Abstract
Mesenchymal stromal cells (MSCs) show promise for treatment of a variety of neurological and other disorders. Cat has a high degree of linkage with the human genome and has been used as a model for analysis of neurological disorders such as stroke, Alzheimer’s disease and motor disorders. The present study was designed to characterize bone marrow-derived MSCs from cats and to investigate the capacity to generate functional peptidergic neurons. MSCs were expanded with cells from the femurs of cats and then characterized by phenotype and function. Phenotypically, feline and human MSCs shared surface markers, and lacked hematopoietic markers, with similar morphology. As compared to a subset of human MSCs, feline MSCs showed no evidence of the major histocompatibility class II. Since the literature suggested Stro-1 as an indicator of pluripotency, we compared early and late passages feline MSCs and found its expression in >90% of the cells. However, the early passage cells showed two distinct populations of Stro-1-expressing cells. At passage 5, the MSCs were more homogeneous with regards to Stro-1 expression. The passage 5 MSCs differentiated to osteogenic and adipogenic cells, and generated neurons with electrophysiological properties. This correlated with the expression of mature neuronal markers with concomitant decrease in stem cell-associated genes. At day 12 induction, the cells were positive for MAP2, Neuronal Nuclei, tubulin βIII, Tau and synaptophysin. This correlated with electrophysiological maturity as presented by excitatory postsynaptic potentials (EPSPs). The findings indicate that the cat may constitute a promising biomedical model for evaluation of novel therapies such as stem cell therapy in such neurological disorders as Alzheimer’s disease and stroke.
Keywords: cat, mesenchymal stem cells, neuronal differentiation, immunohistochemistry, feline, bone marrow
INTRODUCTION
The recent discovery of the self-renewal properties of mesenchymal stromal cells (MSCs) and their capacity to differentiate into multiple lineages such as osteocytes, adipocytes, and chondrocytes (Baddoo et al., 2003) has rendered them a focus of study for cellular replacement therapy and tissue engineering. The data provided by the application of MSCs has been derived from either human or rodent models. The rodent model has allowed major advances in our basic understanding of the biology of several diseases (Mashimo and Serikawa, 2009). However, a near universal reliance on these models has revealed some major deficiencies. For example, implanted or induced tumors in xenograft models do not always behave in the same biological manner as the natural parent tumors in humans, which is especially true of primary tumor growth and metastasis (Husemann and Klein, 2009, Talmadge et al., 2007). In addition, treatments that demonstrate success in rodent models rarely translate into successful therapies in human clinical trials, clearly indicating fundamental differences that are difficult to resolve without utilizing alternative models (Kamb et al., 2007). With respect to the development of highly targeted therapies for specific disease conditions in humans, these problems would appear to become further exaggerated, thus establishing the need to combine rodent studies with comparative studies in other species to optimize the potential of developing the most effective therapies for humans. Therefore, in recent years, efforts have been made to utilize non human primate models to provide further insights into the possible mechanisms underlying these diseases (Shively and Clarkson, 2009).
Of particular interest is the cat, which has revealed a high degree of linkage conservation with human genomes, with the cat showing the least chromosomal changes as compared to human (O’Brien et al., 1999, Murphy et al., 2000). The genome analyses have made the cat an excellent model to study the molecular mechanisms for various disease states. Domestic cats are subject to epidemics of two viruses, FeLV and FIV that cause immunodeficiencies and neoplasias, thus providing a powerful animal model for leukemia and AIDS (Dias et al., 2006). Another example is the occurrence of spontaneous tumors in cat that offer a unique opportunity as models for human cancer biology and translational cancer therapeutics (Vail and MacEwen, 2000). Because of factors such as incidence of some cancers, similar physiological responses, large body size, comparable responses to cytotoxic agents and shorter overall lifespan, the cat offers several advantages as an animal model for these malignancies (Porrello et al., 2006). The tumor types that offer the best comparative interest include lymphoma/leukemia, osteosarcoma, STS, melanoma, and mammary tumors (Vail and MacEwen, 2000). In the study of these cancers, cats have also been used to investigate the interaction of host immune response through studies of the major histocompatibility complex, T-cell receptor loci, immunoglobulin genes and other loci that participate in immune response (Bergman, 2003). Thus with the development of new therapeutic agents (traditional chemotherapy, gene therapy, biologic agents, etc.), the feline model may provide useful populations to test new therapeutic agents whose efficacy and toxicity can be effectively examined.
In addition to these diseases, the cat model has been extensively used for the study of varieties of neurological disorders. Several examples include: aging cats with signs and symptoms of human senility have been utilized for the study of Alzheimer’s disease (Head et al., 2005) and stroke (Schaller et al., 2003) and recently, the cat has been identified as a model of ataxia (Ilyas et al., 2008). In our laboratory, we have used feline model to study the mechanisms of defensive rage and predatory attack, forms of aggressive behavior, expressed both in animals and human (Siegel et al., 1999, Siegel et al., 2007). Because there is a strong similarity between nervous tissue antigens of humans and cats, characterization of feline mesenchymal stem cells may provide specific insights into directions that need to be taken for the development of the therapeutic agents for treatment of various neurological disorders. Accordingly, the aim of the present study was to characterize the markers of feline MSCs, their potential to develop into neurons, and their ability to express neural stem cell markers, GABA receptors and electrophysiological currents.
MATERIALS AND METHODS
Reagents and Antibodies
All tissue culture media, fibroblast growth factor, retinoic acid and Histopaque (Density 1.073 g/mL) were purchased from Sigma, (St. Louis, MO, USA). Premium fetal calf sera were purchased from Atlanta Biologicals (Lawrenceville, GA). Antibodies to CD29 and CD45 were purchased from Serotec (Raleigh, NC, USA), while antibodies against CD14, CD34, CD44, CD105 and MHC-II were purchased from VMRD (Pullman, WA, USA). All primary antibodies were used at 1/1000 final dilution and secondary antibodies at 1/500.
