Abstract
Component signaling in taste mixtures containing both beneficial and dangerous chemicals depends on peripheral processing. Unidirectional mixture suppression of chorda tympani (CT) nerve responses to sucrose by quinine and acid is documented for golden hamsters (Mesocricetus auratus). To investigate mixtures of NaCl and acids, we recorded multifiber responses to 50 mM NaCl, 1 and 3 mM citric acid and acetic acid, 250 μM citric acid, 20 mM acetic acid, and all binary combinations of each acid with NaCl (with and without 30 μM amiloride added). By blocking epithelial Na+ channels, amiloride treatment separated amiloride-sensitive NaCl-specific responses from amiloride-insensitive electrolyte-generalist responses, which encompass all of the CT response to the acids as well as responses to NaCl. Like CT sucrose responses, the amiloride-sensitive NaCl responses were suppressed by as much as 50% by citric acid (P = 0.001). The amiloride-insensitive electrolyte-generalist responses to NaCl + acid mixtures approximated the sum of NaCl and acid component responses. Thus, although NaCl-specific responses to NaCl were weakened in NaCl–acid mixtures, electrolyte-generalist responses to acid and NaCl, which tastes KCl-like, were transmitted undiminished in intensity to the central nervous system. The 2 distinct CT pathways are consistent with known rodent behavioral discriminations.
Keywords: acetic acid, amiloride sensitivity, binary mixtures, chorda tympani, citric acid, NaCl
Introduction
Gustatory systems evolved to deal with natural conditions of foraging and feeding, in which information from multiple chemical stimuli is processed simultaneously. A peripheral gustatory nervous system made up of labeled lines (Yarmolinsky et al. 2009) generates and transmits independent signals, signs for nutrients and toxins, centrally for each taste quality. Components of mixtures using separate pathways remain recognizable qualitatively (Frank et al. 2003; Wang et al. 2009); quantitatively, the independent responses should simply add if it were not for mixture suppression (Bartoshuk 1975; Formaker and Frank 1996). Mixture components using a single pathway are qualitatively confusable (Frank et al. 2003; Wang et al. 2009); quantitatively, the associated responses would be equivalent to concentration increases of either compound, if it were not for mixture interactions (Hyman and Frank 1980b). Mixture interactions (modulation or integration of activated lines) are indicated whenever responses to mixtures significantly deviate from response addition for independent or stimulus addition for associated component stimuli.
Physiological identification of potential peripheral nerve pathways using single stimuli is incomplete (Frank et al. 2008). Three types of physiologically distinct chorda tympani (CT) neurons are identified in rodents, 2 of which are specifically tuned to rodent taste qualities: S-fibers to a sucrose-like taste and N-fibers to a Na+/Li+-specific taste. Other CT neurons, the electrolyte generalists (H/E-fibers), respond to acids, Na+-salts, non-Na+-salts, and ionic bitter stimuli such as quinine HCl (Ninomiya et al. 1987; Frank et al. 1988; Ninomiya and Funakoshi 1988), compounds shown to have distinct tastes with conditioned taste aversion generalization tests (Frank and Nowlis 1989; Ninomiya and Funakoshi 1989). The CT transmits the taste of NaCl via N-fibers and H/E-fibers, whereas, tastes of the other electrolyte categories are primarily transmitted by H/E-fibers, neurons that have been subcategorized in rats (Breza et al. 2006, 2010).
In a series of studies on mixtures of stimulus compounds that activate either S-fibers or H/E-fibers in hamsters, mixture interactions were identified by divergence from response addition. Hamster CT nerve fibers show concentration-dependent directional asymmetric mixture suppression of sucrose responses; sweetener responses are suppressed, but responses to other mixture components are not suppressed. Specifically, quinine hydrochloride (Formaker and Frank 1996; Frank et al. 2005) weakened the effectiveness of sucrose and dulcin; and mineral hydrochloric and organic acetic and citric acids weakened the effectiveness of sucrose (Formaker et al. 2009). The quinine and acids may simply act as chemical reagents that interfere with Tas1R receptor activation; or cells activated by quinine or acids may intervene to inhibit cells activated by sucrose within the taste bud (Herness and Zhao 2009; Chaudhari and Roper 2010).
The current experiments deal with binary mixtures of acids with NaCl, component stimuli that activate overlapping sets of CT fibers in hamsters (Frank et al. 1988). Fortunately, N-fiber Na+-specific responses and H/E-fiber NaCl responses can be separated pharmacologically by treating the tongue with amiloride, which blocks the epithelial Na+-channel (ENaC). ENaC-dependent NaCl-specific N-fibers (Hettinger and Frank 1990) do not respond to organic acids (Frank et al. 1988), making response-addition the appropriate NaCl + acid mixture prediction. ENaC-independent H/E-fibers respond to NaCl and acids (ibid.) making stimulus-addition the proper mixture prediction. We found citric acid reduced ENaC-dependent NaCl responses, documenting a third directional response suppression by H/E-fiber stimuli.
Whereas ENaC-independent responses to acid + NaCl mixtures matched response addition, exceeding the stimulus-addition expected for a single pathway.
Materials and methods
Subjects, surgery, and electrophysiology
Whole-nerve taste responses were recorded from the right CT nerve of 22, 105–160 g adult, male golden hamsters (Mesocricetus auratus). Hamsters were anesthetized with an intraperitoneal injection of sodium pentobarbital (initial dose: 80 mg/kg; subsequent dosages to maintain a surgical level of anesthesia: 40 mg/kg). Body temperature was maintained at ∼37 °C with a Deltaphase isothermal pad. A tracheal cannula was inserted to assist breathing. The CT nerve, exposed using a mandibular approach, was cut near its entrance into the tympanic bulla, desheathed, and placed on a nichrome wire recording electrode. An indifferent electrode was positioned in nearby tissue. Protocols were approved by the Animal Care Committee of the University of Connecticut Health Center.
Multifiber neural activity was differentially amplified, square rectified, and summed (200-ms time constant) before digitization with a Cambridge Electronic Design (CED) Micro 1401 II analog to digital converter. Digitized data were displayed (Figure 1) and saved on a PC for offline analysis using CED Spike2 software.
