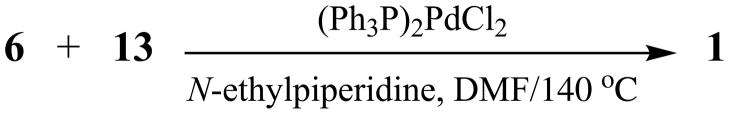Abstract
A series of substituted 2,4-diaminopyrimidines 1 has been prepared and evaluated for activity against Bacillus anthracis using previously reported (±)-3-{5-[(2,4-diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-(1-propyl-2(1H)-phthalazinyl)-2-propen-1-one (1a), with a minimum inhibitory concentration (MIC) value of 1–3 μg/mL, as the standard. In the current work, the corresponding isobutenyl (1e) and phenyl (1h) derivatives displayed the most significant activity in terms of the lowest MICs with values of 0.5 μg/mLand 0.375–1.5 μg/mL, respectively. It is likely that the S isomers of 1 will bind the substrate-binding pocket of dihydrofolate reductase (DHFR) as was found for (S)-1a. The final step in the convergent synthesis of target systems 1 from (±)-1-(1-substituted-2(1H)-phthalazinyl)-2-propen-1-ones 6 with 2,4-diamino-5-(5-iodo-3,4-dimethoxybenzyl)pyrimidine (13) was accomplished via a novel Heck coupling reaction under sealed-tube conditions.
Keywords: 2,4-diaminopyrimidine; antibiotic; DHFR inhibitor; Heck coupling; sealed-tube reaction
1. Introduction
Interest has been mounting to develop agents to treat inhalation anthrax, which is considered an important bioterror threat [1]. Studies by others [2] have revealed the inhibitory capabilities of several substituted 2,4-diaminopyrimidines against Bacillus anthracis, although the minimum inhibitory concentration (MIC) values were higher than those recorded in this work. Our compounds target dihydrofolate reductase (DHFR), a key enzyme in the folate pathway [2–4]. It is well accepted that DHFR plays a significant role in DNA synthesis [5,6] and is important in cancer chemotherapy (methotrexate) [7,8], in antimalarial activity (pyrimethamine) [9], and in antibacterial activity (trimethoprim) [10,11]. Other DHFR inhibitors with antibacterial activity include a related series of dihydrophthalazines [12–14] being pursued by Basilea Pharmaceutica (Switzerland) as well as Iclaprim [15], AR-709 [16] under development at Evolva (Switzerland), and a collection of 7-aryl-2,4-diaminoquinazolines [17] being evaluated by Trius Therapeutics (San Diego, CA). DHFR inhibitors, also under investigation at a research and development level, are represented by work on ω-(carboxyalkoxy)benzylpyrimidines targeted to Pneumocystis carinii [18] as well as a number of broad-spectrum propargyl-based compounds [19]. The common inclusion of a pyrimidine nucleus in the majority of DHFR inhibitors examined to date supports the hypothesis that this ring is essential [2,3,20–22].
The basic structural motif, incorporating a pyrimidine and a phthalazine ring, led to the preparation of racemic 1a (R = n-propyl) [3], which was separated into its enantiomers [23]. Complexation of racemic 1a with DHFR in B. anthracis resulted in co-crystallization where only the S isomerappeared deep in the active site as determined by X-ray analysis [3]. Additionally, both (R)- and (S)-1a were potent in inhibiting the growth of Staphylococcus aureus [24]. The focus of the present work was to determine the antibacterial activity of racemic members of 1 via comparison with (S)-1a with consideration of the docking orientation of this enantiomer in B. anthracis DHFR [3]. As stated previously, correlations have been drawn between antibacterial activity and DHFR inhibition [12–14]. Thus, in the present work, the X-ray analysis on DHFR-(S)-1a provided a molecular basis for the synthesis of additional derivatives of the target heterocycles and served as a guide for the design of related structures with potential antibacterial activity.
2. Chemistry
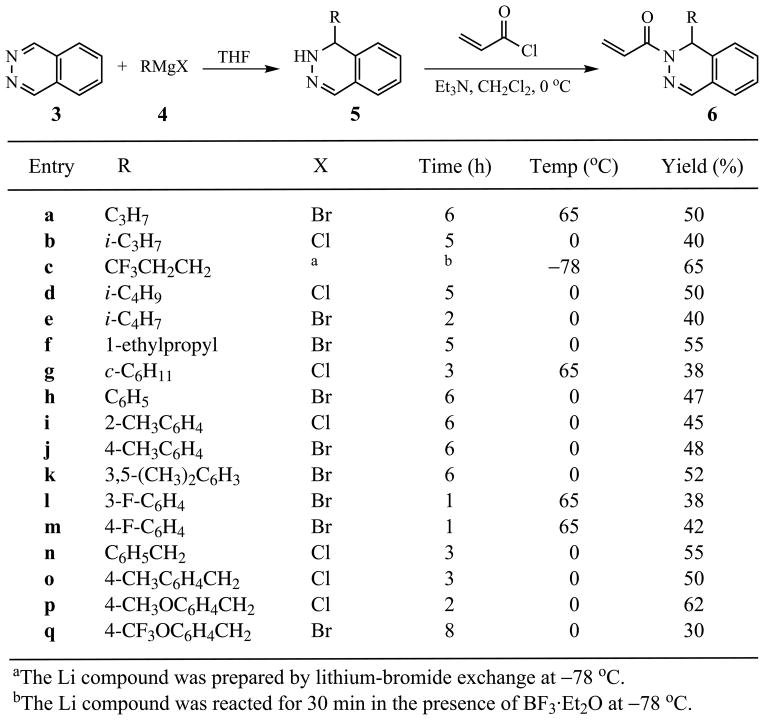
We report herein detailed preparations [22] for racemic members of 1. Phthalazine (2) was added to selected organometallic reagents 3 in THF to yield dihydrophthalazines 4 as viscous oils. Subsequent N-acylation of 4 by acryloyl chloride (5) in dichloromethane in the presence of triethylamine furnished members of 6, which were chromatographed and used immediately in the final coupling step to give the desired targets 1. The preparations leading to 4 utilized Grignard reagents 3 with one exception. The synthesis of 4c required a lithium-bromine exchange using methyllithium in ether to convert 1,1,1-trifluoro-3-iodopropane to the corresponding lithium reagent 3c, which was added to 2 in the presence of boron trifluoride etherate at −78 °C [25] (Scheme 1).
Scheme 1.
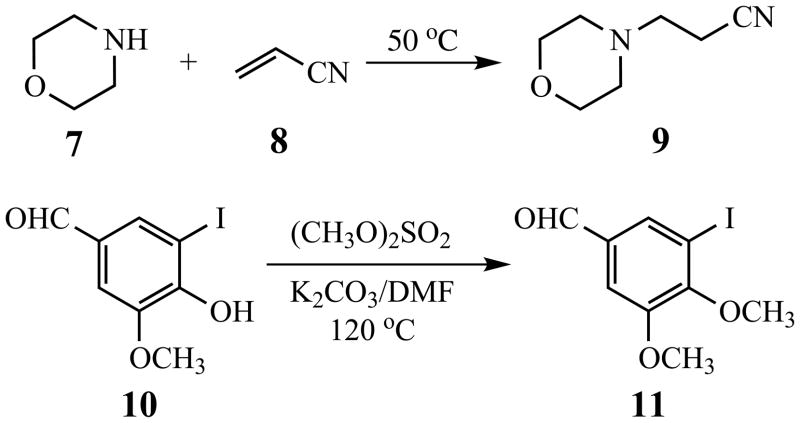
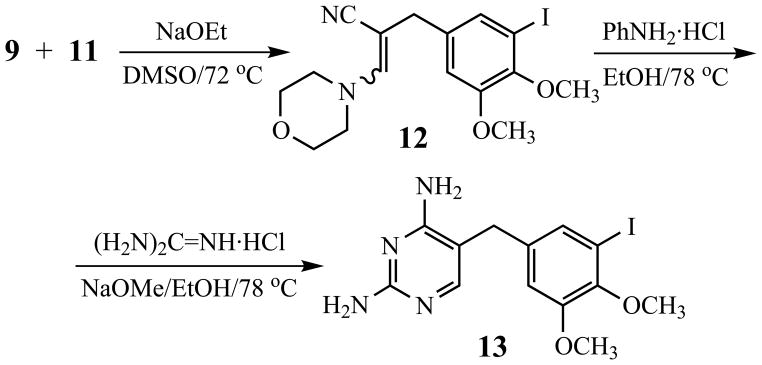
The requisite aryl iodide 13 for coupling with members of 6 was synthesized from 9 and 11 (Scheme 2). Conjugate addition of morpholine (7) to acrylonitrile (8) [26] generated 3-morpholinopropionitrile (9) [27] in 95% yield. O-Methylation of 5-iodovanillin (10) using potassium carbonate and dimethyl sulfate in DMF [28] furnished dimethoxyaldehyde 11 (96%). Conversion of 9 and 11 to 13 was then carried out using a procedure improved from the original synthetic approach [29] (Scheme 3). The presumed intermediate enaminonitrile 12 was a dark brown oil, which was treated at once with aniline hydrochloride in boiling ethanol for 1 h. To the resulting crude, hot mixture was added guanidine hydrochloride, followed by sodium methoxide, and heating was continued for 3 h. Isolation and purification of the product by crystallization afforded 13 (60%), which was characterized by 1H-NMR and 13C-NMR spectral analyses.
Scheme 2.
Scheme 3.
Coupling of each member of 6 with 13 was accomplished via a sealed-tube Heck reaction, promoted by bis(triphenylphosphine)palladium(II) chloride and N-ethylpiperidine in anhydrous DMF at 140 °C under argon, to afford crude oils containing the desired product (Scheme 4). Flash chromatography, using CH2Cl2 and CH3OH (96:4), followed by one recrystallization, then gave racemic targets 1. Isolated yields ranged from 25–71%. Samples of (±)-1a–q were characterized by spectral and elemental analyses. To the best of our knowledge, Heck reactions have not previously been reported using sealed-tube conditions [30].
Scheme 4.
The hydrophilic nature of 1 was clearly revealed by its propensity to retain water and/or polar solvents, such as methanol, in the crystal [31]. All elemental analyses of members of 1 contained varying amounts of water and, in the cases of 1n and 1o, traces of methanol. Exhaustive attempts to remove these polar contaminants, via extensive drying techniques, were unsuccessful. Reaction conditions to prepare 6a–q, as well as (±)-1a–q, varied for each compound, with the optimum methodology given in the Experimental Section.
3. Biological activity
Alterations of “R” in 1 serve to modulate interactions at the interface of the substrate binding site and the solvent (Figure 1) [3]. These observations correlate with the measured MIC values, where shorter and more hydrophobic substitutions [3] exhibit lower MIC values (Table 1). The protein surfaces complexed by (S)-1a have not typically been targeted by therapeutics since (S)-1a protrudes further along and extends to the distal end of the binding site. As stated previously, the dihydrophthalazine moiety is critical to this activity and promotes specificity for the binding sites of bacterial DHFR over human DHFR [3,24]. Prior work [3] clearly demonstrated that the contacts between the phthalazine moiety in the DHFR-(S)-1a complex in B.anthracis were hydrophobic, which was found also for the binding site in S. aureus [24]. Aliphatic portions of Lys33, Thr36, Leu41, Leu55, Pro56, and Arg58 are located around the dihydrophthalazine ring [2], with residues Leu29, Glu30, Lys33, and Arg53 most closely approaching the R group.
