Abstract
Infection of the tobacco cultivar Samsun NN by tobacco mosaic virus (TMV) results in a hypersensitive response. During this defense reaction several host encoded proteins, known as pathogenesis-related proteins (PR-proteins), are induced. Poly(A)+ RNA from TMV infected tobacco plants was used to construct a cDNA library. Thirty two cDNA clones were isolated and after digestion with different restriction endonucleases, twenty clones were found to code for PR-1a, six clones for PR-1b, and four clones for PR-1c. Two independent cDNA clones of each class were further characterized by DNA sequence analysis. All clones analyzed contained the 138 amino acid coding regions of their respective mature proteins, but only partial sequences of the signal peptides. Minor differences between the nucleotide sequences for clones belonging to the same class were detected. Comparison of the amino acid sequence for PR-1a deduced from its nucleotide sequence with published data obtained by Edman degradation of the protein showed four differences. Analysis of the 3' ends of the cDNA clones indicates that various alternate poly(dA) addition sites are used. Southern blot analysis using these cDNAs as probes suggests the presence of multiple PR-protein genes in the genomes of tobacco and tomato plants.
Full text
PDF



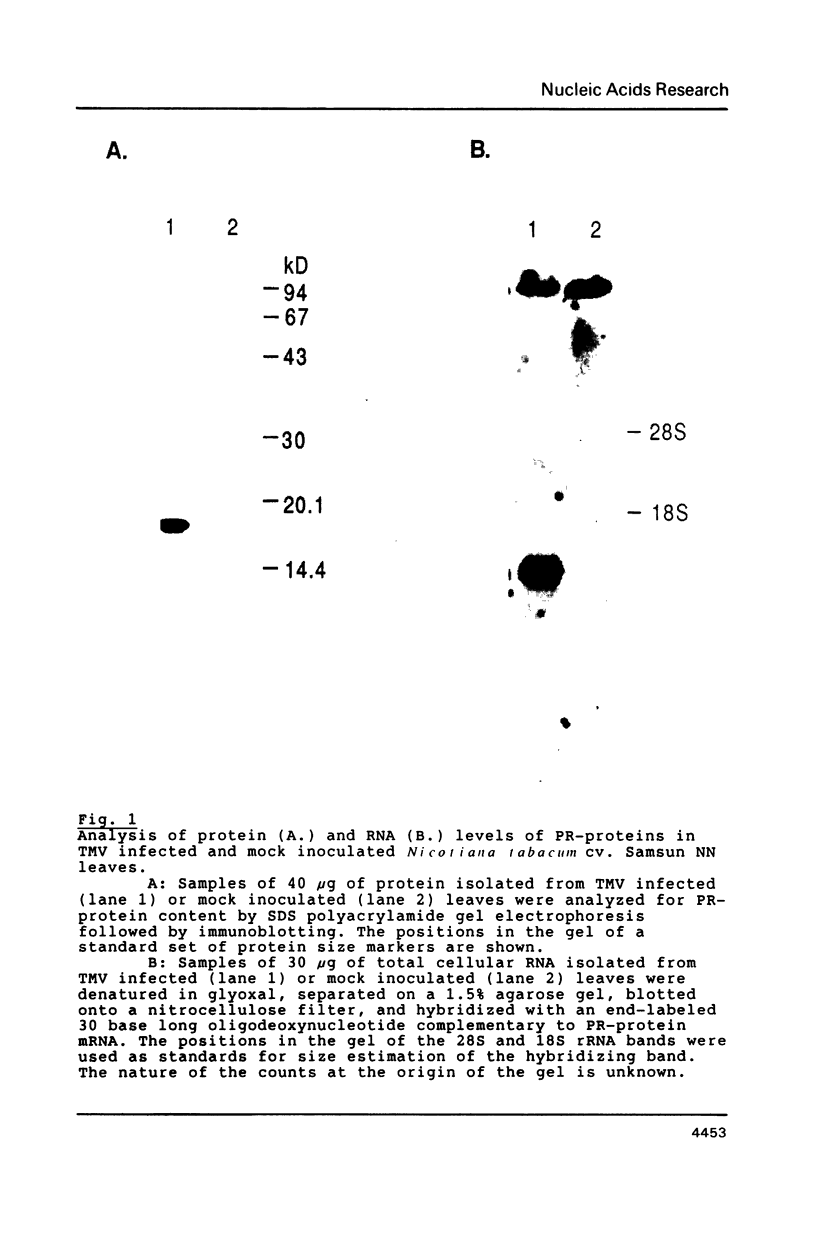

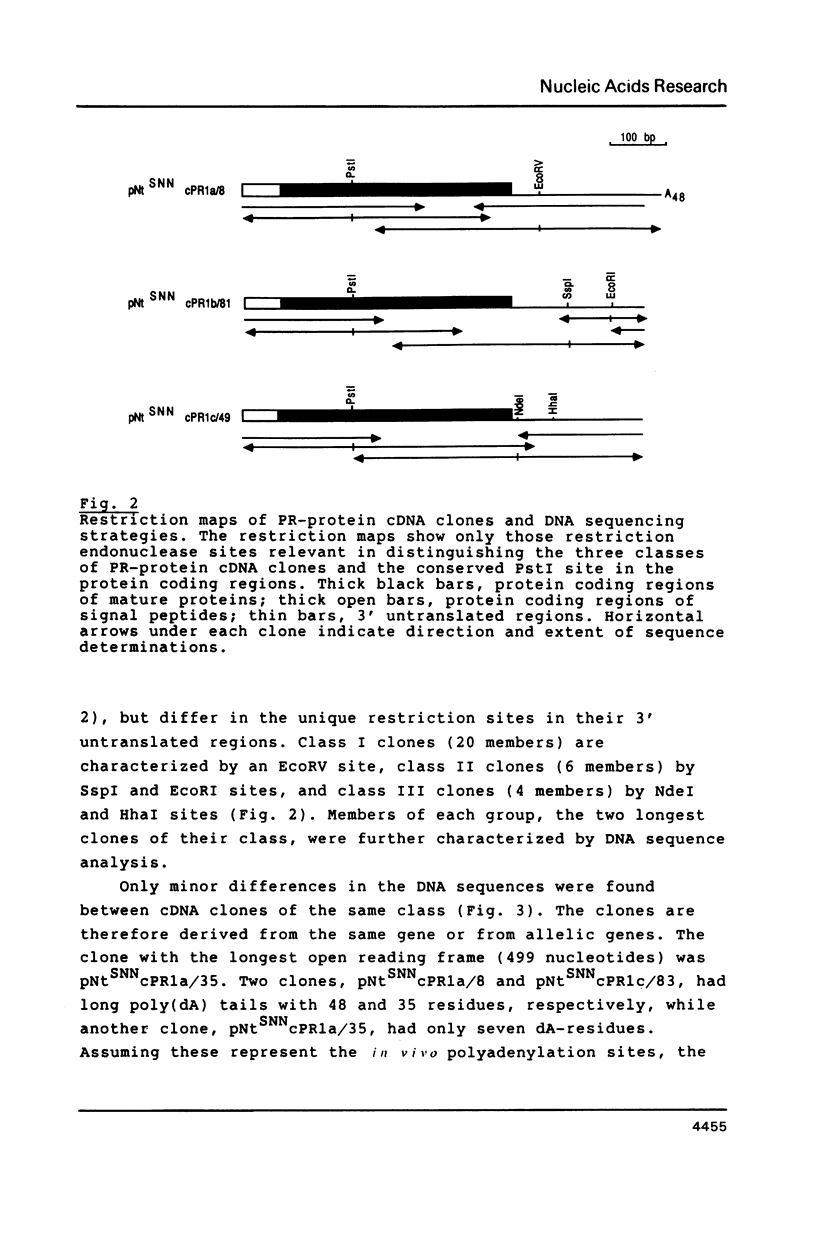




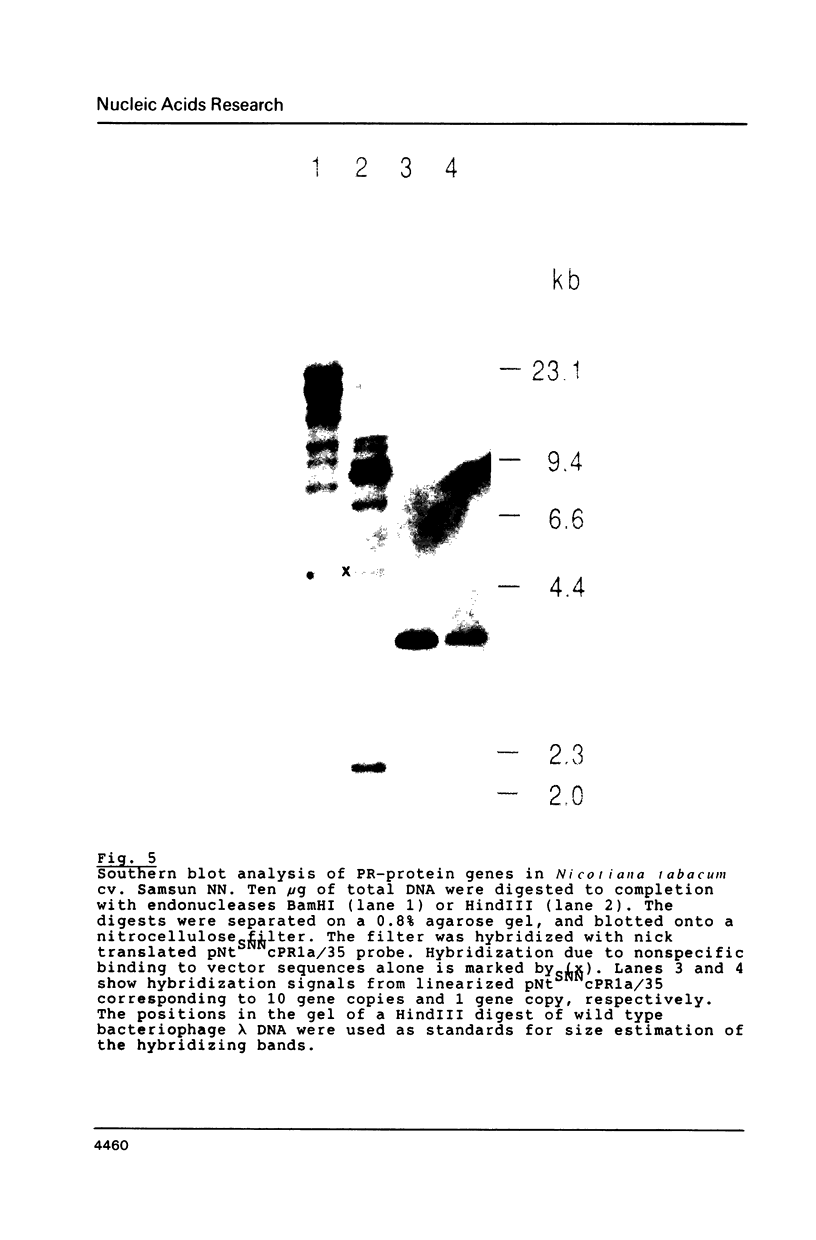
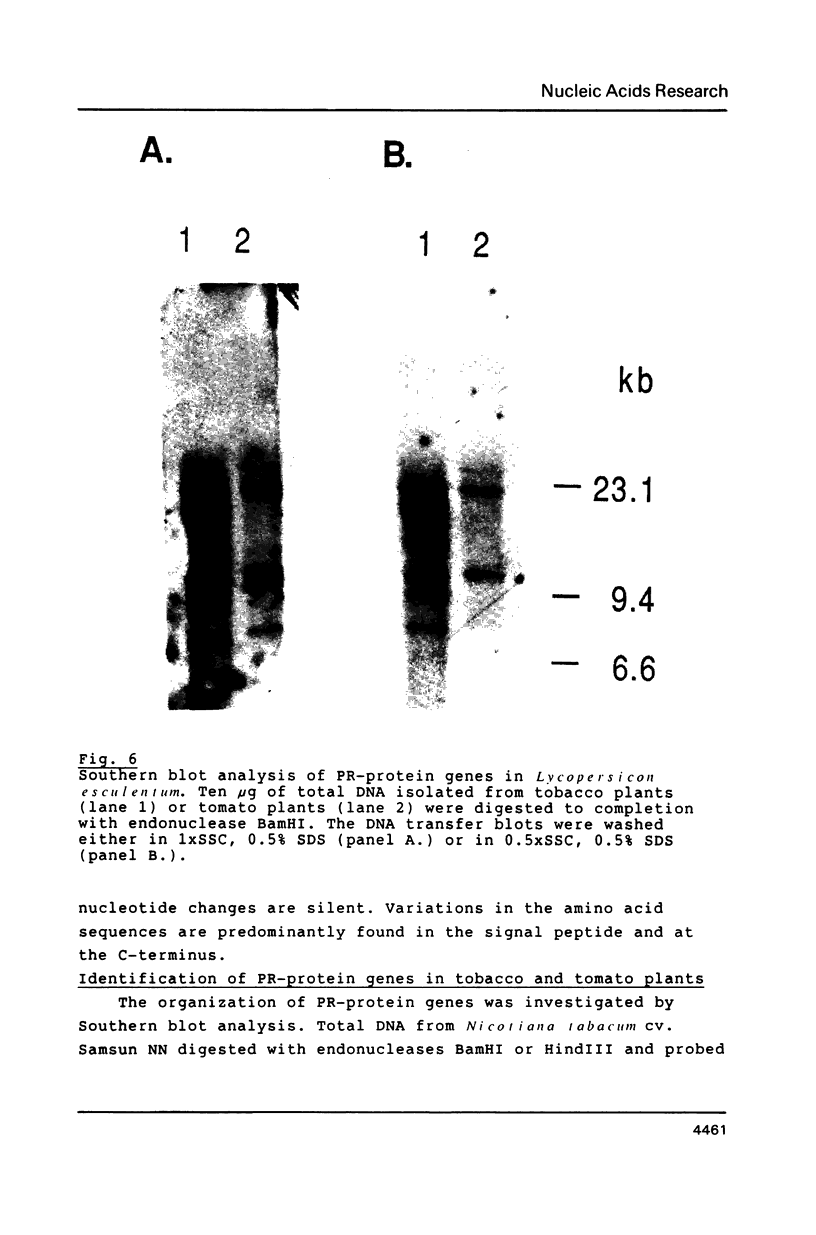




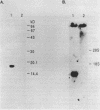
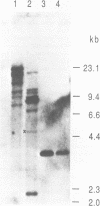
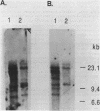
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carr J. P., Dixon D. C., Klessig D. F. Synthesis of pathogenesis-related proteins in tobacco is regulated at the level of mRNA accumulation and occurs on membrane-bound polysomes. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7999–8003. doi: 10.1073/pnas.82.23.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen B. J., Hooft van Huijsduijnen R. A., Van Loon L. C., Bol J. F. Molecular characterization of messenger RNAs for 'pathogenesis related' proteins la, lb and lc, induced by TMV infection of tobacco. EMBO J. 1986 Jan;5(1):37–40. doi: 10.1002/j.1460-2075.1986.tb04174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dean