Abstract
Alarmins are endogenous molecules that are constitutively available and released upon tissue damage and activate the immune system. Current evidence indicates that uncontrolled and excessive release of alarmins contributes to the dysregulated processes seen in many inflammatory and autoimmune conditions, as well as tumorigenesis and cancer spread. Conversely, alarmins have also been found to play a major role in the orchestration of tissue homeostasis, including repair and remodeling in the heart, skin, and nervous system. Here, we provide an update and overview on alarmins, highlighting the areas that may benefit from this clinical translation.
Alarmins in health and disease
The alarmin family comprises structurally diverse and evolutionarily unrelated multifunctional endogenous molecules that are passively released from necrotic cells upon infection or tissue injury or are rapidly secreted by stimulated leukocytes and epithelia. In the absence of injury or infection, alarmins play important intracellular roles (Table 1). However, once released extracellularly, alarmins promote activation of innate immune cells and recruitment and activation of antigen-presenting cells engaged in host defense and tissue repair through pattern recognition receptors such as the TLRs, many of which have a key role in the detection of pathogens (refs. 1–3 and Table 1). In health, inflammation is self-limiting and a vital part of the innate host defense. It occurs in response to sterile injury or infection and involves the recruitment of phagocytes to remove cell debris and microbes. This is followed by resolution, with the recruitment of other cell types, including stem and endothelial cells, to restore tissue homeostasis. As potent mediators of inflammation, alarmins play a fundamental role in the pathogenesis of a wide range of sterile or infection-induced immune and inflammatory disorders (4–6). Crucially, their ability to enhance the adaptive immune response through their effects on antigen-presenting cells, including DCs, makes them a critical link between the innate and adaptive arms of the immune response (7). Hence, the alarmin family represents an intriguing therapeutic target, not only to dampen inflammation but also to uncouple the innate and adaptive immune responses in chronic pathologies, including autoimmune disorders. Furthermore, alarmins may serve as useful diagnostic and prognostic biomarkers in inflammatory disorders. While there is now a rapidly growing list of alarmins in the literature, the best characterized in health and disease are high-mobility group protein B1 (HMGB1), S100 proteins, and heat shock proteins (HSPs). In this Review, we will focus on these alarmins, as they have the clearest and most tangible clinical translational potential to date.
Table 1.
Alarmins: physiological and pathological functions
Modulation of the alarmin response to suppress inflammation
Dysregulation of inflammation underlies the pathophysiological process in many immune and inflammatory disorders. The identification of proinflammatory cytokines, in particular TNF-α, as a therapeutic target in the 1990s heralded a paradigm shift that led to impressive clinical benefits, most notably in patients suffering from RA (8). Unfortunately, cytokine blockade is not effective in a significant proportion of patients (9) and has been disappointing in the treatment of patients with acute inflammatory disorders, such as trauma-induced systemic inflammatory response syndrome (SIRS) or sepsis (10). The recent identification of alarmins as crucial mediators of the inflammatory processes in these disorders and the observation that their release is accompanied by an upregulation of their receptor suggests the alarmin signaling pathway as an alternative target (Figure 1).
Figure 1. Alarmin pathway as potential therapeutic target in the innate inflammatory cascade.

The upstream alarmin signaling pathways are potential therapeutic targets for immunomodulation in both acute and chronic inflammatory diseases. This has been achieved in animal models by directly targeting the alarmins using antibodies or competitive inhibitors, e.g., Box A (HMGB1), or by targeting the pattern recognition receptors (PRR) with antibodies or soluble decoy receptors. The convergence of S100 proteins and HMGB1 onto their receptors may be a rate-limiting step in this pathway and hence represents an attractive therapeutic target. Downstream cytokine blockade, including of TNF-α and IL-6, is used clinically in the treatment of chronic inflammatory diseases such as RA and inflammatory bowel disease, but remains ineffective in a significant proportion of patients. Cytokine blockade has been notably unsuccessful in patients with acute inflammatory disorders.
Acute disorders
SIRS and sepsis.
Severe trauma remains the most common cause of death under the age of 45 years. There is a biphasic distribution of mortality, with the initial peak corresponding to the “first hit,” such as severe injury or hemorrhagic shock. This triggers the host innate immune system to mount an immediate inflammatory response, the magnitude of which depends on the severity of the injuries. If the patient survives, this systemic inflammation is further augmented by a “second hit,” such as ischemia/reperfusion, surgical interventions, and opportunistic infections, accounting for the subsequent peak in mortality (11). The resultant “cytokine storm,” which describes the overwhelming and sustained release of pro/anti-inflammatory mediators, including TNF-α, IL-1β, IL-6, and IL-10, is responsible for organ dysfunction as well as increased susceptibility to sepsis (12–14). Indeed, prognosis correlates with circulating levels of biomarkers of systemic inflammation, such as IL-6 and C-reactive protein (CRP).
The prospect of treating polytrauma and sepsis as an inflammatory and immunological disease by immunomodulation remains unfulfilled (10, 15). Strategies involving blockade of cytokines and their receptors were promising in the preclinical setting, but disappointed in clinical trials (10, 16), perhaps in part due to the very early release of TNF-α precluding timely intervention. Currently, there is no FDA-approved agent for the treatment of SIRS or sepsis after activated droterecogin alfa, the recombinant form of human activated protein C, was recalled by the FDA in 2011.
In their role as proinflammatory mediators, alarmins provide valuable insights into the regulation of traumatic inflammation and the pathogenesis of SIRS and related conditions, including sepsis (17), which lead to multiorgan failure and death in up to 60% of patients (18). The best-characterized alarmin in this context is HMGB1. It is rapidly released into the circulation upon severe mechanical trauma (19) and in related conditions as well as sepsis (6). This is associated with a devastating and self-injurious innate immune response. HMGB1 levels are also elevated in other life-threatening conditions associated with acute inflammation, including stroke and acute myocardial infarction (19–21).