Figure 1.
The surface of the B. anthracis DHFR binding site is depicted in grey and cut into the plane of the page to permit visualization (the backbone of the protein is displayed in blue). Coordinates were taken from the co-crystal structure [3], which resulted from complexation with (S)-1a (yellow).
Table 1.
MIC values for racemic members of 1
| Compound | R | MIC (μg/mL) |
|---|---|---|
| 1a | C3H7 | 1–3 |
| 1b | i-C3H7 | 1–2 |
| 1c | CF3CH2CH2 | 1–2 |
| 1d | i-C4H9 | 1–2 |
| 1e | i-C4H7 | ≤0.5 |
| 1f | 1-ethylpropyl | 4–8 |
| 1g | c-C6H11 | 1–4 |
| 1h | C6H5 | 0.375–1.5 |
| 1i | 2-CH3C6H4 | 8 |
| 1j | 4-CH3C6H4 | 1–2 |
| 1k | 3,5-(CH3)2C6H3 | 4–8 |
| 1l | 3-F-C6H4 | 2 |
| 1m | 4-F-C6H4 | 1–2 |
| 1n | C6H5CH2 | 8 |
| 1o | 4-CH3C6H4CH2 | 4–8 |
| 1p | 4-CH3OC6H4CH2 | 8–16 |
| 1q | 4-CF3OC6H4CH2 | 4 |
4. Results and discussion
Racemic mixtures 1a, 1b, 1c, 1d, and 1e exhibit generally favorable MIC values (Table 1). Interestingly, 1h, 1l and 1m, which incorporate large aryl rings, also possess low MIC values. The most potent derivatives are 1e and 1h, while 1a is the standard for which the co-crystal structure is available [3]. Smaller differences in MIC ranges for 1a, 1b, 1c, 1d, 1j, and 1m may be due, in part, to flexibility around R at C-1 of the dihydrophthalazine ring and/or the unoccupied space around R can accommodate group differences. In contrast, the maximum activity found for 1e (MIC ≤ 0.5 μg/mL) may reflect the restricted motion in the isobutenyl group. Larger groups, such as in 1p, are surmised to protrude into solvent space exterior to the binding site leading to elevated MICs. A similar explanation can be applied to 1f, 1i, 1k, 1n, 1o, 1p, and 1q where possible critical steric factors are unfavorable to allow useful activity. The new data suggest that the double bond of the isobutenyl group in 1e alters its conformation considerably [3], possibly by centering itself more evenly between residues Leu29, Gln30, Lys33, and Arg53, with enhanced activity being the result. Thus, the overall shape and flexibility (or lack thereof) of the substitution also clearly have an effect, with 1e displaying the most favorable MIC value regarding in vitro activity in this assay.
Figure 1 displays the protein:solvent surface arrangements involving the complex of B. anthracis with (S)-1a in which the n-propyl group is somewhat exposed to solvent [3]. This positioning of the R group is undoubtedly an important factor in eliciting activity since hydrogen at this position results in loss of potency [4]. The effect appears to be very subtle since agents 1h (R = phenyl) and 1j (R = p-tolyl) possess large aryl groups but still retain reasonably good MICs. Additionally, interchanging hydrogen and fluorine, as in comparing 1h with 1l and 1m, did not significantly improve potency, nor did replacing a fluorine atom with a methyl group (1m versus 1j). Finally, benzyl substitution, as in 1n, 1o, 1p, and 1q, generally resulted in higher MICs. Thus, refinement of ligand structures to induce greater activity must take into account steric considerations and quite possibly electronic differences in view of the binding site properties [3] of DHFR in B. anthracis. Since selectivity of (S)-1a for B. anthracis over human DHFR has been confirmed [3,24], continued investigations of structure-activity relationships for inhibiting the bacteria are warranted.
In summary, 16 substituted 2,4-diaminopyrimidines have been synthesized using a novel sealed-tube Heck reaction and were evaluated for activity against B. anthracis, with the previously described (±)-(E)-3-{5-[(2,4-diamino-5-pyrimidinylmethyl]-2,3-dimethoxyphenyl}-1-(1-propyl-2(1H)-phthalazinyl)-2-propen-1-one (1a) serving as the reference [3]. The preparative method may well have broad scope with promising utility in terms of simplicity of operation and ease of purifying the products. Agents 1e (R = isobutenyl) and 1h (R = phenyl) exhibited the best activity with MICs of ≤0.5 μg/mL and 0.375–1.5 μg/mL, respectively. Other racemic compounds with activity comparable to that of 1a (1–3 μg/mL) were 1b, 1c, 1d, 1j, and 1m, which showed MICs in the1–2 μg/mL range. It is speculated that the S isomer of each of these systems might possess even lower MICs. Interestingly, each enantiomer of 1a demonstrates distinct binding characteristics with S. aureus DHFR [24], with the S isomer situating in the normal binding pocket while the R isomer resides in a shallow surface cavity. Whether or not the other agents will express similar antibacterial activity and show behavior analogous to (S)-1a with a specific organism requires additional analysis.
5. Experimental section
5.1. Chemistry
5.1.1. General methods
Commercial anhydrous N,N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) were stored under dry nitrogen and transferred by syringe into reactions where they were used. Tetrahydrofuran (THF) was dried over KOH pellets and distilled from LiAlH4 prior to use. All other commercial reagents were used as received from various vendors. Commercial Grignard reagents used were purchased from Aldrich Chemical Co.
Unless otherwise specified, all reactions were run under dry nitrogen in oven-dried glassware. The saturated NH4Cl and NaCl used in work-up procedures were aqueous solutions. Reactions were monitored by thin layer chromatography on silica gel GF plates (Analtech No. 21521). Preparative separations were performed by column chromatography in quartz columns using silica gel (Davisil® grade 62, 60–200 mesh) mixed with UV-active phosphor (Sorbent Technologies, No. UV-5). Band elution, for all chromatographic separations, was monitored using a hand-held UV lamp. Melting points were uncorrected. FT-IR spectra were run as thin films on NaCl disks. Unless otherwise indicated, 1H- and 13C-NMR spectra were measured in CDCl3 on a Varian GEMINI 300 instrument at 300 MHz (1H) and 75 MHz (13C) or a Varian INOVA 400 instrument at 400 MHz (1H) and 100 MHz (13C) and referenced to internal tetramethylsilane. Elemental analyses were performed by Atlantic Microlab, Inc.
5.1.2. Method A. (±)-1-(1-Propyl-2(1H)-phthalazinyl)-2-propen-1-one (6a)
The method of Guerry et al. [22] was modified. Propylmagnesium bromide (3a) was generated on a 19.3-mmol scale from 2.38 g (1.66 mL, 19.3 mmol) of 1-bromopropane and 0.65 g (25.7 mmol) of magnesium metal in 15 mL of THF. To the resulting Grignard reagent at room temperature was added dropwise a solution of 2.00 g (15.4 mmol) of phthalazine (2) in 8 mL of THF with stirring, and the reaction was gently refluxed for 6 h. The mixture was cooled to room temperature, and 10 mL of saturated NH4Cl was cautiously added. The crude mixture was transferred to a separatory funnel containing 40 mL of additional NH4Cl solution and was extracted with diethyl ether (3 × 50 mL). The combined organic extracts were washed with saturated NaCl, dried (MgSO4), and concentrated under vacuum for 30 min to give 2.43 g of dihydrophthalazine 4a as a viscous, brown oil, which was used immediately in the next reaction.
The crude 4a was dissolved in 20 mL of dichloromethane at 0 °C and 1.82 g (2.50 mL, 18.0 mmol) of triethylamine was added. A solution of 1.40 g (1.26 mL, 15.5 mmol) of acryloyl chloride (5) in 10 mL of dichloromethane was then added dropwise over 20 min. The new solution was stirred for 2 h at 0 °C and was then poured into ice water and extracted with dichloromethane (3 × 50 mL). The combined organic extracts were washed with saturated NaCl, dried (MgSO4), and concentrated under vacuum. The resulting brown oil was chromatographed on a 50-cm × 2.5-cm silica gel column eluted with 5–10% ethyl acetate in hexanes to give 1.75 g (50%) of 6a as a viscous, brown oil. IR: 1664, 1616 cm−1; 1H-NMR (300 MHz): δ 7.62 (s, 1H), 7.43 (td, 1H, J = 7.5, 1.5 Hz), 7.35 (td, 1H, J = 7.5, 1.5 Hz), 7.32 (dd, 1H, J = 17.2, 10.4 Hz), 7.27 (d, 1H, J = 7.5 Hz), 7.15 (d, 1H, J = 7.5 Hz), 6.47 (dd, 1H, J = 17.2, 2.2 Hz), 5.84 (t, 1H, J = 6.7 Hz), 5.77 (dd, 1H, J = 10.4, 2.2 Hz), 1.62 (m, 2H), 1.25 (m, 2H), 0.85 (t, 3H, J = 7.3 Hz) ; 13C-NMR (75 MHz): δ 166.2, 142.4, 134.1, 131.4, 128.3, 127.9, 127.1, 126.4, 125.6, 123.8, 51.1, 37.2, 18.2, 13.7.
5.1.3. Method B. (±)-1-(1-Isopropyl-2(1H)-phthalazinyl)-2-propen-1-one (6b)
A solution of 2.00 g (15.0 mmol) of 2 in 20 mL of dry THF was cooled to 0 °C, and 8.0 mL of 2.0 M isopropylmagnesium chloride (3b) (16.0 mmol) in ether was slowly added dropwise over 20 min. The reaction was allowed to stir at 0 °C for 5 h and was then quenched with 50 mL of saturated NH4Cl and extracted with ethyl acetate (3 × 50 mL). The combined organic extracts were washed with saturated NaCl, dried (MgSO4), and concentrated under vacuum to give dihydrophthalazine 4b asa viscous, brown oil. This oil was dried under high vacuum for 30 min and was used without further purification.
The crude 4b in dichloromethane at 0 °C was treated, as above, with 1.82 g (2.50 mL, 18.0 mmol) of triethylamine and 1.40 g (1.26 mL, 15.5 mmol) of 5, and the reaction was stirred for 2 h. Work-up and chromatography gave 1.83 g (40%) of 6b as a viscous,brown oil. IR: 1664, 1615 cm−1; 1H-NMR (300 MHz): δ 7.63 (s, 1H), 7.45 (td, 1H, J = 7.7, 1.6 Hz), 7.37 (t, 1H, J= 7.7 Hz), 7.34 (dd, 1H, J = 17.0, 10.4 Hz), 7.28 (d, 1H, J = 7.7 Hz), 7.17 (d, 1H, J = 7.7 Hz), 6.46 (dd, 1H, J = 17.0, 2.2 Hz), 5.78 (dd, 1H, J = 10.4, 2.2 Hz), 5.67 (d, 1H, J = 7.1 Hz), 1.99 (septet, 1H, J = 6.8 Hz), 0.92 (d, 3H, J = 6.6 Hz), 0.77 (d, 3H, J = 6.6 Hz); 13C-NMR (75 MHz): δ 166.5, 143.2, 132.0, 131.1, 128.3, 128.0, 127.5, 127.2, 125.4, 124.6, 56.3, 33.4, 19.1, 18.0.
