Abstract
Despite the intimate relationship dogs share with humans in Western society, we know relatively little about the variables that produce and maintain dog social behavior towards humans. One possibility is that human social interaction is itself a reinforcer for dog behavior. As an initial assessment of the variables that might maintain dog social behavior, we compared the relative efficacy of brief human social interaction to a small piece of food as a reinforcer for an arbitrary response (nose touch). We investigated this in three populations of canids: shelter dogs, owned dogs, and hand-reared wolves. Across all three canid populations, brief social interaction was a relatively ineffective reinforcer compared to food for most canids, producing lower responding and longer latencies than food.
Keywords: social reinforcement, food reinforcement, dogs, wolves, Canis lupus
Dogs enjoy a unique niche in Western society. Their high level of interaction with humans offers a wealth of research avenues that bear on both basic and applied questions about the dog–human relationship. Two such questions are: What variables produce and maintain dogs' interactions with humans and how can we use our understanding of those variables to improve the welfare of domestic dogs and owner satisfaction with their pets? Recent research has demonstrated the role of operant principles in dogs' exceptional sensitivity to humans, such as gesture following (Udell, Giglio, & Wynne, 2008) and gazing toward humans (Bentosela, Barrera, Jakovcevic, Elgier, & Mustaca, 2008).
Gesture-following and gazing are often components of human–dog social interaction. Beyond these interactions, however, the nature of the social relationship between humans and dogs remains relatively unexplored from a behavior-analytic perspective. Here we are specifically interested in the reinforcers that produce and maintain dog social behavior toward humans; that is, why do dogs interact socially with humans at all?
Understanding whether human social interaction functions as a reinforcer for dog behavior, and, if so, under what conditions, bears on basic questions. For example, is social interaction a primary or conditioned reinforcer? What is the role of early experience in establishing social interaction as a potential reinforcer? More generally, what is the provenance of reinforcers, especially those not of primary survival value? Whether human social interaction is a reinforcer for dog behavior also bears on applied questions, such as how to improve training techniques and facilitate adoptions.
One possibility is that human social interaction is itself a reinforcer for dog behavior, and dogs' observed sociability with humans is the product of this reinforcer. If this is the case, human social interaction might be a primary reinforcer, with the reinforcing function a phylogenetic product of domestication. Alternatively, the reinforcing function of human social interaction might only come about given appropriate early experiences. That is, it is functionally a primary reinforcer (it does not require continued pairing with back up reinforcers) but has to be established as such through early socialization. Finally, human social interaction might have little or no reinforcing function for dogs. Dog social behavior with humans might be produced and maintained mainly through other reinforcers that humans deliver (such as food or access to the outdoors). In this scenario social interaction would be at best a conditioned reinforcer, and (barring any punishment contingencies), at worst, a neutral stimulus.
Earlier research suggests a reinforcing function of humans and human interaction for dogs. Puppies ran faster in a maze when a passive person was present in the endbox than when the endbox was empty (Stanley, Morris, & Trattner, 1965). The puppies' rate of running in a session, however, decreased across trials. The authors suggested that the puppies satiated quickly on the human as a reinforcer. Supporting this possibility, puppies that were given 2 min of access to a passive person prior to running ran more slowly than matched puppies that were not given such access (Bacon & Stanley, 1963).
The reinforcing function of a passive human persisted even in puppies prevented from seeing or directly interacting with humans from the time they could walk and isolated from conspecifics starting at 31 days of age (Stanley, 1966). This result suggested that the presence of a human is a primary reinforcer for dog behavior, as a specific conditioning history did not appear to be necessary.
Despite the intriguing conclusions from Stanley and colleagues, rate of running in a maze is not as sensitive a measure for reinforcer effectiveness as other measures, such as response rate (Skinner, 1969), and introduces possible fatigue as a confounding variable. Two later studies assessed latency to respond using less effortful responses, allowing for a greater number of observations and a closer approximation of response rate.
First, McIntire and Colley (1967) found that adult dogs' latency to respond to five different commands (sit, down, come, stay, or heel) decreased when petting (5-10 s) plus verbal praise (“good dog”) were delivered contingent on the correct response. The dogs' latencies to respond increased, however, when the consequence was only verbal praise (“good dog”). Although this supports the conclusion that petting might function as a reinforcer and parallels research pointing to petting being an unconditioned stimulus (Gantt, Newton, Royer, & Stephens, 1966), the experiment involved other contingencies that confound a clear conclusion. If the dog did not respond within 15 s, the experimenter forced the dog into the desired position, and then delivered the programmed consequence. The forcing of the dog might have functioned as a punisher for long latencies, in which case more than one contingency was acting on shorter latencies. Additionally, the authors only reported averaged data and individual performances cannot be assessed.
Fonberg, Kostarczyk, and Prechtl (1981) reported a similar finding: Dogs in a discrete-trials procedure would lift a paw, sit, or lie down to specific discriminative stimuli when the response was followed by petting (typically 5 s of petting, but up to 30 s during the lying down response). In a second experiment, Fonberg et al. compared social interaction (20–30 s of petting) and food (small piece of boiled meat) as a reinforcer across two groups of dogs. Using percent correct across trials, the two groups did not show differences in acquisition or stable responding. While this result suggests that social interaction is as effective a reinforcer as food, the authors did not report latency, nor provide data on individual performances, such that differences might still exist. Additionally, the same issue of confounding contingencies arose in both of their experiments. Dogs were forced into position if they did not respond within 60 s.
Physiological studies also provide evidence for an effect of human social interaction on dog behavior. After being petted and talked to quietly by a human for 5-23 min (mean 15 min) dogs showed elevated serum levels of hormones and neurotransmitters associated with feelings of euphoria (β-endorphin), intimate bonding (oxytocin), social bonding (prolactin), feelings of attraction and exhilaration (β-phenylethylamine), and pleasurable sensations and exhilaration (dopamine) (Odendaal & Meintjes, 2003). These results demonstrate the physiological changes dogs experience as a result of human social interaction. They also suggest that human interaction might produce concomitant behavioral changes in dogs, including possibly operant behavioral changes as a result of human social interaction functioning as a reinforcer.
As a first attempt at investigating dog–human dyadic relations from a behavior-analytic perspective, we investigated how human social interaction (brief petting and vocal praise) compared in reinforcing efficacy to a small piece of food, without confounding contingencies. We tested dogs living at a local animal shelter, pet dogs living in human homes, and hand-reared wolves.
We hypothesized that human social interaction functions as an effective reinforcer for sociable domestic dogs (i.e., for dogs that do not cower, retreat, or show aggression upon seeing a human). If human interaction is a primary reinforcer, or a conditioned reinforcer that generalizes easily across humans, we predicted that shelter dogs would show increased sensitivity to human social interaction reinforcement because of their relative state of deprivation from human interaction. Barrera, Jakovcevic, Elgier, Mustaca, and Bentosela, (2010) attributed shelter dogs' greater frequency of fear-appeasement behaviors and tendency to stay in proximity to the experimenter to their relative state of deprivation from human contact. Alternatively, if conditioning specific to an individual person is required for that person's interaction to function as a reinforcer, we predicted that shelter dogs would not show increased sensitivity to human social interaction, but that owned dogs would when the owner served as experimenter.
Finally, we also investigated the possibility that any sensitivity to human social interaction as a reinforcer for domestic dog behavior might be a product of domestication. That is, despite being raised similarly to owned dogs, other canids would not show that enhanced sensitivity. Thus, we also tested hand-reared wolves.
We delivered the programmed consequences (food or brief social interaction) contingent on a nose touch by the canid to the experimenter's hand. The number of nose touches and the latency to respond were our metrics of reinforcer effectiveness. We chose a nose touch operant because it is low effort, thus reducing the risk of fatigue. We also chose this response because dogs and hand-reared wolves typically emit a nose touch to a human hand spontaneously, precluding the need to shape the response. That the animals did not need shaping ensured that the animals had no prior reinforcement history with the experimenter. The exceptions to this were the experiments involving owned dogs and hand-reared wolves, for which the experimenter was a known caretaker.
GENERAL PROCEDURE
We recorded nose touches emitted by the dog or wolf to the experimenter's hand. All of our subjects emitted a nose touch and thus contacted the contingencies without shaping.
No animals had received any treats on the day of the experiment. Because we had less control over when the shelter dogs were fed their regular food, the level of overall food deprivation of shelter dogs varied from a minimum of 1 hr to over 14 hr. The majority of shelter dogs had not eaten in over 14 hr (we conducted most of the tests in the morning prior to the morning feeding). Shelter dogs had had no human contact on the day of testing except for being shifted from one side of their kennel to the other for cleaning. Owned dogs had not eaten for at least 2 hr, but they had been at home with their owner immediately prior to the experiment. Wolves had not eaten for at least 2 hr and had had no human contact the day of the experiment except for staff members delivering food earlier in the day. All dogs were tested on a leash because of constraints of the testing room at the shelter (see below). Wolves were tested loose in a fenced enclosure, as they were not accustomed to being on leash. Although this was different than the procedure used for the dogs, we chose not to leash the wolves in an attempt to increase the likelihood that they would engage in the operant task. Furthermore, the similarity between the results of the dogs and wolves indicates that this procedural difference was not a relevant variable.
A trial began with the experimenter presenting one hand at her side at the level of the animal's nose. If the canid touched the experimenter's hand with any part of its muzzle, the experimenter removed her hand by bringing her hand up to her shoulder and subsequently delivering the programmed consequence (food, social interaction, or extinction). After the programmed consequence was delivered, the experimenter presented her hand again. The intertrial interval was approximately 4 s in all conditions. If the animal did not respond within 30 s, the experimenter removed her hand and presented it again. If the canid did not respond again within an additional 30 s, the session ended. Thus, sessions ended if the canid did not respond within 1 min. If the canid continued to respond, sessions ended after a set number of nose touches were emitted (Experiment 1) or after a set amount of time elapsed (Experiments 2–5). Given these procedural differences between experiments, the number of trials per session varied by experiment. Exact details on the number of trials per session are described below in the methods for the individual experiments. Intersession intervals lasted approximately 30 s, during which the experimenter did not interact with the animal except to take a few steps, thus ensuring that the canids were standing at the beginning of each session.
In different conditions, the programmed consequences were social interaction, food, and extinction. During the Social condition, the experimenter engaged with the animal for approximately 4 s after it emitted a nose touch. The interaction consisted of the experimenter scratching the animal around the neck with both hands while praising it verbally. We chose this duration of interaction because it was similar to the low end of interaction duration used by McIntire and Colley (1967), and it was a similar duration as that required to deliver the food reinforcer; thus, intertrial intervals were equivalent across conditions. It also is a duration that would likely be used in training situations in which long intertrial intervals are undesirable.