In sterile injury, HMGB1 is released as an early mediator that activates the later release of TNF-α and other cytokines, and in animals systemic administration of HMGB1 is lethal (22). Numerous animals studies have shown that inhibition of HMGB1 with either neutralizing antibody or the recombinant antagonists Box A or N-terminal domain of thrombomodulin (23) is beneficial in hemorrhagic shock (24, 25), ischemia/reperfusion (26, 27), acute lung (28, 29), myocardial (30), and cerebral ischemic injury (31, 32). By contrast, HMGB1 appears to be a late mediator in sepsis (6, 22, 33), providing a clinically relevant time frame for pharmacological intervention by HMGB1 antagonism, and it has been effective in preclinical models of sepsis (22, 25, 33, 34). Moreover, the presence of autoantibodies to HMGB1 in sepsis is associated with a favorable outcome in critically ill patients (35). Another member of the HMGB family, HMGN1, has also recently been identified as a novel alarmin that is critical for lipopolysaccharide-induced immune responses (36).
S100A8 and S100A9, the most abundant cytoplasmic proteins of neutrophils and monocytes, are also mediators of inflammation (4). S100A8/S100A9 complexes are released during activation of phagocytes and mediate their effects via TLR4, leading to the production of TNF-α and other cytokines (37). Deficiency in these proteins conferred a survival advantage in models of sepsis (37, 38), and blockade of S100A8 and S100A9 suppressed LPS-induced proinflammatory activities (39). Importantly, S100A8/S100A9 levels were elevated on exposure to minimal amounts of LPS. Levels were also increased in septic patients and inversely correlated with survival (40).
An alternative strategy to targeting the upstream regulators of acute inflammation would be to target the pathways that perpetuate the inflammatory process, thereby preventing the “second hit” (41). For instance, TLR4 and receptor for advanced glycation end products (RAGE) have been shown to perpetuate the innate inflammatory response in septic shock (42), and their inhibition offered protection in animal studies (34). However, total abolition of the response may be counterproductive, as many microbial products share pattern recognition receptors such as TLR4 with alarmins, and inhibition may predispose the patient to infection. Therefore, targeting the alarmins directly would avoid the susceptibility to sepsis normally associated with immunosuppressive therapies. In addition, it would be helpful to develop biomarkers to identify those patients who would benefit from immunomodulatory therapy (10). Thus far, the efficacy of targeting the alarmin/receptor axis remains to be validated in controlled clinical trials.
Chronic and autoimmune disorders
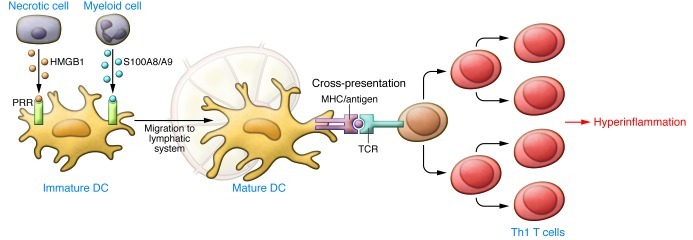
It is now widely accepted that alarmins play a key role in the pathogenesis of inflammatory diseases (refs. 43, 44, and Table 1). They not only initiate but also amplify and sustain the inflammatory processes. Alarmins recruit immature DCs, which take up antigens and home to secondary lymphoid organs, where they present antigenic epitopes to naive T cells, resulting in the induction of the adaptive immune response (45–47). Persistent release of alarmins may lead to upregulation of ectopic MHC type I and II expression and presentation of previously unencountered antigens, as well as proliferation of antigen-specific T lymphocytes, thus preventing their activation-dependent apoptosis and promoting their polarization toward a Th1 phenotype. The overall effect is to drive a local hyperinflammatory environment (Figure 2). More recent data suggest that alarmins may also be able to direct commitment toward a Th2, Th7, or regulatory T cell fate (44).
Figure 2. Induction of chronic and autoimmune inflammation by alarmins by upregulating the adaptive immune response.
Alarmins recruit immature DCs and induce their functional maturation, leading them to take up antigens and home to secondary lymphoid organs. Here, they present antigenic epitopes to naive T cells, driving Th1 T cell polarization and inducing an adaptive immune response. Persistent release of alarmins upregulates ectopic MHC type I and II expression and presentation of previously unencountered antigens as well as uncontrolled proliferation of T cells, driving a hyper-inflammatory environment. Adapted with permission from Immunological Reviews (7).
S100A8, S100A9, and S100A12 in humans are highly expressed by phagocytes within the affected joints in inflammatory arthritis (48–50). They activate the endothelium; recruit and stimulate immune cells such as macrophages to produce proinflammatory cytokines, including TNF-α and IL-1β; and demonstrate cytotoxic effects, leading to tissue destruction (51–53). They also appear to be essential in the development of autoreactive CD8+ T cells and systemic immunity (54). Strategies that target the proinflammatory properties of S100 proteins may therefore provide a novel approach for immunotherapy upstream of TNF-α and NF-κB activation, as illustrated by animal studies of hypersensitivity and chronic bowel inflammation (41). However, unlike S100 proteins, HMGB1 involvement appears to be independent of TNF-α (55). Anti–TNF-α therapy had no effect on HMGB1 expression (56), but anti-HMGB1 antibodies and the antagonist Box A successfully inhibited the development of synovial inflammation and joint swelling in animal models of arthritis (55, 57, 58). Furthermore, binding of HMGB1 to other endogenous partners such as nucleosomes appears to break immunological tolerance and contribute to the pathogenesis of autoimmunity (59). Therefore, alarmins are attractive targets in RA and other chronic inflammatory disorders, especially for patients who do not respond to anti–TNF-α therapy.
In contrast, members of the HSP family, in particular HSP60 and HSP70, have been identified as alarmins whose upregulation may actually be beneficial to patients with inflammatory arthritis (60). HSP60 induces a subtype of regulatory T cells that suppress proinflammatory T cells (61–64). Transfer of these HSP-specific regulatory T cells inhibited inflammation in animal models of arthritis (60, 62). A recent phase II clinical trial yielded promising results (65, 66). This strategy illustrates how targeting the upstream components of the inflammatory cascade that leads to pathological cytokine production may offer a more effective strategy.
Cancer
Chronic inflammation and necrotic cell death are important features of tumorigenesis (67), and alarmins are upregulated in a number of cancers (68–73). The S100A8/S100A9 proteins promote proliferation and survival of tumor cells in vitro (74, 75), and upregulation of S100A9 in myeloid precursors results in the accumulation of cells that suppress T cell proliferation and enable immune evasion (76). S100A8/S100A9 have also been found to promote tumor spread (76–78). Anti–carboxylated glycan antibodies used to inhibit S100 protein-N-glycan binding reduced chronic inflammation and tumorigenesis in an in vivo model of colitis-associated carcinogenesis (75).