5.1.4. Method C. (±)-1-[1-(3,3,3-Trifluoropropyl)-2(1H)-phthalazinyl]-2-propen-1-one (6c)
The procedure of Uno et al. [25] was modified. A solution of 2.00 g (15.0 mmol) of 2 and 3.60 mL (30.0 mmol) of 1,1,1 trifluoro-3-iodopropane in 20 mL of ether was cooled to −78 °C, and 3.90 mL (30.0 mmol) of boron trifluoride etherate was added dropwise over 10 min. The solution was stirred for 5 min before 19.2 mL of 1.6 M methyllithium in ether (30.0 mmol) was added dropwise. The reaction mixture was stirred at −78 °C for 30 min and was then poured into 50 mL of saturated NH4Cl and extracted with ether (3 × 50 mL). The combined ether layers were washed with saturated NaCl, dried (MgSO4), and concentrated under vacuum to give dihydrophthalazine 4c as a viscous, yellow oil. This oil was dried under high vacuum for 30 min and was used without further purification.
The crude 4c in dichloromethane at 0 °C was treated, as above, with 1.82 g (2.50 mL, 18.0 mmol) of triethylamine and 1.34 g (1.20 mL, 15.0 mmol) of 5, and the reaction mixture was stirred for 2 h. Work-up and chromatography gave 2.82 g (65%) of 6c as a yellow liquid. IR: 1666, 1622, 1325 cm−1; 1H-NMR (400 MHz): δ 7.67 (s, 1H), 7.50 (td, 1H, J = 7.4, 1.4 Hz), 7.41 (t, 1H, J = 7.4 Hz), 7.33 (dd, 1H, J = 7.4, 1.4 Hz), 7.30 (dd, 1H, J = 17.2, 10.5 Hz), 7.19 (dd, 1H, J = 7.4, 0.4 Hz), 6.50 (dd, 1H, J = 17.2, 2.1 Hz), 5.96 (t, 1H, J = 6.6 Hz), 5.83 (dd, 1H, J = 10.5, 2.1 Hz), 2.18-1.98 (complex m, 2H), 1.90 (m, 2H); 13C-NMR (100 MHz): δ 166.4, 142.6, 132.6, 132.1, 129.1, 128.7, 126.7, 126.7 (q, JC-F = 276.3 Hz), 126.3, 126.0, 123.7, 49.8, 30.0 (q, JC-F = 29.2 Hz), 27.8 (q, JC-F = 2.6 Hz).
5.1.5. (±)-1-(1-Isobutyl-2(1H)-phthalazinyl)-2-propen-1-one (6d)
This compound was prepared and purified on the same scale using Method B(0 °C, 5 h)to give 1.83 g (50%) of 6d as a viscous, brown oil. IR: 1663, 1616 cm−1; 1H-NMR (300 MHz): δ 7.68 (s, 1H), 7.44 (td, 1H, J = 7.7, 1.6 Hz), 7.36 (td, 1H, J = 7.1, 1.1 Hz), 7.30 (dd, 1H), 7.29 (d, 1H, J = 6.6 Hz), 7.17 (d, 1H, J = 7.1 Hz), 6.47 (dd, 1H, J = 17.0, 2.2 Hz), 5.92 (t, 1H, J = 7.1 Hz), 5.68 (dd, 1H, J = 10.4, 2.2 Hz), 1.58-1.36 (complex m, 3H), 0.98 (d, 3H, J = 6.0 Hz), 0.92 (d, 3H, J = 6.0 Hz); 13C-NMR (75 MHz): δ 166.1, 143.0, 134.6, 131.4, 128.4, 128.0, 127.1, 126.2, 125.8, 123.8, 49.5, 43.7, 23.8, 23.1, 22.2.
5.1.6. (±)-1-(1-Isobutenyl-2(1H)-phthalazinyl)-2-propen-1-one (6e)
This compound was prepared and purified on the same scale using Method B (0 °C, 2 h)to give 1.47 g (40%) of 6e as a viscous, brown oil. IR: 1665, 1622, 1613 cm−1; 1H-NMR (400 MHz): δ 7.58 (s, 1H), 7.43 (td, 1H, J = 7.4, 1.4 Hz), 7.33 (td, 1H, J = 7.4, 1.1 Hz), 7.30 (dd, 1 H, J = 17.2, 10.5 Hz), 7.27 (dm, 1H, J = 7.4 Hz), 7.14 (d, 1H, J = 7.5 Hz), 6.51 (d, 1H, J = 10.0 Hz), 6.46 (dd, 1H, J = 17.2, 2.1 Hz), 5.76 (dd, 1H, J = 10.5, 2.1 Hz), 5.27 (dt, 1H, J = 10.0, 1.2 Hz), 2.02 (d, 3H, J = 1.2 Hz), 1.64 (d, 3H, J = 1.2 Hz); 13C-NMR (75 MHz): δ 165.9, 141.4, 134.3, 134.0, 131.7, 128.0, 127.7, 127.2, 126.0, 125.6, 123.0, 121.9, 49.7, 25.5, 18.4.
5.1.7. (±)-1-[1-(1-Ethylpropyl)-2(1H)-phthalazinyl]-2-propen-1-one (6f)
This compound was prepared and purified on the same scale using Method B (0 °C, 5 h)to give 2.16 g (55%) of 6f as a viscous, brown oil. IR: 1664, 1611 cm−1; 1H-NMR (400 MHz): δ 7.65 (s, 1H), 7.44 (td, 1H, J = 7.4, 1.5 Hz), 7.36 (td, 1H, J = 7.4, 1.5 Hz), 7.32 (dd, 1H, J = 17.2, 10.3 Hz), 7.28 (d, 1H, J = 7.2 Hz), 7.18 (d, 1H, J = 7.5 Hz), 6.46 (dd, 1H, J = 17.2, 2.1 Hz), 5.88 (d, 1H, J = 7.0 Hz), 5.77 (dd, 1H, J = 10.3, 2.1 Hz), 1.56 (m, 1H), 1.42-1.24 (complex m, 3H), 1.10 (m, 1H), 0.96 (t, 3H, J = 7.4 Hz), 0.80 (t, 3H, J = 7.1 Hz); 13C-NMR (100 MHz): δ 166.3, 143.6, 132.7, 131.2, 128.3, 127.9, 127.21, 127.20, 125.5, 124.5, 52.7, 45.4, 21.0, 20.6, 11.1, 10.5.
5.1.8. (±)-1-(1-Cyclohexyl-2(1H)-phthalazinyl)-2-propen-1-one (6g)
This compound was prepared and purified on the same scale using Method A (65 °C, 3 h) to give 1.56 g (38%) of 6g as a viscous, brown oil. IR: 1663, 1614 cm−1; 1H-NMR (400 MHz): δ 7.64 (s, 1H), 7.44 (td, 1H, J = 7.4, 1.1 Hz) 7.37 (td, 1H, J = 7.4, 1.1 Hz), 7.33 (dd, 1H, J = 17.2, 10.5 Hz), 7.29 (dd, 1H, J = 7.4, 0.8 Hz), 7.15 (d, 1H, J = 7.4 Hz), 6.46 (dd, 1H, J = 17.2, 2.1 Hz), 5.78 (dd, 1H, J = 10.5, 2.1 Hz), 5.67 (d, 1H, J = 7.2 Hz), 1.80-1.50 (complex m, 6H), 1.20-0.97 (complex m, 3H), 0.87 (m, 2H); 13C-NMR (75 MHz): δ 166.5, 143.6, 132.3, 131.0, 128.4, 128.0, 127.6, 127.1, 125.4, 124.5, 55.8, 42.7, 29.2, 28.5, 26.01, 25.98, 25.9.
5.1.9. (±)-1-(1-Phenyl-2(1H)-phthalazinyl)-2-propen-1-one (6h)
This compound was prepared and purified on the same scale using Method B (0 °C, 6 h)to give 1.90 g (47%) of 6h as a viscous, brown oil. IR: 1664, 1615 cm−1; 1H-NMR (400 MHz): δ 7.65 (s, 1H), 7.44 (td, 1H, J = 7.4, 1.6 Hz), 7.38 (td, 1H, J = 7.4, 1.1 Hz), 7.32 (dd, 1H, J = 17.2, 10.5 Hz), 7.32 (d, 1H, J = 7.4 Hz), 7.27 (d, 1H, J = 7.4 Hz), 7.25–7.28 (complex m, 5H), 6.98 (s, 1 H), 6.48 (dd, 1H, J = 17.2, 2.1 Hz), 5.79 (dd, 1H, J = 10.5, 2.1 Hz); 13C-NMR (75 MHz): δ 166.4, 141.7, 141.0, 132.9, 132.0, 128.8, 128.5, 128.3, 127.7, 127.2, 127.0, 126.9, 125.9, 123.5, 54.2.
5.1.10. (±)-1-[1-(2-Methylphenyl)-2(1H)-phthalazinyl]-2-propen-1-one (6i)
This compound was prepared and purified on the same scale using Method B (0 °C, 6 h)to give 1.90 g (45%) of 6i as a viscous, brown oil. IR: 1666, 1613 cm−1; 1H-NMR (400 MHz): δ 7.58 (s, 1H), 7.35-7.26 (complex m, 3H), 7.30 (dd, 1H, J = 17.2, 10.5 Hz), 7.19 (dd, 1H, J = 7.4, 1.6 Hz), 7.15-7.00 (complex m, 4H), 6.96 (s, 1H), 6.39 (dd, 1H, J = 17.2, 2.1 Hz), 5.72 (dd, 1H, J = 10.5, 2.0 Hz), 2.74 (s, 3H); 13C-NMR (100 MHz): δ 166.4, 142.5, 139.8, 134.4, 133.9, 132.1, 130.6, 128.4, 128.0, 127.6, 127.4, 127.3, 126.7, 126.5, 126.4, 122.2, 52.1, 20.0.
5.1.11. (±)-1-[1-(4-Methylphenyl)-2(1H)-phthalazinyl]-2-propen-1-one (6j)
This compound was prepared and purified on the same scale using Method B (0 °C, 6 h) to give 2.00 g (48%) of 6j as a viscous, brown oil. IR: 1665, 1615 cm−1; 1H-NMR (300 MHz): δ 7.64 (s, 1H), 7.43 (td, 1H, J = 7.1, 1.6 Hz), 7.39-7.27 (complex m, 3H), 7.24 (d, 1H, J = 7.7 Hz), 7.10 (d, 2H, J = 8.2 Hz), 7.03 (d, 2H, J = 8.2 Hz), 6.94 (s, 1H), 6.47 (dd, 1H, J = 17.0, 1.6 Hz), 5.77 (dd, 1H, J = 10.4, 1.6 Hz), 2.24 (s, 3H); 13C-NMR (75 MHz): δ 166.4, 141.7, 138.2, 137.5, 133.1, 132.0, 129.2, 128.7, 128.2, 127.14, 127.11, 126.9, 125.9, 123.5, 54.0, 21.0.