For all dogs in the Food condition, the experimenter delivered a small piece of Natural Balance®, approximately 10 mm x 7.5 mm x 7.5 mm, to the animal contingent on a nose touch. We chose this reinforcer because it is a commonly used treat in dog training and the size is consistent with that used in training classes, thus providing ecological validity. The one exception was a dog in Experiment 3 (Conner) that had a food allergy and thus received a piece of potato instead. For all wolves, the food was a small piece of summer sausage, approximately 3 cm x 1.5 cm x 1 cm. Although this is a different reinforcer than that used with dogs, the type and size used was based on the request of the Wolf Park staff as a suitable treat for the wolves.
For all canids, the food was always delivered in the hand not used as the stimulus for the nose touch. Because there was no magazine training conducted (we did not want the experimenter associated with any type of food prior to the experiment), the experimenter presented the food from her hand directly in front of the animal's nose during the first five food trials so that the canid would be certain to access the food. Subsequently the experimenter delivered the food from her hand on the side opposite the hand to which the canid emitted nose touches. The experimenter used the same hand for the nose touch for a given subject and always delivered food from the other hand. For experiments involving shelter dogs, dogs emitted a nose touch to the right hand and received the food from the left. For owned dogs and wolves, however, the owner or Wolf Park staff member was allowed to choose whichever hand was used for the nose touch and the other was used for food delivery. Thus, the sides chosen varied by owner. In all cases, however, this arrangement required that the canine emit a nose touch and then move to the other side of the experimenter to obtain the piece of food. During the Extinction condition, the experimenter removed her hand for 4 s (to equate the interval to approximately that of the Social and Food conditions) by bringing it up to her shoulder before presenting it again.
Experiment 1 used an ABABA design (Food vs. Social Interaction). In Experiments 2, 3, and 5, we used an ABCABC design (Food vs. Social Interaction vs. Extinction). The sequence of conditions was counterbalanced across canids. Occasionally an additional condition alternation was added at the end to further substantiate the observed response patterns. In Experiment 4, we used an AB design (12 sessions of social interaction followed by at least six sessions of food). Except in Experiment 4, each dog experienced three sessions of each condition before the condition changed to the next one scheduled. The one exception was the dog Caffrey in Experiment 1 that experienced four social interaction sessions in one condition due to experimenter error.
During the Social condition, the treat bag that the experimenter kept open on the side of her waist during the Food condition was closed and/or rotated to her back. During Extinction the treat bag was removed from the experimenter's waist. Thus, the status of the treat bag could serve as a discriminative stimulus for the dog to facilitate discrimination between conditions and reduce carryover effects between conditions.
All sessions were video-recorded and we used JWatcher™ (version 6.1) to code sessions, either live or using the video. We measured the number of nose touches emitted in each session and the latency to respond to determine reinforcer efficacy. Interobserver agreement (IOA) was determined by having an assistant code sessions from video; IOA was taken during 22% of the sessions across 14 of the 38 (37%) animals and ranged from 91% to 100%. Agreements were scored when the two observers recorded the same event (stimulus presentation, nose touch, or reinforcer delivery) within .5 s of each other. All others were scored as disagreements. The overall IOA was 98%.
EXPERIMENT 1: SOCIAL INTERACTION VS. FOOD
Methods
Subjects
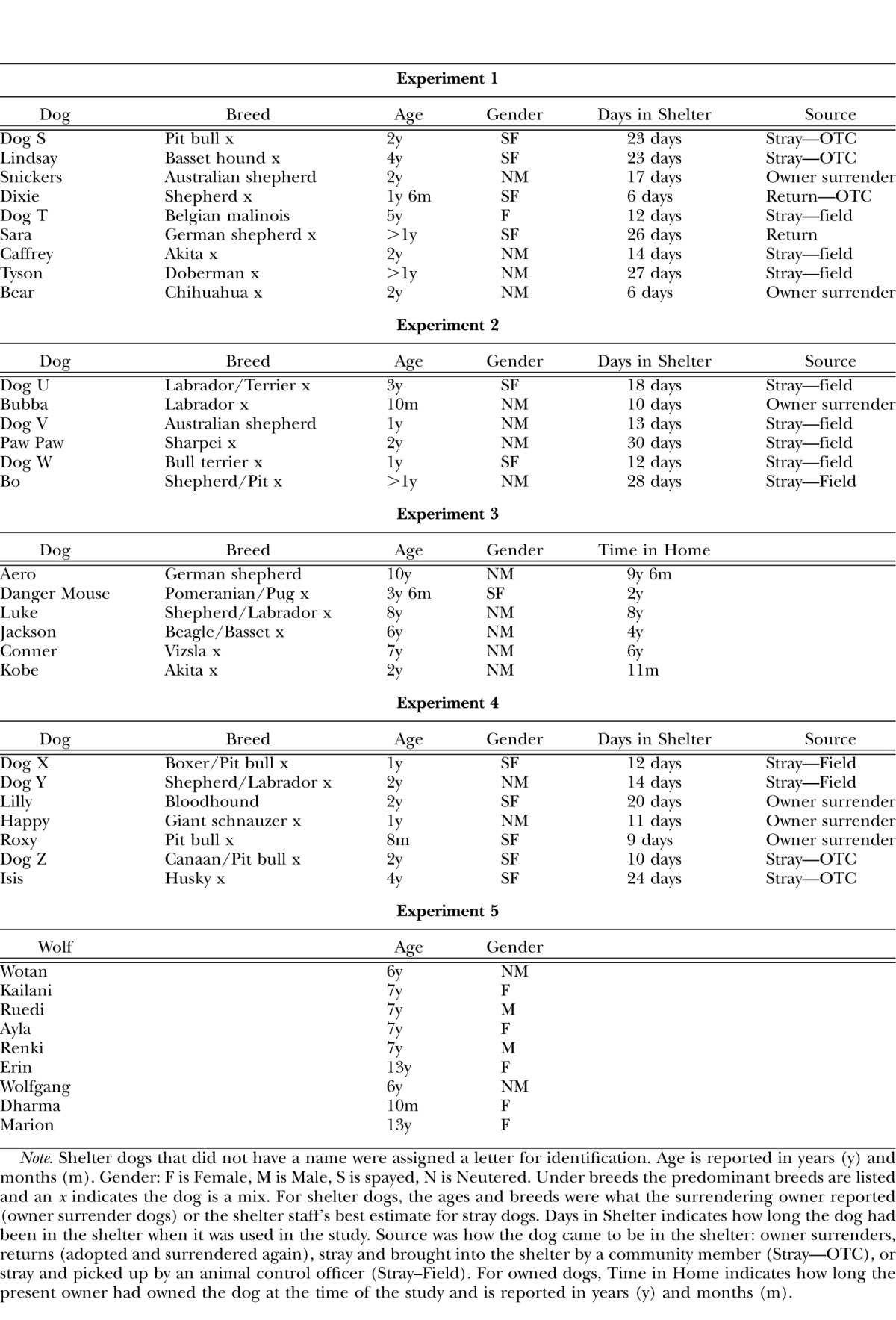
We tested 9 shelter dogs available for adoption at Marion County Animal Services, Ocala, FL. All of the dogs were over 8 months old and were either strays or previously owned dogs surrendered by their owners. All dogs had been in the shelter for at least 5 days (see Table 1 for dog demographics including time in shelter for individual dogs). Dogs were selected based on the criteria that they approach the front of the kennel when the experimenter approached, would allow a leash to be put on them, and would walk out of the kennel.
Table 1.
Canid Demographics
|
Experiment 1 | |||||
|
Dog |
Breed |
Age |
Gender |
Days in Shelter |
Source |
| Dog S | Pit bull x | 2y | SF | 23 days | Stray—OTC |
| Lindsay | Basset hound x | 4y | SF | 23 days | Stray—OTC |
| Snickers | Australian shepherd | 2y | NM | 17 days | Owner surrender |
| Dixie | Shepherd x | 1y 6m | SF | 6 days | Return—OTC |
| Dog T | Belgian malinois | 5y | F | 12 days | Stray—field |
| Sara | German shepherd x | >1y | SF | 26 days | Return |
| Caffrey | Akita x | 2y | NM | 14 days | Stray—field |
| Tyson | Doberman x | >1y | NM | 27 days | Stray—field |
| Bear | Chihuahua x | 2y | NM | 6 days | Owner surrender |
|
Experiment 2 | |||||
|
Dog |
Breed |
Age |
Gender |
Days in Shelter |
Source |
| Dog U | Labrador/Terrier x | 3y | SF | 18 days | Stray—field |
| Bubba | Labrador x | 10m | NM | 10 days | Owner surrender |
| Dog V | Australian shepherd | 1y | NM | 13 days | Stray—field |
| Paw Paw | Sharpei x | 2y | NM | 30 days | Stray—field |
| Dog W | Bull terrier x | 1y | SF | 12 days | Stray—field |
| Bo | Shepherd/Pit x | >1y | NM | 28 days | Stray—Field |
|
Experiment 3 | |||||
|
Dog |
Breed |
Age |
Gender |
Time in Home |
|
| Aero | German shepherd | 10y | NM | 9y 6m | |
| Danger Mouse | Pomeranian/Pug x | 3y 6m | SF | 2y | |
| Luke | Shepherd/Labrador x | 8y | NM | 8y | |
| Jackson | Beagle/Basset x | 6y | NM | 4y | |
| Conner | Vizsla x | 7y | NM | 6y | |
| Kobe | Akita x | 2y | NM | 11m | |
|
Experiment 4 | |||||
|
Dog |
Breed |
Age |
Gender |
Days in Shelter |
Source |
| Dog X | Boxer/Pit bull x | 1y | SF | 12 days | Stray—Field |
| Dog Y | Shepherd/Labrador x | 2y | NM | 14 days | Stray—Field |
| Lilly | Bloodhound | 2y | SF | 20 days | Owner surrender |
| Happy | Giant schnauzer x | 1y | NM | 11 days | Owner surrender |
| Roxy | Pit bull x | 8m | SF | 9 days | Owner surrender |
| Dog Z | Canaan/Pit bull x | 2y | SF | 10 days | Stray—OTC |
| Isis | Husky x | 4y | SF | 24 days | Stray—OTC |
|
Experiment 5 | |||||
|
Wolf |
Age |
Gender |
|||
| Wotan | 6y | NM | |||
| Kailani | 7y | F | |||
| Ruedi | 7y | M | |||
| Ayla | 7y | F | |||
| Renki | 7y | M | |||
| Erin | 13y | F | |||
| Wolfgang | 6y | NM | |||
| Dharma | 10m | F | |||
| Marion | 13y | F | |||
Note. Shelter dogs that did not have a name were assigned a letter for identification. Age is reported in years (y) and months (m). Gender: F is Female, M is Male, S is spayed, N is Neutered. Under breeds the predominant breeds are listed and an x indicates the dog is a mix. For shelter dogs, the ages and breeds were what the surrendering owner reported (owner surrender dogs) or the shelter staff's best estimate for stray dogs. Days in Shelter indicates how long the dog had been in the shelter when it was used in the study. Source was how the dog came to be in the shelter: owner surrenders, returns (adopted and surrendered again), stray and brought into the shelter by a community member (Stray—OTC), or stray and picked up by an animal control officer (Stray–Field). For owned dogs, Time in Home indicates how long the present owner had owned the dog at the time of the study and is reported in years (y) and months (m).