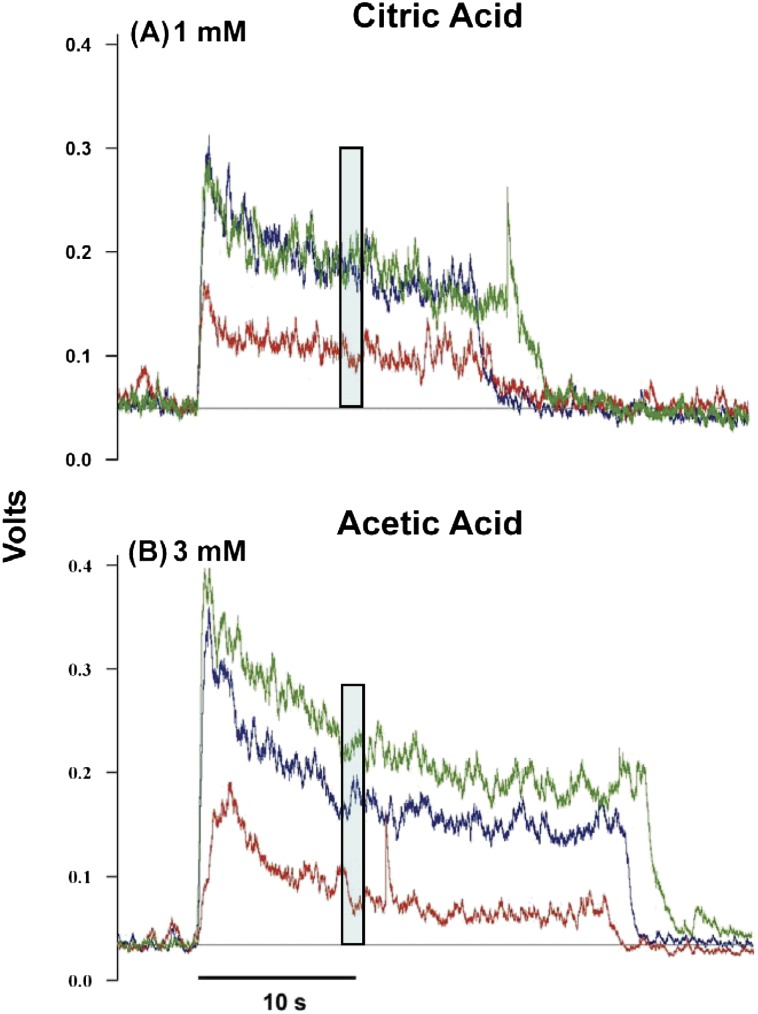
Figure 1.
Raw individual data tracings show effects of (A) citric acid (1 mM) and (B) acetic acid (3 mM) at equal titratable acidity on control hamster CT responses to 50 mM NaCl. Traces for the NaCl + acid mixture (green), acid (red), and NaCl (blue) are overlaid. Dotted lines are mean baseline voltages. The blue–gray rectangle indicates our 9.5–10.5 s window of response measurement. Note the citric acid + NaCl mixture and NaCl responses superimpose (A), whereas the acetic acid + NaCl mixture response falls well above the NaCl response (B). The citric acid reduces the NaCl response more than the acetic acid does.
Stimulation
Gustatory stimuli were 250 μM (pH 3.7), 1 mM (pH 3.2), and 3 mM (pH 2.9) citric acid; 1 mM (pH 3.9), 3 mM (pH 3.7), and 20 mM (pH 3.2) acetic acid, 50 mM NaCl, and all binary combinations of each acid with NaCl. The organic acids represent sour stimuli that specifically activate hamster CT H/E-fibers (Frank et al. 1988). HCl was not used because it also activates hamster CT N-fibers, especially after stimuli are repeatedly applied to the tongue (ibid.). NaCl was used at 50 mM, the lowest concentration at which CT responses to quinine–NaCl mixtures were suppressed (Formaker and Frank 1996), to strike a balance between response size and interactive concentrations of NaCl.
Solutions of reagent grade compounds were prepared so binary mixture components and separately presented stimuli were equal in concentration. Room-temperature (∼21 °C) solutions were applied to the anterior tongue, which projected through a rubber dam into a glass flow chamber. Stimuli, introduced into a funnel, were presented at ∼2 mL/s for ∼15 to 20 s (ensuring stable gentle flow rates at 10-s time points) followed by a rinse for ≥45 s (to reestablish baseline response levels) with a gravity flow system.
The single components of each binary mixture and the mixture were separately applied to the tongue for every mixture studied. Mixtures were presented in order of ascending acid concentration and 500 mM NH4Cl applied at the beginning and end of each mixture set (the 2 component stimuli and binary mixture) to monitor preparation stability over time. Stimulus order between NH4Cl presentations varied.
Amiloride treatment
Compounds were dissolved in distilled water or an aqueous solution of 30 μM amiloride hydrochloride (Formaker et al. 2009). Treatment with 30 μM amiloride optimizes concentration-dependent specific inhibition of amiloride-sensitive hamster CT responses, eliminating N-fiber without affecting H/E-fiber steady-state responses to ≤100 mM NaCl (Hettinger and Frank 1990). Stimuli were presented first using water rinses and then again using amiloride rinses with amiloride prerinsed over the tongue for 2 min.
The 2 series of measurements on the control- (water-rinsed) and amiloride-treated tongue (amiloride-insensitive responses) allowed us to separately and directly evaluate the impact of acid on Na+-specific NaCl responses and electrolyte-generalist responses. Amiloride-sensitive Na+-specific responses were obtained by subtracting responses for amiloride-treated tongues from corresponding responses for water-rinsed tongues. For example, the response to 1 mM citric acid + 50 mM NaCl in the presence of amiloride was subtracted from the response to 1 mM citric acid + 50 mM NaCl in the presence of water. In the pharmacologically treated amiloride-insensitive component, excitatory and any possible inhibitory amiloride-sensitive processing is absent. The amiloride-sensitive component (obtained by subtraction of the amiloride-insensitive) encompasses everything that is amiloride-sensitive.
Response measurement
Responses were recorded from 2 independent groups of 8 hamsters, 1 for citric acid mixtures and 1 for acetic acid mixtures. A supplemental group of 6 hamsters was added at the end of the experiment to study mixture effects with 250 μM citric acid and 20 mM acetic acid. Taste-stimulated responses were quantified as the mean increase in voltage above baseline for a 1-s period beginning 9.5 s after stimulus onset. This time point represents the mean steady-state response at 10 s (±0.5 s) (Figure 1) that is relatively free from differential diffusion of various chemical species to receptor populations (Ye et al. 1994) and the time point for which amiloride specificity was established in hamsters (Hettinger and Frank 1990). Note that stable average electrophysiological responses at 10 s in A and B records in Figure 1. Previously digitized data (sample rate: 25 KHz) were sampled from this 1-s period at 100 Hz, making each response measure an average of 100 voltage points. This response point, used for analysis in earlier analog recordings (Formaker and Frank 1996), also permits direct comparison of several data sets. All responses were expressed relative to the standard NH4Cl response, an average of 2 values presented before and after a mixture set. Relative data are presented as response means ± standard errors of means.
Response addition or stimulus addition?