Like the S100 proteins, HMGB1 has both intra- and extracellular roles in carcinogenesis (79) and tumor spread (80–83), probably through its ability to promote cell migration (51) and angiogenesis (84). Members of the S100 family interact with cytoskeletal elements, including microtubules and actin, leading to increased cellular motility and invasion (85, 86). Upregulation of HMGB1 expression has been found in melanoma and cancers of the prostate, pancreas, and breast (80, 87, 88) and is associated with invasion and metastasis. Blockade of HMGB1 and RAGE suppressed tumor growth and metastasis in murine models of lung and colon cancer (79, 89).
Other alarmins implicated in the promotion of cancer include defensins and cathelicidins. Defensins are a family of cysteine-rich, cationic peptides produced by cells involved in host defense against microbial infections. Expression of some defensins is constitutive, whereas for others it is regulated by damage-associated molecular patterns (DAMPs), cytokines, and growth factors in a tissue-specific manner. Human β-defensin–4 (DEFB4), the homolog of murine Defb29 that has been shown to have proangiogenic and protumorigenic functions, is upregulated seven-fold in stage III compared with stage I tumors (90). Human cationic antimicrobial protein 18 (hCAP-18) is the only known member of the cathelicidin family in humans. The active peptide LL-37 is overexpressed in breast, lung, and ovarian tumors, probably functioning as an autocrine survival factor (91–93). It may also promote tumor growth through angiogenesis and recruitment of CD45+ cells (69).
Paradoxically, alarmins also display antitumor characteristics. Dying tumor cells following chemotherapy and radiotherapy release HMGB1, which can induce the maturation of DCs via TLR2 and TLR4 to promote a cytotoxic T lymphocyte response through cross-presentation of tumor antigens (94–96). Various defensins have been found to have tumor suppressor properties (97–99) and are significantly downregulated in various carcinomas, including DEFB1 in renal cell and prostate carcinomas and DEFB4A and α-defensin HNP-2 in cervical squamous cell carcinomas. Furthermore, although data on the role of α-defensins in cancer biology are currently lacking, they may be antiangiogenic, acting by disrupting fibronectin signaling via the α5β1 integrin (100). The apparently contradictory role of alarmins in cancers requires further investigation.
Alarmins as biomarkers
Similar to inflammatory biomarkers such as CRP, erythrocyte sedimentation rate (ESR), and IL-6, the levels of the S100 proteins correlate with disease activity in a number of inflammatory conditions (Table 2 and refs. 51, 101–103). However, S100 proteins show important advantages over traditional clinical or laboratory markers for specific indications, probably due to their local expression and release in direct response to tissue damage. Serum levels of S100A8/S100A9 were found to correlate better with disease activity and joint destruction in various inflammatory arthritides than classical markers of inflammation (104–106). In addition, serum S100A8/S100A9 and S100A12 can precisely stage severity and response to therapy (107) as well as predicting relapse (108, 109) and clinical progression, such as the development of erosive disease (110) and radiographic progression of joint damage (111). The suitability of both S100 proteins and HMGB1 as biomarkers for acute systemic inflammatory conditions such as sepsis and following major surgery is also currently under investigation (e.g., ref. 112). Last but not least, S100A8/S100A9 is the only parameter so far that allows early and reliable diagnosis of systemic onset juvenile idiopathic arthritis (SOJIA) and differentiates from infection, which is critical in instigating the appropriate treatment (50).
Table 2.
Alarmins as biomarkers

Alarmins as regenerative therapy
The effects of alarmins, whether beneficial or detrimental, appear to depend on timing of release, dose, and context. Excessive and chronic presence of alarmins and unremitting alarmin-induced events exacerbate injury, but when expressed in a transient and self-limited manner upon injury and acute inflammation, they mediate repair (113). This dual role is exemplified by the proinflammatory cytokine TNF-α. While sustained upregulation has a destructive role in many inflammatory conditions, TNF-α also acts as a growth factor for myelin-producing cells (114), differentiation factor for mesenchymal stem cells (115), and potential therapeutic in the infarcted myocardium (116) or bone fractures (117).
The regenerative role of extracellular HMGB1 is largely mediated by its chemoattractant effects (84, 118–121) as well as its ability to promote cell proliferation (120, 122) and neo-angiogenesis (84, 120, 121). The β-defensin family and cathelicidins also exhibit proangiogenic (90), chemotactic, and proliferative properties (123, 124). Thus far, research on the use of alarmins in regenerative therapy is limited to preclinical studies, the greatest challenge being to understand how to enhance the regenerative processes in postnatal human tissues, where most cells are terminally differentiated and tissues heal with fibrosis following injury.
Exogenous application of alarmins has shown promise in cutaneous wounds. Skin wound repair is problematic in diabetes mellitus due to a dysregulated inflammatory response compounded by an increased microbial load, excessive protease activity, and vascular compromise (125). The antimicrobial alarmins are particularly attractive for cutaneous wound healing due to their additional antimicrobial activities. The pre-form hCAP18 is upregulated in human skin upon wounding, but its levels are low in chronic ulcers. Moreover, antibodies against LL-37 inhibited re-epithelialization (126). Human DEFB3 expression through viral transfection led to accelerated wound closure in Staphylococcus aureus–infected diabetic wounds in a pig model (127). HMGB1 expression is reduced in diabetic skin (125). Topical application of HMGB1 to wounds accelerated healing in diabetic mice but not normoglycemic mice, whereas topical Box A impaired wound healing in normoglycemic mice, suggesting that the latter may already have optimal levels of HMGB1 (125). S100A8 and S100A9 also appear to promote skin wound healing (128), and wound fluid from nondiabetic patients with non-healing venous leg ulcers showed that S100A8 and S100A9 were significantly reduced (129).
The use of exogenous alarmins to recruit and induce proliferation and differentiation of resident stem cells to enhance wound healing was demonstrated initially in a murine model of myocardial infarction (122, 130). Local administration of HMGB1 led to improved structural and functional outcomes after infarction (131). Furthermore, cardiac-specific overexpression of HMGB1 conferred significant protection against tissue damage and was associated with improved cardiac function (132), while anti-HMGB1 antibodies exacerbated injury (133).