Antibodies to neuronal antigens used for immunohistochemistry were as follows: mouse anti-βIII-tubulin, anti-synaptophysin, anti-Sox2, anti-MAP2, anti-Tau, anti-Neuronal Nuclei (NeuN) and anti-nestin were purchased from EMD Millipore Corporation (Billerica, Massachusetts). All neuronal primary antibodies were used at 1/1000 dilutions and secondary antibodies at 1/500.
Anti-murine stro-1 was purchased from R&D Systems (Minneapolis, MN), APC-rabbit anti-mouse IgG/IgM/IgA from Open Biosystems (Huntsville, AL), FITC-goat anti-mouse IgG from Santa Cruz Biotechnology Inc (Santa Cruz, CA). Anti-stro-1 was used at 1/100 final dilution and secondary antibody at 1/200.
Isolation of feline marrow/culture of feline mesenchymal stromal cells (MSCs)
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey-New Jersey Medical School. The studies used female cats, weighing between 2 and 4 kg (Liberty laboratories, Waverly, NY). Each animal was housed individually in its home cage and maintained on an ad libitum feeding and drinking schedule.
Cats were perfused transcardially under deep anesthesia (pentobarbital 100 mg/kg body weight) with 9.25% sucrose solution in PBS (w/v) (pH 7.2). Feline bone marrow was harvested from the femur or humerus of the cat by flushing the shaft of a femur under sterile conditions. The ends of each humerus and femur were clipped off to expose the marrow. A syringe was inserted into the bone and complete Iscove’s modified Dulbecco’s medium (IMDM) containing 200 units/mL heparin was pushed through the bone to collect the marrow. Bone marrow was collected into 1–5 volumes. Ten ml of cell suspension was loaded onto 3 ml of Histopaque solution and then centrifuged at 500 g for 30 minutes. Mononuclear cells were collected at the interface of PBS and Histopaque. Cells were washed 2x with phosphate-buffered saline (PBS) and seeded at 2 × 105/cm2 in Dulbecco’s Modified Eagle’s Medium (DMEM) (1 g/L glucose) with 10% fetal bovine serum and incubated at 37°C, 5%CO2. Previously it was determined that selected fetal bovine serum has been shown to have least toxicity for the cells. After 72 h incubation, non-adherent cells were removed and 2/3 of media was replaced with fresh medium. After 7 to 12 days in culture, the adherent cells reached 80% confluence and were then trypsinized and replated at 8000/cm2. At weekly intervals, 2/3 of medium was replaced with fresh medium. The passages continued and at passage five, the cells were analyzed by flow cytometry for CD45, CD105, CD44 and CD29. Each batch of cells were also studied for adipogenic and osteogenic potential as described (Potian et al., 2003).
Culture of Human MSCs
The method to culture MSCs were previously described (Greco et al., 2007a). Briefly, MSCs were grown from bone marrow aspirates of healthy individuals between 20–30 years. The use of human bone marrow aspirates was approved by the Institutional Review Board of University of Medicine and Dentistry of New Jersey (Newark, NJ) Aspirates were added to vacuum-gas plasma treated, tissue culture Falcon 3003 petri dishes (BD biosciences) in DMEM containing 10% FBS. After 3 days, RBCs and neutrophils were removed by Histopaque density gradient. At passage 4, the MSCs were symmetric, CD29+, CD44+, CD105+, CD14−, CD34−, CD45−, prolyl-4-hydroxylase (−) (Potian et al., 2003); generated electrophysiologically active dopaminergic and peptidergic neurons (Greco et al., 2007b, Trzaska et al., 2009); and differentiated into osteogenic and adipogenic cells (Potian et al., 2003). All antibodies were used at 1/500 dilution.
Flow cytometry
Flow cytometry for membrane-bound proteins were performed for CD29, CD14, CD44, CD45, CD34, CD105 and MHC II. MSCs were resuspended in 1% bovine serum albumin with 0.2% sodium azide in PBS and then labeled on ice with the antibodies, at 1/500 dilution. Non-specific binding was determined in parallel with FITC-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology Inc, Santa Cruz, CA). The cells were analyzed on a BD FACSCanto™ II fluorescence-activated cell sorter.
Intracellular flow cytometry for stro-1 was performed by the following consecutive treatments: fixed in 4% formaldehyde for 15 min at 4°C, permeabilized in 0.1% Triton X-100 for 30 min, incubated with anti-stro-1 for 30 min at 4°C, washed once with cold PBS and then incubated with APC-goat anti-mouse Ig for 30 min in the dark at 4°C. After this, cells were washed and immediately analyzed with the FACSCalibur (BD Biosciences).
Neuronal induction of feline MSCs
The neuronal induction protocol was conducted as described (Greco et al., 2008, Greco et al., 2007b). Briefly, at 70–80% confluence, MSCs were trypsinized and subcultured in either 35 or 60 mm3 petri dishes (Falcon 3003) with neuronal induction medium (NIM). NIM consisted of Ham’s DMEM/F12, 2% FBS, B27 supplement, 20 μM RA, and 12.5 ng/ml of basic fibroblast growth factor (bFGF). The induced and uninduced cells were analyzed with neuron-specific antibodies (see below for method) and co-labeling with phalloidin for structure and DAPI to identify the nuclei, as described (Trzaska et al., 2009). In addition, the cells were studied for Nestin and Sox-2 to identify neuronal stem cells and, βIII-tubulin, Tau-3, and MAP-2 for mature neurons. Antibodies for synaptic vesicles anti-synaptophysin were used to identify formation of synaptic vesicles in neurons and functional synaptic junctions among them.
Immunocytochemistry for neuronal markers
Uninduced and induced MSCs were labeled with antibodies to nestin, Sox-2, NeuN, βIII-tubulin, Tau-3, Map-2, synaptic vesicles or synaptophysin at 4°C overnight. Positive cells were visualized with a FITC- or Rhodamine-conjugated secondary antibody (Santa Cruz Biotechnology Inc, Santa Cruz, CA) and counterstained with DAPI (Vector Laboratories, Burlingame, CA, USA). The secondary antibodies were incubated for two hours at room temperature. The uninduced MSCs served as controls for the stem cell markers and day 12 induction served as controls for the neuronal markers. The images were taken on at 100x magnification with EVOS digital microscope (Advanced Microscopy Group, Bothell, WA).