5.1.12. (±)-1-[1-(3,5-Dimethylphenyl)-2(1H)-phthalazinyl]-2-propen-1-one (6k)
This compound was prepared and purified on the same scale using Method B (0 °C, 6 h) to give 2.32 g (52%) of 6k as a viscous, brown oil. IR: 1665, 1610 cm−1; 1H-NMR (400 MHz): δ 7.65 (s, 1H), 7.42 (td, 1H, J = 7.4, 1.6 Hz), 7.34 (td, 1H, J = 7.4, 1.2 Hz), 7.34 (dd, 1H, J = 17.2, 10.3 Hz), 7.32 (dd, 1H, J = 7.4, 1.6 Hz), 7.25 (d, 1H, J = 7.4 Hz), 6.87 (s, 1H), 6.81 (s, 1H), 6.82 (s, 2H), 6.46 (dd, 1H, J = 17.2, 2.1 Hz), 5.77 (dd, 1H, J = 10.3, 2.1 Hz), 2.20 (s, 6H); 13C-NMR (100 MHz): δ 166.3, 141.7, 141.2, 138.1, 133.3, 132.0, 129.5, 128.7, 128.2, 127.15, 127.13, 125.9, 124.5, 123.7, 54.3, 21.3.
5.1.13. (±)-1-[1-(3-Fluorophenyl)-2(1H)-phthalazinyl]-2-propen-1-one (6l)
A mixture of 2.80 g (16.0 mmol) of 1-bromo-3-fluorobenzene, 25 mL of THF and 0.49 g (19.0 mmol) of magnesium metal was stirred vigorously at room temperature for 5 min and then refluxed for 1.5 h to give a yellow solution. Heating was discontinued, and a solution of 2.00 g (15.0 mmol) of 2 in 8 mL of THF was added dropwise with stirring over a period of 10 min. The reaction was then refluxed for 1 h and carried forward as in Method A (65 °C, 1 h)to give 1.63 g (38%) of 6l as a viscous, brown oil. IR: 1666, 1611 cm−1; 1H-NMR (400 MHz): δ 7.66 (s, 1H), 7.45 (td, 1H, J = 7.4, 1.6 Hz), 7.38 (td, 1H, J = 7.4, 1.1 Hz), 7.33 (dd, 1H, J = 7.4, 1.6 Hz), 7.32 (dd, 1H, J = 17.2, 10.5 Hz), 7.25 (dt, 1H, J = 7.4, 0.6 Hz), 7.18 (m, 1H), 7.03 (dm, 1H, J = 7.4 Hz), 6.96 (s, 1H), 6.89 (dd, 2H, J = 9.2, 1.2 Hz), 6.49 (dd, 1H, J = 17.2, 2.1 Hz), 5.80 (dd, 1H, J = 10.5, 2.1 Hz); 13C-NMR (100 MHz): δ 166.4, 162.7 (d, JC-F = 246.6 Hz), 143.3 (d, JC-F = 5.9 Hz), 141.7, 132.3, 132.1, 130.1 (d, JC-F = 8.1 Hz), 129.1, 128.6, 127.1, 126.8, 126.1, 123.4, 122.5 (d, JC-F = 2.9 Hz), 114.7 (d, JC-F = 21.4 Hz), 114.0 (d, JC-F = 22.1 Hz), 53.7 (d, JC-F = 1.5 Hz).
5.1.14. (±)-1-[1-(4-Fluorophenyl)-2(1H)-phthalazinyl]-2-propen-1-one (6m)
Using 4-fluorophenylmagnesium bromide, generated as above, this compound was prepared and purified on the same scale using Method A (65 °C, 1 h)to give 1.80 g (42%) of 6m as a viscous, brown oil. IR: 1666, 1604 cm−1; 1H-NMR (400 MHz): δ 7.67 (s, 1H), 7.47 (td, 1H, J = 7.1, 1.6 Hz), 7.40 (td, 1H, J = 7.4, 1.4 Hz), 7.36 (dd, 1H, J = 7.4, 1.4 Hz), 7.31 (dd, 1H, J = 17.2, 10.5 Hz), 7.24 (d, 1H, J = 7.4 Hz), 7.18 (m, 2H), 6.96 (s, 1H), 6.92 (m, 2H), 6.49 (dd, 1H, J = 17.2, 1.6 Hz), 5.78 (dd, 1H, J = 10.5, 1.6 Hz); 13C-NMR (75 MHz): δ 166.4, 162.1 (d, JC-F = 246.8 Hz), 141.7, 136.9, 132.6, 132.0, 128.9, 128.8, 128.4, 127.1, 126.9, 126.0, 123.4, 115.3 (d, JC-F = 21.5 Hz), 53.4.
5.1.15. (±)-1-(1-Benzyl-2(1H)-phthalazinyl)-2-propen-1-one (6n)
This compound was prepared and purified on the same scale using Method B (0 °C, 3 h)to give 2.35 g (55%) of 6n as a viscous, brown oil. IR: 1663, 1612 cm−1; 1H-NMR (400 MHz): δ 7.49 (s, 1H), 7.31 (dd, 1H, J = 17.2, 10.4 Hz), 7.34-7.21 (complex m, 3H), 7.20-7.14 (complex m, 3H), 6.88 (m, 2H), 6.60 (d, 1H, J = 7.4 Hz), 6.48 (dd, 1H, J = 17.2, 2.1 Hz), 5.99 (dd, 1H, J = 8.6, 4.9 Hz), 5.79 (dd, 1H, J = 10.3, 2.1 Hz), 2.97 (dd, 1H, J = 12.9, 4.9 Hz), 2.89 (dd, 1H, J = 12.9, 8.6 Hz); 13C-NMR (100 MHz): δ 166.2, 141.9, 136.1, 132.5, 130.9, 129.9, 128.5, 128.1, 128.0, 127.1, 127.0, 126.6, 125.5, 123.9, 53.1, 40.8.
5.1.16. (±)-1-[1-(4-Methylbenzyl)-2(1H)-phthalazinyl]-2-propen-1-one (6o)
This compound was prepared and purified on the same scale using Method B (0 °C, 3 h)to give 2.25 g (50%) of 6o as a viscous, brown oil. IR: 1665, 1613 cm−1; 1H-NMR (400 MHz): δ 7.48 (s, 1H), 7.30 (dd, 1H, J = 17.2, 10.3 Hz), 7.30-7.19 (complex m, 3H), 6.97 (d, 2H, J = 7.6 Hz), 6.76 (d, 2H, J = 7.6 Hz), 6.61 (d, 1H, J = 7.4 Hz), 6.48 (dd, 1H, J = 17.2, 2.1 Hz), 5.96 (dd, 1H, J = 8.6, 4.7 Hz), 5.78 (dd, 1H, J = 10.3, 2.1 Hz), 2.92 (dd, 1H, J = 12.9, 4.7 Hz), 2.85 (dd, 1H, J = 12.9, 8.6 Hz), 2.28 (s, 3H); 13C-NMR (100 MHz): δ 166.2, 141.9, 136.0, 133.0, 132.6, 130.9, 129.7, 128.7, 128.4, 128.0, 127.1, 127.0, 125.5, 123.8, 53.1, 40.4, 21.0.
5.1.17. (±)-1-[1-(4-Methoxybenzyl)-2(1H)-phthalazinyl]-2-propen-1-one (6p)
This compound was prepared and purified on the same scale using Method B (0 °C, 2 h)to give 2.90 g (62%) of 6p as a viscous, brown oil. IR: 2835, 1663, 1611 cm−1; 1H-NMR (400 MHz): δ 7.49 (s, 1H), 7.35-7.22 (complex m, 4H), 7.31 (dd, 1H, J = 17.2, 10.3 Hz), 6.78 (d, 2H, J = 8.6 Hz), 6.71 (d, 2H, J = 8.6 Hz), 6.48 (dd, 1H, J = 17.2, 2.1 Hz), 5.94 (dd, 1H, J = 8.5, 4.8 Hz), 5.80 (dd, 1H, J = 10.3, 2.1 Hz), 3.77 (s, 3H), 2.90 (dd, 1H, J = 13.1, 4.8 Hz), 2.84 (dd, 1H, J = 13.1, 8.5 Hz); 13C-NMR (100 MHz): δ 166.2, 158.4, 141.9, 132.7, 130.93, 130.89, 128.5, 128.2, 128.1, 127.14, 127.06, 125.5, 123.9, 113.5, 55.1, 53.2, 40.0.
5.1.18. (±)-1-[1-(4-Trifluoromethoxybenzyl)-2(1H)-phthalazinyl]-2-propen-1-one (6q)
This compound was prepared and purified on the same scale using Method B (0 °C, 8 h)to give 1.66 g (30%) of 6q as a viscous, brown oil. IR: 1665, 1612 cm−1; 1H-NMR (400 MHz): δ 7.47 (s, 1H), 7.36-7.23 (complex m, 3H), 7.29 (dd, 1H, J = 17.2, 10.5 Hz), 7.02 (d, 2H, J = 8.4 Hz), 6.88 (d, 2H, J = 8.4 Hz), 6.65 (d, 1H, J = 7.4 Hz), 6.48 (dd, 1H, J = 17.2, 1.6 Hz), 5.99 (apparent t, 1H, J = 7.0 Hz), 5.80 (dd, 1H, J = 10.5, 1.6 Hz), 2.98-2.86 (complex m, 2H); 13C-NMR (100 MHz): δ 166.2, 148.1 (q, JC-F = 1.5 Hz), 141.7, 135.0, 132.1, 131.2, 131.1, 128.7, 128.3, 126.9, 126.8, 125.7, 123.9, 120.5, 120.4 (q, JC-F = 257.0 Hz), 52.8, 40.2.
5.1.19. 3-Morpholinopropionitrile (9)
This compound was prepared on a 0.47-mol scale according to the literature procedures [26,27]. The crude product was distilled at 88–90 °C/0.5 mm Hg (lit [27] bp 149 °C/20 mm Hg) to give 38.2 g (95%) of 9 as a colorless liquid. IR: 2253 cm−1; 1H-NMR (300 MHz): δ 3.72 (t, 4H, J = 4.7 Hz), 2.68 (t, 2H, J = 6.8 Hz), 2.52 (t, 2H, J = 7.0 Hz), 2.50 (t, 4H, J = 4.7 Hz); 13C-NMR (75 MHz): δ 118.6, 66.7, 53.6, 53.0, 15.7.
5.1.20. 5-Iodo-3,4-dimethoxybenzaldehyde (11)
This compound was prepared on a 0.27-mol scale using the method of Nimgirawath [28]. The crude product was recrystallized (4:1 ethanol:water) to give 25.2 g (96%) of 11 as a white solid, mp 71–72 °C (lit [28] mp 71–72 °C). IR: 2832, 2730, 2693 cm−1; 1H-NMR (300 MHz): δ 9.83 (s, 1H), 7.85 (d, 1H, J = 1.7 Hz), 7.41 (d, 1H, J = 1.7 Hz), 3.93 (s, 3H), 3.92 (s,3 H); 13C-NMR (75 MHz): δ 189.7, 154.2, 153.0, 134.7, 133.9, 111.0, 92.1, 60.7, 56.1.