The idea behind additivity of mixture components is that the response to a mixture will be equal to the sum of the responses to the separate components when the components are acting independently. Independence is excluded when there is inhibition or synergy in the responses of the components or if the components share a common transduction pathway. Inhibition and synergy are easy to identify as suppression or enhancement of one stimulus by another and readily observed if the response to the mixture is less than the response to either component (inhibition) or greater than the responses of both components combined (synergy). The condition of sharing a common pathway is more difficult to conceptualize, but the idea is that the stimulus components can replace one another in the response. This requires knowledge of the component response-concentration function, which usually shows nonlinearity or a tendency to saturate with increasing concentration (compression). Mixture stimuli sharing a common pathway are predicted to follow the same compressive function. From the responses of one stimulus component (A), an equivalent concentration of the other component (B) is calculated. The equivalent concentration (B) is added to the known concentration of the mixture component (A) and the response to the mixture predicted.
Thus, CT responses to mixtures containing components with completely independent effects should equal the sum of the CT responses to the separately presented components. Since amiloride-sensitive Na+-specific CT responses to organic acids approximate zero, any deviation in NaCl + acid mixture responses from the response to the NaCl component alone would be indicative of mixture interactions. Whereas CT responses to mixtures containing components with effects associated with a common pathway should equal the sum of stimulus concentrations predicted by concentration-response functions for single mixture components that can substitute for one another. Deviation of electrolyte-generalist NaCl + acid mixture responses from stimulus addition would indicate mixture interactions. Hyman and Frank (1980b) illustrate how the predicted response for stimulus addition is computed. For the present experiment, the acid concentration eliciting a response equaling the magnitude of the response to the NaCl component is derived from plots of CT response versus acid concentration and added to the actual concentration of the acid component. The sum of derived and actual acid concentrations is used to predict the mixture response from the plots of CT response versus acid concentration. Average slopes of response functions were determined to be 0.23 ± 0.03 for acetic acid and 0.40 ± 0.08 for citric acid. If the slope was 1, the prediction for stimulus addition and response addition would be identical. The acid response slopes used for determining stimulus addition yielded predicted mixture responses much smaller than response addition.
Data analysis
Paired t-test contrasts were used to compare each actual mixture response to predicted values and the mixture components, with α adjusted by a Bonferroni correction. Entire nerve (no-amiloride) responses and amiloride-sensitive responses were compared with response addition and the NaCl component response, whereas amiloride-insensitive responses were compared with response addition and, when possible, stimulus addition. Stimulus addition values could not be computed for 250 μM citric acid or 20 mM acetic acid because the requisite second concentration of neither acid was recorded in the supplemental group of hamsters.
Results
Amiloride-sensitive and amiloride-insensitive responses combined: entire CT
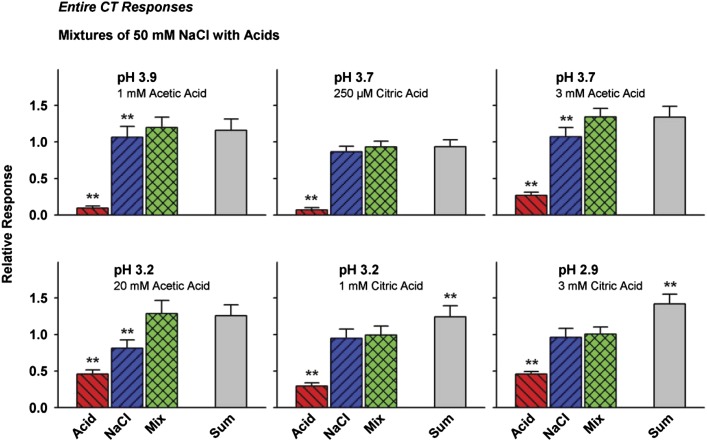
Mixtures of 50 mM NaCl with citric and acetic acid affected the entire CT differently. Figure 1 illustrates this difference with 1 of 8 raw electrophysiological response traces recorded with each acid presented at equal, 3 mEq/L, titratable acidity. CT responses to NaCl + 1 mM citric acid and the NaCl component superimpose (A), whereas CT responses to NaCl + 3 mM acetic acid are well above responses to individual mixture components (B), indicating mixture responses were reduced more by citric than acetic acid. A similar difference in effectiveness of the acids in suppressing sucrose responses occurs at equal acidity (Formaker et al. 2009). In group data presented versus pH for the entire nerve (Figure 2 and Table 1A), note that citric and acetic acid responses increase with concentration (Table 2) and, at pH ≤ 3.2, mean responses to citric acid + NaCl are smaller than response addition (approaching responses to NaCl itself); when at pH ≥ 3.2, mean responses to acetic acid + NaCl (greater than responses to NaCl itself) approximate response addition. Amiloride-insensitive values were subtracted from the entire-nerve responses to evaluate mixtures of the nonstimulatory acids on amiloride-sensitive NaCl responses.
Figure 2.
Effects of mixing 50 mM NaCl with citric and acetic acids on hamster entire-nerve CT responses. Responses to mixtures of NaCl with citric acid approximated responses to NaCl itself, falling well short of response addition (Sum) at pH ≤ 3.2. Responses to mixtures of NaCl with acetic acid approximate response addition. **P < 0.01 versus mixtures.
Table 1.