Osteogenic Induction
Osteogenic induction of MSCs was performed using Poietics Human Mesenchymal Stem Cells kit (Lonza, Switzerland). Briefly, MSCs were seeded in complete media at a density of 3 × 103 per cm2 on vacuum gas plasma-treated plates. Cells were allowed for adhere for 24 h, and media was replaced with osteogenic induction media (containing β-glycerophosphate, ascorbic acid, and dexamethasone). Media was replaced every 72 h with fresh osteogenic induction media for 21 days, and cells were stained in 2% alizarin red solution for 5 mins. Detection of mineralization of bone was observed by brightfield microscopy.
Adipogenic Induction
Adipogenic induction of MSCs was performed using Poietics Human Mesenchymal Stem Cells kit (Lonza). Briefly, MSCs were seeded in complete media at a density of 2 × 104 per cm2 on vacuum gas plasma-treated plates. Cells were allowed for adhere for 24 h, and media was replaced every 72 hours until cultures reached 100% confluence. Cells were incubated in adipogenic induction media (containing indomethacin, isobutylmethylxanthene, insulin, and dexamethasone) for 72 h then incubated in adipogenic maintenance media (containing insulin) for 48 h. After three cycles of induction and maintenance, complete media was added for 7 days. Cells were fixed in 10% formalin and incubated 0.18% Oil Red O (Sigma) solution for 5 min and observed by bright field microscopy.
Whole-cell Patch Clamp Electrophysiology
Electrical signals were obtained in a conventional whole-cell configuration with a Multiclamp 700A amplifier (Molecular Devices, Union City, CA), a Digidata 1440A digitizer (Molecular Devices) and pCLAMP 10.2 software (Molecular Devices). Data were filtered at 1 kHz and sampled at 5 kHz. The patch electrodes had a resistance of 4–6 MΩ when filled with the pipette solution containing (in mM): 135 KCl, 12 NaCl, 0.5 EGTA, 10 HEPES, 2 Mg-ATP, and 0.3 Tris-GTP, pH7.3 with KOH. Stem cells in a cover slip were placed into a 0.4 ml recording chamber filled with artificial cerebrospinal fluid containing (in mM) 125 NaCl, 1.6 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 25 NaHCO3, and 11 glucose, and saturated with 95%O2/5%CO2. Stem cells were identified under visual guidance using infrared-differential interference contrast (IR-DIC) video microscopy with a ×40 water immersion objective. The image was detected with an IR-sensitive CCD camera and displayed on a monitor. Membrane potentials were recorded from current clamped stem cells. All recordings were made at 32°C, maintained by an automatic temperature controller (Warner Instruments, Hamden, CT).
RESULTS
Growth and morphology of cultured MSCs
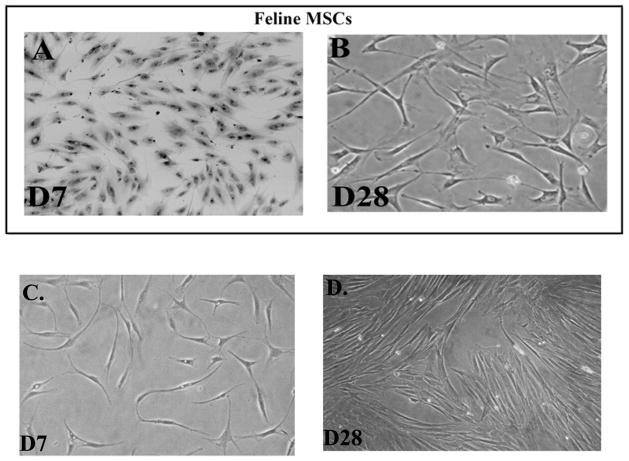
At each passage of the feline MSC culture, we examined the cells with an inverted microscope. We expect mixed cultures of adherent cells after the first passage. Therefore, we begin to compare the morphology of feline MSCs with human MSCs, begi at week nning 1 of passage 2 cultures. Human MSCs are expected to be spindle shaped (Potian et al., 2003). At the early time period, the feline MSCs were decidedly spindle shaped in their morphology (Figure 1A), but with broader spindles (Figure 1A inset) as compared to human MSCs (Figure 1C). At passage 4, the feline MSCs lose their spindle shaped morphology and began to express various sizes and shapes, with cytoplasm appearing granular (Figure 1B). In contrast, the equivalent human MSCs retained their symmetrical shape with long spindle (Figure 1D).
Fig 1. Morphology of Feline and Human MSCs at equivalent passages.
A. Culture at day 7 of passage 2 feline MSCs. Inset shows the morphology of the early passage MSCs. B. Passage of MSCs of the donor, shown in ‘A’, at day 28, which is equivalent to Passage 4. C and D. Shown are human MSCs from bone marrow aspirates, at comparable passages as feline MSCs.