Despite the evidence that alarmins promote tissue homeostasis, there are also data suggesting the contrary. For instance, although HMGB1 is a potent neurotrophic mediator, it also contributes to neuronal cell death in cerebral ischemia (32), and downregulation conferred significant protection (31, 32, 134). Activation of proinflammatory pathways by HMGB1 also exacerbated myocardial injury. Serum HMGB1 levels are elevated in patients with myocardial infarction and correlate with poor clinical outcomes (21). Treatment with HMGB1 inhibitor, Box A, significantly reduced infarct size and tissue damage in an ischemia/reperfusion injury model of the murine heart, and systemically administered rHMGB1 increased the severity of damage (30). While these findings appear to conflict with other studies that suggest that exogenous HMGB1 promoted cardiac regeneration (30, 122), this discrepancy may be explained by the low dose of HMGB1 being administered during a critical time window when its expression was low in the latter studies.
The harmful role of alarmins is particularly evident in chronic conditions. For example, activated macrophages promote destruction and impair regeneration via secretion of S100A8 and S100A9 in inflammatory muscle diseases (135), and blockade of RAGE restores effective cutaneous wound healing in diabetic mice (136). Comparison of acute and chronic wounds in humans identified elevated levels of S100A8 and S100A9 from the exudate of non-healing wounds (137). However, this may again be attributed to a dose-dependent effect: for example, low doses of S100B have been found to promote neurite outgrowth, whereas high doses led to apoptosis (138, 139).
Challenges and future directions
The therapeutic potential for immunomodulation by targeting alarmins and their signaling pathways appears promising and needs to be tested in clinical trials. However, there are several key issues that remain to be addressed.
First, it remains unclear whether there exists a hierarchy of dominance in the inflammatory effects of alarmins. For example, both HMGB1 and S100 proteins have been shown to be critical inflammatory mediators in RA, but which is the master regulator, and how do we decide which to target?
Second is the practical issue of how to inhibit alarmins in inflammatory conditions. While neutralizing antibodies or antagonists have been successful in experimental models, effective blockade of their proinflammatory activities clinically may prove challenging given the high extracellular concentrations encountered at sites of inflammation, such as in the synovial joints. An approach that targets the steps that are rate limiting may prove more effective — for example, inhibition of the S100A8/S100AA9-dependent transendothelial leukocyte migration by blocking S100-N-glycan binding to endothelial cells (140, 141) or inhibition of the active release of S100A8/S100A9 by selectively targeting the “alternative pathway” in phagocytes. Furthermore, the concomitant release of multiple alarmins, all of which appear to drive and perpetuate inflammation, presents a challenge, as their inhibition may lead to differential effects, as exemplified by the beneficial effects of S100A8/S100A9 and HMGB1 inhibition versus that of HSP upregulation in inflammatory arthritis. Furthermore, the convergence of alarmins on pattern recognition receptors, for example S100A8/S100A9 and HMGB1 on TLR4 and RAGE, may mean that inhibitors of these receptors or their downstream intracellular signaling cascades may represent a more efficient approach (refs. 142–144 and Figure 2). However, a potential drawback of this approach is that many microbial products share patterns recognition receptors such as TLR4, and hence therapeutic approaches directed against the receptors may increase the risk of infection.
The third and greatest challenge concerns the issue of balance. Like many signaling molecules, alarmins exhibit both harmful and beneficial effects within the same disease context. While dampening of inflammation may be desirable in certain pathological contexts, total abolition of the host defense is detrimental, leaving the patient susceptible to opportunistic infections and tumorigenesis as well as impairing repair and remodeling pathways. Conversely, the alarmin signaling axis can be manipulated to activate transient and self-limited inflammation and pathways that orchestrate tissue homeostasis. This is likely to be achieved by a better understanding of how the microenvironment and dosage contribute to the net effects of alarmins. For example, it has been found that low doses of S100B induce trophic effects in neurites, whereas high doses induce apoptosis (138). Furthermore, we must understand how to clinically modulate the local environment so as to activate the innate protective pathways that initiate regenerative and repair processes while downregulating the self-injurious pathways that inhibit repair and drive excessive and deleterious cytokine release. An area that deserves particular attention is the mechanism by which alarmins mediate the interaction between alarmin-stimulated DCs and tissue-repair macrophages, as this would offer the prospect of a rational, mechanism-based approach to promote wound repair.
The translational potential of alarmins and their signaling pathways is not restricted to the clinical conditions mentioned in this Review. For example, the immunological adjuvant activity of all alarmins and antimicrobial properties of many also mean that they have therapeutic potential in the development of therapies and vaccines against viruses, fungi, and cancer (145–147). However, this is relatively new territory, and such advances are more distant on the translational horizon.
Over the past century, tremendous strides have been made in understanding the immune system, leading to significant translational successes, including the development of immunosuppressive drugs in the field of organ transplantation (148) and, more recently, biologic therapies, including anti–TNF-α therapy in the treatment of RA and other chronic autoimmune conditions (149). Further understanding of apparently conflicting roles of alarmins in inflammation and repair is beginning to yield novel approaches for translation to the clinical arena.
Supplementary Material
Acknowledgments
James Chan was supported by the Wellcome Trust [096035] and the Royal College of Surgeons of England. Project no. F-09-23N was supported by the AO Foundation.
Footnotes
Conflict of interest: Marc Feldmann is a shareholder in Merck, Johnson & Johnson, Maimonidex, Xenexus, and BioAtrix. He is a consultant for Halozyme TetraLogic Pharmaceuticals and GlaxoSmithKline (GSK) and receives research support from Novo Nordisk, Merck, Wyeth, and GSK.
Citation for this article: J Clin Invest. 2012;122(8):2711–2719. doi:10.1172/JCI62423.