5.1.21. 2,4-Diamino-5-(5-iodo-3,4-dimethoxybenzyl)pyrimidine (13)
The general method of Roth, et al. [29] was modified. A solution of 6.92 g (54.1 mmol) of 9 in 20 mL of anhydrous DMSO was treated with 0.29 g (5.40 mmol) of sodium methoxide, and heated at 70–72 °C. A warm solution of 12.2 g (41.8 mmol) of 11 in 15 mL of DMSO was added to the solution of 9 over 15 min, and stirring was continued at 72 °C for 45 min. The crude reaction mixture was cooled in an ice bath, and 40 mL of ice-cold water was added. The mixture was extracted with dichloromethane (3 × 100 mL), and the combined organic layers were washed with saturated NaCl, dried (MgSO4), and concentrated under vacuum to give 3-morpholino-2-(5-iodo-3,4-dimethoxybenzyl)acrylonitrile (12) as dark red oil. This crude material was dissolved in dry ethanol, 6.76 g (52.2 mmol) of aniline hydrochloride was added, and the mixture was refluxed for 1 h. While the mixture was still hot, 9.55 g (100 mmol) of guanidine hydrochloride was added, followed by 9.00 g (167 mmol) of sodium methoxide (Caution! The initial addition must be done very slowly). The reaction mixture was then heated under reflux for 3 h and concentrated under vacuum to one-third volume. The mixture was cooled to 0 °C for 30 min, and 40 mL of ice-cold water was added. The resulting crude product was filtered, washed with water and recrystallized twice (4:1 ethanol:water) to give 13 (9.68 g, 60%) as a tan solid, mp 217–218 °C. IR: 3467, 3315, 3140, 1638 cm−1; 1H- NMR (DMSO-d6, 300 MHz): δ 7.57 (s, 1H), 7.14 (d, 1H, J = 1.8 Hz), 6.98 (d, 1H, J = 1.8 Hz), 6.16 (br s, 2H), 5.77 (br s, 2H), 3.77 (s,3 H), 3.66 (s, 3H), 3.54 (s, 2H); 13C-NMR (DMSO-d6, 75 MHz): δ 162.4, 162.1, 156.0, 152.0, 146.3, 138.9, 129.1, 113.8, 105.2, 92.4, 59.8, 55.8, 31.7.
5.1.22. (±)-(E)-3-{5-[(2,4-Diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-(1-propyl-2(1H)-phthalazinyl)-2-propen-1-one (1a)
A procedure of Guerry et al. [22] was significantly modified. A solution of 800 mg (2.07 mmol) of 13, 472 mg (2.07 mmol) of 6a, 18 mg (0.026 mmol) of bis(triphenylphosphine)palladium(II) dichloride [(Ph3P)2PdCl2] and 257 mg (0.30 mL, 2.27 mmol) of N-ethylpiperidine in 4 mL of anhydrous DMF was added to a 15-mL Pyrex pressure vessel (Chemglass CG-1880-01 with O-ring CG-309-210) equipped with a magnetic stirrer. The solution was purged with argon for 2 min, and the tube was tightly closed. The reaction was heated at 140 °C for 18 h, during which time the mixture turned a bright red color. After cooling, the crude reaction mixture was transferred directly to a 30 cm × 2 cm silica gel column slurry packed in dichloromethane. Impurities were eluted using dichloromethane, and the final product was eluted using 4% methanol in dichloromethane. Evaporation of the solvent gave a pale yellow solid, which was rechromatographed using a 15-cm × 2-cm silica gel column packed with 5% triethylamine in dichloromethane and eluted with 4% methanol in dichloromethane. Recrystallization of the isolated material (methanol) gave 422 mg (42%) of 1a as a white powder, mp 121–124 °C (shrinks to a glass-like bead). IR: 3474, 3341, 3182, 1645, 1608 cm−1; 1H-NMR (DMSO-d6, 300 MHz): δ 8.07 (d, 1H, J = 16.1 Hz), 7.80 (s, 1H), 7.66 (s, 1H), 7.64 (d, 1H, J = 16.1 Hz), 7.45 (td, 1H, J = 7.3, 1.5 Hz), 7.36 (td, 1H, J = 7.5, 1.3 Hz), 7.28 (dd, 1H, J = 7.5, 1.5 Hz), 7.18 (dd, 1H, J = 7.3, 1.5 Hz), 7.13 (d, 1H, J = 1.9 Hz), 6.66 (d, 1H, J = 1.9 Hz), 5.91 (t, 1H, J = 6.8 Hz), 4.84 (br s, 2H), 4.66 (br s, 2H), 3.84 (s, 3H), 3.80 (s, 3H), 3.68 (s, 2H), 1.64 (m, 2H), 1.27 (m, 2H), 0.87 (t, 3H, J = 7.1 Hz); 13C-NMR (DMSO-d6, 75 MHz): δ 166.5, 162.6, 162.3, 156.8, 153.5, 147.3, 142.3, 137.1, 134.2, 134.0, 131.4, 129.7, 128.0, 126.5, 125.6, 124.0, 118.7, 118.6, 112.9, 106.3, 61.4, 55.9, 51.3, 37.3, 34.4, 18.3, 13.8. Anal. Calcd for C27H30N6O3·2.0 H2O·0.5 CH3OH: C, 61.34; H, 6.69; N, 15.61. Found: C, 61.56; H, 6.42; N, 15.81.
5.1.23. (±)-(E)-3-{5-[(2,4-Diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-(1-isopropyl-2(1H)-phthalazinyl)-2-propen-1-one (1b)
This compound was prepared and purified on the same scale as described above. Work-up, chromatography and recrystallization (methanol) gave 400 mg (40%) of 1b as an off-white powder, mp 130–132 °C. IR: 3474, 3337, 3183, 1648, 1604 cm−1; 1H-NMR (DMSO-d6, 300 MHz): δ 7.95 (s, 1H), 7.86 (d, 1H, J = 16.2 Hz), 7.66 (d, 1H, J = 16.2 Hz), 7.60 (s, 1H), 7.59-7.42 (complex m, 3H), 7.38 (d, 1H, J = 7.1 Hz), 7.26 (s, 1H), 6.99 (s, 1H), 6.21 (br s, 2H), 5.76 (br s, 2H), 5.66 (d, 1H, J = 7.1 Hz), 3.79 (s, 3H), 3.73 (s, 3H), 3.59 (s, 2H), 1.89 (octet, 1H, J = 6.6 Hz), 0.84 (d, 3H, J = 6.6 Hz), 0.72 (d, 3H, J = 6.6 Hz); 13C-NMR (DMSO-d6, 75 MHz): δ 165.8, 162.2 (2C), 155.5, 152.4, 145.9, 143.6, 136.6 (2C), 131.4, 131.3, 128.3, 127.8, 127.5, 125.9, 124.3, 118.3, 117.9, 114.7, 105.8, 60.8, 55.7, 55.5, 32.9, 32.4, 18.9, 17.9. Anal. Calcd for C27H30N6O3·1.5 H2O: C, 63.16; H, 6.43; N, 16.37. Found: C, 63.14; H, 6.48; N, 16.36.
5.1.24. (±)-(E)-3-{5-[(2,4-Diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-[1-(3,3,3-trifluoropropyl)-2(1H)-phthalazinyl]-2-propen-1-one (1c)
This compound was prepared and purified on the same scale as described above. Work-up, chromatography and recrystallization (methanol) gave 790 mg (71%) of 1c as an off-white powder, mp 118–120 °C. IR: 3467, 3341, 3186, 1649, 1609 cm−1; 1H-NMR (DMSO-d6, 300 MHz): δ 8.00 (s, 1H), 7.89 (d, 1H, J = 16.2 Hz), 7.63-7.53 (complex m, 3H), 7.58 (d, 1H, J= 16.2 Hz), 7.50 (t, 2H, J = 6.6 Hz), 7.26 (s, 1H), 7.02 (s, 1H), 6.36 (br s, 2H), 6.01 (t, 1H, J = 6.6 Hz), 5.91 (br s, 2H), 3.80 (s, 3H), 3.75 (s, 3H), 3.60 (s, 2H), 2.18 (m, 2H), 1.79 (q, 2H, J = 7.7 Hz); 13C-NMR (DMSO-d6, 75 MHz): δ 165.9, 162.3, 161.5, 154.3, 152.5, 146.1, 143.0, 136.9, 136.3, 132.2, 132.1, 128.8, 127.7, 127.0 (q, JC-F = 274.0 Hz), 126.6, 126.4, 123.5, 118.4, 117.6, 114.9, 106.0, 60.8, 55.8, 49.1, 32.3, 29.0 (q, JC-F = 28.3 Hz), 26.8. Anal. Calcd for C27H27F3N6O3·1.5 H2O: C, 57.14; H, 5.29; N, 14.81. Found: C, 57.39; H, 4.98; N, 14.88.
5.1.25. (±)-(E)-3-{5-[(2,4-Diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-(1-isobutyl-2(1H)-phthalazinyl)-2-propen-1-one (1d)
This compound was prepared and purified on the same scale as described above. Work-up, chromatography and recrystallization (methanol) gave 670 mg (65%) of 1d as an off-white powder, mp 127–129 °C. IR: 3443, 3352, 3173, 1644, 1596 cm−1; 1H-NMR (DMSO-d6, 300 MHz): δ 8.00 (s, 1H), 7.86 (d, 1H, J = 16.5 Hz), 7.58 (d, 1H, J = 16.5 Hz), 7.57 (s, 1H), 7.55 (overlapping d and t, 2H, J ≈ 7.7 Hz), 7. 46 (t, 1H, J = 7.4 Hz), 7.38 (d, 1H, J = 7.1 Hz), 7.27 (s, 1H), 7.02 (s, 1H), 6.87 (br s, 2H), 6.38 (br s, 2H), 5.89 (t, 1H, J = 6.6 Hz), 3.80 (s, 3H), 3.74 (s, 3H), 3.62 (s, 2H), 1.38 (m, 3H), 0.93 (d, 3H, J = 4.9 Hz), 0.88 (d, 3H, J = 4.9 Hz); 13C-NMR (DMSO-d6, 75 MHz): δ 165.4, 162.8, 159.3, 152.5, 150.1, 146.1, 143.3, 136.6, 135.5, 134.0, 131.8, 128.3, 127.9, 126.2 (2C), 123.6, 118.6, 117.9, 114.9, 106.9, 60.8, 55.8, 48.8, 43.3, 32.1, 23.5, 22.9, 22.2. Anal. Calcd for C28H32N6O3·1.5 H2O: C, 63.76; H, 6.64; N, 15.94. Found: C, 63.85; H, 6.44; N, 15.94.
5.1.26. (±)-(E)-3-{5-[(2,4-Diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-(1-isobutenyl-2(1H)-phthalazinyl)-2-propen-1-one (1e)
This compound was prepared and purified on the same scale as described above. Work-up, chromatography and recrystallization (methanol) gave 671 mg (65%) of 1e as an off-white powder, mp 130–132 °C. IR: 3474, 3339, 3172, 1642, 1603 cm−1; 1H-NMR (DMSO-d6, 400 MHz): δ 7.91 (s, 1H), 7.86 (d, 1H, J = 16.0 Hz), 7.59 (d, 1H, J = 16.0 Hz), 7.57 (s, 1H), 7.52 (m, 2H), 7.42 (t, 1H, J = 7.4 Hz), 7.31 (d, 1H, J = 7.7 Hz), 7.25 (s, 1H), 7.01 (s, 1H), 6.72 (br s, 2H), 6.50 (d, 1H, J = 8.8 Hz), 6.24 (br s, 2H), 5.24 (d, 1H, J = 8.8 Hz), 3.80 (s, 3H), 3.74 (s, 3H), 3.61 (s, 2H), 1.96 (s, 3H), 1.60 (s, 3H); 13C-NMR (DMSO-d6, 100 MHz): δ 165.3, 162.7, 159.9, 155.9, 152.5, 151.2, 146.1, 142.0, 136.7, 135.7, 133.8, 133.5, 132.1, 128.2, 127.9, 126.1 (2C), 123.0, 122.1, 118.5, 118.0, 114.8, 106.6, 60.8, 55.8, 49.2, 25.2, 18.4. Anal. Calcd for C28H30N6O3·1.5 H2O: C, 64.00; H, 6.29; N, 16.00. Found: C, 64.37; H, 6.35; N, 16.07.
