/Akt signaling pathway in pancreatic acinar cells is a reduction of PI3K p85α. To examine the effects of the p85α0 reduction on downstream signaling, pancreatic acinar cells from young mice were transfected with p85α siRNA before stimulation with IGF-1, and activation of PI3K/Akt downstream signaling proteins was examined. Transfection with p85α siRNA reduced the cellular protein levels of p85α to approximately 30% of the original level (Fig. 4A), which was equivalent to the age-associated reduction (~35%) observed in Figure 3B. Treatment with IGF-1 increased phosphorylation of Akt, mTOR, p70S6K, and GSK3β in pancreatic acinar cells transfected with non-targeting control (NTC) siRNA (3.5-, 1.4-, 1.7- and 1.6-fold, respectively). The IGF-1-induced phosphorylation of Akt, mTOR, p70S6K, and GSK3β was significantly reduced by transfection with p85α siRNA (p<0.001, p=0.041, p=0.044, p=0.046, respectively; Fig. 4B and 4C). Similar results were obtained in experiments using the pharmacological PI3K/Akt inhibitor wortmannin; IGF-1-mediated phosphorylation of Akt, mTOR, p70S6K and GSK3β in acinar cells from young mice was reduced by wortmannin treatment (Fig. 4D and 4E). We have previously reported that IGF-1-mediated pancreatic acinar cell proliferation in vitro was reduced by p85a siRNA and wortmannin (Watanabe et al. 2005). Taken together, our results clearly indicate that reduction of p85a level causes suppression of Akt and downstream signal activation and reduction of acinar cell proliferation in young mice, suggesting that age-associated reduction of p85α is causally related to suppression of PI3K/Akt activation and acinar cell proliferation in aged mice.
Figure 4. Down-regulation of p85α suppresses IGF-1-mediated PI3K/Akt downstream signaling in pancreatic acinar cells.
(A–C) The effect of p85α siRNA on IGF-1-mediated PI3K/Akt downstream signaling in isolated pancreatic acinar cells was assessed by Western blotting and densitometric analysis. Acinar cells were transfected with either p85α or NTC siRNA 2 days before treatment with IGF-1 (10 nM) for 2 h. (A) Cellular protein extracts were subjected to Western blot analyses of p85α to confirm successful knockdown. Levels of p85α were normalized by the corresponding density of β-actin. (B) Cellular protein extracts were subjected to Western blot analyses of p-Akt, Akt, p-mTOR, mTOR, p-p70S6K, p70S6K, p-GSK3β, and GSK3β. (C) Densitometric analysis of (B). Levels of p-Akt, p-mTOR, p-p70S6K, and p-GSK30β, were normalized by the corresponding density of total Akt, mTOR, p70S6K and GSK3β respectively. Similar results were obtained from 3 independent experiments. Values are mean ± SD; n=3. *p<0.05 and ***p<0.001 comparing p85α siRNA with NTC siRNA treatment. †p<0.05 and ††p<0.01 comparing IGF-1 with control PBS treatment in pancreatic acinar cells transfected with NTC siRNA. (D) The effect of wortmannin on IGF-1-mediated PI3K/Akt downstream signaling in isolated pancreatic acinar cells was assessed by Western blotting. Isolated pancreatic acinar cells were treated with wortmannin (100 nM) 30 min prior to IGF-1 (10 nM) stimulation and harvested 120 min later. Cellular protein samples were subjected to Western blot analyses of p-mTOR, mTOR, p-p70S6K, p70S6K, p-GSK3β, and GSK3β. (E) Densitometric analysis of (D). Levels of p-Akt, p-mTOR, p-p70S6K, and p-GSK3β were normalized by the corresponding density of total Akt, mTOR, p70S6K, and GSK3β, respectively. Similar results were obtained from 2 additional independent experiments. Wort:wortmannin.