Mixture responses: tests of predictions
| Obtained | Predicted | ||||
| mM | Mixture | Response addition | P | NaCl component | P |
| A. Entire CT nerve | |||||
| Citric acid | |||||
| 0.250 | 0.93 ± 0.08 | 0.93 ± 0.10 | ns | 0.87 ± 0.08 | 0.03 |
| 1 | 0.99 ± 0.12 | 1.24 ± 0.15 | 0.002 | 0.95 ± 0.13 | ns |
| 3 | 1.00 ± 0.10 | 1.42 ± 0.13 | 0.0002 | 0.96 ± 0.12 | ns |
| Acetic acid | |||||
| 1 | 1.20 ± 0.14 | 1.16 ± 0.16 | ns | 1.06 ± 0.15 | ns |
| 3 | 1.34 ± 0.12 | 1.34 ± 0.15 | ns | 1.07 ± 0.13 | 0.01 |
| 20 | 1.29 ± 0.18 | 1.27 ± 0.14 | ns | 0.81 ± 0.12 | 0.002 |
| B. Amiloride-sensitive CT | |||||
| Citric acid | |||||
| 0.250 | 0.59 ± 0.07 | 0.68 ± 0.08 | 0.009 | 0.68 ± 0.07 | ns |
| 1 | 0.49 ± 0.12 | 0.71 ± 0.12 | 0.0014 | 0.79 ± 0.12 | 0.001 |
| 3 | 0.36 ± 0.10 | 0.72 ± 0.12 | 0.0008 | 0.82 ± 0.12 | 0.002 |
| Acetic acid | |||||
| 1 | 0.75 ± 0.12 | 0.83 ± 0.14 | ns | 0.85 ± 0.14 | ns |
| 3 | 0.75 ± 0.12 | 0.89 ± 0.15 | ns | 0.85 ± 0.12 | ns |
| 20 | 0.49 ± 0.10 | 0.60 ± 0.12 | ns | 0.67 ± 0.11 | 0.002 |
| Obtained | Predicted | ||||
| mM | Mixture | Response addition | P | Stimuli substitute | P |
| C. Amiloride-insensitive CT | |||||
| Citric acid | |||||
| 0.250 | 0.34 ± 0.03 | 0.26 ± 0.04 | 0.038 | Nd | |
| 1 | 0.50 ± 0.04 | 0.53 ± 0.06 | ns | 0.42 ± 0.04 | 0.003 |
| 3 | 0.65 ± 0.04 | 0.70 ± 0.06 | ns | 0.58 ± 0.05 | 0.02 |
| Acetic acid | |||||
| 1 | 0.45 ± 0.05 | 0.33 ± 0.04 | 0.04 | 0.26 ± 0.02 | 0.006 |
| 3 | 0.59 ± 0.04 | 0.44 ± 0.04 | 0.06 | 0.30 ± 0.03 | 0.002 |
| 20 | 0.80 ± 0.10 | 0.67 ± 0.08 | ns | Nd | |
Means ± standard errors of CT mixture responses (relative to 0.5 M NH4Cl responses) are tabled. P values are for contrasts of obtained mixture responses and predicted responses. ns = not significant and Nd = Not determined. P values > 0.025 < 0.10 suggestive of trends.
Table 2.
Chorda tympani responses to acetic and citric acid
| Entire nerve | Amiloride sensitive | Amiloride insensitive | ||||
| pH | Acetic-H | Citric-H | Acetic-H | Citric-H | Acetic-H | Citric-H |
| 3.9 | 0.09 ± 0.03 | –– | −0.02 ± 0.02 | –– | 0.12 ± 0.02 | –– |
| 3.7 | 0.27 ± 0.04 | 0.07 ± 0.03 | 0.04 ± 0.05 | 0 ± 0.05 | 0.23 ± 0.03 | 0.07 ± 0.03 |
| 3.2 | 0.46 ± 0.06 | 0.29 ± 0.04 | −0.06 ± 0.03 | −0.08 ± 0.04 | 0.52 ± 0.06 | 0.37 ± 0.05 |
| 2.9 | –– | 0.46 ± 0.04 | –– | −0.10 ± 0.04 | –– | 0.56 ± 0.05 |
Means ± standard errors of CT responses (relative to 0.5 M NH4Cl responses) are tabled.
Amiloride-sensitive CT responses
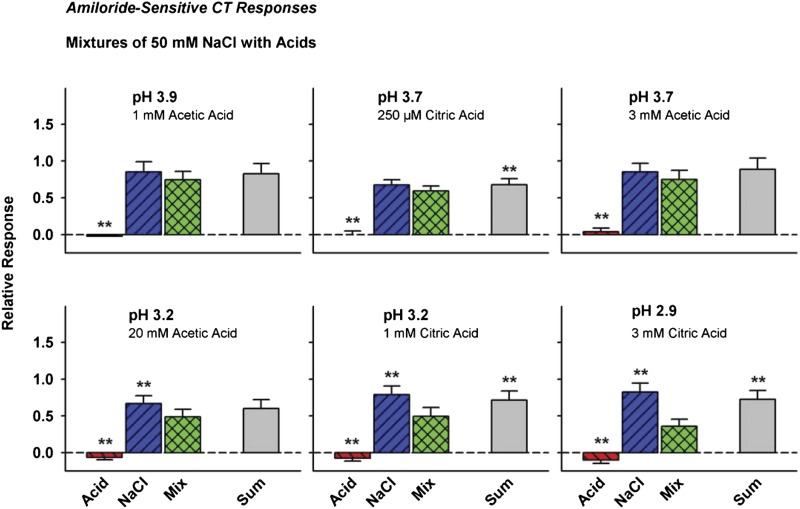
As anticipated, amiloride reduced hamster entire CT responses to stimuli containing NaCl (Hettinger and Frank 1990). Amiloride treatment reduced mean responses to NaCl on average 81% (P < 0.001) in the 3 groups of hamsters, leaving ∼20% of NaCl-evoked CT neural activity in the amiloride-insensitive component. Mean responses to NaCl + citric acid mixtures were lowered by 53% (P < 0.001), and mean responses to NaCl + acetic acid mixtures were lowered by 48% (P < 0.001) by amiloride treatment.
Amiloride-sensitive CT NaCl responses were reduced when mixed with citric acid (Table 1B). Figure 3 shows mean responses to NaCl + citric acid each falling below response addition; the responses to NaCl + 1 and 3 mM citric acid were smaller than responses to NaCl itself. The mean response with adding pH 2.9 (3 mM) citric acid to NaCl tended to be smaller (P < 0.08) than the response with adding either pH 3.2 (1 mM) or pH 3.7 (250 μM) citric acid, which did not differ significantly from one another. This result suggests that NaCl response suppression increases with citric acid concentration in the amiloride-sensitive CT nerve response.
Figure 3.
Effects of mixing 50 mM NaCl with citric and acetic acids on hamster amiloride-sensitive CT responses. Citric acid suppressed NaCl responses from pH 3.7 to pH 2.9. Although the response to pH 3.2 acetic acid was less than the response to NaCl itself, the mixture response was not significantly lower than the response sum nor were any of the responses to NaCl–acetic acid mixtures. **P < 0.01 versus mixtures.
Amiloride-sensitive CT NaCl responses were unaffected when mixed with pH 3.9 (1 mM) and pH 3.7 (3 mM) acetic acid. Neither mixture response differed significantly from either the mean response to the NaCl component or mean response to the sum of the mixture components (Table 1B). However, adding pH 3.2 (20 mM) acetic acid reduced the NaCl + acid mixture response to below the NaCl response component itself but not below the slightly smaller sum of NaCl and acetic acid responses. Acids themselves appear to reduce ongoing neural activity (Table 2, Figure 3) in the amiloride-sensitive CT component.