5.1.27. (±)-(E)-3-{5-[(2,4-Diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-[1-(1-ethylpropyl)-2(1H)-phthalazinyl]-2-propen-1-one (1f)
This compound was prepared and purified on the same scale as described above. Work-up, chromatography and recrystallization (methanol) gave 575 mg (54%) of 1f as an off-white powder, mp 134–136 °C. IR: 3479, 3332, 3184, 1656, 1605 cm−1; 1H-NMR (DMSO-d6, 300 MHz): δ 8.00 (s, 1H), 7.85 (d, 1H, J = 16.0 Hz), 7.65 (d, 1H, J = 16.0 Hz), 7.59 (s, 1H), 7.56 (d, 1H, J = 7.4 Hz), 7.53 (t, 1H, J = 7.2 Hz), 7.46 (t, 1H, J = 7.4 Hz), 7.36 (d, 1H, J = 7.4 Hz), 7.27 (s, 1H), 7.01 (d, 1H, J = 1.2 Hz), 6.57 (br s, 2H), 6.11 (br s, 2H), 5.83 (d, 1H, J = 7.4 Hz), 3.79 (s, 3H), 3.74 (s, 3H), 3.61 (s, 2H), 1.48 (m, 1H), 1.26 (m, 3H), 1.01 (m 1H), 0.89 (t, 3H, J = 7.2 Hz), 0.74 (t, 3H, J = 7.2 Hz); 13C-NMR (DMSO-d6, 75 MHz): δ 165.6, 162.5, 160.6, 152.5 (2C), 146.0, 144.0, 136.6, 136.0, 132.1, 131.5, 128.2, 127.8, 127.2, 126.0, 124.3, 118.5, 117.9, 114.8, 106.4, 60.8, 55.8, 52.1, 45.4, 32.2, 20.6, 20.1, 10.8, 10.4. Anal. Calcd for C29H34N6O3·1.5 H2O: C, 64.32; H, 6.84; N, 15.52. Found: C, 63.95; H, 7.10; N, 15.27.
5.1.28. (±)-(E)-3-{5-[(2,4-Diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-(1-cyclohexyl-2(1H)-phthalazinyl)-2-propen-1-one (1g)
This compound was prepared and purified on the same scale as described above. Work-up, chromatography and recrystallization (methanol) gave 510 mg (47%) of 1g as an off-white powder, mp 177–178 °C. IR: 3480, 3340, 3180, 1645, 1604 cm−1; 1H-NMR (DMSO-d6, 300 MHz): δ 7.96 (s, 1H), 7.85 (d, 1H, J = 16.2 Hz), 7.66 (d, 1H, J = 16.2 Hz), 7.59 (s, 1H), 7.59-7.42 (complex m, 3H), 7.36 (d, 1H, J = 7.7 Hz), 7.26 (s, 1H), 7.00 (d, 1H, J = 1.6 Hz), 6.48 (br s, 2H), 6.01 (br s, 2H), 5.67 (d, 1H, J = 7.1 Hz), 3.79 (s, 3H), 3.74 (s, 3H), 3.60 (s, 2H), 1.74-1.37 (complex m, 6H), 1.10-0.84 (complex m, 5H); 13C-NMR (DMSO-d6, 75 MHz): δ165.8, 162.5, 160.9, 153.1, 152.5, 146.0, 143.8, 136.6, 136.1, 131.7, 131.3, 128.3, 127.8, 127.5, 125.9, 124.2, 118.5, 117.9, 114.8, 106.3, 60.8, 55.8, 54.9, 42.1, 32.3, 28.8, 28.1, 25.6, 25.5, 25.3. Anal. Calcd for C30H34N6O3·2.5 H2O: C, 63.05; H, 6.83; N, 14.71. Found: C, 63.06; H, 6.53; N, 14.47.
5.1.29. (±)-(E)-3-{5-[(2,4-Diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-(1-phenyl-2(1H)-phthalazinyl)-2-propen-1-one (1h)
This compound was prepared and purified on the same scale as described above. Work-up, chromatography and recrystallization (methanol) gave 647 mg (60%) of 1h as a light pink powder, mp 142–144 °C. IR: 3478, 3340, 3178, 1653, 1607 cm−1; 1H-NMR (DMSO-d6, 300 MHz): δ 7.98 (s, 1H), 7.87 (d, 1H, J = 15.9 Hz), 7.70 (d, 1H, J = 15.9 Hz), 7.64-7.50 (complex m, 3H), 7.60 (s, 1 H), 7.45 (t, 1H, J = 7.1 Hz), 7.33-7.18 (complex m, 7H), 6.99 (s, 1H), 6.19 (br s, 2H), 5.74 (br s, 2H), 3.78 (s, 3H), 3.72 (s, 3H), 3.59 (s, 2H); 13C-NMR (DMSO-d6, 75 MHz): δ 165.8, 162.3, 162.1, 155.8, 152.4, 146.0, 142.0, 141.7, 137.1, 136.6, 132.9, 132.2, 128.58, 128.51, 127.6, 127.5, 127.2, 126.4, 126.0, 122.9, 118.4, 117.6, 114.9, 105.7, 60.8, 55.7, 53.8, 32.4. Anal. Calcd for C30H28N6O3·0.5 H2O: C, 68.05; H, 5.48; N, 15.87. Found: C, 68.41; H, 5.49; N, 15.93.
5.1.30. (±)-(E)-3-{5-[(2,4-Diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-[1-(2-methylphenyl)-2(1H)-phthalazinyl]-2-prop-2-en-1-one (1i)
This compound was prepared and purified on the same scale as described above. Work-up, chromatography and recrystallization (methanol) gave 638 mg (58%) of 1i as an off-white solid, mp 143–145 °C. IR: 3474, 3344, 3178, 1650, 1607 cm−1; 1H-NMR (DMSO-d6, 400 MHz): δ 7.92 (s, 1H), 7.79 (d, 1H, J = 16.1 Hz), 7.65 (d, 1H, J = 16.1 Hz), 7.60 (s, 1H), 7.54 (dd, 1H, J = 7.4, 1.2 Hz), 7.43 (td, 1H, J = 7.4, 1.6 Hz), 7.39 (td, 1H, J = 7.4, 1.2 Hz), 7.25 (d, 1H, J = 1.6 Hz), 7.23 (d, 1H, J = 7.6 Hz), 7.15 (m, 2H), 7.09 (td, 1H, J = 7.2, 1.8 Hz), 7.05 (td, 1H, J = 7.6, 1.6 Hz), 6.98 (d, 1H, J = 1.6 Hz), 6.94 (s, 1H), 6.18 (br s, 2H), 5.74 (br s, 2H), 3.77 (s, 3H), 3.70 (s, 3H), 3.59 (s, 2H), 2.71 (s, 3H); 13C-NMR (DMSO-d6, 100 MHz): δ 165.6, 162.3, 162.2, 155.8, 152.4, 146.0, 143.2, 140.2, 136.8, 136.6, 133.9, 133.7, 132.3, 130.3, 128.3, 127.7, 127.4, 127.0, 126.83, 126.78, 126.6, 121.9, 118.2, 117.8, 114.8, 105.7, 60.8, 55.7, 51.4, 32.4, 19.6. Anal. Calcd for C31H30N6O3·1.0 H2O: C, 67.39; H, 5.80; N, 15.22. Found: C, 67.17; H, 5.53; N, 15.17.
5.1.31. (±)-(E)-3-{5-[(2,4-Diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-[1-(4-methylphenyl)-2(1H)-phthalazinyl]-2-propen-1-one (1j)
This compound was prepared and purified on the same scale as described above. Work-up, chromatography and recrystallization (methanol) gave 740 mg (67%) of 1j as a light pink powder, mp 178–180 °C. IR: 3442, 3329, 3149, 1652, 1603 cm−1; 1H-NMR (DMSO-d6, 300 MHz): δ 7.97 (s, 1H), 7.86 (d, 1H, J = 15.9 Hz), 7.69 (d, 1H, J = 15.9 Hz), 7.60 (s, 1H), 7.56 (m, 3H), 7.44 (m, 1H), 7.27 (s, 1H), 7.11 (d, 2H, J = 8.8 Hz), 7.07 (d, 2H, J = 8.8 Hz), 6.99 (s, 1H), 6.94 (s, 1H), 6.19 (br s, 2H), 5.74 (br s, 2H), 3.78 (s, 3H), 3.72 (s, 3H), 3.59 (s, 2H), 2.20 (s, 3H); 13C-NMR (DMSO-d6, 75 MHz): δ 165.8, 162.3, 162.1, 155.8, 152.4, 146.0, 142.0, 138.8, 137.1, 136.8, 136.6, 133.1, 132.2, 129.1, 128.5, 127.7, 127.1, 126.4, 126.0, 123.0, 118.4, 117.7, 114.8, 105,7, 60.8, 55.7, 53.5, 32.4, 20.5. Anal. Calcd for C31H30N6O3·0.5 H2O: C, 68.51; H, 5.71; N, 15.47. Found: C, 68.55; H, 5.58; N, 15.48.
5.1.32. (±)-(E)-3-{5-[(2,4-Diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-[1-(3,5-dimethylphenyl)-2(1H)-phthalazinyl]-2-propen-1-one (1k)
This compound was prepared and purified on the same scale as described above. Work-up, chromatography and recrystallization (methanol) gave 480 mg (42%) of 1k as a light pink powder, mp 215–217 °C. IR: 3440, 3336, 3169, 1658, 1601 cm−1; 1H-NMR (DMSO-d6, 400 MHz): δ 7.96 (s, 1H), 7.85 (d, 1H, J = 16.1 Hz), 7.70 (d, 1H, J = 16.1 Hz), 7.59 (s, 1H), 7.54 (complex m, 1H), 7.52 (m, 2H), 7.43 (m, 1H), 7.29 (d, 1H, J = 1.6 Hz), 7.00 (d, 1H, J = 1.6 Hz), 6.87 (s, 1H), 6.84 (s, 2H), 6.45 (br s, 2H), 5.98 (br s, 2H), 3.79 (s, 3H), 3.72 (s, 3H), 3.60 (s, 2H), 2.18 (s, 6H); 13C-NMR (DMSO-d6, 100 MHz): δ 165.7, 162.4, 161.1, 153.5, 152.5, 146.1, 142.0, 141.9, 137.6, 137.0, 136.2, 133.1, 132.2, 129.0, 128.4, 127.7, 127.1, 126.4, 125.6, 122.7, 118.4, 117.7, 114.9, 106.2, 60.8, 55.8, 54.0, 32.3, 21.0. Anal. Calcd for C32H32N6O3·1.75 H2O: C, 66.26; H, 6.13; N, 14.50. Found: C, 66.22; H, 6.00; N, 14.46.