Age-Dependent Decrease in p85α Subunit Expression in Mouse and Human Pancreas
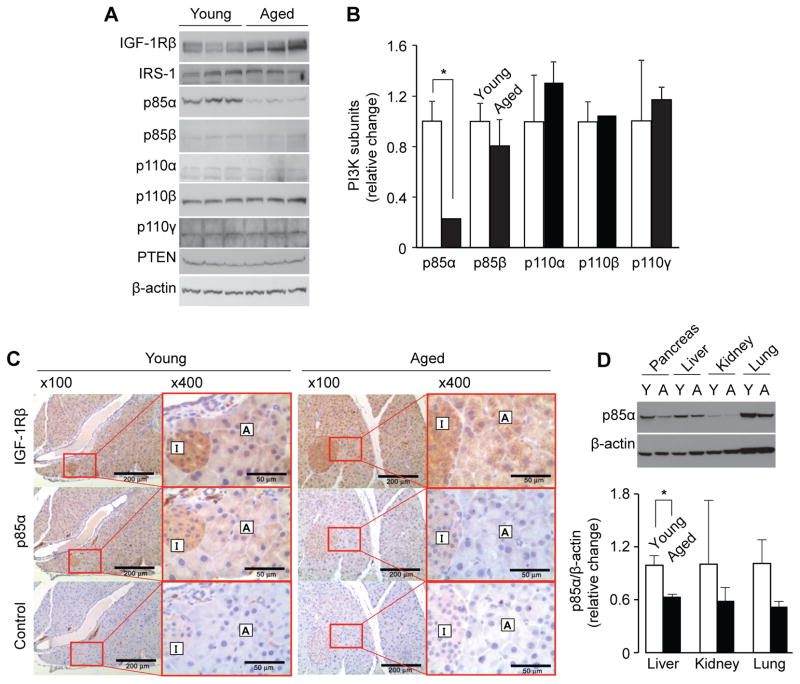
We further examined the expression of various PI3K subunits (p85α, p85β, p110α, p110β and p110γ) and PI3K-related factors (IGF-1Rβ, IRS-1 and PTEN) in whole pancreas tissues from young and aged mice (Fig. 5A and 5B). Expression of the p85α subunit was decreased approximately 5-fold by aging (p=0.011); however, expression of other PI3K subunits (p85β, p110α, p110β and p110γ) as well as IRS-1 and PTEN did not show age-associated alterations. Expression of IGF-1Rβ in aged mice was approximately 2-fold higher than that in young mice although statistical significance was not obtained (p = 0.059). Expression of p85α and IGF-1Rβ was also assessed by immunohistochemistry (Fig. 5C). Both acinar cells and islet cells in young mice expressed IGF-1Rβ and p85α, whereas p85α expression was markedly reduced in aged mice.
Figure 5. Age-associated decreases in PI3Kp85α subunit in mouse pancreas.
(A) The protein levels of IGF-1Rβ, IRS-1, PI3K subunits (p85α, p85β, p110α, p110β, p110γ), and PTEN in whole pancreas from young and aged mice were compared by Western blot analysis. (B) Densitometric analysis of (A). Each lane represents a pancreas protein sample from an individual mouse. All data were normalized by β-actin. Values are mean ± SD; n=3. *p<0.05 comparing aged with young mice. (C) The expression of IGF-1Rβ and p85α in pancreas serial sections from young and aged mice were analyzed by immunohistochemical staining. For negative controls, sections were incubated with control IgG instead of primary antibodies. Results are representative of at least 3 animals. A, acinar cells; I, islet (original magnification 100×, scale bar indicates 200 μm; enlarged magnification 400×, scale bar indicates 50 μm). (D) The levels of p85α in various tissues from young and aged mice were assessed by Western blot analysis. Values are mean ± SD; n=3. *p<0.05 comparing aged with young mice.