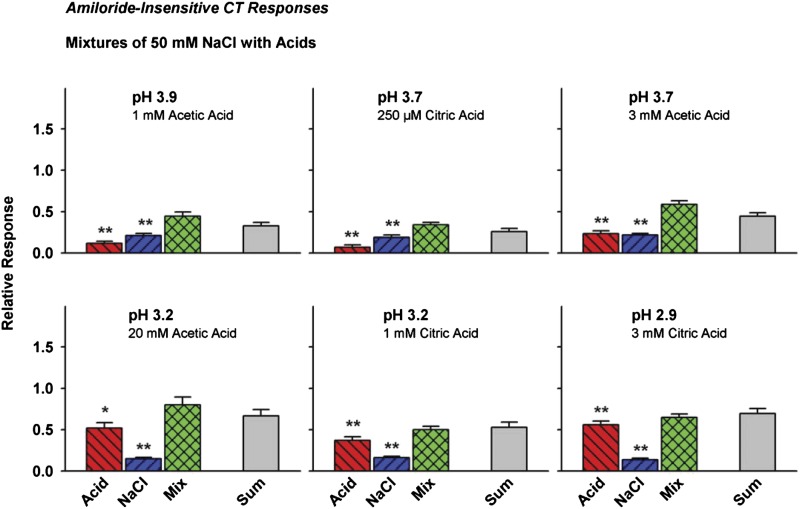
Amiloride-insensitive CT responses
Figure 4 and Tables 1C and 2 reveal responses to 50 mM NaCl and concentration-dependent responses to citric and acetic acids in the amiloride-insensitive CT. Acetic acid responses increased with increasing concentration (decreasing pH) (P = 0.002); as did citric acid responses (P = 0.0002), although at equal pH (3.7 or 3.2), acetic acid was more effective than citric acid (P = 0.005 and 0.07, respectively) (Figure 4, Table 2). Mean responses to NaCl + 1 or 3 mM citric and acetic acids exceeded stimulus-concentration addition (Table 1C). Predictions were based on concentration values for NaCl derived from relationships between the 1 and 3 mM acids, which increased by slopes well below 1.00: 0.23 for acetic and 0.40 for citric. By approximating response addition, responses to NaCl + acetic acid mixtures in the CT amiloride-insensitive component were heightened above values expected for stimuli that share the same pathway.
Figure 4.
Effects of mixing 50 mM NaCl with citric acid and acetic acid on hamster amiloride-insensitive CT responses. Responses to mixtures of NaCl with either acid approximated response addition (Sum). **P < 0.01 and *P < 0.05 versus mixtures.
Discussion
Entire CT responses to NaCl + acid mixtures
Entire hamster CT nerve responses measured from a water baseline fell below response addition for NaCl mixed with pH 2.9–3.2 citric acid. Similar effects were seen for entire rat CT nerve responses for NaCl mixed with pH 2.9–3.3 hydrochloric acid (Ogawa 1969). Thus, organic and mineral acids alike reduce rodent entire CT responses to NaCl + acid mixtures at low pH. The entire nerve response, however, obscures the contributions of separate specific amiloride-sensitive and -insensitive response elements. After subtraction of the amiloride-insensitive contribution to the mixture, organic acid responses in this pH range fell below baseline, and NaCl response suppression was segregated to this Na+/Li+-specific CT component. The halving of Na+-specific responses approximated maximal suppressions of sucrose responses in mixtures with acids or quinine reported earlier (Formaker and Frank 1996; Frank et al. 2005; Formaker et al. 2009). Consistent with absence of a pH effect on entire CT responses to mixtures of acid + amiloride-insensitive KCl or NH4Cl (Lyall et al. 2002), CT amiloride-insensitive responses to acid + salt mixture components were additive regardless of acid or pH.
Amiloride-sensitive NaCl responses and organic acid responses are generated in separate CT nerve-fiber populations. The cation-specific N-fiber response to a 100 mM NaCl stimulus (Frank et al. 1988) is silenced by treatment with 10 μM amiloride (Hettinger and Frank 1990). However, the anion-sensitive electrolyte-generalist H/E-fiber responses to 100 mM NaCl and non-Na+ salts (Formaker and Hill 1988; Rehnberg et al. 1993) are unchanged by amiloride treatment (Ninomiya and Funakoshi 1988; Hettinger and Frank 1990). Electrolyte-generalist hamster CT fibers (Frank 1973; Frank et al. 1988), not specific for electrolytes of one taste quality (Frank and Nowlis 1989), respond to acids, salts, and ionic bitters (Frank et al. 1988; Ninomiya and Funakoshi 1988). Thus, amiloride treatment, in essence, knocks out the contribution of CT neurons that are Na+-specific from the entire CT nerve recording.
NaCl + acid mixture response suppression in Na+-specific CT N-fibers
Compared with additivity, amiloride-sensitive responses to 50 mM NaCl were reduced in NaCl + acid mixtures, a suppression dependent on the particular acid (acetic or citric) and its pH (hydronium concentration). Suppression was evident in mixture responses that were smaller than the NaCl response itself at pH 3.2 (20 mM) acetic acid and citric acid (1 mM) but not at higher pH. This contrasts with the distinct response patterns at equal 3 mEq/L acidity; NaCl + 3 mM acetic acid (pH 3.7) shows no suppression, but NaCl + 1 mM citric acid (pH 2.9) shows >50% suppression (Figure 1). Citric acid also suppressed sucrose responses in acid + sucrose mixtures much more than acetic acid at equal 30 mEq/L acidity (Formaker et al. 2009). However, complicating the amiloride-sensitive picture, responses to higher 3.7–3.9 pH acids alone approached zero, whereas responses to lower 2.9–3.2 pH acids alone fell below zero, decreases approaching significance (P = 0.06) at the lowest pH (Figure 5, Table 2). Compared with response additivity (Sum, obtained by summing acid decrement and NaCl response), only response decrements to NaCl + citric acid mixtures attained significance (Figure 3).
Figure 5.
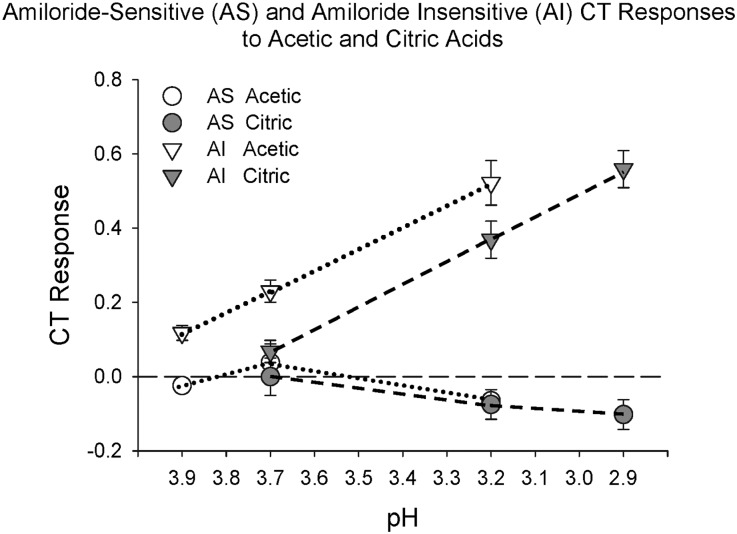
Responses of amiloride-sensitive and amiloride-insensitive CT response components to acetic and citric acids as a function of pH. At equal pH, 3.7 or 3.2, acetic acid tended to be more effective than citric acid (P = 0.005 and 0.07, respectively). The responses to 2.9 pH citric acid tended to fall below zero (P = 0.06).