5.1.33. (±)-(E)-3-{5-[(2,4-Diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-[1-(3-fluorophenyl)-2(1H)-phthalazinyl]-2-propen-1-one (1l)
This compound was prepared and purified on the same scale as described above. Work-up, chromatography and recrystallization (methanol) gave 667 mg (60%) of 1l as an off-white powder, mp 153–155 °C. IR: 3495, 3433, 3375, 3165, 1647, 1600 cm−1; 1H-NMR (DMSO-d6, 400 MHz): δ 7.99 (s, 1H), 7.88 (d, 1H, J = 16.1 Hz), 7.70 (d, 1H, J = 16.1 Hz), 7.65 (d, 1H, J= 7.4 Hz), 7.60 (s, 1H), 7.57 (m, 1H), 7.56 (d, 1H, J = 7.2 Hz), 7.47 (m, 1H), 7.34 (m, 1H), 7.29 (d, 1H, J = 1.6 Hz), 7.07 (m, 3H), 7.02 (s, 1H), 7.01 (d, 1H, J = 1.6 Hz), 6.31 (br s, 2H), 5.85 (br s, 2H), 3.79 (s, 3H), 3.73 (s, 3H), 3.60 (s, 2H); 13C-NMR (DMSO-d6, 100 MHz): δ 165.9, 162.3, 162.1 (d, JC-F = 244.9 Hz), 161.8, 154.7, 152.5, 146.1, 144.5 (d, JC-F = 6.6 Hz), 142.0, 137.4, 136.4, 132.41, 132.38, 130.8 (d, JC-F = 8.1 Hz), 128.8, 127.6, 127.2, 126.6, 122.8, 121.9 (d, JC-F = 2.2 Hz), 118.4, 117.5, 114.9, 114.4 (d, JC-F = 20.6 Hz), 112.9 (d, JC-F = 22.8 Hz), 105.9, 60.8, 55.8, 53.4, 32.4. Anal. Calcd for C30H27FN6O3·0.7 H2O: C, 65.38; H, 5.16; N, 15.26. Found: C, 65.21; H, 5.21; N, 15.32.
5.1.34. (±)-(E)-3-{5-[(2,4-Diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-[1-(4-fluorophenyl)-2(1H)-phthalazinyl]-2-propen-1-one (1m)
This compound was prepared and purified on the same scale as described above. Work-up, chromatography and recrystallization (methanol) gave 608 mg (55%) of 1m as an off-white powder, mp 143–145 °C. IR: 3479, 3347, 3180, 1649, 1600 cm−1; 1H-NMR (DMSO-d6, 300 MHz): δ 8.00 (s, 1H), 7.88 (d, 1H, J = 15.9 Hz), 7.69 (d, 1H, J = 15.9 Hz), 7.61-7.56 (complex m, 3H), 7.60 (s, 1H), 7.46 (m, 1H), 7.27 (complex m, 3H), 7.12 (t, 2H, J = 8.8 Hz), 7.02 (s, 2H), 6.19 (br s, 2H), 5.75 (br s, 2H), 3.79 (s, 3H), 3.73 (s, 3H), 3.59 (s, 2H); 13C-NMR (DMSO-d6, 75 MHz): δ 165.8, 162.3, 162.2, 161.4 (d, JC-F = 243.9 Hz), 155.8, 152.5, 146.0, 142.1, 137.9 (d, JC-F = 2.9 Hz), 137.3, 136.6, 132.7, 132.3, 128.7, 128.3 (d, JC-F = 8.3 Hz), 127.6, 127.2, 126.5, 122.9, 118.4, 117.5, 115.4 (d, JC-F = 21.5 Hz), 114.4, 105.7, 60.8, 55.7, 53.0, 32.4. Anal. Calcd for C30H27FN6O3·0.5 H2O: C, 65.81; H, 5.12; N, 15.36. Found: C, 65.88; H, 5.14; N, 15.24.
5.1.35. (±)-(E)-1-(1-Benzyl-2(1H)-phthalazinyl)-3-{5-[(2,4-diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-2-propen-1-one (1n)
This compound was prepared and purified on the same scale as described above. Work-up, chromatography and recrystallization (methanol) gave 620 mg (56%) of 1n as an off-white powder, mp 143–145 °C. IR: 3479, 3336, 3179, 1658, 1604 cm−1; 1H-NMR (DMSO-d6, 400 MHz): δ 7.83 (d, 1H, J = 16.0 Hz), 7.78 (s, 1H), 7.61 (s, 1H), 7.60 (d, 1H, J = 16.0 Hz), 7.47-7.37 (complex m, 3H), 7.24 (s, 1H), 7.17 (m, 3H), 7.00 (d, 1H, J = 1.2 Hz), 6.96 (m, 1H), 6.86 (m, 2H), 6.19 (br s, 2H), 6.04 (t, 1H, J = 6.4 Hz), 5.73 (br s, 2H), 3.79 (s, 3H), 3.74 (s, 3H), 3.60 (s, 2H), 2.90 (dd, 1H, J = 13.0, 7.6 Hz), 2.83 (dd, 1H, J = 13.0, 5.7 Hz); 13C-NMR (DMSO-d6, 100 MHz): δ 165.6, 162.3, 162.2, 155.8, 152.5, 146.0, 142.3, 136.64, 136.57, 136.2, 132.4, 131.3, 129.6, 128.4, 127.9, 127.8, 126.6, 126.5, 125.9, 123.8, 118.3, 117.8, 114.7, 105.7, 60.8, 55.7, 52.3, 48.6, 32.4. Anal. Calcd for C31H30N6O3·1.0 CH3OH: C, 67.84; H, 6.01; N, 14.84. Found: C, 68.13; H, 5.86; N, 15.12.
5.1.36. (±)-(E)-3-{5-[(2,4-Diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-[1-(4-methylbenzyl)-2(1H)-phthalazinyl]-2-propen-1-one (1o)
This compound was prepared and purified on the same scale as described above. Work-up, chromatography and recrystallization (methanol) gave 545 mg (48%) of 1o as an off-white powder, mp 135–137 °C. IR: 3477, 3338, 3176, 1657, 1605 cm−1; 1H-NMR (DMSO-d6, 400 MHz): δ 7.83 (d, 1H, J = 16.1 Hz), 7.78 (s, 1H), 7.61 (s, 1H), 7.60 (d, 1H J = 16.1 Hz), 7.44 (m, 1H), 7.41 (dd, 2H, J = 5.6, 3.4 Hz), 7.24 (d, 1H J = 1.6 Hz), 6.99 (s, 1H), 6.98 (d, 2H, J = 8.0 Hz), 6.96 (m, 1H), 6.73 (d, 2H, J = 8.0 Hz), 6.18 (br s, 2H), 6.00 (t, 1H, J = 6.4 Hz), 5.73 (br s, 2H), 3.79 (s, 3H), 3.74 (s, 3H), 3.60 (s, 2H), 2.85 (dd, 1H, J = 13.2, 7.6 Hz), 2.78 (dd, 1H, J= 13.2, 5.5 Hz), 2.23 (s, 3H); 13C-NMR (DMSO-d6, 100 MHz): δ 165.5, 162.3, 162.2, 155.8, 152.5, 146.0, 142.2, 136.6, 136.5, 135.4, 133.1, 132.5, 131.3, 129.5, 128.6, 128.3, 127.8, 126.7, 125.9, 123.8, 118.3, 117.8, 114.7, 105.7, 60.8, 55.7, 52.4, 48.6, 32.4, 20.7. Anal. Calcd for C31H30N6O3·1.0 CH3OH: C, 67.84; H, 6.01; N, 14.84. Found: C, 68.17; H, 6.11; N, 14.78.
5.1.37. (±)-(E)-3-{5-[(2,4-Diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-[1-(4-methoxybenzyl)-2(1H)-phthalazinyl]-2-propen-1-one (1p)
This compound was prepared and purified on the same scale as described above. Work-up, chromatography and recrystallization (methanol) gave 595 mg (51%) of 1p as an off-white powder, mp 253–255 °C. IR: 3478, 3333, 3174, 1657, 1605 cm−1; 1H-NMR (DMSO-d6, 400 MHz): δ 7.83 (d, 1H, J = 16.1 Hz), 7.78 (s, 1H), 7.60 (s, 1H), 7.60 (d, 1H, J = 16.1 Hz), 7.42 (m, 3H), 7.24 (s, 1H), 6.99 (s, 1H), 6.97 (m, 1H), 6.76 (d, 2H, J = 9.1 Hz), 6.74 (d, 2H, J = 9.1 Hz), 6.18 (br s, 2H), 5.97 (t, 1H, J = 6.2 Hz), 5.73 (br s, 2H), 3.79 (s, 3H), 3.73 (s, 3H), 3.68 (s, 3H), 3.59 (s, 2H), 2.83 (dd, 1H, J = 13.3, 7.4 Hz), 2.76 (dd, 1H, J = 13.3, 5.5 Hz); 13C-NMR (DMSO-d6, 100 MHz): δ 165.5, 162.3, 162.2, 157.9, 155.8, 152.5, 146.0, 142.2, 136.6, 136.5, 132.5, 131.3, 130.6, 128.3, 128.0, 127.8, 126.7, 125.9, 123.8, 118.3, 117.8, 114.2, 113.4, 105.7, 60.8, 55.7, 54.9 (2C), 52.4, 32.4. Anal. Calcd for C32H32N6O4·0.5 H2O: C, 67.02; H, 5.76; N, 14.66. Found: C, 67.15; H, 5.69; N, 14.66.
5.1.38. (±)-(E)-3-{5-[(2,4-Diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-[1-(4-trifluoromethoxybenzyl)-2(1H)-phthalazinyl]-2-propen-1-one (1q)
This compound was prepared and purified on the same scale as described above. Work-up, chromatography and recrystallization (methanol) gave 320 mg (25%) of 1q as an off-white powder, mp 125–127 °C. IR: 3481, 3335, 3183, 1657, 1607 cm−1; 1H-NMR (DMSO-d6, 400 MHz): δ 7.81 (d, 1H, J = 16.1 Hz), 7.80 (s, 1H), 7.60 (s, 1H), 7.58 (d, 1H, J = 16.1 Hz), 7.44 (m, 3H), 7.24 (s, 1H), 7.17 (d, 2H, J = 8.0 Hz), 7.08 (m, 1H), 6.99 (s, 1H), 6.98 (d, 2H, J = 8.0 Hz), 6.18 (br s, 2H), 6.08 (t, 1H, J = 6.3 Hz), 5.73 (br s, 2H), 3.79 (s 3H), 3.73 (s, 3H), 3.59 (s, 2H), 2.95 (dd, 1H, J = 13.2, 7.0 Hz), 2.85 (dd, 1H, J = 13.2, 6.1 Hz); 13C-NMR (DMSO-d6, 100 MHz): δ 165.6, 162.3, 162.2, 155.7, 152.5, 147.1, 146.0, 142.4, 136.6, 135.8, 132.3, 131.5, 131.4, 128.5, 127.7, 126.6, 126.0, 123.8, 120.5, 120.0 (q, JC-F = 256.2 Hz), 118.8, 118.3, 117.7, 114.8, 105.7, 60.8, 55.7 (2C), 52.0, 32.4. Anal. Calcd for C32H29F3N6O4·2.5 H2O: C, 57.92; H, 5.13; N, 12.67. Found: C, 57.74; H, 4.91; N, 12.37.