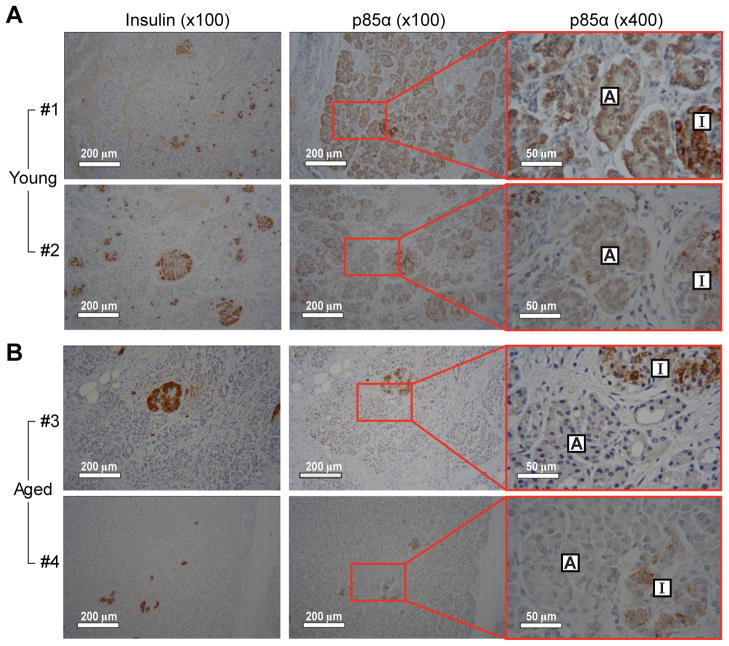
To examine whether aging attenuates expression of p85α in other organs, we compared p85α levels in various tissues including pancreas, liver, kidney, and lung from young and aged mice (Fig. 5D). The age-associated decrease in p85α expression was most clearly observed in the pancreas compared to the other tissues examined; however, the protein levels of p85α in liver, lung, and kidney were also notably decreased by aging (approximately 64%, 59% and 51%, respectively). These results indicate that p85α expression is reduced by aging in several tissues and that the change was most dramatic in the pancreas. Finally, we examined whether the age-associated decrease in p85α expression also occurs in the human pancreas. For this study, human pancreas tissue sections from young (2 and 4 days old) and aged (73 and 81 years old) subjects were immunohistochemically stained with an antibody against p85α. To distinguish islet cells from acinar cells in the pancreas, a serial section was stained using an insulin specific antibody. As shown in Fig. 6A, strong expression of the p85α subunit was detected within pancreatic acinar cells of young subjects. In contrast, expression of p85α was markedly decreased in acinar cells from aged subjects (Fig. 6B) From these results, we conclude that, similar to expression patterns in mouse pancreas, p85α expression in pancreatic acini is decreased by aging in humans as well.
Figure 6. Age-associated decreases in PI3Kp85α subunit in human pancreas.
The expression of p85α in the pancreas from human was determined by immunohistochemical staining. Pancreas tissue sections were obtained from 2 young (A; #1 and #2) and 2 aged (B; #3 and #4) individuals described in Table S2. To distinguish islet cells from acinar cells, a slide sample from the same set of serial sections was stained by an insulin specific antibody. A, acinar cells; I, islet (original magnification 100×, scale bar indicates 200 μm; enlarged magnification 400×, scale bar indicates 50 μm).
DISCUSSION
We previously reported an age-associated decrease in pancreatic regeneration and Akt phosphorylation after partial Px in mice (Watanabe et al. 2005), however, the underlying cellular mechanisms for these alterations were not defined. Major questions regarding whether these age-associated decreases in physiological response are caused by a loss of extracellular signals or an alteration of intracellular pathways with aging remain. To begin to address these questions, we performed in vitro analyses of isolated pancreatic acini from young and aged mice and demonstrated that IGF-1-induced activation of PI3K/Akt pathway and cell proliferation were completely absent in acinar cells from aged mice. Importantly, our results also showed that aging suppressed IGF-1-induced PI3K/Akt signaling without affecting IGF-1 receptor activation. These results indicate that aging causes intracellular changes in pancreatic acinar cells leading to the suppression of PI3K/Akt activation and the loss of cell proliferation capability. The results further suggest that the age-associated loss of pancreatic regeneration after partial Px in our previous study (Watanabe et al. 2005), is also caused by intrinsic acinar cell aging rather than age-associated changes in signals from the extra-pancreatic environment.
A second major finding of our current study is that age-associated loss of PI3K/Akt activation in pancreatic acinar cells is due to a dramatic and unexpected decrease in the protein levels of the PI3K regulatory subunit p85α. We demonstrated that total protein levels of p85α in pancreatic acinar cells are reduced to approximately 35% with aging. Importantly, this age-associated decrease in p85α was confirmed in both mouse and human pancreas in vivo by immunohistochemical staining. Further, knockdown of p85α in cultured acinar cells to 30% of its original level caused a significant decrease in IGF-1-induced activation of the PI3K downstream signaling factors mTOR, p70S6K, and GSK3β, which are important for regulating cell growth and cell cycling. Taken together, we conclude that loss of pancreatic acinar cell proliferation in old age is caused by an intrinsic age-associated dysfunction of the PI3K/Akt pathway characterized by the reduction of PI3K regulatory subunit p85α.