Function and Phenotype of Feline MSCs
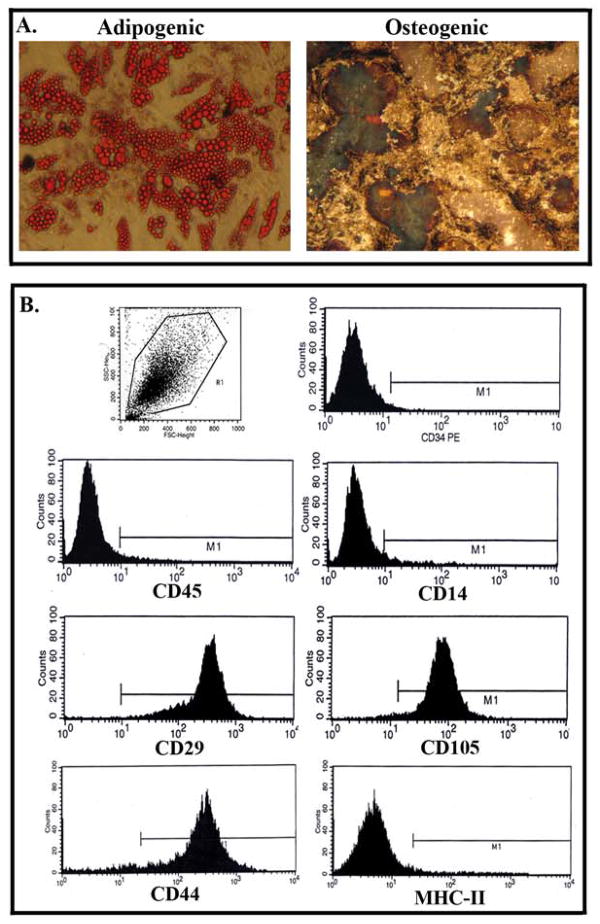
Due to the morphological differences between feline and human MSCs, we asked if passage 4 feline cells were multipotent. This was addressed by inducing the cells with adipogenic and osteogenic induction media as described (Potian et al., 2003). Indeed, passage 4 cells showed adipogenic as well as osteogeneic cells (Figure 2A). We next asked if the feline MSCs show phenotype that was consistent for human MSCs by FACS analysis with passage 4 cells. The results revealed that >90% of cells were positive for CD29, CD44, and CD105 (Figure 2B), consistent with markers of MSCs (Potian et al., 2003). In contrast, all cells were negative for the monocytes/macrophage marker CD14, hematopoietic cell marker CD34 and CD45. A subset of human MSCs expressed MHC-II (Francois et al., 2009, Chan et al., 2006). However, similar analyses with feline cells showed no evidence of MHC-II expression.
Fig 2. Flow cytometry analysis of feline MSCs and lineage differentiation.
A. MSCs were induced with adipogenic and chondrogenic media. Images are shown for three different experiments at 40x magnification. B. Representative of three analyses of MSCs by flow cytometry. Uninduced MSCs were labeled for the following: hematopoietic cell surface marker CD34, monocytes /macrophage cell surface marker CD14, and further developmental stages of blood cells (CD45, MHC-II); MSC marker, CD105, CD29 and CD44.
Neuronal-like cells from feline MSCs
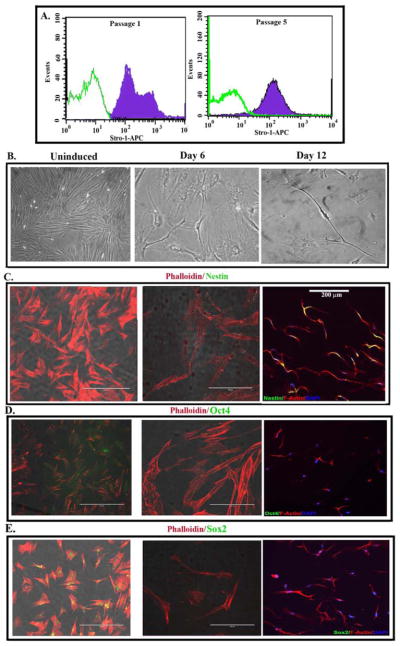
Although the fetal development of MSCs remains a question, it is believed that they could be neuroectodermal in origin (da Silva Meirelles et al., 2008). However, MSCs also show smooth muscle pattern, consistent with mesodermal origin (Caplan, 2009). Stro-1 has been suggests as a marker that defines the function of MSCs (Bensidhoum et al., 2004, Psaltis et al., 2010, Dennis et al., 2002). We therefore compared early (passage 1) and with passage 5 feline MSCs for stro-1 expression by immunofluorescence. The results showed stro-1 expression at each passage (Figure 3A). However at passage 1, there were two subsets, based on stro-1 expression (Figure 3A). By passage 5, the cells show homogeneity with regards to stro-1 expression (Figure 3A). We next determined whether passage 5 feline MSCs can differentiate into neurons. Figure 3B showed the timeline change in morphology by induced cells. This correlated with an increase in Nes (Figure tin 3C) and concomitant loss of the stem cell associated genes, Oct4 (Figure 3D) and Sox2 (Figure 3E).
Fig 3. Stem cell markers in uninduced and induced MSCs.
A. Uninduced MSCs at passages 1 and 5 were labeled for Stro-1 by immunofluorescence. B. Images of uninduced and induced MSCs, at 6 and 12 days. The images are shown at 100x magnification. C. Representative of three experiments of immunofluorescence for nestin, Oct4 and Sox2 (green). Cells were co-labeled for DAPI and phalloidin. The image is shown at 100x magnification.