References
- 1.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17(4):359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi ME, Manfredi AA. Immunology. Dangers in and out. Science. 2009;323(5922):1683–1684. doi: 10.1126/science.1172794. [DOI] [PubMed] [Google Scholar]
- 3.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 4.Ehrchen JM, Sunderkotter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009;86(3):557–566. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- 5.Zhu S, Li W, Ward MF, Sama AE, Wang H. High mobility group box 1 protein as a potential drug target for infection- and injury-elicited inflammation. Inflamm Allergy Drug Targets. 2010;9(1):60–72. doi: 10.2174/187152810791292872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianchi ME, Manfredi AA. High–mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 8.Taylor PC, Feldmann M. Anti-TNF biologic agents: still the therapy of choice for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):578–582. doi: 10.1038/nrrheum.2009.181. [DOI] [PubMed] [Google Scholar]
- 9.Feldmann M, Maini RN. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9(10):1245–1250. doi: 10.1038/nm939. [DOI] [PubMed] [Google Scholar]
- 10.Reinhart K, Karzai W. Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit Care Med. 2001;29(7 suppl):S121–S125. doi: 10.1097/00003246-200107001-00037. [DOI] [PubMed] [Google Scholar]
- 11.Rotstein OD. Modeling the two-hit hypothesis for evaluating strategies to prevent organ injury after shock/resuscitation. J Trauma. 2003;54(5 suppl):S203–S206. doi: 10.1097/00005373-200301000-00032. [DOI] [PubMed] [Google Scholar]
- 12.Hensler T, et al. Association between injury pattern of patients with multiple injuries and circulating levels of soluble tumor necrosis factor receptors, interleukin-6 and interleukin-10, and polymorphonuclear neutrophil elastase. J Trauma. 2002;52(5):962–970. doi: 10.1097/00005373-200205000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 14.Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36(6):691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 15.Martin TR. Cytokines and the acute respiratory distress syndrome (ARDS): a question of balance. Nat Med. 1997;3(3):272–273. doi: 10.1038/nm0397-272. [DOI] [PubMed] [Google Scholar]
- 16.Tracey KJ, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 17.Zedler S, Faist E. The impact of endogenous triggers on trauma-associated inflammation. Curr Opin Crit Care. 2006;12(6):595–601. doi: 10.1097/MCC.0b013e3280106806. [DOI] [PubMed] [Google Scholar]
- 18.Neunaber C, et al. Immunomodulation in polytrauma and polymicrobial sepsis — where do we stand? Recent Pat Inflamm Allergy Drug Discov. 2011;5(1):17–25. doi: 10.2174/187221311794474892. [DOI] [PubMed] [Google Scholar]
- 19.Peltz ED, et al. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock. 2009;32(1):17–22. doi: 10.1097/SHK.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohno T, et al. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovasc Res. 2009;81(3):565–573. doi: 10.1093/cvr/cvn291. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein RS, et al. Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock. 2006;25(6):571–574. doi: 10.1097/01.shk.0000209540.99176.72. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 23.Abeyama K, et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005;115(5):1267–1274. doi: 10.1172/JCI22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang R, et al. Anti–HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12(4–6):105–114. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JY, et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005;288(5):L958–L965. doi: 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- 26.Tsung A, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201(7):1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe T, et al. The role of HMGB-1 on the development of necrosis during hepatic ischemia and hepatic ischemia/reperfusion injury in mice. J Surg Res. 2005;124(1):59–66. doi: 10.1016/j.jss.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa EN, et al. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am J Respir Crit Care Med. 2006;174(4):400–407. doi: 10.1164/rccm.200605-699OC. [DOI] [PubMed] [Google Scholar]
- 29.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165(6):2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 30.Andrassy M, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117(25):3216–3226. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 31.Liu K, et al. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 2007;21(14):3904–3916. doi: 10.1096/fj.07-8770com. [DOI] [PubMed] [Google Scholar]
- 32.Muhammad S, et al. The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci. 2008;28(46):12023–12031. doi: 10.1523/JNEUROSCI.2435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101(1):296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutterloh EC, et al. Inhibition of the RAGE products increases survival in experimental models of severe sepsis and systemic infection. Crit Care. 2007;11(6):R122. doi: 10.1186/cc6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnay-Verdier S, Fattoum L, Borde C, Kaveri S, Gibot S, Marechal V. Emergence of autoantibodies to HMGB1 is associated with survival in patients with septic shock. Intensive Care Med. 2011;37(6):957–962. doi: 10.1007/s00134-011-2192-6. [DOI] [PubMed] [Google Scholar]
- 36.Yang D, et al. High-mobility group nucleosome-binding protein 1 acts as an alarmin and is critical for lipopolysaccharide-induced immune responses. J Exp Med. 2012;209(1):157–171. doi: 10.1084/jem.20101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogl T, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13(9):1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 38.van Zoelen MA, et al. Expression and role of myeloid-related protein-14 in clinical and experimental sepsis. Am J Respir Crit Care Med. 2009;180(11):1098–1106. doi: 10.1164/rccm.200810-1552OC. [DOI] [PubMed] [Google Scholar]
- 39.Vandal K, Rouleau P, Boivin A, Ryckman C, Talbot M, Tessier PA. Blockade of S100A8 and S100A9 suppresses neutrophil migration in response to lipopolysaccharide. J Immunol. 2003;171(5):2602–2609. doi: 10.4049/jimmunol.171.5.2602. [DOI] [PubMed] [Google Scholar]
- 40.Payen D, et al. Gene profiling in human blood leucocytes during recovery from septic shock. Intensive Care Med. 2008;34(8):1371–1376. doi: 10.1007/s00134-008-1048-1. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108(7):949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liliensiek B, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113(11):1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamaki Y, et al. Expression of Toll-like receptors and their signaling pathways in rheumatoid synovitis. J Rheumatol. 2011;38(5):810–820. doi: 10.3899/jrheum.100732. [DOI] [PubMed] [Google Scholar]