Age-associated attenuation of PI3K/Akt signaling occurs in various tissues other than the pancreas. An earlier study demonstrated an age-associated reduction of p85α levels in murine cardiac but not skeletal muscle, suggesting that these two tissues may have different mechanisms for altered PI3K signaling with aging (Martineau et al. 1999); the age-associated decrease in cardiac p85α protein level was later confirmed in rats (Centurione et al. 2002). Another study of mouse skeletal muscle showed an age-associated reduction of total IGF-1 receptor protein and its phosphorylation; the same study also reported that phosphorylation of p70S6K, but not Akt-1, in response to IGF-1 was suppressed by aging (Li et al. 2003). Shay and Hagen (Shay & Hagen 2009) recently reported that cultured primary hepatocytes from aged rats had lower basal p-Akt (Ser473) levels than hepatocytes from young rats. Another study reported that aging decreased the basal and IGF-1-stimulated BrdU incorporation in mouse osteoblasts; a 10-fold increase in IGF-1 receptor expression was also identified with aging (Cao et al. 2007). In our present study, we also demonstrated that basal p85α protein levels were reduced approximately 40% in the liver; lung and kidney also showed a similar trend though not statistically significant. In contrast to these observations, Majumdar and Du (Majumdar & Du 2006) have demonstrated an increased phosphorylation of p85α and Akt in the colonic mucosa of aged rats compared to expression levels in the colon of young rats. Collectively, although the mechanisms for the age-associated alterations in PI3K/Akt signaling are not exactly the same among various tissues, reduction of p85α protein levels appears to be an important underlying mechanism associated with aging in various organs including the pancreas and liver.
Because IGF-1Rβ and IRβ have significant homology at regions surrounding their tyrosine residues, all available anti-p-IGF-1β antibodies also recognize p-IRβ (Fig. 2). The total protein levels of IRβ in pancreatic acinar cells appeared to be low and showed no age-associated differences. Taking into consideration that IGF-1 binds with strong affinity to IGF-1R and with lower affinity to insulin receptor (Gauguin et al. 2008), it is likely that the phosphorylated receptor detected by our Western blot analysis (Fig. 2A) is IGF-1Rβ rather than IRβ.
In addition to IGF-1, we showed that EGF-induced BrdU incorporation in pancreatic acinar cells was decreased by aging. EGF activates the PI3K/Akt pathway through its own receptor, EGFR (Henson & Gibson 2006), and EGF-mediated activation of p70S6K and DNA synthesis in hepatocytes is decreased with aging (Ohtake et al. 2008). Williams et al. (Williams et al. 2002; Williams 2006) reported that CCK activates the PI3K/Akt-mTOR pathway to regulate protein synthesis at the translational level in pancreatic acinar cells. Pancreatic growth in rats in response to the CCK analogue caerulein is decreased by aging (Greenberg et al. 1988). Together, these reports and our current study suggest that the age-dependent reduction of p85α, a fairly common phenomenon in multiple organs, results in attenuated PI3K/Akt signaling in response to several hormones and growth factors, which may be causally related to age-associated dysfunction.
Activation of the PI3K/Akt pathway is important in pancreatic endocrine functions such as insulin signaling, insulin-stimulated glucose transport, and glycogen synthesis (White 1997; Hugl et al. 1998; Burks & White 2001; Williams 2001; Zawalich et al. 2002). In addition, we previously reported that the PI3K/Akt pathway is essential for pancreatic duct cell differentiation into insulin-producing cells both in vitro and in vivo during pancreatic regeneration (Watanabe et al. 2008a; Watanabe et al. 2008b). Both endocrine and exocrine pancreatic secretion appears to decrease with aging (Khalil et al. 1985; Elahi et al. 2002). Our immunohistochemical studies show that mouse pancreatic p85α expression is decreased with aging not only in acini but also in islets. In human pancreas, p85α expression in insulin-positive cells in aged subjects is decreased compared to those in young. Therefore, it is likely that p85α levels in pancreatic islet cells are also decreased with aging which could explain, in part, the age-associated decrease in pancreatic endocrine secretion. This is an area of focus in our laboratory.
In summary, our present study identifies an age-dependent reduction of PI3K/Akt regulatory subunit p85α in pancreatic acinar cells that is causally associated with a loss of proliferative response of these cells with aging. We demonstrate that: (1) aging decreases the expression of the p85α PI3K subunit in pancreatic acinar cells; (2) IGF-1-induced activation of PI3K/Akt downstream signaling is reduced by aging; (3) IGF-1-induced pancreatic acinar cell proliferation is suppressed by aging; and (4) p85α knockdown suppresses IGF-1-induced PI3K/Akt downstream signaling. The reduction of p85α expression in old age is not limited to pancreatic acinar cells but also islet cells and several extra-pancreatic tissues. These findings provide a potential mechanism for the age-related changes in the physiologic and regenerative function of various GI tissues.