Phenotype and electrophysiology of induced MSCs
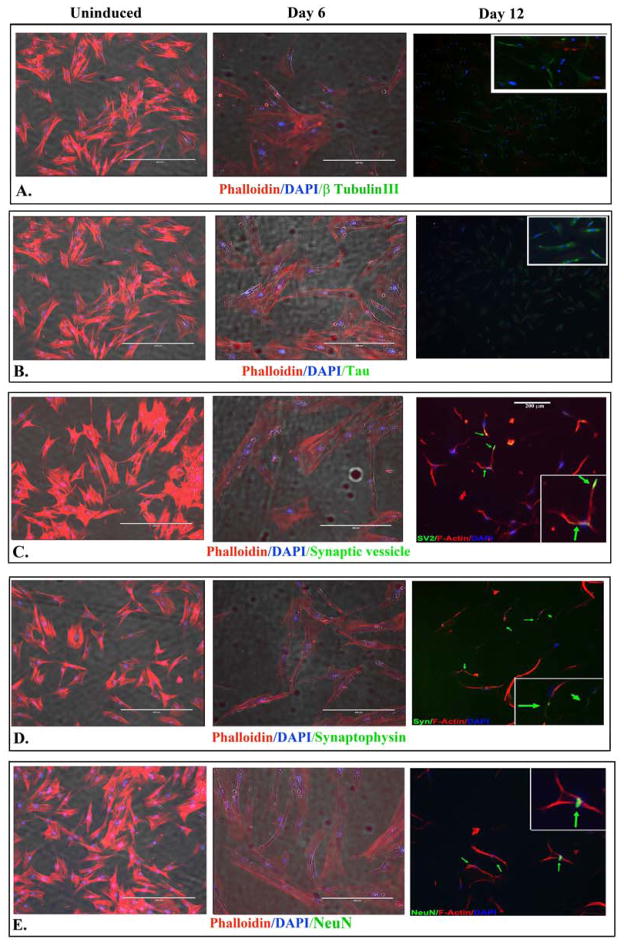
In this set of studies, we performed immunofluorescence for markers of mature neurons. The expression of nestin at Day 12 induced feline MSCs (Figure 3C) led us to ask if these cells also co-expressed mature neuronal markers. We examined the cells for Neuronal Nuclei (NeuN), βIII-tubulin, and Tau-3. We also asked if the expression of mature markers correlated with synaptic proteins, synaptic vesicles and synaptophysin. Synapic vesicle was observed throughout the neuronal cell and synaptophysin was visualized as axonal bouton–limited puctate, corresponding to mature neurotransmitter-secreting terminals. Although there is faint evidence of their expressions at day 6 induction, there were marked expression in >90% of the cells at day 12 (Figure 4).
Fig 4. Immunofluorescence labeling for neuronal markers.
Shown are representative images (100x) of three different experiments for the following: β-tubulin III (A); Tau (B); Synaptic vesicle (C); Synaptophysin (D) and NeuN (E). The labelings were performed for uninduced MSCs and cells induced for 6 and 12 days. Co-labelings were performed with DAPI (blue) and phalloidin (red).
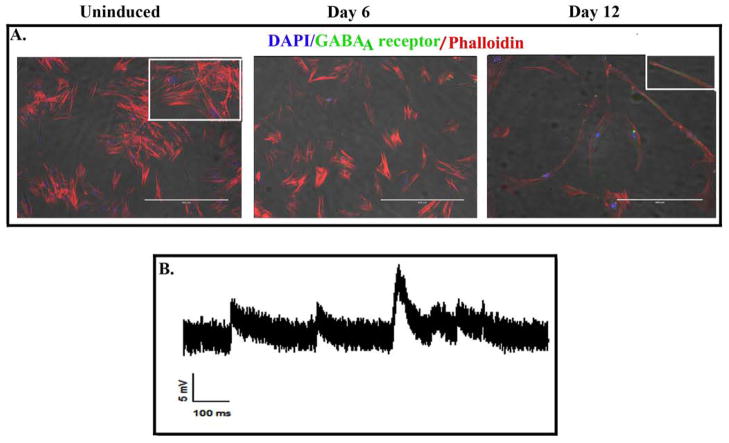
The day 12 induced MSCs were also positive for the neurotransmitter receptor, GABAA. The expression pattern for these neurotransmitter receptors indicated that the majority of cells were positive for the receptor and that the receptors were uniformly distributed on the cells (Fig 5A). Figure 5B is a representative voltage trace recorded from day 12 induced MSCs, showing some post-synaptic potential like activities with rapid rising phase and slow decay phase; average amplitude of 4.7 mV (range between 4.5 an 10.4 d mV). This indicated neuronal intrinsic functionality of feline MSC-derived neurons.
Fig 5. Expression of GABA receptor and electrophysiological properties in day 12 induced MSCs.
A. Uninduced and induced (6 and 12 days) MSCs were labeled for the neurotransmitter receptor GABAA by immunofluorescence. The image represents three experiments, each with cells from a different cat. B. Representative voltage trace recorded from a stem cell, showing some postsynaptic potential like activities, which have a rapid rising phase and a slow decay phase and an average amplitude of 4.7 mV (range from 4.5 to 10.4 mV).
DISCUSSION
This study provides evidence of feline MSCs to differentiate into neuronal lineage. After the fourth passage, the cell populations were more than 90% homogeneous with regards to the expression of CD44, CD105 and CD29 and negative for hematopoietic markers, CD45, CD34 and CD14. Unlike human MSCs, murine counterparts were negative for MHC class II (not shown) (Potian et al., 2003).
The expansion potential of feline MSCs in the present study was measured only up to the 4th passage after which they were induced for neuronal morphology. Previous in vitro differentiation studies of MSCs to neuronal phenotype have been shown using cells expanded beyond passage 20 (Woodbury et al., 2000) and MSCs expansion for at least 25 population doublings was suggested to be a prerequisite for neuronal differentiation (Wislet-Gendebien et al., 2003). The present study utilized cells at passage 4 for neuronal differentiation and demonstrated no difference of expression pattern for any of the stem cell markers, suggesting that cell age may not be a crucial factor in affecting differentiation ability.
Because of the lack of specific phenotypic markers, the identification of MSCs has been reserved to cell populations fulfilling several identifying criteria, based on certain negative and positive cell surface markers. The population of cells following culture of feline bone marrow fully corresponded to the basic feature of adherent character and rapid growth at low seeding densities, characteristics of MSCs obtained from rodent, human and other species (Colter et al., 2001, Sekiya et al., 2002). The cell cultures from cats examined in this study were negative for markers of hematopoietic progenitors (CD34), markers for the monocytes /macrophage lineage (CD14) and markers for developmental stages of blood cells (CD45, HLA-DR). The cell population further revealed high expression of other generally accepted MSC markers (CD44, CD105 and CD29) (Arai et al., 2002, Docheva et al., 2007, Kemp et al., 2005). In addition to the general morphogenic and phenotypic characteristics, feline MSCs were negative for MHC class II markers (not shown), as reported for feline MSCs by Martin et al. (Martin et al., 2002).