Setting
For shelter dogs, all sessions took place in a small room (3 m x 4 m) at Marion County Animal Shelter during times when the shelter was closed. The room was novel to the dogs, which were allowed to acclimate to the room for 2 min before the experimental sessions began. The room had glass walls, and contained several chairs and a small table along the periphery. Dogs could sometimes hear shelter staff in a nearby office, or see staff passing by on the far side of the front counter in an adjacent room, but the only people in the experimental room were the experimenter and sometimes an assistant who coded the sessions live. During sessions the experimenter stood directly in front of a chair along one wall such that dogs could maneuver in front and to the side of the experimenter, but could only go behind the experimenter by crawling under or jumping up on a chair, which occurred only rarely. One half of one wall in the room was open to a hallway. For this reason, we tested dogs on leash to prevent them from going down the hall. If the dog moved to the end of the leash (approximately 2 m long), the experimenter held the leash stationary and the only pressure was that produced by the dog. That is, the experimenter did not redirect the dog with the leash in any way. Dogs were free to maneuver toward or away from the experimenter; some dogs did, in fact, move to the end of the leash and lie down away from the experimenter during certain conditions.
Procedures
Sessions consisted of a maximum of 15 trials (i.e., the dog could maximally emit 15 nose touches), but were terminated earlier if a nose touch had not occurred for at least 1 min. Dogs alternated between Social and Food conditions, and the sequence of conditions was counterbalanced across dogs. Each condition consisted of three sessions of the same programmed reinforcer. Each dog experienced a minimum of five conditions (e.g., ABABA), although some were exposed to more sessions to further demonstrate experimental control.
One dog (Dog S) showed consistently high responding in both conditions and thus we exposed this dog to two sessions of extinction (Figure 1) to ensure that the nose touch was not simply elicited behavior.
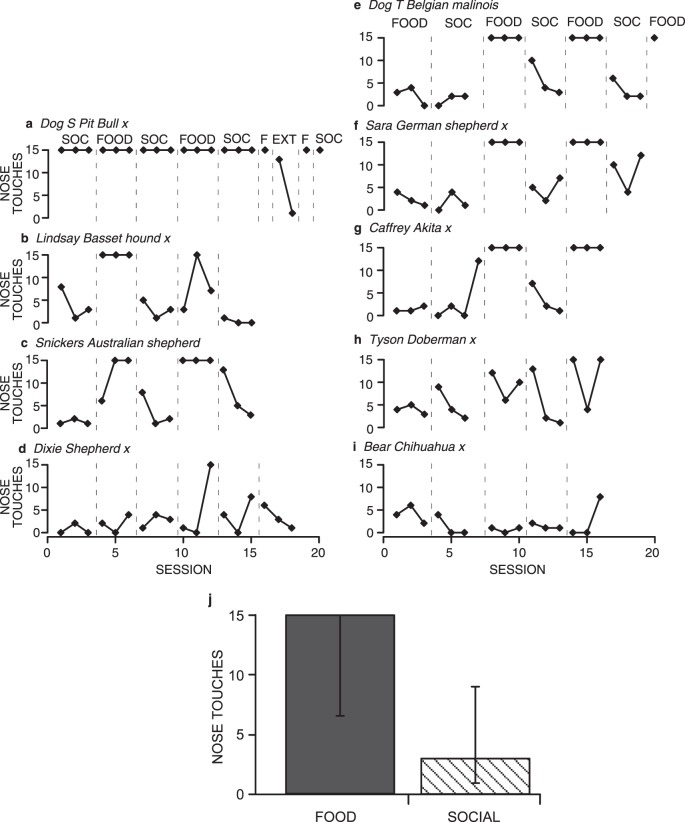
Fig. 1.
Total number of nose touches emitted by shelter dogs in each session under Food or Social (SOC) conditions (and Extinction—EXT—in the case of Dog S) (a-i). A total of 15 nose touches was possible in each session. Dogs that experienced the Social condition first are graphed on the left; dogs that experienced the Food condition first are graphed on the right. Graph j shows the medians (height of bar) and interquartile ranges (whiskers) of the total number of nose touches per session emitted by all dogs in the last complete Food (dark gray) and Social (diagonal hatching) conditions for each dog (i.e., three sessions of each condition).
Results & Discussion
From the 9 dogs tested, three patterns of responding emerged. As Figure 1 shows, these were not affected by the order of conditions (Food–Social vs. Social–Food). The most common pattern, displayed by 5 dogs, was a high number of nose touches emitted in the food sessions and a low number of nose touches in social interaction sessions (Figure 1b, c, e-g). Restricting our analysis to the last three food sessions of each of these 5 dogs, 4 out of the 5 dogs emitted the maximum of 15 nose touches in all three sessions. The other dog emitted the maximum of 15 nose touches in one out of the three sessions.
The second most common pattern of responding was overall low and variable responding in both Food and Social conditions (Figure 1d, h, i). The final pattern of responding was shown by only 1 dog (Figure 1a). This dog emitted the maximal number of nose touches in both the Food and Social conditions. In the Extinction condition, within two sessions of extinction, the number of nose touches for this dog decreased to one. The response immediately recovered when the food contingency was reinstated.
Figure 1j shows the medians (level of the bars) and interquartile ranges (whiskers) of the total number of nose touches per session emitted by all dogs in the last complete Social and Food conditions for each dog (i.e., three sessions of each condition are graphed). We restricted our analysis to the last complete condition to eliminate any differences attributable to acquisition. The median number of nose touches per session in the Food condition was 15, whereas the median number of nose touches per session in the Social condition was three, although the interquartile ranges of the two conditions overlapped slightly. This analysis further substantiates the differences in responding between Food and Social conditions.
We also measured latency to respond after the experimenter's hand was presented. The three patterns for total nose touches emitted correspond to three patterns of latency to respond, which are represented in Figures 2 and 3. Figure 2 shows the cumulative record for all sessions for 3 individual dogs. The time during reinforcer delivery was removed. Figure 3 shows the medians (levels of the bars) and interquartile range (whiskers) of the latency to respond for all 9 dogs. The data graphed are the latencies from the last complete Social and Food conditions for each dog (i.e., three sessions of each condition are graphed) to eliminate any differences attributable to acquisition.
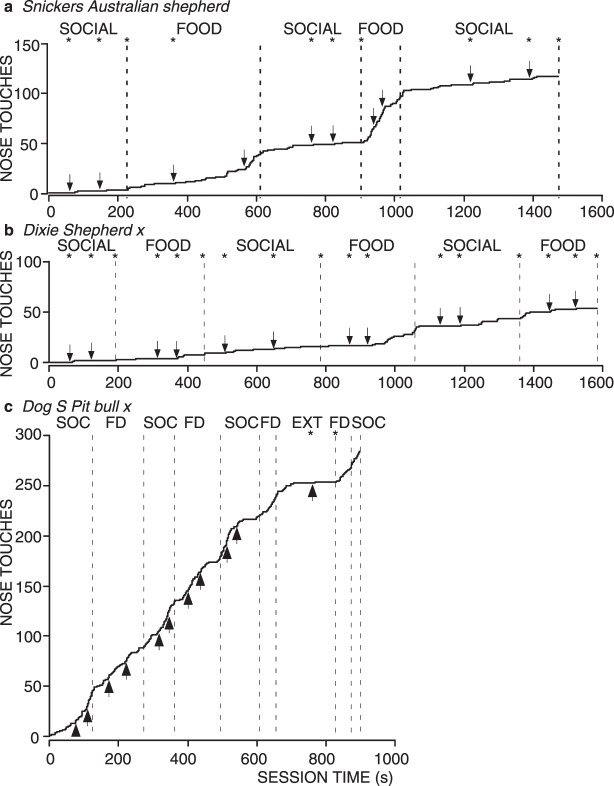
Fig. 2.
Cumulative records for all sessions for 3 individual shelter dogs from Experiment 1, each corresponding to one of three patterns of responding our study revealed. Time during reinforcer delivery was subtracted, leaving a cumulative record of time from stimulus onset to response. The dashed vertical lines show when conditions changed (condition type noted at the top of each graph). Arrows show where each individual session ended. Asterisks at the top of the graph indicate when a session ended before the dog emitted all 15 possible nose touches (i.e., the dog stopped responding for at least 1 min). Graph a shows the most common pattern of responding (short latencies in the Food condition and long latencies in the Social condition). Graph b shows the next most common pattern of responding (low and variable responding in both conditions). Graph c shows the pattern of responding for Dog S, who was unique in our study for responding equivalently to food (FD) and social interaction (SOC) consequences.
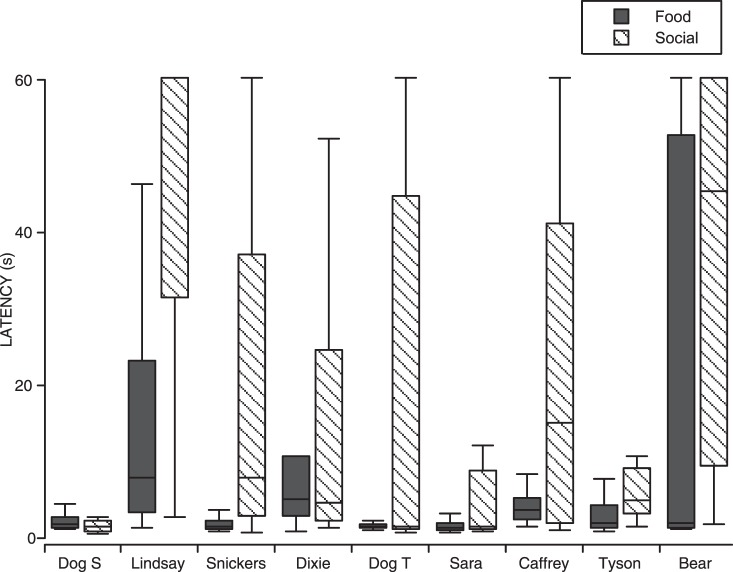
Fig. 3.
Medians (height of bar) and interquartile ranges (whiskers) of the latencies to respond for shelter dogs in Experiment 1. The data graphed are the latencies from the last complete Food (dark gray) and Social (diagonal hatching) conditions for each dog (i.e., three sessions of each condition). The maximum latency for any trial was 60 s.
Figure 2a shows the most common pattern of latency to respond. This pattern consisted of short latencies during Food conditions, and long latencies during Social conditions. As is shown in Figure 3, this pattern of latencies was produced by the same 5 dogs that consistently emitted more nose touches in the food sessions than in the social interaction sessions, the most common pattern of responding for total nose touches emitted, as well as one dog (Tyson), which showed more low and variable numbers of nose touches between the two conditions. For these dogs, the median latency was greater for all except Dog T, and all had minimal overlap of interquartile ranges between the Food and Social conditions.