- 44.Manfredi AA, Capobianco A, Bianchi ME, Rovere-Querini P. Regulation of dendritic- and T-cell fate by injury-associated endogenous signals. Crit Rev Immunol. 2009;29(1):69–86. doi: 10.1615/critrevimmunol.v29.i1.30. [DOI] [PubMed] [Google Scholar]
- 45.Yang D, Tewary P, de la Rosa G, Wei F, Oppenheim JJ. The alarmin functions of high–mobility group proteins. Biochim Biophys Acta. 2010;1799(1–2):157–163. doi: 10.1016/j.bbagrm.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dumitriu IE, et al. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174(12):7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 47.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81(1):59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 48.Frosch M, et al. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2000;43(3):628–637. doi: 10.1002/1529-0131(200003)43:3<628::AID-ANR20>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 49.Odink K, et al. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330(6143):80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- 50.Frosch M, Roth J. New insights in systemic juvenile idiopathic arthritis — from pathophysiology to treatment. Rheumatology (Oxford). 2008;47(2):121–125. doi: 10.1093/rheumatology/kem271. [DOI] [PubMed] [Google Scholar]
- 51.Foell D, Roth J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 2004;50(12):3762–3771. doi: 10.1002/art.20631. [DOI] [PubMed] [Google Scholar]
- 52.van Lent PL, et al. Myeloid-related proteins S100A8/S100A9 regulate joint inflammation and cartilage destruction during antigen-induced arthritis. Ann Rheum Dis. 2008;67(12):1750–1758. doi: 10.1136/ard.2007.077800. [DOI] [PubMed] [Google Scholar]
- 53.van Lent PL, et al. Stimulation of chondrocyte-mediated cartilage destruction by S100A8 in experimental murine arthritis. Arthritis Rheum. 2008;58(12):3776–3787. doi: 10.1002/art.24074. [DOI] [PubMed] [Google Scholar]
- 54.Loser K, et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat Med. 2010;16(6):713–717. doi: 10.1038/nm.2150. [DOI] [PubMed] [Google Scholar]
- 55.Pullerits R, Jonsson IM, Kollias G, Tarkowski A. Induction of arthritis by high mobility group box chromosomal protein 1 is independent of tumour necrosis factor signalling. Arthritis Res Ther. 2008;10(3):R72. doi: 10.1186/ar2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sundberg E, et al. Systemic TNF blockade does not modulate synovial expression of the pro-inflammatory mediator HMGB1 in rheumatoid arthritis patients--a prospective clinical study. Arthritis Res Ther. 2008;10(2):R33. doi: 10.1186/ar2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kokkola R, et al. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 2003;48(7):2052–2058. doi: 10.1002/art.11161. [DOI] [PubMed] [Google Scholar]
- 58.Ostberg T, et al. Protective targeting of high mobility group box chromosomal protein 1 in a spontaneous arthritis model. Arthritis Rheum. 2010;62(10):2963–2972. doi: 10.1002/art.27590. [DOI] [PubMed] [Google Scholar]
- 59.Urbonaviciute V, et al. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med. 2008;205(13):3007–3018. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5(4):318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- 61.Kamphuis S, et al. Tolerogenic immune responses to novel T-cell epitopes from heat-shock protein 60 in juvenile idiopathic arthritis. Lancet. 2005;366(9479):50–56. doi: 10.1016/S0140-6736(05)66827-4. [DOI] [PubMed] [Google Scholar]
- 62.Prakken BJ, et al. Inhibition of adjuvant-induced arthritis by interleukin-10-driven regulatory cells induced via nasal administration of a peptide analog of an arthritis-related heat-shock protein 60 T cell epitope. Arthritis Rheum. 2002;46(7):1937–1946. doi: 10.1002/art.10366. [DOI] [PubMed] [Google Scholar]
- 63.de Kleer IM, et al. CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol. 2004;172(10):6435–6443. doi: 10.4049/jimmunol.172.10.6435. [DOI] [PubMed] [Google Scholar]
- 64.Prakken BJ, et al. Epitope-specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2004;101(12):4228–4233. doi: 10.1073/pnas.0400061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Broere F, van der Zee R, van Eden W. Heat shock proteins are no DAMPs, rather ‘DAMPERs’. Nat Rev Immunol. 2011;11(8):565. doi: 10.1038/nri2873-c1. [DOI] [PubMed] [Google Scholar]
- 66.Koffeman EC, et al. Epitope-specific immunotherapy of rheumatoid arthritis: clinical responsiveness occurs with immune deviation and relies on the expression of a cluster of molecules associated with T cell tolerance in a double-blind, placebo-controlled, pilot phase II trial. Arthritis Rheum. 2009;60(11):3207–3216. doi: 10.1002/art.24916. [DOI] [PubMed] [Google Scholar]
- 67.Srikrishna G, Freeze HH. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009;11(7):615–628. doi: 10.1593/neo.09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 69.Coffelt SB, Scandurro AB. Tumors sound the alarmin(s). Cancer Res. 2008;68(16):6482–6485. doi: 10.1158/0008-5472.CAN-08-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang D, Kang R, Zeh HJ, 3rd, Lotze MT. High–mobility group box 1 and cancer. Biochim Biophys Acta. 2010;1799(1–2):131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salama I, Malone PS, Mihaimeed F, Jones JL. A review of the S100 proteins in cancer. Eur J Surg Oncol. 2008;34(4):357–364. doi: 10.1016/j.ejso.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 72.Gebhardt C, Nemeth J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol. 2006;72(11):1622–1631. doi: 10.1016/j.bcp.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 73.Fages C, Nolo R, Huttunen HJ, Eskelinen E, Rauvala H. Regulation of cell migration by amphoterin. J Cell Sci. 2000;113(pt 4):611–620. doi: 10.1242/jcs.113.4.611. [DOI] [PubMed] [Google Scholar]
- 74.Arumugam T, Simeone DM, Schmidt AM, Logsdon CD. S100P stimulates cell proliferation and survival via receptor for activated glycation end products (RAGE). J Biol Chem. 2004;279(7):5059–5065. doi: 10.1074/jbc.M310124200. [DOI] [PubMed] [Google Scholar]