To assess the true proportion of progenitor cells within this heterogeneous culture we used Stro-1, a stromal cell marker (Simmons and Torok-Storb, 1991, Stewart et al., 2003) that recognizes non-hematopoietic bone marrow progenitor cells with stable undifferentiated phenotype. These cells proliferate extensively while retaining the potential to differentiate even when cultured long-term (Gronthos et al., 1999, Tuli et al., 2003). Our results demonstrated that the expression of Stro-1 antigen on mesenchymal stem cells increased from passage 1 to passage 4.
Feline MSCs showed the potential to differentiate into neurons with retinoic acid stimulation. Morphological changes were evident within 24 h of culture in retinoic acid containing medium, with some cells exhibiting classic bipolar morphology within 3 days. By day 6, these changes were uniform and when grown to confluence, the differentiated cells showed evidence of synapse-like formation. However, it was only day 12 induced cells that showed labeling for synaptophysin and synaptic vesicles (Figure 4). Following neuronal induction morphological changes were observed resembling the initial steps of neuronal differentiation—especially neurite outgrowth. In brief, after formation of the original round shape, the neuron was broken down to make a bud that was ultimately transformed into a neurite and was further transformed into an axon or dendrite like structure (da Silva and Dotti, 2002). This finding is in accordance with observations made in the course of neuritogenesis (da Silva and Dotti, 2002, Dehmelt and Halpain, 2004).
It has been shown that morphological changes alone may be stimulated only by the addition of β-mercaptoethanol which can induce the formation of neurite-like processes in neuronal and MSCs cultures (Ishii et al., 1993, Woodbury et al., 2000). However, our induction protocol was different. Immunostaining for Sox-2 and Nestin, neuronal stem cell marker indicated that differentiated cells had undergone a phenotypic change. Nestin is expressed by neural stem cells (Dahlstrand et al., 1992) and identifies adult neuronal and glial progenitor cells in culture (Dahlstrand et al., 1995, Mignone et al., 2004). Some studies have reported that Nestin expression is an essential prerequisite for MSCs to progress toward a neural lineage (Wislet-Gendebien et al., 2003) and is indicative of the potential of the cell to develop into neuronal precursors (Mezey et al., 2000). Various other reports that found Nestin expression in progenitor cells of diverse tissue types may suggest that this marker is indicative of a general precursor cell rather than one specifically committed to a neural lineage (About et al., 2000, Ha et al., 2003, Mokry and Nemecek, 1998, Niki et al., 1999, Sejersen and Lendahl, 1993, Zulewski et al., 2001). Immunohistochemical data from our study revealed that expression of Nestin on differentiated MSCs declined rapidly with the maturation of neurons. Similar to Nestin, Sox-2 is also expressed by neural stem cells (Cai et al., 2002, Zappone et al., 2000). Sox-2 followed an expression pattern similar to Nestin, with small number of cells expressing Sox-2 within 72 hr of neuronal induction as compared to 24 h to its complete disappearance at day 6. These expression patterns for Nestin and Sox-2 suggest that MSCs following induction undergo neuronal differentiation. The expression of markers for mature neurons further confirmed the terminal neural differentiation of MSCs into mature neural cell populations. After a 12 day period, up to 80% of differentiated MSCs demonstrated a mature neuronal phenotype expressing β Tubulin, Tau, MAP2 and Neuronal Nuclei antigens.
Although robust studies were not conducted on signaling between neurons, the expression of synaptophysin – that increases in parallel to the formation of synapses (Andressen et al., 1996, Knaus et al., 1986), as well as electrophysiological studies, indicate mature neurons (Jiang et al., 2002, Kohyama et al., 2001). A shift of immunostaining from the cell bodies to the processes was observed as neurons matured because synaptic vesicle antigens are synthesized in the cell bodies before they are transported down the processes and ultimately become located in presynaptic terminals. Electrical currents are an intrinsic property of fully functional neuronal cells. In an effort to confirm the neuronal maturity of MSC-derived cells, we performed Whole-Cell Patch clamp electrophysiology. These cells not only formed synaptic connections, but exhibited EPSPs at these junctions. In conclusion, our results show that MSCs from feline bone marrow differentiated into cells expressing both morphologic and phenotypic progression along a neuronal cell lineage. A high proliferation potential and differentiation capacity make MSCs a promising tool for medical applications involving reconstruction of damaged neurons as well as gene delivery for correction of inherited diseases.
HIGHLIGHTS.
Feline bone marrow-derived mesenchymal stem cells (MSCs) can be harvested and expanded in vitro.
The markers of MSCs were similar to those reported for human MSCs.
Feline MSCs can differentiate into functional neurons as determined electrophysiologically.
The neurons express GABA receptors.
Feline MSCs appear to show similar properties as human bone marrow-derived MSCs.