Figure 2b shows a more variable pattern of latency to respond in which Social and Food conditions are not distinguishable. This pattern of latencies was typical of the dogs that showed low and variable numbers of total nose touches emitted and, as shown in Figure 3, was produced by 2 out of those 3 dogs (Dixie and Bear, Figure 3).
Finally, Figure 2c shows the latencies to respond for the one dog (Dog S) that showed a high total number of nose touches in both the Food and Social conditions. Interestingly, the latencies to respond were nearly identical between both Food and Social conditions, so that this dog's behavior was indistinguishable across Food and Social conditions in both the number of nose touches emitted and the latency to respond.
Overall, these results show that, compared to food, social interaction as administered in this experiment is a relatively poor reinforcer for most dogs. This pattern was evident whether the dog experienced the Social or the Food condition first. Dogs not only emitted more nose touches when food was the consequence, but they emitted the nose touches after a much shorter latency when food was the consequence rather than social interaction.
The pattern of responding in the Social conditions, however, fails to distinguish between three possibilities: 1) social interaction does not function as a reinforcer at all (i.e., it is functionally extinction); 2) social interaction is a low value reinforcer that maintains only low levels of responding; or 3) social interaction is a potent reinforcer but rapidly satiates and thus loses its function quickly.
Additionally, these results point to individual variability across dogs. While there was a clear and consistent pattern in the majority of the dogs, 3 dogs showed overall low and variable levels of responding. For these dogs, other environmental stimuli (e.g., olfactory or visual stimulation) might have functioned as more potent reinforcers in that environment than the food or the social interaction. Finally, Dog S showed high levels of responding in both Social and Food conditions, demonstrating that social interaction can function as a reinforcer for some dogs. Nevertheless, it remains possible that food was a more potent reinforcer than social interaction even for Dog S. We arbitrarily ended sessions after 15 nose touches, leaving open the possibility that if we had allowed the sessions to continue beyond 15 nose touches, the behavior of Dog S in the Food and Social conditions would have differentiated and shown the same pattern as the majority of dogs (more nose touches in the Food than in the Social condition).
EXPERIMENT 2: SOCIAL INTERACTION VS. FOOD VS. EXTINCTION—SHELTER DOGS
Because responding in the Social condition in Experiment 1 was typically at low rates for most dogs, we wanted to assess how the dogs' behavior in the Social condition compared to their behavior in an Extinction condition.
Methods
Subjects
We tested 6 shelter dogs selected using the same criteria as detailed in Experiment 1 (see Table 1 for the dogs' demographic information).
Procedures
The only differences between Experiment 2 and Experiment 1 were: 1) rather than terminating after the dog emitted 15 nose touches, sessions lasted for 3 min or until the dog had not responded for 1 min, and 2) we added Extinction conditions. We limited the sessions by time instead of number of nose touches to allow for greater variability in the number of nose touches emitted. Thus, in Experiment 2, there was no a priori maximum number of trials. The number of trials administered depended on the rate of the dogs' responding. That is, as long as dogs continued to respond, the experimenter continued to present her hand after the 4-s intertrial interval until 3 min had elapsed. Dogs alternated between Social, Food, and Extinction conditions. The sequence of conditions was counterbalanced across dogs, except that Extinction was always the last condition the dogs experienced. Each dog experienced its sequence of conditions twice (e.g., ABCABC). To further substantiate the observed trends, all dogs were exposed a third time to the A condition for 1–3 sessions. Except for the last A condition, in which the number of sessions varied across dogs, each condition consisted of three sessions.
Results & Discussion
Figure 4 a-f shows the total number of nose touches emitted by the 6 shelter dogs in Food, Social, and Extinction conditions. In all 6 shelter dogs, social interaction again produced overall low responding, as did extinction. For the 3 dogs that experienced the food → social interaction → extinction sequence, the Extinction condition appears to be an indistinguishable continuation of the Social condition, providing no evidence that the Social condition is functionally different from extinction. For the 3 dogs that experienced the social interaction→ food→ extinction sequence, however, all 3 dogs showed a small increase in the number of nose touches emitted in the first social interaction session that occurred after Extinction (i.e., the second Social condition overall). When these 3 dogs were exposed to a third Social condition, the initial increase after Extinction was not observed, although 2 dogs (Bubba and Dog V) did show an increase in number of nose touches emitted in the second (Dog V) and the second and third (Bubba) sessions in that social interaction condition.
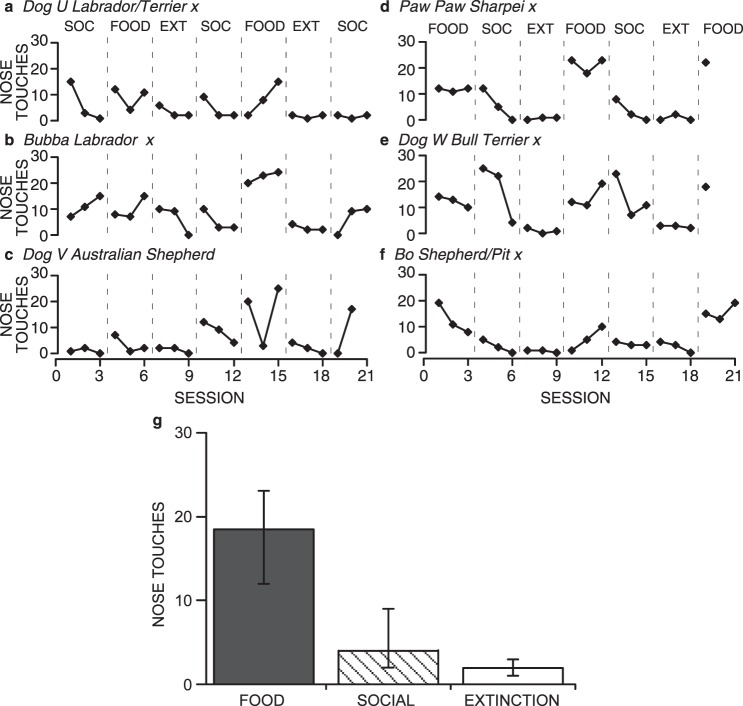
Fig. 4.
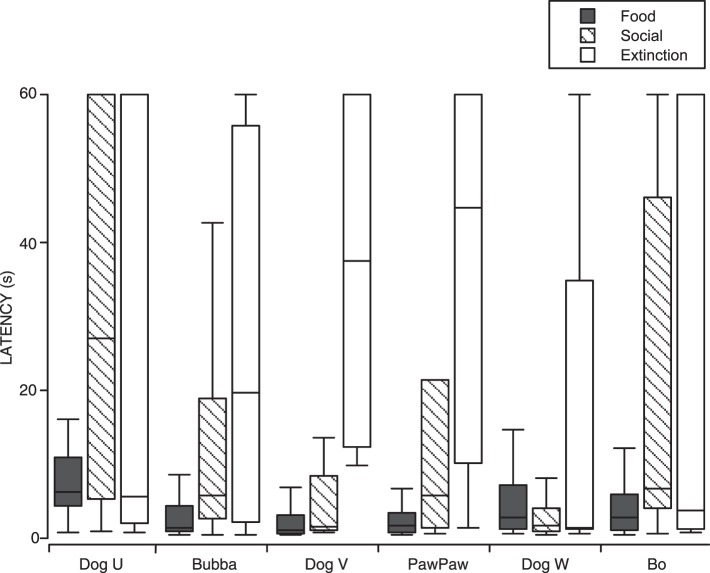
Total number of nose touches emitted by shelter dogs in each session under Food, Social (SOC), and Extinction (EXT) conditions (a-f). Dogs that experienced the Social condition first are graphed on the left; dogs that experienced the Food condition first are graphed on the right. Graph g shows the medians and interquartile ranges of the total number of nose touches per session emitted by all shelter dogs in the last complete Food (dark gray), Social (diagonal hatching), and Extinction (white) conditions for each dog (i.e., three sessions of each condition).
Figure 4g shows the medians (level of the bars) and interquartile ranges (whiskers) of the total number of nose touches per session emitted by all dogs from the last complete Social, Food, and Extinction conditions for each dog (i.e., three sessions of each condition are graphed). The median number of nose touches per session in the Food condition was 18, whereas the median in the Social condition was 4, and in the Extinction condition was 3. The interquartile range of the Food condition did not overlap with either that of the Social or Extinction conditions, although the interquartile ranges of the Social and Extinction conditions did overlap. This analysis further demonstrates the differences in responding between the Food condition and the Social and Extinction conditions.
The primacy of food as a reinforcer was not as pronounced in this experiment as in Experiment 1. It is still evident, however, in the later food sessions for all dogs, either as an increasing trend in the last Food session (Dog U), or high consistent responding in later Food sessions, (PawPaw and Bo). The moderated effect of food was likely due to the dogs experiencing both a Social condition and an Extinction condition between successive Food conditions. Both of these conditions produced very low levels of responding, which may have retarded how quickly the dog contacted the contingency change during the Food condition.
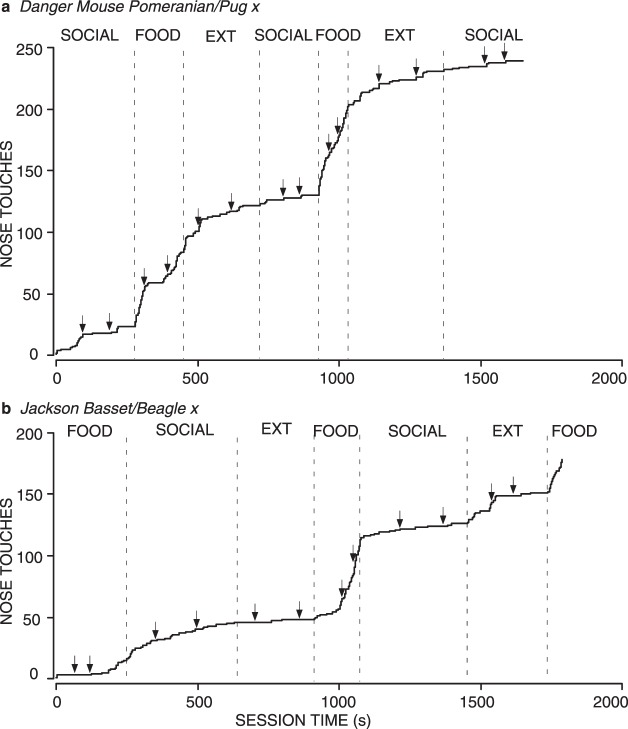
The results for total nose touches emitted correspond to the patterns of latency to respond, which are shown in Figures 5 and 6. Figure 5 shows the cumulative record of all sessions for 2 representative dogs (1 dog that experienced food first and the other that experienced social interaction first) and is analogous to Figure 2 of Experiment 1. Figure 6 shows the medians and interquartile range of the latency to respond for all 6 dogs. The data graphed are the latencies from the last complete Social, Food, and Extinction conditions for each dog (i.e., three sessions of each condition). The data from both of the individual dogs plotted in Figure 5 show that latencies are relatively long in both the Social and Extinction conditions, and that the shorter latencies typical of Food conditions emerge in the second exposure to food, mirroring the total nose touch data.