EXPERIMENTAL PROCEDURES
Materials
All reagents, materials and antibodies used in this study are described in Table S1 of the Supporting Information.
Animals
Young adult (4–6 months old) and aged (22–26 months old) male C57BL/6 mice were obtained from a colony of the National Institute on Aging. Before experiments, mice were acclimated for at least 7 days in an environment with controlled temperature (21–23°C), and lighting (14 h light/10 h dark) with free access to water and regular chow diet (Rodent Diet No. 2500 from LabDiet, St. Louis, MO). All procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Isolation of Pancreatic Acinar Cells and siRNA Transfection
Isolation of pancreatic acinar cells and siRNA transfections were performed as previously described (Watanabe et al. 2005) with some modifications. Briefly, isolated pancreatic acinar cells were cultured on laminin-coated plates in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum overnight at 37°C in 5% CO2. In order to minimize the effect of growth factors in serum, cells were washed with phosphate buffered saline (PBS) and further incubated in serum-free DMEM for 3h followed by stimulation with IGF-1 (1–100 nM). For experiments utilizing siRNA, siSTABLE SMARTpool reagents for p85α and non-targeting control (NTC) siRNA were synthesized by Dharmacon (Lafayette, CO). Isolated pancreatic acinar cells were seeded on laminin-coated 6-well plates. Cells were washed 24 h later with fresh DMEM and transfected with p85α or NTC siRNA (final concentration 200 nmol/L) using Trans IT TKO Transfection Reagent. Cells were washed 48 h after siRNA transfection with PBS and incubated with serum free DMEM for 3 h and treated with IGF-1 for 2h. To confirm results from these siRNA studies, isolated pancreatic acinar cells were independently treated with PI3K inhibitor wortmannin (100 nM) 30 min prior to IGF-1 (10 nM) stimulation as previously described (Watanabe et al. 2005), and cell lysate was collected 120 min later. This dose of wortmannin was previously shown to block acinar cell proliferation effectively (Watanabe et al. 2005).
Protein Extraction and Western Blot Analysis
Protein samples were extracted from whole pancreas or isolated acinar cells as previously described (Watanabe et al. 2005). An equal amount of protein was resolved on NuPAGE 4–12% Bis-Tris gels and electrophoretically transferred to polyvinylidene difluoride membranes. After blocking with 5% dried skimmed milk dissolved in Tris-buffered saline with 0.05% Tween 20 for 1 h at room temperature, the membranes were incubated with primary antibodies overnight at 4°C. The membranes were then incubated with a secondary antibody conjugated with horseradish peroxidase for 1 h. The immunoreaction was visualized using electrochemiluminescence (ECL) or ECL Plus Western blotting detection reagents.
Cell Proliferation Assay
Cell proliferation was assessed by measuring 5-bromo-2′-deoxyuridine (BrdU) incorporation and DNA content as previously described (Watanabe et al. 2005) with a few modifications. Isolated acinar cells were cultured in laminin-coated 96-well plates overnight, incubated with serum free DMEM for 3 h, and stimulated with IGF-1 or epidermal growth factor (EGF). BrdU (10 μmol/L final concentration) was added 2 h later and the cells were further incubated for 14 h. BrdU incorporation was measured using a BrdU enzyme-linked immunosorbent assay (ELISA) kit. To measure total DNA content, cells were lysed at 42°C overnight with a lysis buffer containing proteinase K (100 μg/mL), 0.5% SDS, 10 mmol/L Tris-HCl (pH 8.0), and 10 mmol/L EDTA. DNA concentration in lysates was determined with PicoGreen dsDNA kit. Briefly, diluted samples and standard DNA were incubated with Quant-iT™ PicoGreen® for 5 min at room temperature. Sample fluorescence was measured by fluorescence microplate reader at 480 nm. BrdU incorporation and DNA content in IGF-1-treated cells were normalized by DNA content in non-treated control cells.