- 75.Turovskaya O, et al. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis. 2008;29(10):2035–2043. doi: 10.1093/carcin/bgn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheng P, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205(10):2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181(7):4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hiratsuka S, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10(11):1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 79.Maeda S, et al. Essential roles of high-mobility group box 1 in the development of murine colitis and colitis-associated cancer. Biochem Biophys Res Commun. 2007;360(2):394–400. doi: 10.1016/j.bbrc.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 80.Ellerman JE, et al. Masquerader: high mobility group box-1 and cancer. Clin Cancer Res. 2007;13(10):2836–2848. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- 81.Chung HW, et al. Serum high mobility group box–1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancer. J Transl Med. 2009;7:38. doi: 10.1186/1479-5876-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nestl A, et al. Gene expression patterns associated with the metastatic phenotype in rodent and human tumors. Cancer Res. 2001;61(4):1569–1577. [PubMed] [Google Scholar]
- 83.Poser I, Golob M, Buettner R, Bosserhoff AK. Upregulation of HMG1 leads to melanoma inhibitory activity expression in malignant melanoma cells and contributes to their malignancy phenotype. Mol Cell Biol. 2003;23(8):2991–2998. doi: 10.1128/MCB.23.8.2991-2998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schlueter C, et al. Angiogenetic signaling through hypoxia: HMGB1: an angiogenetic switch molecule. Am J Pathol. 2005;166(4):1259–1263. doi: 10.1016/S0002-9440(10)62344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33(7):637–668. doi: 10.1016/S1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 86.Vogl T, et al. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood. 2004;104(13):4260–4268. doi: 10.1182/blood-2004-02-0446. [DOI] [PubMed] [Google Scholar]
- 87.Sparvero LJ, et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brezniceanu ML, et al. HMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinoma. FASEB J. 2003;17(10):1295–1297. doi: 10.1096/fj.02-0621fje. [DOI] [PubMed] [Google Scholar]
- 89.Taguchi A, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405(6784):354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 90.Conejo-Garcia JR, et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10(9):950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 91.Coffelt SB, et al. Ovarian cancers overexpress the antimicrobial protein hCAP-18 and its derivative LL-37 increases ovarian cancer cell proliferation and invasion. Int J Cancer. 2008;122(5):1030–1039. doi: 10.1002/ijc.23186. [DOI] [PubMed] [Google Scholar]
- 92.Heilborn JD, et al. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int J Cancer. 2005;114(5):713–719. doi: 10.1002/ijc.20795. [DOI] [PubMed] [Google Scholar]
- 93.von Haussen J, et al. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer. 2008;59(1):12–23. doi: 10.1016/j.lungcan.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 94.Curtin JF, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6(1):e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 96.Campana L, Bosurgi L, Rovere-Querini P. HMGB1: a two-headed signal regulating tumor progression and immunity. Curr Opin Immunol. 2008;20(5):518–523. doi: 10.1016/j.coi.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 97.Sun CQ, et al. Human beta-defensin-1, a potential chromosome 8p tumor suppressor: control of transcription and induction of apoptosis in renal cell carcinoma. Cancer Res. 2006;66(17):8542–8549. doi: 10.1158/0008-5472.CAN-06-0294. [DOI] [PubMed] [Google Scholar]
- 98.Bullard RS, et al. Functional analysis of the host defense peptide Human Beta Defensin-1: new insight into its potential role in cancer. Mol Immunol. 2008;45(3):839–848. doi: 10.1016/j.molimm.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hubert P, et al. Defensins induce the recruitment of dendritic cells in cervical human papillomavirus-associated (pre)neoplastic lesions formed in vitro and transplanted in vivo. FASEB J. 2007;21(11):2765–2775. doi: 10.1096/fj.06-7646com. [DOI] [PubMed] [Google Scholar]
- 100.Economopoulou M, et al. Inhibition of pathologic retinal neovascularization by alpha-defensins. Blood. 2005;106(12):3831–3838. doi: 10.1182/blood-2005-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 102.Claus RA, Otto GP, Deigner HP, Bauer M. Approaching clinical reality: markers for monitoring systemic inflammation and sepsis. Curr Mol Med. 2010;10(2):227–235. doi: 10.2174/156652410790963358. [DOI] [PubMed] [Google Scholar]
- 103.Mease PJ. The potential roles for novel biomarkers in rheumatoid arthritis assessment. Clin Exp Rheumatol. 2011;29(3):567–574. [PubMed] [Google Scholar]
- 104.Hammer HB, et al. Calprotectin (a major leucocyte protein) is strongly and independently correlated with joint inflammation and damage in rheumatoid arthritis. Ann Rheum Dis. 2007;66(8):1093–1097. doi: 10.1136/ard.2006.064741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brun JG, Jonsson R, Haga HJ. Measurement of plasma calprotectin as an indicator of arthritis and disease activity in patients with inflammatory rheumatic diseases. J Rheumatol. 1994;21(4):733–738. [PubMed] [Google Scholar]
- 106.Kane D, Roth J, Frosch M, Vogl T, Bresnihan B, FitzGerald O. Increased perivascular synovial membrane expression of myeloid-related proteins in psoriatic arthritis. Arthritis Rheum. 2003;48(6):1676–1685. doi: 10.1002/art.10988. [DOI] [PubMed] [Google Scholar]
- 107.Foell D, et al. S100A12 (EN-RAGE) in monitoring Kawasaki disease. Lancet. 2003;361(9365):1270–1272. doi: 10.1016/S0140-6736(03)12986-8. [DOI] [PubMed] [Google Scholar]
- 108.Foell D, Frosch M, Schulze zur Wiesch A, Vogl T, Sorg C, Roth J. Methotrexate treatment in juvenile idiopathic arthritis: when is the right time to stop? Ann Rheum Dis. 2004;63(2):206–208. doi: 10.1136/ard.2003.005686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Foell D, et al. Methotrexate withdrawal at 6 vs 12 months in juvenile idiopathic arthritis in remission: a randomized clinical trial. JAMA. 2010;303(13):1266–1273. doi: 10.1001/jama.2010.375. [DOI] [PubMed] [Google Scholar]
- 110.Liao H, et al. Use of mass spectrometry to identify protein biomarkers of disease severity in the synovial fluid and serum of patients with rheumatoid arthritis. Arthritis Rheum. 2004;50(12):3792–3803. doi: 10.1002/art.20720. [DOI] [PubMed] [Google Scholar]
- 111.Hammer HB, et al. Calprotectin (a major S100 leucocyte protein) predicts 10-year radiographic progression in patients with rheumatoid arthritis. Ann Rheum Dis. 2010;69(1):150–154. doi: 10.1136/ard.2008.103739. [DOI] [PubMed] [Google Scholar]
- 112. University Hospital, Clermont-Ferrand. Soluble Forms and Ligands of RAGE in ALI/ARDS (SoLiRAGE). ClinicalTrials.gov. NIH Web site. http://www.clinicaltrials.gov/ct2/show/NCT01270295?term=NCT01270295&rank=1 . Updated January 4, 2011. Accessed March 26, 2012.