Fig. 5.
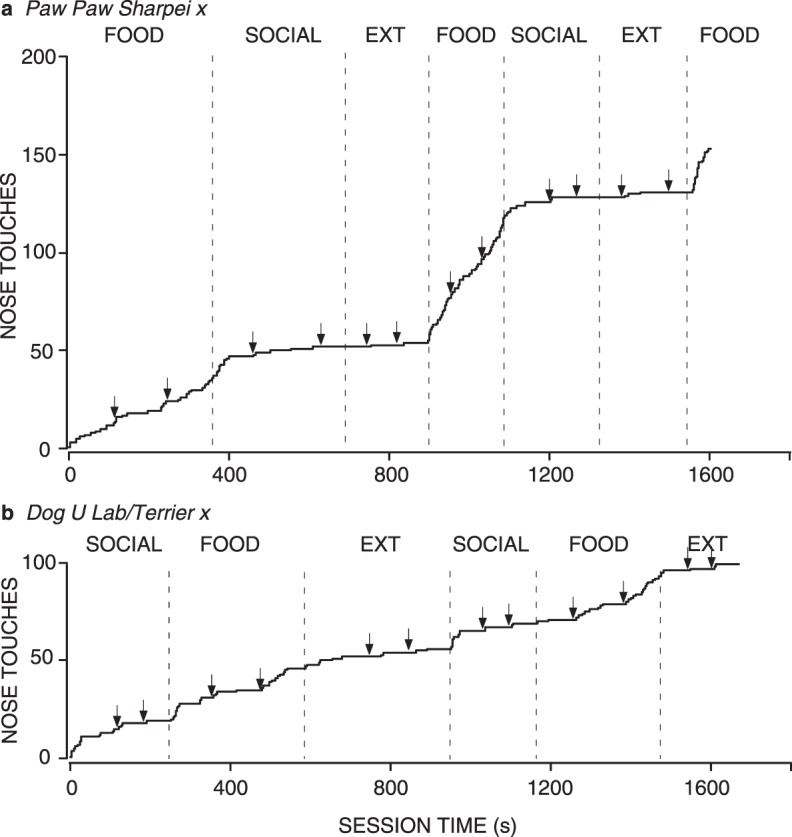
Cumulative records for all sessions for 2 individual shelter dogs from Experiment 2, both corresponding to the most common pattern of responding our study revealed. Other details are the same as for Figure 2. Graph a shows the most common pattern of responding (short latencies in the Food condition and long latencies in the Social condition). Graph b shows the next most common pattern of responding (low and variable responding in both conditions).
Fig. 6.
Medians and interquartile ranges of the latencies to respond for shelter dogs in Experiment 2. The data graphed are the latencies from the last complete Food (dark gray), Social (diagonal hatching), and Extinction (white) conditions for each dog (i.e., three sessions of each condition). Other details are the same as for Figure 3.
When the data from all 6 dogs are considered (Figure 6), the Food condition produced shorter and more consistent latencies than the other conditions. The exception was Dog W for which the median latency in the Food condition was nearly equal to that of the Social condition and slightly higher than that of Extinction. The variability was still greater in the Extinction condition than either the Food or Social conditions. For 3 dogs (Bubba, Dog V, and PawPaw), the median latencies in the Social condition were shorter than those in Extinction. For the other 3 dogs, the median latency in the Social and Extinction conditions were similar (Dog W) or greater in the Social condition (Dog U and Bo). Nevertheless, the variability in the Extinction condition was greater than in the Social condition for all 6 dogs.
These results give a more nuanced view of social interaction as a consequence compared to extinction. Social interaction might not be functionally equivalent to extinction, given the greater variability of latencies for all 6 dogs. The results, however, still point to the conclusion that, if social interaction does function as a reinforcer, it is either a relatively low-value reinforcer, or its function rapidly decreases due to satiation.
EXPERIMENT 3: SOCIAL INTERACTION VS. FOOD VS. EXTINCTION—OWNED DOGS
We wanted to compare how shelter dogs in Experiment 2 would compare to owned dogs. The shelter dogs were relatively deprived of social interaction with a relatively meager recent history of human-mediated reinforcement and none with the experimenter. The owned dogs, alternatively, were not deprived of social interaction but did have a history of reinforcement with the experimenter (who was their owner).
Methods
Subjects
We tested 6 owned dogs, all of which were at least 8 months old and had lived with their owner for at least 6 months (see Table 1 for the dogs' demographic information).
Setting
All sessions took place in a quiet room at the house where the dog lived. The people present were the experimenter (the owner of the dog) and the first author.
Procedures
The procedures were the same as those used in Experiment 2 for shelter dogs with the following variations: The experimenter was the dog's owner who, prior to conducting the experiment, received training from the first author. One owned dog, Conner, had food allergies; thus his food item was pieces of potato.
Results & Discussion
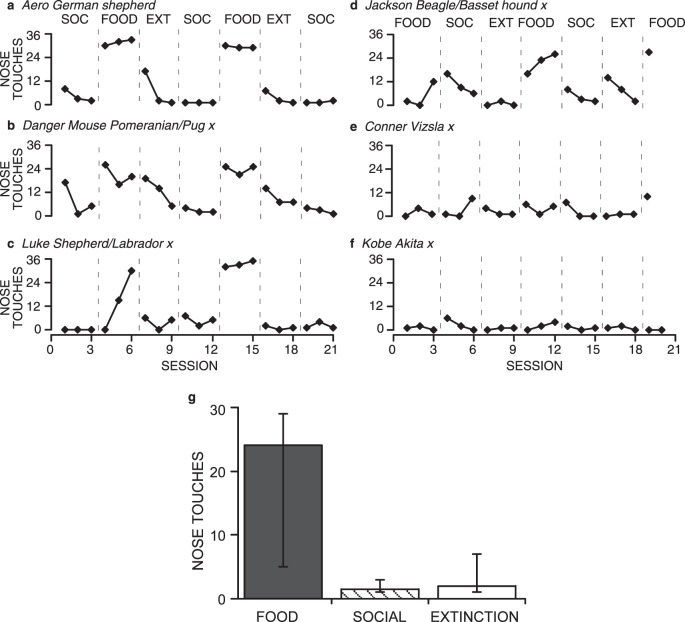
Figure 7 shows that the pattern of total nose touches emitted by the owned dogs across conditions is consistent with the results of the shelter dogs. Four of the dogs showed the most common pattern of high responding in the Food condition and low responding in the Social condition and Extinction condition (Figure 7 a-d); 2 of the dogs showed overall low levels of responding (e-f). This individual variability is similar to that of the shelter dogs seen in Experiments 1 and 2. For those 4 dogs that did show differential responding across conditions, food produced much higher numbers of nose touches than did social interaction. It did so in the first exposure to the Food condition for 3 dogs, and in the second exposure to the Food condition for the 4th dog (Jackson). This response differentiation between Food and Social conditions occurred with less exposure to the conditions than it did for shelter dogs. This is perhaps a result of the owned dogs' greater recent exposure to the various contingencies (social interaction vs. food vs. extinction) allowing the dog to discriminate between them more rapidly.
Fig. 7.
Total number of nose touches emitted by owned dogs in each session under Food, Social (SOC), and Extinction (EXT) conditions (a-f). Dogs that experienced the Social condition first are graphed on the left; dogs that experienced the Food condition first are graphed on the right. Graph g shows the medians and interquartile ranges of the total number of nose touches per session emitted by all owned dogs. Other details are the same as for Figure 4g.
It is also possible that the owned dogs had emitted nose touches in their day-to-day lives with their owners, which produced consequences and thus might account for their faster acquisition of the response than the shelter dogs. Such a scenario might also produce the observed pattern that, even in the first conditions of food and social interaction, the owned dogs showed large differences in the number of nose touches emitted. One owned dog (Aero) had been trained to emit a nose touch to a human hand, and some of the other dogs, although not explicitly trained to do so, might have had a recent history of nose touches producing reinforcing consequences. Countering this argument, however, at least 3 dogs (Luke, Jackson, and Conner) had been trained that an extended hand did not produce any consequences and touches to the hand had been previously extinguished by the owners. Luke, however, produced results similar to those of Aero.
The absolute maximum number of nose touches emitted by each of these 4 owned dogs was higher than the maximum emitted by any shelter dog, again indicating that the owned dogs' recent history of reinforcement may have impacted their results. In terms of the owned dogs' behavior in the Social condition, no dogs showed the temporary increase in responding when Social followed an Extinction condition. Overall, the responses in the Social conditions for owned dogs were consistently lower than those seen in the shelter dogs, making Social and Extinction less distinguishable in owned dogs than in shelter dogs.
Figure 7g shows the medians (levels of the bars) and interquartile ranges (whiskers) of the total number of nose touches per session emitted by all dogs and is analogous to Figure 4g. The median number of nose touches per session in the Food condition was 24, whereas the median in the Social condition was 1.5, and in the Extinction condition was 2. The interquartile range of the Food condition was much greater than that from Experiment 2 and overlapped with the interquartile range of the Extinction but not of the Social condition. The interquartile ranges of the Social and Extinction conditions also overlapped. This analysis further parallels the differences between conditions seen in shelter dogs. It also corroborates the greater number of nose touches per session by owned dogs compared to shelter dogs, although the variability for owned dogs in the Food condition was greater than that of the shelter dogs.
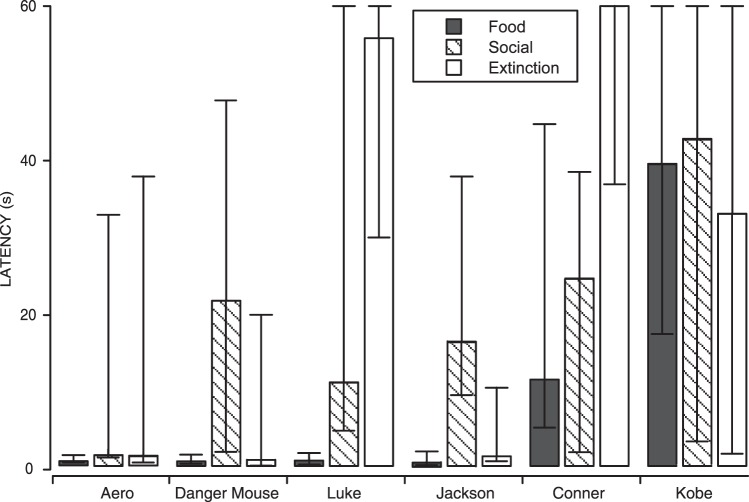
The results for total nose touches emitted correspond to the patterns of latency to respond, which are shown in Figures 8 and 9. Figure 8 shows the cumulative record of all sessions for 2 representative dogs. Both show short latencies characteristic of the Food condition, and relatively longer latencies in the Social and Extinction conditions, regardless of the order in which the conditions were experienced. Figure 9 shows the medians and interquartile ranges of the latency to respond for all 6 dogs. The 4 dogs that emitted a high number of nose touches in the Food condition all had very short and consistent latencies in the Food condition compared to Social and Extinction. Of these 4 dogs, 2 dogs had shorter median latencies in Social than in Extinction conditions, but the other 2 dogs had shorter median latencies in Extinction. There was substantial variability in both conditions, however. This result differed from the shelter dogs, in which the latency in the Social condition was less variable than that in the Extinction condition for all 6 dogs. The 2 owned dogs that had low overall responding also had long and variable latencies in all three conditions.