5.2. Biology
5.2.1. Biological assay-determinations of the MICs
The minimum inhibitory concentration (MIC in μg/mL) was evaluated for each agent against B. anthracis Sterne strain using the broth microdilution method following CLSI protocols [4,32]. Compounds were screened using two-fold dilutions, typically starting with 16 μg/mL, except for racemic compounds 1a and 1h, which were also screened with 12 μg/mL as a starting concentration. The MIC values were determined as the nearest dilution that inhibited growth to 80% of the positive growth control, as evaluated visually and by optical density (turbidity) at 600 nm. The data were derived from two independent experiments performed in duplicate and are presented in Table 1.
*Highlights.
An improved synthesis of 2,4-diaminopyrimidine-based antibiotics is reported.
Antibiotic synthesis includes a novel sealed-tube Heck reaction.
The agents synthesized show activity in the μg/mL range.
The size and planarity of the phthalazine C-1 substituent influences the activity.
Steric and electronic factors affect the binding of the agents to DHFR.
Acknowledgments
We gratefully acknowledge support of this work by the National Institutes of Allergy and Infectious Diseases [1-R01-AI090685-01] of the NIH/NIAID to WWB. We are also pleased to acknowledge funding for the Oklahoma Statewide NMR Facility by the National Science Foundation (BIR-9512269), the Oklahoma State Regents for Higher Education, the W. M. Keck Foundation, and Conoco, Inc. Finally, molecular graphics images were produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California-San Francisco [supported by NIH P41 RR001081].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Hauer J, McDade J, Osterholm MT, O’Toole T, Parker G, Perl TM, Russell PK, Tonat K. Anthrax as a biological weapon: Medical and public health management. Working group on civilian biodefense. JAMA. 1999;281:1735–1745. doi: 10.1001/jama.281.18.1735. [DOI] [PubMed] [Google Scholar]
- 2.Beierlien JM, Frey KM, Bolstad DB, Pelphey PM, Joska TM, Smith AE, Priestley ND, Wright DL, Anderson AC. Synthetic and crystallographic studies of a new inhibitor series targeting Bacillusanthracis dihydrofolate reductase. J Med Chem. 2008;51:7532–7540. doi: 10.1021/jm800776a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourne CR, Bunce RA, Bourne PC, Berlin KD, Barrow EW, Barrow WW. Crystal structure of Bacillus anthracis dihydrofolate reductase with the dihydrophthalazine-based trimethoprim derivative RAB1 provides a structural explanation of potency and selectivity. Antimicrob Agents Chemother. 2009;53:3065–3073. doi: 10.1128/AAC.01666-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrow EW, Drier J, Reinelt S, Bourne PC, Barrow WW. In vitro efficacy of new antifolates against trimethoprim-resistant Bacillus anthracis. Antimicrob Agents Chemother. 2007;51:4447–4452. doi: 10.1128/AAC.00628-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson RC. In: In Antifolate Drugs in Cancer Therapy. Jackman AL, editor. Humana Press; Totowa, NJ: 1999. [Google Scholar]
- 6.Berman EM, Werbel LM. The renewed potential for folate antagonists in contemporary cancer chemotherapy. J Med Chem. 1991;34:479–485. doi: 10.1021/jm00106a001. [DOI] [PubMed] [Google Scholar]
- 7.Takimoto CH. Antifolates in clinical development. Semin Oncol. 1997;24:S18-40–S18-51. [PubMed] [Google Scholar]
- 8.Bleyer WA. The clinical pharmacology of methotrexate. New applications of an old drug. Cancer Treat Rev. 1978;41:36–51. doi: 10.1002/1097-0142(197801)41:1<36::aid-cncr2820410108>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Adane L, Bharatam PV. Modelling and informatics in the analysis of P. falciparum DHFR enzyme inhibitors. Curr Med Chem. 2008;15:1552–1569. doi: 10.2174/092986708784911551. [DOI] [PubMed] [Google Scholar]
- 10.Finland M, Kass SF. Symposium on trimethoprim-sulfmethoxazole. J Infect Dis. 1973;128:S425–S816. doi: 10.1093/infdis/128.supplement_3.s792. [DOI] [PubMed] [Google Scholar]
- 11.Suling WJ, Seitz LE, Pathak V, Westbrook L, Barrow EW, Zywno-Van-Ginkel S, Reynolds RC, Piper JR, Barrow WW. Antimycobacterial activities of 2,4-diamino-5-deazapteridine derivatives and effects on mycobacterial dihydrofolate reductase. Antimicrob Agents Chemother. 2000;44:2784–2793. doi: 10.1128/aac.44.10.2784-2793.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark C, Ednie LM, Lin G, Smith K, Kosowska-Shick K, McGhee P, Dewasse B, Beachel L, Caspers P, Gaucher B, Mert G, Shapiro S, Appelbaum PC. Antistaphylococcal activity of dihydrophthalazine antifolates, a family of novel antibacterial drugs. Antimicrob Agents Chemother. 2009;53:1353–1361. doi: 10.1128/AAC.01619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caspers P, Bury L, Gaucher B, Heim J, Shapiro S, Siegrist S, Schmitt-Hoffmann A, Thenoz L, Urwyler H. In vitro and in vivo properties of dihydrophthalazine antifolates, a novel family of antibacterial drugs. Antimicrob Agents Chemother. 2009;53:3620–3627. doi: 10.1128/AAC.00377-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowker KE, Caspers P, Gaucher B, MacGowan AP. In vitro activities of three new dihydrofolate reductase inhibitors against clinical isolates of gram-positive bacteria. Antimicrob Agents Chemother. 2009;53:4949–4952. doi: 10.1128/AAC.00845-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawser S, Lociuro S, Islam K. Dihydrofolate reductase inhibitors as antibacterial agents. Biochem Pharm. 2006;71:941–948. doi: 10.1016/j.bcp.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 16.Smith K, Ednie LM, Appelbaum PC, Hawser S, Lociuro S. Antistreptococcal activity of AR-709 compared to that of other agents. Antimicrob Agents Chemother. 2008;52:2279–2282. doi: 10.1128/AAC.01620-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Hilgers M, Cunningham M, Chen Z, Trzoss M, Zhang J, Kohnen L, Lam T, Creighton C, Kedar GC, Nelson K, Kwan B, Stidham M, Brown-Driver V, Shaw KJ, Finn J. Structure-based design of new DHFR-based antibacterial agents: 7-aryl-2,4-diaminoquinazolines. Bioorg Med Chem Lett. 2011;21:5171–5176. doi: 10.1016/j.bmcl.2011.07.059. [DOI] [PubMed] [Google Scholar]
- 18.Cody V, Pace J. Structural analysis of Pneumocystis carinii and human DHFR complexes with NADPH and a series of five potent 6-[5′-(ω-carboxyalkoxy)benzyl]pyrido[2,3-d]-pyrimidine derivatives. Acta Cryst. 2011;D67:1–7. doi: 10.1107/S0907444910041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beierlein JM, Karri NG, Anderson AC. Targeted mutations of Bacillus anthracis dihydrofolate reductase condense complex structure-activity relationships. J Med Chem. 2010;53:7327–7336. doi: 10.1021/jm100727t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gangjee A, Yu J, McGuire JJ, Cody V, Galitsky N, Kisliuk RL, Queener SF. Design, synthesis and X-ray crystal structure of a potent dual inhibitor of thymidine synthase and dihydrofolate reductase as an antitumor agent. J Med Chem. 2000;43:3837–3851. doi: 10.1021/jm000200l. [DOI] [PubMed] [Google Scholar]
- 21.Gangjee A, Viwdans A, Elzein E, McGuire JJ, Queener SF, Kiliuk RL. Synthesis, antifolate, and antitumor activities of classical and nonclassical 2-amino-4-oxo-5-substituted pyrrolo[2,3-d]pyridmidines. J Med Chem. 2001;44:1993–2003. doi: 10.1021/jm0100382. [DOI] [PubMed] [Google Scholar]
- 22.A patent in this area contained citations to 2, but little experimental detail and very few properties were revealed; see Guerry P, Hubschwerlen C, Jolidon S, Specklin J-L, Wyss PC. Substituted 2,4-diaminopyrimidines. World Patent WO9839328. 1998Chem Abstr. 1998;129:230736.
- 23.Bunce RA, Berlin KD, Bourne CR, Bourne PC, Barrow EW, Barrow WW. Identification and enantiomeric separation of (+)- and (−)-(E)-3-[5-(2,4-diaminopyrimidin-5-ylmethyl)-2,3-dimethoxyphenyl]-1-(1-propyl-1H-phthalazin-2-yl)propenone by supercritical fluid chromatography. Proc Okla Acad Sci. 2010;90:139–142. [Google Scholar]
- 24.Bourne CR, Bunce RA, Barrow EW, Bourne PC, Berlin KD, Barrow WW. Inhibition of antibiotic resistant Staphylococcus aureus by the broad-spectrum dihydrofolate reductase inhibitor RAB1. Antimicrob Agents Chemother. 2010;54:3825–3833. doi: 10.1128/AAC.00361-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uno H, Okada S, Shiraishi Y, Shimokawa K, Suzuki H. Boron trifluoride-assisted Ziegler-Zeiser reaction of perfluoroalkyllithiums. An efficient synthesis of perfluoroalkylated heterocycles. Chem Lett. 1988:1165–1168. [Google Scholar]
- 26.Holcomb WF, Hamilton CS. Derivatives of 4-amino-6-methoxyquinaldine. J Am Chem Soc. 1942;64:1309–1311. [Google Scholar]
- 27.Whitmore FC, Mosher HS, Adams RR, Taylor RB, Chapin EC, Weisel C, Yanko W. Basically substituted aliphatic nitriles and their catalytic reduction to amines. J Am Chem Soc. 1944;66:725–731. [Google Scholar]
- 28.Nimgirawath S. Synthesis of (±)-isoautumnaline and (±)-dysoxylin. Aust J Chem. 1994;47:957–962. [Google Scholar]
- 29.Stuart A, Paterson T, Roth B, Aig E. 2,4-Diamino-5-benzylpyrimidine and analogues as antibacterial Agents. 6. A one-step synthesis of trimethoprim derivatives and activity analysis by molecular modeling. J Med Chem. 1983;26:667–673. doi: 10.1021/jm00359a009. [DOI] [PubMed] [Google Scholar]
- 30.No examples were found in a recent comprehensive review; see Oestreich M, editor. The Mizoroki-Heck Reaction. Wiley & Sons; Chichester, West Sussex, UK: 2009.
- 31.X-ray data on related compounds shows the 2,4-diaminopyrimidine ring H-bonded with water in the crystal structure; see Frey KM, Lombardo MN, Wright DL, Anderson AC. Towards the understanding of resistance mechanisms in clinically isolated trimethoprim-resistant, methicillin-resistant Staphylococcus aureus dihydrofolate reductase. J Struc Biol. 2010;170:93–97. doi: 10.1016/j.jsb.2009.12.011.
- 32.CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard. 8. Vol. 29. Wayne, PA: 2009. pp. 1–65. [Google Scholar]