- 113.Glaros T, Larsen M, Li L. Macrophages and fibroblasts during inflammation, tissue damage and organ injury. Front Biosci. 2009;14:3988–3993. doi: 10.2741/3506. [DOI] [PubMed] [Google Scholar]
- 114.Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4(11):1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 115.Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T. TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone. 2009;45(2):367–376. doi: 10.1016/j.bone.2009.04.252. [DOI] [PubMed] [Google Scholar]
- 116.Kim YS, et al. TNF–alpha enhances engraftment of mesenchymal stem cells into infarcted myocardium. Front Biosci. 2009;14:2845–2856. doi: 10.2741/3417. [DOI] [PubMed] [Google Scholar]
- 117.Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011;108(4):1585–1590. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rouhiainen A, et al. Regulation of monocyte migration by amphoterin (HMGB1). Blood. 2004;104(4):1174–1182. doi: 10.1182/blood-2003-10-3536. [DOI] [PubMed] [Google Scholar]
- 119.Degryse B, et al. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152(6):1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Palumbo R, et al. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164(3):441–449. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mitola S, et al. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol. 2006;176(1):12–15. doi: 10.4049/jimmunol.176.1.12. [DOI] [PubMed] [Google Scholar]
- 122.Limana F, et al. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ Res. 2005;97(8):e73–e83. doi: 10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- 123.Koczulla R, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111(11):1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang D, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192(7):1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Straino S, et al. High-mobility group box 1 protein in human and murine skin: involvement in wound healing. J Invest Dermatol. 2008;128(6):1545–1553. doi: 10.1038/sj.jid.5701212. [DOI] [PubMed] [Google Scholar]
- 126.Heilborn JD, et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120(3):379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 127.Hirsch T, et al. Human beta-defensin-3 promotes wound healing in infected diabetic wounds. J Gene Med. 2009;11(3):220–228. doi: 10.1002/jgm.1287. [DOI] [PubMed] [Google Scholar]
- 128.Wu N, Davidson JM. Migration inhibitory factor-related protein (MRP)8 and MRP14 are differentially expressed in free-electron laser and scalpel incisions. Wound Repair Regen. 2004;12(3):327–336. doi: 10.1111/j.1067-1927.2004.012313.x. [DOI] [PubMed] [Google Scholar]
- 129.Trostrup H, et al. S100A8/A9 deficiency in nonhealing venous leg ulcers uncovered by multiplexed antibody microarray profiling. Br J Dermatol. 2011;165(2):292–301. doi: 10.1111/j.1365-2133.2011.10384.x. [DOI] [PubMed] [Google Scholar]
- 130.Germani A, Limana F, Capogrossi MC. Pivotal advances: high-mobility group box 1 protein — a cytokine with a role in cardiac repair. J Leukoc Biol. 2007;81(1):41–45. doi: 10.1189/jlb.0306165. [DOI] [PubMed] [Google Scholar]
- 131.Limana F, et al. HMGB1 attenuates cardiac remodelling in the failing heart via enhanced cardiac regeneration and miR-206-mediated inhibition of TIMP-3. PLoS One. 2011;6(6):e19845. doi: 10.1371/journal.pone.0019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kitahara T, et al. High-mobility group box 1 restores cardiac function after myocardial infarction in transgenic mice. Cardiovasc Res. 2008;80(1):40–46. doi: 10.1093/cvr/cvn163. [DOI] [PubMed] [Google Scholar]
- 133.Oozawa S, et al. Effects of HMGB1 on ischemia-reperfusion injury in the rat heart. Circ J. 2008;72(7):1178–1184. doi: 10.1253/circj.72.1178. [DOI] [PubMed] [Google Scholar]
- 134.Maroso M, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16(4):413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 135.Seeliger S, et al. Expression of calcium-binding proteins MRP8 and MRP14 in inflammatory muscle diseases. Am J Pathol. 2003;163(3):947–956. doi: 10.1016/S0002-9440(10)63454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goova MT, et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol. 2001;159(2):513–525. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Eming SA, et al. Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from normal healing and chronic wounds. J Proteome Res. 2010;9(9):4758–4766. doi: 10.1021/pr100456d. [DOI] [PubMed] [Google Scholar]
- 138.Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem. 2000;275(51):40096–40105. doi: 10.1074/jbc.M006993200. [DOI] [PubMed] [Google Scholar]
- 139.Donato R, et al. S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta. 2009;1793(6):1008–1022. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 140.Srikrishna G, Panneerselvam K, Westphal V, Abraham V, Varki A, Freeze HH. Two proteins modulating transendothelial migration of leukocytes recognize novel carboxylated glycans on endothelial cells. J Immunol. 2001;166(7):4678–4688. doi: 10.4049/jimmunol.166.7.4678. [DOI] [PubMed] [Google Scholar]
- 141.Srikrishna G, et al. Carboxylated glycans mediate colitis through activation of NF-kappa B. J Immunol. 2005;175(8):5412–5422. doi: 10.4049/jimmunol.175.8.5412. [DOI] [PubMed] [Google Scholar]
- 142.Travis S, et al. RDP58 is a novel and potentially effective oral therapy for ulcerative colitis. Inflamm Bowel Dis. 2005;11(8):713–719. doi: 10.1097/01.MIB.0000172807.26748.16. [DOI] [PubMed] [Google Scholar]
- 143.Liu W, Deyoung BR, Chen X, Evanoff DP, Luo Y. RDP58 inhibits T cell-mediated bladder inflammation in an autoimmune cystitis model. J Autoimmun. 2008;30(4):257–265. doi: 10.1016/j.jaut.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liacini A, Sylvester J, Li WQ, Zafarullah M. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors downregulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 2002;21(3):251–262. doi: 10.1016/S0945-053X(02)00007-0. [DOI] [PubMed] [Google Scholar]
- 145.Yang D, Oppenheim JJ. Alarmins and antimicrobial immunity. Med Mycol. 2009;47(suppl 1):S146–S153. doi: 10.1080/13693780902721416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Biragyn A, et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167(11):6644–6653. doi: 10.4049/jimmunol.167.11.6644. [DOI] [PubMed] [Google Scholar]
- 147.Biragyn A, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298(5595):1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 148.Ramanathan V, Goral S, Helderman JH. Renal transplantation. Semin Nephrol. 2001;21(2):213–219. doi: 10.1053/snep.2001.21213. [DOI] [PubMed] [Google Scholar]
- 149.Feldmann M, Maini RN. Anti-TNF therapy, from rationale to standard of care: what lessons has it taught us? J Immunol. 2010;185(2):791–794. doi: 10.4049/jimmunol.1090051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.