Acknowledgments
This study was supported by NIH grant NS 07941-36 and FM Kirby Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- About I, Laurent-Maquin D, Lendahl U, Mitsiadis TA. Nestin expression in embryonic and adult human teeth under normal and pathological conditions. Am J Pathol. 2000;157:287–295. doi: 10.1016/S0002-9440(10)64539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andressen C, Moertter K, Mai JK. Spatiotemporal expression of CD15 in the developing chick retina. Brain Res Dev Brain Res. 1996;95:263–271. doi: 10.1016/0165-3806(96)00096-x. [DOI] [PubMed] [Google Scholar]
- Arai F, Ohneda O, Miyamoto T, Zhang XQ, Suda T. Mesenchymal stem cells in perichondrium express activated leukocyte cell adhesion molecule and participate in bone marrow formation. J Exp Med. 2002;195:1549–1563. doi: 10.1084/jem.20011700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003;89:1235–1249. doi: 10.1002/jcb.10594. [DOI] [PubMed] [Google Scholar]
- Bensidhoum M, Chapel A, Francois S, Demarquay C, Mazurier C, Fouillard L, Bouchet S, Bertho JM, Gourmelon P, Aigueperse J, Charbord P, Gorin NC, Thierry D, Lopez M. Homing of in vitro expanded Stro-1- or Stro-1+ human mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. Blood. 2004;103:3313–3319. doi: 10.1182/blood-2003-04-1121. [DOI] [PubMed] [Google Scholar]
- Bergman PJ. Mechanisms of anticancer drug resistance. Vet Clin North Am Small Anim Pract. 2003;33:651–667. doi: 10.1016/s0195-5616(03)00004-4. [DOI] [PubMed] [Google Scholar]
- Cai J, Wu Y, Mirua T, Pierce JL, Lucero MT, Albertine KH, Spangrude GJ, Rao MS. Properties of a fetal multipotent neural stem cell (NEP cell) Dev Biol. 2002;251:221–240. doi: 10.1006/dbio.2002.0828. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, Rameshwar P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-{gamma} Blood. 2006;107:4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Meirelles L, Caplan AI, Nardi NB. In Search of the In Vivo Identity of Mesenchymal Stem Cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- da Silva JS, Dotti CG. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat Rev Neurosci. 2002;3:694–704. doi: 10.1038/nrn918. [DOI] [PubMed] [Google Scholar]
- Dahlstrand J, Collins VP, Lendahl U. Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res. 1992;52:5334–5341. [PubMed] [Google Scholar]
- Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res. 1995;84:109–129. doi: 10.1016/0165-3806(94)00162-s. [DOI] [PubMed] [Google Scholar]
- Dehmelt L, Halpain S. Actin and microtubules in neurite initiation: are MAPs the missing link? J Neurobiol. 2004;58:18–33. doi: 10.1002/neu.10284. [DOI] [PubMed] [Google Scholar]
- Dennis JE, Carbillet JP, Caplan AI, Charbord P. The ST -1+ RO marrow cell population is multipotential. Cells Tissues Organs. 2002;170:73–82. doi: 10.1159/000046182. [DOI] [PubMed] [Google Scholar]
- Dias AS, Bester MJ, Britz RF, Apostolides Z. Animal models used for the evaluation of antiretroviral therapies. Curr HIV Res. 2006;4:431–446. doi: 10.2174/157016206778560045. [DOI] [PubMed] [Google Scholar]
- Docheva D, Popov C, Mutschler W, Schieker M. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. J Cell Mol Med. 2007;11:21–38. doi: 10.1111/j.1582-4934.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois M, Romieu-Mourez R, Stock-Martineau S, Boivin MN, Bramson JL, Galipeau J. Mesenchymal stromal cells cross-present soluble exogenous antigens as part of their antigen-presenting cell properties. Blood. 2009;114:2632–2638. doi: 10.1182/blood-2009-02-207795. [DOI] [PubMed] [Google Scholar]
- Greco SJ, Liu K, Rameshwar P. Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem Cells. 2007a;25:3143–3154. doi: 10.1634/stemcells.2007-0351. [DOI] [PubMed] [Google Scholar]
- Greco SJ, Zhou C, Ye JH, Rameshwar P. An interdisciplinary approach and characterization of neuronal cells transdifferentiated from human mesenchymal stem cells. Stem Cells Dev. 2007b;16:811–826. doi: 10.1089/scd.2007.0011. [DOI] [PubMed] [Google Scholar]
- Greco SJ, Zhou C, Ye JH, Rameshwar P. A method to generate human mesenchymal stem cell-derived neurons which express and are excited by multiple neurotransmitters. Biol ProcedOnline. 2008;10:90–101. doi: 10.1251/bpo147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Zannettino AC, Graves SE, Ohta S, Hay SJ, Simmons PJ. Differential cell surface expression of the STRO-1 and alkaline phosphatase antigens on discrete developmental stages in primary cultures of human bone cells. J Bone Miner Res. 1999;14:47–56. doi: 10.1359/jbmr.1999.14.1.47. [DOI] [PubMed] [Google Scholar]
- Ha Y, Lee JE, Kim KN, Cho YE, Yoon DH. Intermediate filament nestin expressions in human cord blood monocytes (HCMNCs) Acta Neurochir. 2003;145:483–487. doi: 10.1007/s00701-003-0023-4. [DOI] [PubMed] [Google Scholar]
- Head E, Moffat K, Das P, Sarsoza F, Poon WW, Landsberg G, Cotman CW, Murphy MP. Beta-Amyloid deposition and tau phosphorylation in clinically characterized aged cats. Neurobiol of Aging. 2005;26:749–763. doi: 10.1016/j.neurobiolaging.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Husemann Y, Klein CA. The analysis of metastasis in transgenic mouse models. Transgenic Res. 2009;18:1–5. doi: 10.1007/s11248-008-9225-0. [DOI] [PubMed] [Google Scholar]
- Ilyas AA, Gu Y, Dalakas MC, Quarles RH, Bhatt S. Induction of experimental ataxic sensory neuronopathy in cats by immunization with purified SGPG. J Neuroimmunol. 2008;193:87–93. doi: 10.1016/j.jneuroim.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Katayama M, Hori K, Yodoi J, Nakanishi T. Effects of 2-mercaptoethanol on survival and differentiation of fetal mouse brain neurons cultured in vitro. Neurosci Lett. 1993;163:159–162. doi: 10.1016/0304-3940(93)90371-q. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Kamb A, Wee S, Lengauer C. Why is cancer drug dis covery so difficult? Nat Rev Drug Discov. 2007;6:115–120. doi: 10.1038/nrd2155. [DOI] [PubMed] [Google Scholar]