Fig. 8.
Cumulative records for all sessions for 2 individual owned dogs from Experiment 3, both corresponding to the most common pattern of responding our study revealed. One dog experienced the Social condition first (a) and 1 dog experienced the Food condition first (b). Other details are the same as for Figure 5.
Fig. 9.
Medians and interquartile ranges of the latencies to respond for owned dogs. Details are the same as for Figure 6.
Together with the results from Experiment 2, these results suggest that 1) the extended history of reinforcement with the owner did not affect the reinforcing effectiveness of social interaction and 2) the temporary slight increase in responding and the reduced variability in latency (compared to Extinction) in the Social condition by shelter dogs might be a product of their greater level of deprivation from social interaction.
EXPERIMENT 4: EXTENDED EXPOSURE TO SOCIAL INTERACTION PRIOR TO FOOD
In Experiments 1 and 2, dogs had at most three sessions with social interaction as the consequence before experiencing sessions in which food was the consequence, and food generally sustained higher levels of behavior than did social interaction. Thus, we wanted to investigate whether, given more exposure to social interaction as the consequence prior to exposure to food, social interaction would begin to function as a stronger reinforcer.
Methods
Subjects
We tested 7 shelter dogs selected using the same criteria as detailed in Experiment 1 (see Table 1 for the dogs' demographic information).
Procedures
The procedures followed the general procedures outlined in Experiment 2. The variation in Experiment 4 was that dogs experienced 12 sessions of the social interaction condition before experiencing a minimum of six sessions of the Food condition.
Results and Discussion
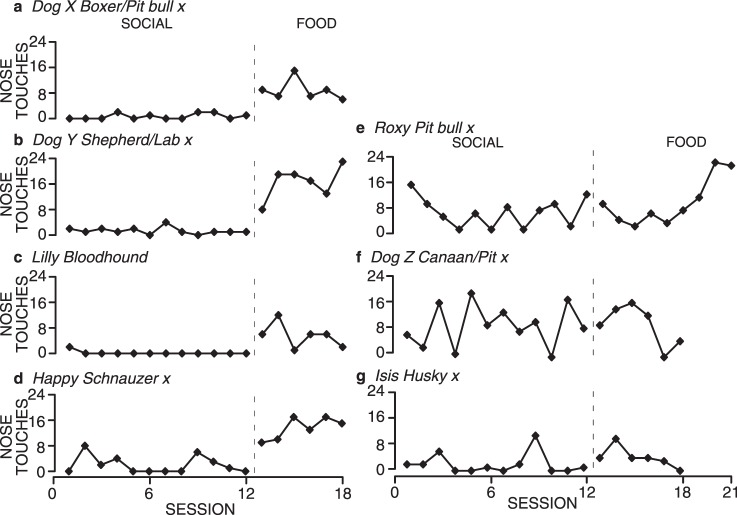
Figure 10 shows the total number of nose touches emitted by the 7 dogs. Five of the dogs showed little or no responding in the Social condition across twelve sessions, but showed a subsequent increase in nose touches emitted when the Food condition was initiated (Figure 10 a-e). Four of these 5 dogs showed an immediate increase in responding when the Food condition was implemented, but the 5th (Roxy) only showed a consistent increase in responding beginning in the sixth food session (Session 18 overall). The total number of nose touches emitted in each session for all 5 dogs was generally lower than that seen in Experiment 2, and is likely a result of the extended exposure to the Social condition. The extended exposure produced a longer history of nose touches not producing food, which may have delayed the dogs' contact with the new contingency in the Food condition. The other 2 dogs' behavior differentiated less between the Social and Food conditions. One of these dogs (Isis) showed low responding across both conditions, and the other dog (Dog Z) emitted a mid-level but more variable number of nose touches across both conditions. Dog Z also produced the most nose touches in the social condition of any of these 7 dogs.
Fig. 10.
Total number of nose touches emitted by shelter dogs in each session under extended Social conditions, followed by a Food condition.
These results again point to some individual variability in responsiveness between dogs. Nevertheless, they highlight the results of earlier experiments: Social interaction functioned as a low-value reinforcer, if it functioned as a reinforcer at all. These results also demonstrate that the low level of responding in Social conditions was not due to a contrast effect from the dogs' contact with the Food condition. Even with extended exposure to the social interaction contingency and no exposure to a food contingency, dogs did not emit any greater number of nose touches in the social interaction sessions nor did they show any trend in that direction.
EXPERIMENT 5: SOCIAL INTERACTION VS. FOOD VS. EXTINCTION IN CAPTIVE WOLVES
Some researchers maintain that domestication has made dogs especially sensitive to humans largely through phylogenetic mechanisms (e.g., Hare & Tomasello, 2005; Miklósi, Topál, & Csányi, 2004, but see Udell, Dorey, & Wynne, 2008, 2010). Dogs' reported enhanced sensitivity to humans includes dogs' greater ability to correctly follow human gestures than wolves (Hare, Brown, Williamson, & Tomasello, 2010, but see Udell, Dorey & Wynne, 2008), dogs' ability to correctly respond to human attentional focus (Virányi, Topál, Gácsi, Miklósi, & Csányi, 2004), as well as dogs' greater tendency than hand-reared wolves to seek help from a human when confronted with an insoluble task (Miklósi et al. 2003). From an operant perspective, we can extend this argument and ask whether domestication has made dogs especially sensitive to human social interaction as a reinforcer such that we would not see the same level of sensitivity in hand-reared wolves. In light of these arguments we replicated Experiment 3 to compare domestic dogs to captive wolves in terms of the relative efficacy of social interaction as a reinforcer.
Methods
Subjects
We tested 9 socialized, captive wolves housed at Wolf Park, Battleground, IN (see Table 1 for the wolves' demographic information). All of the wolves were hand-reared, had regular interaction with humans, and had been trained to emit certain behaviors for food, including touching their nose to a human hand.
Setting
Except for Erin, Ayla, and Renki, each wolf was tested individually in a long, narrow holding pen (approximately 12′ x 40′). Erin and Ayla were tested individually in their home pens, and Renki was tested in a large, square pen (an empty home pen) adjacent to his own home pen.
Procedures
Wolves were tested individually by Wolf Park staff members. One staff member served as experimenter; the other was present as a safety precaution. These staff had a long history of reinforcing the wolves' behavior, similar to Experiment 3 with the owned dogs. The first author remained on the outside of the enclosures and video-recorded the sessions.
Because the wolves were not on leash and the enclosures in which the experiments were conducted were larger than those in which the dogs had been tested in prior experiments, if the wolf was at least 6 m from the experimenter, the experimenter called the wolf's name to begin the session. All of the wolves responded by approaching the experimenter.
The procedures followed those outlined above in Experiment 2. For all wolves, the food was a small piece of summer sausage, approximately 3 cm x 1.5 cm x 1 cm. During the Social condition, the experimenter delivered approximately 4 s of petting and verbal praise to the wolf, matching as closely as possible the procedures used with the dogs. The only exception was wolf Erin, which did not readily accept physical contact, and thus the experimenter delivered only verbal praise.
Results and Discussion
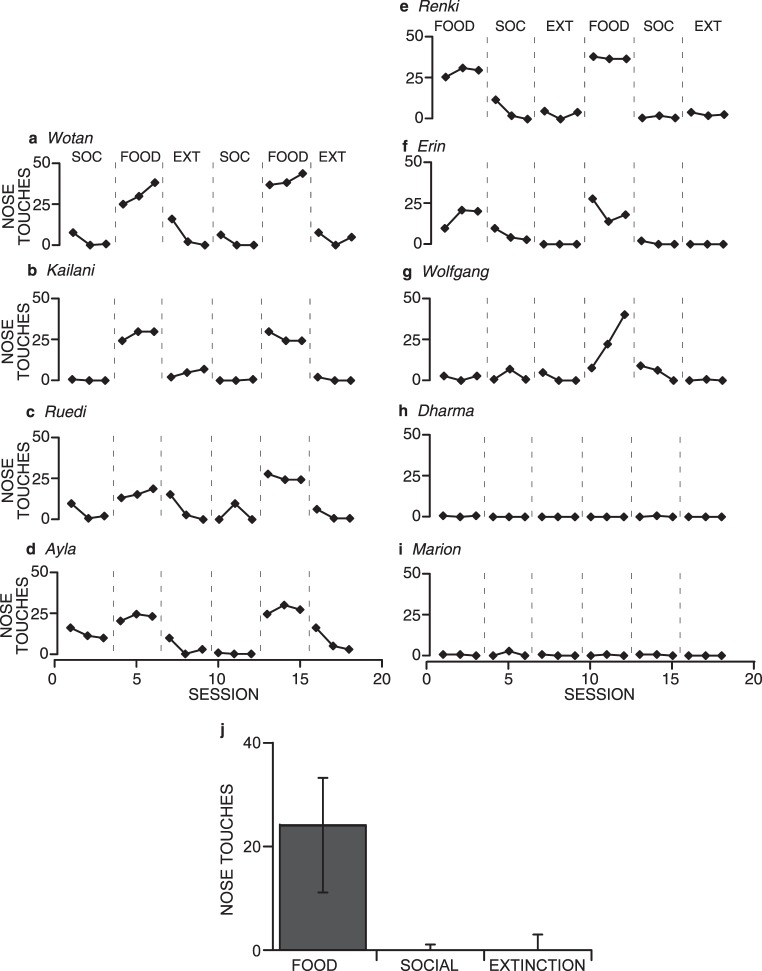
Figure 11 shows data for the 9 wolves we tested. These results closely replicate those obtained from the earlier experiments with domestic dogs. The patterns of responding in the wolves are especially similar to the patterns seen in the owned dogs. Seven of the wolves showed the typical pattern of high responding in the Food condition and low responding in Social and Extinction conditions (Figure 11 a-g); 2 wolves showed overall low levels of responding (h-i). This individual variability is similar to that seen in the previous experiments with domestic dogs. For those 7 wolves that did show differential responding across conditions, food produced much greater numbers of nose touches than did social interaction, and did so in the first exposure to the Food condition for 6 wolves, and in the second exposure to the Food condition for the 7th wolf (Wolfgang). This response differentiation between Food and Social conditions occurred with less exposure to the conditions than it did for shelter dogs but similar to the owned dogs.