Immunohistochemical and Immunocytochemical Analyses
Formalin-fixed paraffinized pancreas tissues were used for immunohistochemical staining according to our previously published methods (Chen et al. 2009) with several modifications. Specimens from young and aged human subjects were obtained from the Department of Pathology at the University of Texas Medical Branch. The clinical details of these samples are described in Table S2. For these studies, only tissue sections from normal (i.e., non-cancerous) parts of the pancreas were used. Immunohistochemical analysis was performed by the dextran polymer method using Dako EnVision+ system. For immunofluorescent cytochemistry, isolated acinar cells were cultured on laminin-coated glass cover slips and fixed with 4% paraformaldehyde for 20 min at 37°C. Fixed cells were permeabilized with 0.3% Triton-X100, incubated with primary antibodies for 1 h at room temperature, washed, and incubated with secondary antibodies (Alexa-488 or Alexa-594 conjugated) for 30 min. The cover slips were mounted on glass slides with Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI). The immunofluorescence was detected by laserscanning confocal microscopy with Leica TSC SP5 inverted microscope (Leica, Wetzlar, Germany).
Statistical Analysis
Data were analyzed by Student’s t-test or two-way ANOVA followed by the Bonferroni adjustment for multiple comparison using SigmaStat Statistical Software version 2.0 (Systat Software, San Jose, CA). A p value less than 0.05 was considered statistically significant.
Supplementary Material
Figure S1. Western blot analysis of insulin receptor β and pro-insulin receptor in isolated pancreatic acinar cells.
Table S1. Materials used in this study
Table S2. Clinical details of human pancreas samples
Acknowledgments
This work was supported by grants P01 DK035608, R01 AG025273, R01 AG025908 and R01 AG039732. We thank Dr. Suimin Qiu at the University of Texas Medical Branch for providing human tissue samples. We also thank Karen Martin and Donna Gilbreath for manuscript preparation.
ABBREVIATIONS
- BrdU
5-bromo-2′-deoxyuridine
- CCK
cholecystokinin
- EGF
epidermal growth factor
- GSK3β
glycogen synthase kinase 3β
- IGF-1
insulin-like growth factor-1
- IGF-1R
insulin-like growth factor-1 receptor
- IRβ
insulin receptor β
- IRS
insulin receptor substrate
- mTOR
mammalian target of rapamycin
- NTC
non-targeting control
- PI3K
phosphatidylinositol 3-kinase
- p-Akt
phosphorylated Akt
- p70S6K
p70 S6 Kinase
- Px
pancreatectomy
- PTEN
phosphatase and tensin homolog
- siRNA
small interfering RNA
Footnotes
AUTHOR CONTRIBUTIONS
The research was designed by H.S. and B.M.E., experiments, data analysis, and statistical analysis were performed by H.T., D.O. and M.E.S., manuscript was written by H.T., H.S. and B.M.E. Funding for this research was obtained by B.M.E. and H.S.
Contributor Information
Hitoshi Takahashi, Email: hitakaha@kensei-hospital.or.jp.
Daiki Okamura, Email: daiki.okamura@uky.edu.
Marlene E. Starr, Email: marlene.starr@uky.edu.
Hiroshi Saito, Email: hiroshi.saito@uky.edu.
References
- Baserga R, Hongo A, Rubini M, Prisco M, Valentinis B. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim Biophys Acta. 1997;1332:F105–126. doi: 10.1016/s0304-419x(97)00007-3. [DOI] [PubMed] [Google Scholar]
- Burks DJ, White MF. IRS proteins and beta-cell function. Diabetes. 2001;50(Suppl 1):S140–145. doi: 10.2337/diabetes.50.2007.s140. [DOI] [PubMed] [Google Scholar]
- Calvo EL, Bernatchez G, Pelletier G, Iovanna JL, Morisset J. Downregulation of IGF-I mRNA expression during postnatal pancreatic development and overexpression after subtotal pancreatectomy and acute pancreatitis in the rat pancreas. J Mol Endocrinol. 1997;18:233–242. doi: 10.1677/jme.0.0180233. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Cao JJ, Kurimoto P, Boudignon B, Rosen C, Lima F, Halloran BP. Aging impairs IGF-I receptor activation and induces skeletal resistance to IGF-I. J Bone Miner Res. 2007;22:1271–1279. doi: 10.1359/jbmr.070506. [DOI] [PubMed] [Google Scholar]