- Kemp KC, Hows J, Donaldson C. Bone marrow-derived mesenchymal stem cells. Leuk Lymphoma. 2005;46:1531–1544. doi: 10.1080/10428190500215076. [DOI] [PubMed] [Google Scholar]
- Knaus P, Betz H, Rehm H. Expression of synaptophysin during postnatal development of the mouse brain. J Neurochem. 1986;47:1302–1304. doi: 10.1111/j.1471-4159.1986.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Kohyama J, Abe H, Shimazaki T, Koizumi A, Nakashima K, Gojo S, Taga T, Okano H, Hata J, Umezawa A. Brain from bone: efficient “meta-differentiation” of marrow stroma-derived mature osteoblasts to neurons with Noggin or a demethylating agent. Differentiation. 2001;68:235–244. doi: 10.1046/j.1432-0436.2001.680411.x. [DOI] [PubMed] [Google Scholar]
- Martin DR, Cox NR, Hathcock TL, Niemeyer GP, Baker HJ. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp Hematol. 2002;30:879–886. doi: 10.1016/s0301-472x(02)00864-0. [DOI] [PubMed] [Google Scholar]
- Mashimo T, Serikawa T. Rat resources in biomedical research. Curr Pharm Biotechnol. 2009;10:214–220. doi: 10.2174/138920109787315105. [DOI] [PubMed] [Google Scholar]
- Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nes -GFP tin transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Mokry J, Nemecek S. Immunohistochemical detection of intermediate filament nestin. Acta Medica. 1998;41:73–80. [PubMed] [Google Scholar]
- Murphy WJ, Sun S, Chen Z, Yuhki N, Hirschmann D, Menotti-Raymond M, O’Brien SJ. A radiation hybrid map of the cat genome: implications for comparative mapping. Genome Res. 2000;10:691–702. doi: 10.1101/gr.10.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki T, Pekny M, Hellemans K, Bleser PD, Berg KV, Vaeyens F, Quartier E, Schuit F, Geerts A. Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells. Hepatology. 1999;29:520–527. doi: 10.1002/hep.510290232. [DOI] [PubMed] [Google Scholar]
- O’Brien SJ, Menotti-Raymond M, Murphy WJ, Nash WG, Wienberg J, Stanyon R, Copeland NG, Jenkins NA, Womack JE, Marshall Graves JA. The promise of comparative genomics in mammals. Science. 1999;286:458–481. doi: 10.1126/science.286.5439.458. [DOI] [PubMed] [Google Scholar]
- Porrello A, Cardelli P, Spugnini EP. Oncology of companion animals as a model for humans an overview of tumor histotypes. J Exp Clin Cancer Res. 2006;25:97–105. [PubMed] [Google Scholar]
- Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-Like Activity of Mesenchymal Stem Cells: Functional Discrimination Between Cellular Responses to Alloantigens and Recall Antigens. J Immunol. 2003;171:3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- Psaltis PJ, Paton S, See F, Arthur A, Martin S, Itescu S, Worthley SG, Gronthos S, Zannettino ACW. Enrichment for STRO-1 expression enhances the cardiovascular paracrine activity of human bone marrow-derived mesenchymal cell populations. J Cell Physiol. 2010;223:530–540. doi: 10.1002/jcp.22081. [DOI] [PubMed] [Google Scholar]
- Schaller B, Graf R, Wienhard K, Heiss WD. A new animal model of cerebral venous infarction: ligation of the posterior part of the superior sagittal sinus in the cat. Swiss Med Wkly. 2003;133:412–418. doi: 10.4414/smw.2003.10236. [DOI] [PubMed] [Google Scholar]
- Sejersen T, Lendahl U. Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci. 1993;1106:1291–1300. doi: 10.1242/jcs.106.4.1291. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- Shively CA, Clarkson TB. The unique value of primate models in translational research. Am J Primatol. 2009;71:1–7. doi: 10.1002/ajp.20720. [DOI] [PubMed] [Google Scholar]
- Siegel A, Bhatt S, Bhatt R, Zalcman SS. The neurobiological bases for development of pharmacological treatments of aggressive disorders. Curr Neuropharmacol. 2007;5:135–147. doi: 10.2174/157015907780866929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A, Roeling TA, Gregg TR, Kruk MR. Neuropharmacology of brain-stimulation-evoked aggression. Neurosci Biobehav Rev. 1999;23:359–389. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- Stewart K, Monk P, Walsh S, Jefferiss CM, Letchford J, Beresford JN. STRO-1, HOP-26 (CD63), CD49a and SB-10 (CD166) as markers of primitive human marrow stromal cells and their more differentiated progeny: a comparative investigation in vitro. Cell Tissue Res. 2003;313:281–290. doi: 10.1007/s00441-003-0762-9. [DOI] [PubMed] [Google Scholar]
- Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 2007;170:793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzaska KA, King CC, Li KY, Kuzhikandathil EV, Nowycky MC, Ye JH, Rameshwar P. Brain-derived neurotrophic factor facilitates maturation of mesenchymal stem cell-derived dopamine progenitors to functional neurons. J Neurochem. 2009;110:1058–1069. doi: 10.1111/j.1471-4159.2009.06201.x. [DOI] [PubMed] [Google Scholar]
- Tuli R, Tuli S, Nandi S, Wang ML, Alexander PG, Haleem-Smith H, Hozack WJ, Manner PA, Danielson KG, Tuan RS. Characterization of multipotential mesenchymal progenitor cells derived from human trabecular bone. Stem Cells. 2003;21:681–693. doi: 10.1634/stemcells.21-6-681. [DOI] [PubMed] [Google Scholar]
- Vail DM, MacEwen EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000;18:781–792. doi: 10.3109/07357900009012210. [DOI] [PubMed] [Google Scholar]
- Wislet-Gendebien S, Leprince P, Moonen G, Rogister B. Regulation of neural markers nestin and GFAP expression by cultivated bone marrow stromal cells. J Cell Sci. 2003;116:3295–3302. doi: 10.1242/jcs.00639. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Zappone MV, Galli R, Catena R, Meani N, De BS, Mattei E, Tiveron C, Vescovi AL, Lovell-Badge R, Ottolenghi S, Nicolis SK. Sox2 regulatory sequences direct expression of a (beta)-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development. 2000;127:2367–2382. doi: 10.1242/dev.127.11.2367. [DOI] [PubMed] [Google Scholar]
- Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Muller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pan endocrine, creatic exocrine, and hepatic phenotypes. Diabetes. 2001;50:521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]