C., Tamaki S., Dunsmuir P., Favreau M., Katayama C., Dooner H., Bedbrook J. mRNA transcripts of several plant genes are polyadenylated at multiple sites in vivo. Nucleic Acids Res. 1986 Mar 11;14(5):2229–2240. doi: 10.1093/nar/14.5.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hooft van Huijsduijnen R. A., Cornelissen B. J., van Loon L. C., van Boom J. H., Tromp M., Bol J. F. Virus-induced synthesis of messenger RNAs for precursors of pathogenesis-related proteins in tobacco. EMBO J. 1985 Sep;4(9):2167–2171. doi: 10.1002/j.1460-2075.1985.tb03911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leberman R. The isolation of plant viruses by means of "simple" coacervates. Virology. 1966 Nov;30(3):341–347. doi: 10.1016/0042-6822(66)90112-7. [DOI] [PubMed] [Google Scholar]
- Lucas J., Henriquez A. C., Lottspeich F., Henschen A., Sänger H. L. Amino acid sequence of the ;pathogenesis-related' leaf protein p14 from viroid-infected tomato reveals a new type of structurally unfamiliar proteins. EMBO J. 1985 Nov;4(11):2745–2749. doi: 10.1002/j.1460-2075.1985.tb03998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M., Ohashi Y. Induction of pathogenesis-related proteins in tobacco leaves. Plant Physiol. 1986 Feb;80(2):505–510. doi: 10.1104/pp.80.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Ross J., Aviv H., Scolnick E., Leder P. In vitro synthesis of DNA complementary to purified rabbit globin mRNA (RNA-dependent DNA polymerase-reticulocyte-hemoglobin-density gradient centrifugation-oligo(dT) primer). Proc Natl Acad Sci U S A. 1972 Jan;69(1):264–268. doi: 10.1073/pnas.69.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somssich I. E., Schmelzer E., Bollmann J., Hahlbrock K. Rapid activation by fungal elicitor of genes encoding "pathogenesis-related" proteins in cultured parsley cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2427–2430. doi: 10.1073/pnas.83.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timko M. P., Kausch A. P., Castresana C., Fassler J., Herrera-Estrella L., Van den Broeck G., Van Montagu M., Schell J., Cashmore A. R. Light regulation of plant gene expression by an upstream enhancer-like element. Nature. 1985 Dec 12;318(6046):579–582. doi: 10.1038/318579a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]