- Centurione L, Antonucci A, Miscia S, Grilli A, Rapino M, Grifone G, Di Giacomo V, Di Giulio C, Falconi M, Cataldi A. Age-related death-survival balance in myocardium: an immunohistochemical and biochemical study. Mech Ageing Dev. 2002;123:341–350. doi: 10.1016/s0047-6374(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Chen LA, Li J, Silva SR, Jackson LN, Zhou Y, Watanabe H, Ives KL, Hellmich MR, Evers BM. PKD3 is the predominant protein kinase D isoform in mouse exocrine pancreas and promotes hormone-induced amylase secretion. J Biol Chem. 2009;284:2459–2471. doi: 10.1074/jbc.M801697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi D, Muller DC, Egan JM, Andres R, Veldhuist J, Meneilly GS. Glucose tolerance, glucose utilization and insulin secretion in ageing. Novartis Found Symp. 2002;242:222–242. discussion 242–226. [PubMed] [Google Scholar]
- Evers BM, Townsend CM, Jr, Thompson JC. Organ physiology of aging. Surg Clin North Am. 1994;74:23–39. doi: 10.1016/s0039-6109(16)46226-2. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- Gauguin L, Klaproth B, Sajid W, Andersen AS, McNeil KA, Forbes BE, De Meyts P. Structural basis for the lower affinity of the insulin-like growth factors for the insulin receptor. J Biol Chem. 2008;283:2604–2613. doi: 10.1074/jbc.M709220200. [DOI] [PubMed] [Google Scholar]
- Greenberg RE, McCann PP, Holt PR. Trophic responses of the pancreas differ in aging rats. Pancreas. 1988;3:311–316. doi: 10.1097/00006676-198805000-00012. [DOI] [PubMed] [Google Scholar]
- Hayakawa H, Kawarada Y, Mizumoto R, Hibasami H, Tanaka M, Nakashima K. Induction and involvement of endogenous IGF-I in pancreas regeneration after partial pancreatectomy in the dog. J Endocrinol. 1996;149:259–267. doi: 10.1677/joe.0.1490259. [DOI] [PubMed] [Google Scholar]
- Henson ES, Gibson SB. Surviving cell death through epidermal growth factor (EGF) signal transduction pathways: implications for cancer therapy. Cell Signal. 2006;18:2089–2097. doi: 10.1016/j.cellsig.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Hugl SR, White MF, Rhodes CJ. Insulin-like growth factor I (IGF-I)-stimulated pancreatic beta-cell growth is glucose-dependent. Synergistic activation of insulin receptor substrate-mediated signal transduction pathways by glucose and IGF-I in INS-1 cells. J Biol Chem. 1998;273:17771–17779. doi: 10.1074/jbc.273.28.17771. [DOI] [PubMed] [Google Scholar]
- Khalil T, Fujimura M, Townsend CM, Jr, Greeley GH, Jr, Thompson JC. Effect of aging on pancreatic secretion in rats. Am J Surg. 1985;149:120–125. doi: 10.1016/s0002-9610(85)80020-9. [DOI] [PubMed] [Google Scholar]
- Li M, Li C, Parkhouse WS. Age-related differences in the des IGF-I-mediated activation of Akt-1 and p70 S6K in mouse skeletal muscle. Mech Ageing Dev. 2003;124:771–778. doi: 10.1016/s0047-6374(03)00124-6. [DOI] [PubMed] [Google Scholar]
- Ludwig CU, Menke A, Adler G, Lutz MP. Fibroblasts stimulate acinar cell proliferation through IGF-I during regeneration from acute pancreatitis. Am J Physiol. 1999;276:G193–198. doi: 10.1152/ajpgi.1999.276.1.G193. [DOI] [PubMed] [Google Scholar]
- Majumdar AP, Du J. Phosphatidylinositol 3-kinase/Akt signaling stimulates colonic mucosal cell survival during aging. Am J Physiol Gastrointest Liver Physiol. 2006;290:G49–55. doi: 10.1152/ajpgi.00106.2005. [DOI] [PubMed] [Google Scholar]
- Majumdar AP, Jaszewski R, Dubick MA. Effect of aging on the gastrointestinal tract and the pancreas. Proc Soc Exp Biol Med. 1997;215:134–144. doi: 10.3181/00379727-215-44120. [DOI] [PubMed] [Google Scholar]
- Martineau LC, Chadan SG, Parkhouse WS. Age-associated alterations in cardiac and skeletal muscle glucose transporters, insulin and IGF-1 receptors, and PI3-kinase protein contents in the C57BL/6 mouse. Mech Ageing Dev. 1999;106:217–232. doi: 10.1016/s0047-6374(98)00106-7. [DOI] [PubMed] [Google Scholar]