An effect of low stimulus pH on receptor function would provide a direct mechanism for the suppression of CT amiloride-sensitive NaCl responses. Amiloride-sensitive N-fibers are matched to a population of amiloride-sensitive taste cells (Yoshida et al. 2009) expressing ENaC function that may be altered by acidic pH (Palmer and Frindt 1987; Awayda et al. 2000). Also, NaCl responses of fungiform papillae shift from adapting to sustained when NaCl is mixed with amiloride or with citric acid that is possibly related to permeating hydronium ions binding within ENaC (Gilbertson et al. 1992). In the amiloride-sensitive CT (Figure 3), NaCl suppression at low pH appeared greater for citric acid, which is less likely to increase intracellular H+ than the more lipophilic acetic acid.
NaCl + acid mixture response addition in electrolyte-generalist CT H/E-fibers
The entire CT nerve's activation to acetic and citric acids is contained in the CT amiloride-insensitive component (Figure 4). CT amiloride-insensitive responses to acetic and citric acid increased with hydronium concentration (decreasing pH), and from pH 3.2 to 3.7 (Figure 5), acetic (corresponding to 3 and 20 mEq/L titratable acidity), the weaker acid, was more effective than citric acid (corresponding to 0.75 and 3 mEq/L acidity). This result is consistent with the well-known dependence of human sourness on pH and total acidity (Beatty and Cragg 1935), suggesting both effects are carried in the CT taste nerve.
CT amiloride-insensitive responses to H+ concentration may be associated with entry of extracellular protons into a distinct population of taste receptor cells (Chang et al. 2010). The resulting rapid cellular depolarization mimics the time course of CT responses to unbuffered acids applied to a water-rinsed tongue (Figure 1), which is not the same as slowly accumulating H+ in cells after prior exposure to buffered solutions for minutes (Lyall et al. 2001). Rodent CT amiloride-insensitive responses for acid + NaCl mixtures, shown here, as well as for KCl + NH4Cl (other amiloride-insensitive stimuli), approximated response addition (Stewart et al. 1998, Lyall et al. 2002) as would be expected for independent stimuli. Component response intensities to binary mixtures of bitter salts were also quantitatively retained in mixtures (Hyman and Frank 1980a; Frank 1989). If electrolyte-generalist responses were generated in a dedicated taste cell population, mixing of the different classes of amiloride-insensitive compounds would be expected to be equivalent to increasing the concentration of a single compound, that is, stimulus addition, not response addition. Also, unlike amiloride-sensitive NaCl response suppression in the amiloride-insensitive CT component acid responses to the more lipophilic acetic acid, which more readily increases intracellular H+, exceed responses to citric acid (Figure 5).
Coding of mixture components in NaCl + acid taste mixtures
Citric acid and NaCl have distinct taste qualities to humans (Bartoshuk 1975; Wang et al. 2009) and rodents (Frank and Nowlis 1989; Hill et al. 1990). Hamster CT acid responses are transmitted to the central nervous system (CNS) in generalist H/E-fibers, and NaCl responses are transmitted simultaneously in N-fibers and H/E-fibers. NaCl is associated with 2 taste qualities in rodents (Hettinger and Frank 1990; Hill et al. 1990; Spector et al. 1996). When the Na+-specific taste, transduced in a distinct population of taste bud cells (Chandrashekar et al. 2010), is inhibited by amiloride (Hill et al. 1990; Spector et al. 1996), remaining electrolyte-generalist responses dominate, giving NaCl the quinine-like taste of KCl and NH4Cl (Frank and Nowlis 1989), also transmitted to the CNS in H/E-fibers. In mixtures, stronger components dominate leaving weakened components unidentifiable (Livermore and Laing 1996; Laing et al. 2002; Frank et al. 2010a, 2010b); this reciprocal symmetric suppression of independent tastes is likely based in the CNS (Chen and Di Lorenzo 2008). The cells/circuits responsible for rodent electrolyte-generalist taste(s) remain unidentified.
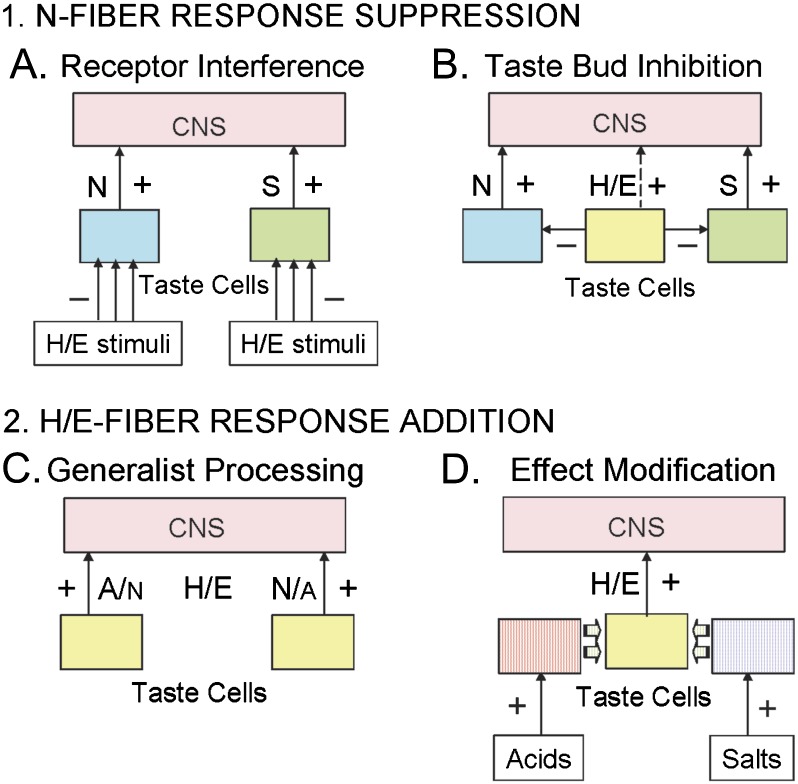
In Figure 6, we explore how circuits may be organized incorporating current knowledge on Na+-specific and electrolyte-generalist tastes. Response suppression for NaCl + acid mixtures in amiloride-sensitive N-fibers is modeled in Figure 6-1. Rodent CT Na+-specific response suppression, like sucrose (Formaker et al. 2009) response suppression by acids, may result from a disruption of ENaC channels, or TR1 receptors, respectively, by low pH. However, sucrose responses are also suppressed by quinine HCl (Formaker and Frank 1996; Formaker et al. 1997; Frank et al. 2005) another H/E stimulus. (A) Each H/E stimulus may individually disrupt receptors; or (B) H/E stimuli may inhibit N-fibers and S-fibers, as well as excite H/E-fibers (Figure 6B), through intervening taste bud circuitry (Dvoryanchikov et al. 2011). Regardless of peripheral mechanism, nutrient identification is muted before transmission to the CNS when combined with potentially harmful aversive stimuli.