Fig. 11.
Total number of nose touches emitted by hand-reared, captive wolves in each session under Food, Social (SOC), and Extinction (EXT) conditions (a-i). Wolves that experienced the Social condition first are graphed on the left; wolves that experienced the Food condition first are graphed on the right. Graph j shows the medians and interquartile ranges of the total number of nose touches per session emitted by all wolves. Other details are the same as for Figure 4g.
The rapid differentiation between conditions is likely a product of the wolves already having an operant nose touch in their repertoires, making acquisition in this setting very rapid. Additionally, it might again be a result of greater recent exposure to the various contingencies (social interaction vs. food vs. extinction) allowing the wolves to discriminate between the contingencies more rapidly. Only 1 wolf, Wotan, showed a temporary increase in responding when Social followed an Extinction condition. Overall, the responses in the Social conditions for wolves were lower and more consistently low than seen in the shelter dogs, but were functionally comparable to the results of the owned dogs.
Figure 11j shows the medians (level of the bars) and interquartile ranges (whiskers) of the total number of nose touches per session emitted by all wolves and is analogous to Figure 4g. The median number of nose touches per session in the Food condition was 24, whereas the median number of nose touches per session in both the Social and Extinction conditions was zero. The interquartile range of the Food condition did not overlap with either that of the Social or Extinction conditions, although the interquartile ranges of the Social and Extinction conditions overlapped with each other. This analysis further substantiates the differences in responding between the Food condition and the Social and Extinction conditions, and little if any difference between the Social and Extinction conditions.
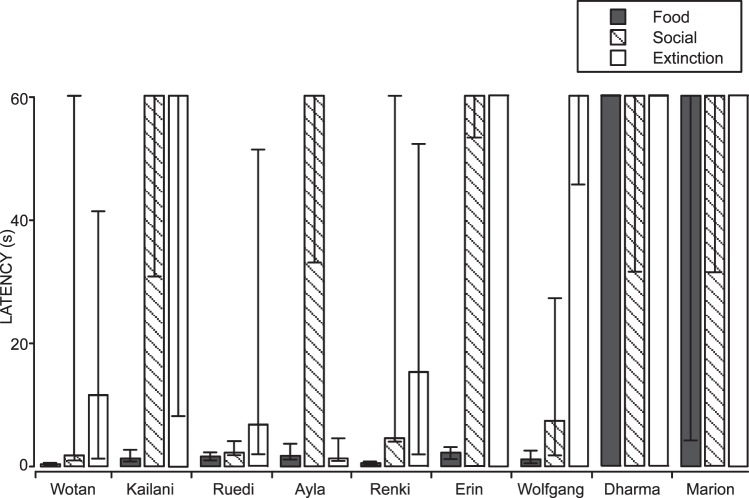
The results for total nose touches emitted correspond to the patterns of latency to respond, which are shown in Figures 12 and 13. Figure 12 shows the cumulative record of all sessions for 2 representative wolves. Both show short latencies characteristic of the Food condition, and relatively longer latencies in the Social and Extinction conditions, regardless of the order in which the conditions were experienced. Figure 13 shows the medians and interquartile ranges of the latency to respond for all 9 wolves. The 7 wolves that emitted a high number of nose touches in the Food condition all had very short and consistent latencies in the Food condition, compared to Social and Extinction conditions, although it varied whether the Social or Extinction condition had a shorter median latency. Of these 7 wolves, 4 had shorter median latencies in the Social condition than in Extinction, 1 had shorter median latencies in extinction, and 2 had equal latencies in Social and Extinction conditions (both were maximal at 60 s). The degree of variability in Social and Extinction conditions varied greatly, as well. However, low variability of latencies was only observed when all or most of the latencies were at the maximum of 60 s.
Fig. 12.
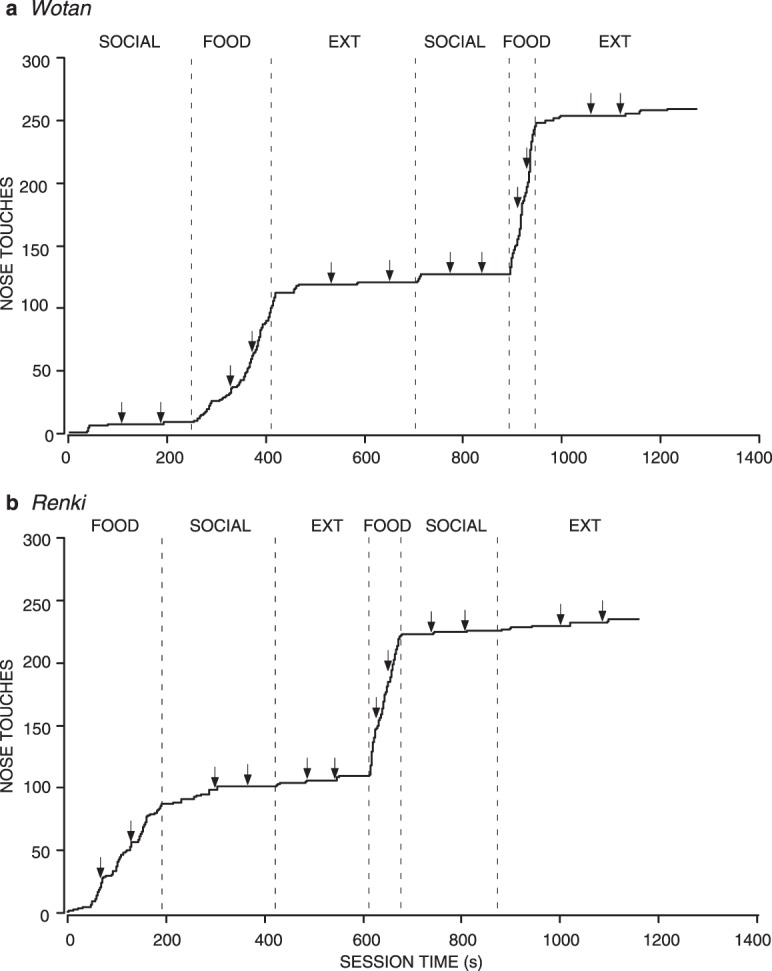
Cumulative records for all sessions for 2 individual wolves from Experiment 5, both corresponding to the most common pattern of responding. One wolf experienced the Social condition first (a) and the other experienced the Food condition first (b). Other details are the same as for Figure 5.
Fig. 13.
Medians and interquartile ranges of the latencies to respond for hand-reared, captive wolves. Details are the same as for Figure 6.
These results again paralleled those of the owned dogs, but differed slightly from the shelter dogs, in which the latency in the Social condition was less variable than in Extinction for all 6 dogs. The 2 wolves that had low overall responding also had long latencies in all three conditions (the median for all three conditions in both wolves was the maximal 60 s), but the degree of variability ranged from no variability (e.g., Food and Extinction conditions for Dharma) to a high level of variability (e.g., Social condition for Dharma and Food condition for Marion).
The only notable difference between the dogs and wolves is the maximum number of nose touches emitted. The wolves emitted the highest number of nose touches of any of the animals we tested. Comparing the maximum number of nose touches in one session, the fastest responding wolf (Wotan) emitted over 33% more nose touches than did the fastest responding dog (Aero). The wolves were very efficient in their responding and the greater number of nose touches emitted occurred concomitantly with shorter latencies (Figure 13). We cannot determine, however, whether this was due to wolves' enhanced performance on problem-solving tasks (Frank & Frank, 1982), the product of the wolves' prior experience with the nose touch task, or the type of food used as reinforcement. These results show, however, that domestication does not appear to have an effect on the relative effectiveness of social interaction in this preparation. For dogs, just as for wolves, food is a stronger and more consistent reinforcer than social interaction as arranged in these experiments.
GENERAL DISCUSSION
As an initial foray into identifying the maintaining reinforcers for sociality in dog–human dyads, we investigated the relative efficacy of brief dog–human social interaction. We had predicted that human social interaction would function as an effective reinforcer in at least one population (shelter or owned) of domestic dogs. Our results revealed that across an array of different canid populations (shelter dogs, owned dogs, and hand-reared wolves) human social interaction typically maintained little responding (few responses and long latencies to respond) compared to food, which maintained a high number of responses with short latencies.
Despite shelter dogs' relative state of deprivation for human social interaction, except for Dog S, human social interaction did not function as a very potent reinforcer, especially compared to food, even when given additional exposure to the Social condition (Experiment 4). There was some evidence that shelter dogs were slightly more sensitive to human social interaction as reinforcer than owned dogs, which might be due to shelter dogs' relative state of deprivation: shelter dogs had less variable latencies in the Social condition compared to extinction than did owned dogs and showed a slight increase in responding in the first Social session following an Extinction condition. Nevertheless, the overall response patterns in both groups of dogs indicate that human social interaction is not an effective reinforcer compared to food. Additionally, having a history of reinforcement with the experimenter, as in the case of owned dogs, also did not affect the efficacy of human social interaction as a reinforcer. It remains to be explored, however, whether owner attention would function as a potent reinforcer for owned dogs if those dogs were subjected to the same level of deprivation from social interaction as were the shelter dogs (e.g., had been at a boarding facility).
The results from the domestic dogs obviated the argument that domestication has made dogs particularly sensitive to humans. This argument stems from reports that dogs outperform hand-reared wolves on tasks related to responding correctly to humans. For example, Hare et al. (2010) reported that dogs were better at following human gestures to find food or other desirable objects than wolves. Other researchers have reported that this difference emerges so early in a dog's life that dogs' ability to correctly follow human gestures cannot be learned and must be a special evolutionary adaptation of dogs (Riedel, Schumann, Kaminski, Call, & Tomasello, 2008, but see Dorey, Udell, & Wynne, 2010 for a reanalysis of the same data). Results from Udell et al. (2008) and Dorey et al. do not support the view that domestication has imbued dogs with special abilities to respond correctly to human behavior and instead suggest that this ability is a product of a specific history of reinforcement. Our results support the latter view that domestication has not necessarily resulted in dogs being more sensitive to human behavior than wolves. The comparison with wolves confirmed that the relative effectiveness of social interaction for hand-reared wolves was the same as for dogs.
We will discuss four possible explanations for our results. First, brief human social interaction might not function as a reinforcer. The initial responding in social interaction sessions might be due to carryover effects from preceding food sessions. We addressed this possibility in the experiments in which we presented Food, Social, and Extinction sessions. Two pieces of evidence suggested that social interaction functioned as a reinforcer. First, in Experiment 2, all 3 of the shelter dogs that experienced the Social condition after Extinction showed brief increases in responding in the first Social session that followed the first Extinction condition. Second, shelter dogs' latency to respond in the Social condition was less variable than in the Extinction condition. Otherwise, social interaction was indistinguishable from extinction.