- Ohtake Y, Maruko A, Ohishi N, Fukumoto M, Ohkubo Y. Effect of aging on EGF-induced proliferative response in primary cultured periportal and perivenous hepatocytes. J Hepatol. 2008;48:246–254. doi: 10.1016/j.jhep.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- Sanchez-Margalet V, Zoratti R, Sung CK. Insulin-like growth factor-1 stimulation of cells induces formation of complexes containing phosphatidylinositol-3-kinase, guanosine triphosphatase-activating protein (GAP), and p62 GAP-associated protein. Endocrinology. 1995;136:316–321. doi: 10.1210/endo.136.1.7828547. [DOI] [PubMed] [Google Scholar]
- Shao J, Evers BM, Sheng H. Roles of phosphatidylinositol 3′-kinase and mammalian target of rapamycin/p70 ribosomal protein S6 kinase in K-Ras-mediated transformation of intestinal epithelial cells. Cancer Res. 2004;64:229–235. doi: 10.1158/0008-5472.can-03-1859. [DOI] [PubMed] [Google Scholar]
- Shay KP, Hagen TM. Age-associated impairment of Akt phosphorylation in primary rat hepatocytes is remediated by alpha-lipoic acid through PI3 kinase, PTEN, and PP2A. Biogerontology. 2009;10:443–456. doi: 10.1007/s10522-008-9187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Shao J, Townsend CM, Jr, Evers BM. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52:1472–1478. doi: 10.1136/gut.52.10.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FE, Rosen KM, Villa-Komaroff L, Weir GC, Bonner-Weir S. Enhanced insulin-like growth factor I gene expression in regenerating rat pancreas. Proc Natl Acad Sci U S A. 1991;88:6152–6156. doi: 10.1073/pnas.88.14.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger JW, Betz M. Insulin receptors and signal transduction proteins in the hypothalamo-hypophyseal system: a review on morphological findings and functional implications. Histol Histopathol. 1998;13:1215–1224. doi: 10.14670/HH-13.1215. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–254. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang X, Hernandez A, Kim S, Evers BM. Inhibition of the phosphatidylinositol 3-kinase pathway contributes to HT29 and Caco-2 intestinal cell differentiation. Gastroenterology. 2001;120:1381–1392. doi: 10.1053/gast.2001.24044. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Saito H, Nishimura H, Ueda J, Evers BM. Activation of phosphatidylinositol-3 kinase regulates pancreatic duodenal homeobox-1 in duct cells during pancreatic regeneration. Pancreas. 2008a;36:153–159. doi: 10.1097/MPA.0b013e318157753e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Saito H, Rychahou PG, Uchida T, Evers BM. Aging is associated with decreased pancreatic acinar cell regeneration and phosphatidylinositol 3-kinase/Akt activation. Gastroenterology. 2005;128:1391–1404. doi: 10.1053/j.gastro.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Saito H, Ueda J, Evers BM. Regulation of pancreatic duct cell differentiation by phosphatidylinositol-3 kinase. Biochem Biophys Res Commun. 2008b;370:33–37. doi: 10.1016/j.bbrc.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MF. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40(Suppl 2):S2–17. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- Williams JA. Intracellular signaling mechanisms activated by cholecystokinin-regulating synthesis and secretion of digestive enzymes in pancreatic acinar cells. Annu Rev Physiol. 2001;63:77–97. doi: 10.1146/annurev.physiol.63.1.77. [DOI] [PubMed] [Google Scholar]
- Williams JA. Regulation of pancreatic acinar cell function. Curr Opin Gastroenterol. 2006;22:498–504. doi: 10.1097/01.mog.0000239863.96833.c0. [DOI] [PubMed] [Google Scholar]
- Williams JA, Sans MD, Tashiro M, Schafer C, Bragado MJ, Dabrowski A. Cholecystokinin activates a variety of intracellular signal transduction mechanisms in rodent pancreatic acinar cells. Pharmacol Toxicol. 2002;91:297–303. doi: 10.1034/j.1600-0773.2002.910606.x. [DOI] [PubMed] [Google Scholar]
- Zawalich WS, Tesz GJ, Zawalich KC. Inhibitors of phosphatidylinositol 3-kinase amplify insulin release from islets of lean but not obese mice. J Endocrinol. 2002;174:247–258. doi: 10.1677/joe.0.1740247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Western blot analysis of insulin receptor β and pro-insulin receptor in isolated pancreatic acinar cells.
Table S1. Materials used in this study
Table S2. Clinical details of human pancreas samples