Figure 6.
Routes of peripheral mixture interaction. 1. N-fiber response suppression: (A) Multiple stimuli, which also activate H/E-fibers, independently obstruct function of individual receptors by various mechanisms. (B) Taste bud cells that activate H/E-fibers inhibit taste bud cells that activate S- and N-fibers. 2. H/E-fiber response addition: (C) Generalist H/E-fibers contain 2 distinct subcategories of fibers, acting independently, they contribute responses that add in the entire nerve. (D) Categories of electrolytes activate distinct taste bud cells that secrete modulators onto nearby cells to adjust H/E-fiber activation before submission to the CNS. Taste bud cells, excited by taste stimuli, activate CT fibers. N and S = NaCl and Sucrose specialists; H/E = Acid sensitive Electrolyte generalists; A/N and N/A = Acid-best and NaCl-best generalist fibers activated more by acid or more by NaCl, respectively.
Response addition for NaCl + acid mixtures in amiloride-insensitive H/E-fibers is modeled in Figure 6-2. Electrolyte generalists are simultaneously excited by the NaCl + acid mixture components in rodents. Responses show additivity, which is inconsistent with 2 doses of a compound activating a single receptor. The stimuli may activate independent subsets of generalists, some more responsive to acid and some more responsive to NaCl (Breza et al. 2006, 2010) having activity spectra (Figure 6C) different from identified rodent receptors (Yarmolinsky et al. 2009). Or responses to distinct stimulus categories (e.g., acids or salts) may be combined or adjusted in taste buds via pannexin hemichannels as in the mouse circumvallate papillae (Dando and Roper 2009) to conserve or even intensify individual stimulus effects in mixtures (Figure 6D). In either case, combined electrolyte-generalist stimulus signals remain at least as strong within mixtures as they are individually. These models require quantitative empirical confirmation attainable from focused CT recordings and behavioral discrimination studies.
Thus, reactions to NaCl–acid mixtures suggest that electrolyte coding in taste buds generates 2 separate pathways with distinct biological meaning when activated by NaCl. Output from a cell population dedicated to the Na+-specific pathway (Chandrashekar et al. 2010) may be modified by chemical signaling within the taste bud (Chaudhari and Roper 2010) that may also generate electrolyte-generalist pathways.
Funding
This work was supported by the National Institutes of Health [5R01 DC004099 to M.E.F.].
References
- Awayda MS, Boudreaux MJ, Reger RL, Hamm LL. Regulation of the epithelial Na+ channel by extracellular acidification. Am J Physiol Cell Physiol. 2000;279:C1896–C1905. doi: 10.1152/ajpcell.2000.279.6.C1896. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM. Taste mixtures: is mixture suppression related to compression? Physiol Behav. 1975;14:643–649. doi: 10.1016/0031-9384(75)90193-6. [DOI] [PubMed] [Google Scholar]
- Beatty RM, Cragg LH. The sourness of acids. J Am Chem Soc. 1935;57:2347–2351. [Google Scholar]
- Breza JM, Curtis KS, Contreras RJ. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol. 2006;95:674–685. doi: 10.1152/jn.00793.2005. [DOI] [PubMed] [Google Scholar]
- Breza JM, Nikonov AA, Contreras RJ. Response latency to lingual taste stimulation distinguishes neuron types within the geniculate ganglion. J Neurophysiol. 2010;103:1771–1784. doi: 10.1152/jn.00785.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJP, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RB, Waters H, Liman ER. A proton current drives action potentials in genetically identified sour taste cells. Proc Natl Acad Sci U S A. 2010;107:22320–22325. doi: 10.1073/pnas.1013664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-Y, Di Lorenzo PM. Responses to binary taste mixtures in the nucleus of the solitary tract: neural coding with firing rate. J Neurophysiol. 2008;99:2144–2157. doi: 10.1152/jn.01020.2007. [DOI] [PubMed] [Google Scholar]
- Dando R, Roper SD. Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol. 2009;587:5899–5906. doi: 10.1113/jphysiol.2009.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoryanchikov G, Huang YA, Barro-Soria R, Chaudhari N, Roper SD. GABA, its receptors, and GABAergic inhibition in mouse taste buds. J Neurosci. 2011;31:5782–5791. doi: 10.1523/JNEUROSCI.5559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formaker BK, Frank ME. Responses of the hamster chorda tympani nerve to binary component taste stimuli: evidence for peripheral gustatory mixture interactions. Brain Res. 1996;727:79–90. doi: 10.1016/0006-8993(96)00356-3. [DOI] [PubMed] [Google Scholar]
- Formaker BK, Hill DL. An analysis of residual NaCl taste response after amiloride. Am J Physiol. 1988;255:R1002–R1007. doi: 10.1152/ajpregu.1988.255.6.R1002. [DOI] [PubMed] [Google Scholar]
- Formaker BK, Lin H, Hettinger TP, Frank ME. Responses of the hamster chorda tympani nerve to sucrose + acid and sucrose + citrate taste mixtures. Chem Senses. 2009;34:607–616. doi: 10.1093/chemse/bjp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formaker BK, MacKinnon BI, Hettinger TP, Frank ME. Opponent effects of quinine and sucrose on single-fiber taste responses of the chorda tympani nerve. Brain Res. 1997;772:239–242. doi: 10.1016/s0006-8993(97)00845-7. [DOI] [PubMed] [Google Scholar]
- Frank M. An analysis of hamster afferent taste nerve response functions. J Gen Physiol. 1973;61:588–618. doi: 10.1085/jgp.61.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME. Processing of mixtures of stimuli with different tastes by primary mammalian taste neurons. In: Laing DG, Cain WS, McBride RL, Ache BW, editors. Perception of complex smells and tastes. Australia: Academic Press; 1989. pp. 127–147. [Google Scholar]
- Frank ME, Bieber SL, Smith DV. The organization of taste sensibilities in hamster chorda tympani nerve fibers. J Gen Physiol. 1988;91:861–896. doi: 10.1085/jgp.91.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME, Formaker BK, Hettinger TP. Taste responses to mixtures: analytic processing of quality. Behav Neurosci. 2003;117:228–235. doi: 10.1037/0735-7044.117.2.228. [DOI] [PubMed] [Google Scholar]