A second explanation for our results is that human social interaction might need to be conditioned to function as a reinforcer. This possibility could explain why social interaction was not a reinforcer for most shelter dogs (although Dog S data argue against this). Our results from owned dogs, however, indicated that a history of reinforcement did not impact the reinforcing effectiveness of social interaction. Nevertheless, this does not discount the possibility that the conditioning must be very specific (e.g., Isaksen & Holth, 2009) or that human social interaction quickly extinguishes if not tightly paired with another reinforcer.
A third possibility for our results is that human social interaction might function as a reinforcer, and possibly a potent one, but dogs might satiate very rapidly on this reinforcer, as suggested by Stanley, Morris and Trattner (1965). Alternatively, it could function as a reinforcer but only a very weak reinforcer that maintains low levels of responding and possibly only in environments relatively devoid of other reinforcers. It leaves open the possibility that over longer time periods, with less frequent delivery of social interaction, we might detect a reinforcing function. Whether social interaction might maintain responding in a more naturalistic schedule of reinforcement (e.g., interspersed with delivery of other reinforcers, such as food) remains to be explored.
A fourth possibility is that the extreme difference between food and social interaction might be an example of behavioral contrast; that is, alternating between a weak and effective reinforcer may have enhanced the performance difference observed across the Social and Food conditions above that which would be observed had only one reinforcer been arranged in long duration sessions separated by a much longer intersession interval. It is also possible that in Experiments 1 and 2 the dogs had not fully habituated to the experimental room during the early social interaction sessions and that other stimuli in the room controlled behavior in the dogs and interfered with their responding on the operant task. If by the time they had habituated they experienced the food contingency, the lower responding in the subsequent social conditions could be an example of negative behavioral contrast. However, even when given more opportunities to respond under the social interaction contingency before contacting the food contingency (Experiment 4), dogs generally showed consistently low responding. Thus, our results are not simply a product of negative behavioral contrast, although we could not rule out a complementary possibility that the higher responding in Food conditions after Social conditions was due to positive behavioral contrast.
Our results contrast with those of Fonberg et al. (1981) and McIntire and Colley (1967), whose experimental paradigms most closely resemble ours. In both studies, the researchers found that human social interaction in the form of petting maintained responding. Here we note two procedural differences that may account for the different results. First, Fonberg et al. and McIntire and Colley both forced their dogs into position when they failed to respond. This may have punished long latencies and established a negative reinforcement contingency (i.e., escape from forced positioning) that produced short latencies independent of social interaction. Second, we delivered 4 s of social interaction as a consequence whereas McIntire and Colley delivered 5-10 s of interaction and Fonberg et al. delivered 20–30 s. Perhaps durations greater than 4 s are required for social interaction to function as a reinforcer; parametric analyses of duration and reinforcer function should be explored.
Our results parallel those of Mason, Saxon, and Sharpe (1963). They found that chimpanzees (Pan troglodytes) showed a similar preference for food over petting by a human or play with a human in a concurrent choice paradigm. However, in 3–6 month old human infants in an orphanage, Haugan and McIntire (1972) found that food maintained the lowest rate of infant vocalization, whereas vocal imitation by the experimenter maintained the highest rate, followed by tactile stimulation. Perhaps social contact is more reinforcing when delivered by a conspecific (e.g., horses will emit operant responses to gain access to a conspecific: Søndergaard, Jensen, & Nicol, 2011) or there might be phylogenetic or ontogenetic differences that affect sensitivity to social reinforcement.
Our goal was to identify the reinforcers that maintain social behavior between dogs and humans. We hypothesized that social interaction might function as a reinforcer that could maintain dogs' social interactions with humans. Although there were some individual differences, our results suggest that social interaction did not reinforce canid behavior as well as did food. If social interaction functions as a reinforcer, it may do so only under specific conditions not explored in the present experiments. The greater efficacy of food as a reinforcer parallels the evolutionary origins of dogs as scavengers of human refuse (Coppinger & Coppinger, 2001) and supports the use of food as a reinforcer for training. The present findings might provide empirical evidence for trainers to give clients who object to using food to train canid behavior (e.g., Donaldson, 1996).
Acknowledgments
We thank Marion County Animal Services in Ocala, FL for allowing us to use their facility and work with their shelter dogs. We also thank the owners of the pet dogs for allowing us to work with their dogs and for acting as experimenters. Finally we thank Wolf Park in Battleground, IN for allowing us to use their facility, work with their wolves, and for the staff volunteering their time to act as experimenters.
REFERENCES
- Bacon W. E, Stanley W. C. Effect of deprivation level in puppies on performance maintained by a passive person reinforcer. Journal of Comparative and Physiological Science. 1963;56:783–785. doi: 10.1037/h0043821. [DOI] [PubMed] [Google Scholar]
- Barrera G, Jakovcevic G, Elgier A. M, Mustaca A, Bentosela M. Responses of shelter and pet dogs to an unknown human. Journal of Veterinary Behavior. 2010;5:339–344. doi: 10.1016/j.jveb.2010.08.012. [Google Scholar]
- Bentosela M, Barrera G, Jakovcevic A, Elgier A. M, Mustaca A. E. Effect of reinforcement, reinforcer omission and extinction on a communicative response in domestic dogs (Canis familiaris) Behavioural Processes. 2008;78:464–469. doi: 10.1016/j.beproc.2008.03.004. doi: 10.1016/j.beproc.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Coppinger R, Coppinger L. Dogs: A startling new understanding of canine origin, behavior & evolution. New York, NY: Scribner; 2001. [Google Scholar]
- Donaldson J. The culture clash. Berkeley, CA: James & Kenneth Publishers; 1996. [Google Scholar]
- Dorey N, Udell M. A. R, Wynne C. D. L. When do domestic dogs, Canis familiaris, start to understand human pointing? The role of ontogeny in the development of interspecies communication. Animal Behaviour. 2010;79:37–41. doi: 10.1016/j.anbehav.20 09.09.032. [Google Scholar]
- Fonberg E, Kostarczyk E, Prechtl J. Training of instrumental responses in dogs socially reinforced by humans. Pavlovian Journal of Biological Science. 1981;16:183–193. doi: 10.1007/BF03003358. [DOI] [PubMed] [Google Scholar]
- Frank H, Frank M. G. Comparison of problem-solving performance in six-week-old wolves and dogs. Animal Behaviour. 1982;30:95–98. doi:10.1016/S00033472(82)80241.8. [Google Scholar]
- Gantt W. H, Newton J. E. O, Royer F. L, Stephens J. H. Effect of person. Conditional Reflex. 1966;1(1):146–160. [Google Scholar]
- Hare B, Brown M, Williamson C, Tomasello M. The domestication of social cognition in dogs. Science. 2010;298:1634–1636. doi: 10.1126/science.1072702. doi: 10.1126/science.1072702. [DOI] [PubMed] [Google Scholar]
- Hare B, Tomasello M. Human-like social skills in dogs. Trends in Cognitive Sciences. 2005;9:439–444. doi: 10.1016/j.tics.2005.07.003. doi: 10.1016/j.tics.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Haugan G. M, McIntire R. W. Comparisons of vocal imitation, tactile stimulation, and food as reinforcers for infant vocalizations. Developmental Psychology. 1972;6(2):201–209. [Google Scholar]
- Isaksen J, Holth P. An operant approach to teaching joint attention skills to children with autism. Behavioral Interventions. 2009;24:215–236. doi: 10.1002/bin.292. [Google Scholar]
- Mason W. A, Saxon S. V, Sharpe L. G. Preferential responses of young chimpanzees to food and social rewards. Psychological Record. 1963;13:341–345. [Google Scholar]
- McIntire R, Colley T. A. Social reinforcement in the dog. Psychological Reports. 1967;20:843–846. doi: 10.2466/pr0.1967.20.3.843. [DOI] [PubMed] [Google Scholar]
- Miklósi Á, Kubinyi E, Topál J, Gácsi M, Virányi Z, Csányi V. A simple reason for a big difference: Wolves do not look back humans, but dogs do. Current Biology. 2003;9:763–766. doi: 10.1016/s0960-9822(03)00263-x. doi:10.1016/S0960-9822(03)00263-X. [DOI] [PubMed] [Google Scholar]
- Miklósi Á, Topál J, Csányi V. Comparative social cognition: what can dogs teach us. Animal Behaviour. 2004;67:995–1004. doi: 10.1016/j.anbehav.2003.10.008. [Google Scholar]
- Odendaal J. S. J, Meintjes R. A. Neurphysiological correlates of affiliative behavior between humans and dogs. Veterinary Journal. 2003;165:296–301. doi: 10.1016/s1090-0233(02)00237-x. doi:10.1016/S1090-0233(02)00237-X. [DOI] [PubMed] [Google Scholar]
- Riedel J, Schumann K, Kaminski J, Call J, Tomasello M. The early ontogeny of human–dog communication. Animal Behaviour. 2008;75:1003–1014. doi: 10.1016/j.anbehav.2007.08.010. [Google Scholar]
- Skinner B. F. Contingencies of reinforcement. East Norwalk, CT: Appleton-Century-Crofts; 1969. [Google Scholar]
- Søndergaard E, Jensen M. B, Nicol C. J. Motivation for social contact in horses measured by operant conditioning. Applied Animal Behaviour Science. 2011;132:131–137. doi: 10.1016/j.applanim.2011.04.007. [Google Scholar]
- Stanley W. C, Morris D. D, Trattner A. Conditioning with a passive person reinforcer and extinction in Shetland sheep dog puppies. Psychonomic Science. 1965;2:19–20. [Google Scholar]
- Stanley W. C. The passive person as reinforcer in isolated beagle puppies. Psychonomic Science. 1966;2:21–22. [Google Scholar]
- Udell M. A. R, Giglio R. F, Wynne C. D. L. Domestic dogs (Canis familiaris) use human gestures but not nonhuman tokens to find human food. Journal of Comparative Psychology. 2008;122(1):84–93. doi: 10.1037/0735-7036.122.1.84. doi: 10.1037/0735–7036.122.1.84. [DOI] [PubMed] [Google Scholar]
- Udell M. A. R, Dorey N. R, Wynne C. D. L. Wolves outperform dogs in following human social cues. Animal Behaviour. 2008;76(6):1767–1773. doi: 10.1016/j.anbehav.2008.07.028. [Google Scholar]
- Udell M. A. R, Dorey N. R, Wynne C. D. L. The performance of stray dogs (Canis familiaris) living in a shelter on human-guided object-choice tasks. Animal Behaviour. 2010;79(3):717–725. doi: 10.1016/j.anbehav.2009.12.027. [Google Scholar]
- Virányi Z, Topál J, Gácsi M, Miklósi Á, Csányi V. Dogs respond appropriately to cues of humans' attentional focus. Behavioural Processes. 2004;66:161–172. doi: 10.1016/j.beproc.2004.01.012. doi: 10.1016/j.beproc.2004.01.012. [DOI] [PubMed] [Google Scholar]