- Frank ME, Formaker BK, Hettinger TP. Peripheral gustatory processing of sweet stimuli by golden hamsters. Brain Res Bull. 2005;66:70–84. doi: 10.1016/j.brainresbull.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Frank ME, Goyert HF, Hettinger TP. Time and intensity factors in identification of components of odor mixtures. Chem Senses. 2010a;35:777–787. doi: 10.1093/chemse/bjq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME, Goyert HF, Hettinger TP. Coding mixture components in sucrose-NaCl mixtures. 2010b. AChemS Abstract 294. Chem Senses. 31: A88. [Google Scholar]
- Frank ME, Lundy RF, Jr, Contreras RJ. Cracking taste codes by tapping into sensory neuron impulse traffic. Prog Neurobiol. 2008;86:245–263. doi: 10.1016/j.pneurobio.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME, Nowlis GH. Learned aversions and taste qualities in hamsters. Chem Senses. 1989;14:379–394. [Google Scholar]
- Gilbertson TA, Avenet P, Kinnamon SC, Roper SD. Proton currents through amiloride-sensitive Na channels in hamster taste cells. Role in acid transduction. J Gen Physiol. 1992;100:803–824. doi: 10.1085/jgp.100.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herness S, Zhao FL. The neuropeptides CCK and NPY and the changing view of cell-to-cell communication in the taste bud. Physiol Behav. 2009;97:581–591. doi: 10.1016/j.physbeh.2009.02.043. [DOI] [PubMed] [Google Scholar]
- Hettinger TP, Frank ME. Specificity of amiloride inhibition of hamster taste responses. Brain Res. 1990;513:24–34. doi: 10.1016/0006-8993(90)91085-u. [DOI] [PubMed] [Google Scholar]
- Hill DL, Formaker BK, White KS. Perceptual characteristics of the amiloride-suppressed sodium chloride taste response in the rat. Behav Neurosci. 1990;104:734–741. doi: 10.1037//0735-7044.104.5.734. [DOI] [PubMed] [Google Scholar]
- Hyman AM, Frank ME. Sensitivities of single nerve fibers in the hamster chorda tympani to mixtures of taste stimuli. J Gen Physiol. 1980a;76:143–173. doi: 10.1085/jgp.76.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AM, Frank ME. Effects of binary taste stimuli on the neural activity of the hamster chorda tympani. J Gen Physiol. 1980b;76:125–142. doi: 10.1085/jgp.76.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing DG, Link C, Jinks AL, Hutchinson I. The limited capacity of humans to identify the components of taste mixtures and taste-odour mixtures. Perception. 2002;31:617–635. doi: 10.1068/p3205. [DOI] [PubMed] [Google Scholar]
- Livermore A, Laing DG. Influence of training and experience on the perception of multicomponent odor mixtures. J Exp Psychol Hum Percept Perform. 1996;22:267–277. doi: 10.1037//0096-1523.22.2.267. [DOI] [PubMed] [Google Scholar]
- Lyall V, Alam RI, Phan DQ, Ereso GL, Phan TH, Malik SA, Montrose MH, Chu S, Heck GL, Feldman GM, et al. Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. Am J Physiol Cell Physiol. 2001;281:C1005–C1013. doi: 10.1152/ajpcell.2001.281.3.C1005. [DOI] [PubMed] [Google Scholar]
- Lyall V, Alam RI, Phan THT, Russell OF, Malik SA, Heck GL, DeSimone JA. Modulation of rat chorda tympani NaCl responses and intracellular Na+ activity in polarized taste receptor cells by pH. J Gen Physiol. 2002;120:793–815. doi: 10.1085/jgp.20028656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. Amiloride inhibition of responses of rat single chorda tympani fibers to chemical and electrical tongue stimulations. Brain Res. 1988;451:319–325. doi: 10.1016/0006-8993(88)90777-9. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. Behavioral discrimination between glutamate and the four basic taste substances in mice. Comp Biochem Physiol A Comp Physiol. 1989;92:365–370. doi: 10.1016/0300-9629(89)90577-x. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Mizukoshi T, Nishikawa T, Funakoshi M. Ion specificity of rat chorda tympani fibers to chemical and electrical tongue stimulations. Brain Res. 1987;404:350–354. doi: 10.1016/0006-8993(87)91393-x. [DOI] [PubMed] [Google Scholar]
- Ogawa H. Effects of pH on taste responses in the chorda tympani nerve of rats. Jpn J Physiol. 1969;19:670–681. doi: 10.2170/jjphysiol.19.670. [DOI] [PubMed] [Google Scholar]
- Palmer LG, Frindt G. Effects of cell Ca and pH on Na channels from rat cortical collecting tubule. Am J Physiol. 1987;253:F333–F339. doi: 10.1152/ajprenal.1987.253.2.F333. [DOI] [PubMed] [Google Scholar]
- Rehnberg BG, MacKinnon BI, Hettinger TP, Frank ME. Anion modulation of taste responses in sodium-sensitive neurons of the hamster chorda tympani nerve. J Gen Physiol. 1993;101:453–465. doi: 10.1085/jgp.101.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Guagliardo NA, St. John SJ. Amiloride disrupts NaCl versus KCl discrimination performance: implications for salt taste coding in rats. J Neurosci. 1996;16:8115–8122. doi: 10.1523/JNEUROSCI.16-24-08115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RE, Lyall V, Feldman GM, Heck GL, DeSimone JA. Acid-induced responses in hamster chorda tympani and intracellular pH tracking by taste receptor cells. Am J Physiol. 1998;44:C227–C238. doi: 10.1152/ajpcell.1998.275.1.C227. [DOI] [PubMed] [Google Scholar]
- Wang M-F, Marks LE, Frank ME. Taste coding after selective inhibition by chlorhexidine. Chem Senses. 2009;34:653–666. doi: 10.1093/chemse/bjp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky DA, Zuker CS, Ryba NJP. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Heck GL, DeSimone JA. Effects of voltage perturbation of the lingual receptive field on chorda tympani responses to Na+ and K+ salts in the rat: implications for gustatory transduction. J Gen Physiol. 1994;104:885–907. doi: 10.1085/jgp.104.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Horio N, Murata Y, Yasumatsu K, Shigemura N, Ninomiya Y. NaCl responsive taste cells in the mouse fungiform taste buds. Neuroscience. 2009;159:795–803. doi: 10.1016/j.neuroscience.2008.12.052. [DOI] [PubMed] [Google Scholar]