Abstract
Objective
The mechanisms of restenosis in autogenous vein bypass grafts placed for peripheral artery disease are not completely understood. We seek to investigate the role of hemodynamic stress in a case study of a revised bypass graft that failed due to restenosis.
Methods
The morphology of the lumen is reconstructed from a custom 3D ultrasound system. Scans were taken at one, six, and sixteen months after a patch angioplasty procedure. Computational hemodynamic simulations of the patient-specific model provide the blood flow features and the hemodynamic stresses on the vessel wall at the three time points studied.
Results
The vessel was initially free of any detectable lesions, but a 60% diameter reducing stenosis developed over the 16 month interval of study. As determined from the simulations, chaotic and recirculating flow occurred downstream of the stenosis due to the sudden widening of the lumen at the patch location. Curvature and a sudden increase in the lumen cross-sectional area induce these flow features that are hypothesized to be conducive to intimal hyperplasia. Favorable agreement was found between simulation results and in vivo Doppler ultrasound velocity measurements.
Conclusions
Transitional and chaotic flow occurs at the site of the revision, inducing a complex pattern of wall shear are computed with the hemodynamic simulations. This supports the hypothesis that the hemodynamic stresses in the revised segment, produced by the coupling of vessel geometry and chaotic flow, led to the intimal hyperplasia and restenosis of the graft.
Keywords: Hemodynamics, Peripheral Artery Disease, Intimal Hyperplasia
Introduction
The failure rate of autogenous vein bypass grafting for the treatment of peripheral artery disease remains high —up to 20 % within the first year of implantation and up to 50% within five years [1, 2, 3]. Graft failure usually follows development of stenotic lesions brought on by intimal hyperplasia. Up to 70% of lower extremity bypass grafts require reintervention to correct stenotic lesions [4].
Autogenous venous grafts will adapt to the conditions of the arterial circulation in the first few months following surgery (i.e. arterialization). The morphological and structural changes that occur during the remodeling process include an increase in graft lumen cross-section [5, 6], as well as graft wall medial and intimal thickening [7, 8]. The adaptive remodeling of the graft is widely understood to be stimulated by hemodynamic forces [9].
The remodeling in the intima can degenerate into an uncontrolled proliferation process (i.e. intimal hyperplasia). Vascular medial smooth muscle cells and adventitial fibroblasts proliferate [10], migrate into the intima, and deposit extracellular matrix, locally inducing a stenotic lesion that jeopardizes graft patency. Although the exact biological mechanisms that trigger intimal hyperplasia remain unknown, hemodynamic wall shear stress has consistently been shown to influence the outcome of intimal thickening. A clinical study of morphological changes in revised peripheral vein bypass grafts [11] showed a broad range of remodeling responses. Many of the grafts experienced significant lumen narrowing and/or stenoses post-revision. However, most stenoses were seen to regress while some continued to progress and required clinical intervention. Animal models of vein arterialization, however, have shown that the extent of intimal hyperplasia in grafts is inversely proportional to the wall shear stress magnitude [8, 12, 13, 14, 15].
Direct detailed measurements of in-vivo hemodynamics remain challenging, even with recent advances in Doppler ultrasound and phase-contrast magnetic resonance imaging. Limitations in spatial and temporal resolution result in high uncertainty in the estimation of shear stress, particularly in regions of transitional or chaotic flow. Advances in computational fluid dynamics provide useful tools to quantify the complex flow features and shear stress patterns present in bypass grafts. The hemodynamic forces in both idealized [16, 17, 18, 19, 20] and patient-specific geometries [21, 22, 23] have been examined via computational fluid simulations. These simulations achieve a high level of temporal resolution (0.1ms) and spatial resolution (0.1mm) not attainable in either in-vivo or in-vitro experiments [24]. This level of resolution is essential to understand the details of the interaction between blood flow and tissue remodeling. These studies have shown that some regions of low wall shear stress (< 0.5Pa), such as anastomoses, correlate with regions of intimal thickening as determined by histological examinations [25].
Despite the attention focused on this problem, no definitive link has been established between hemodynamic forces and long-term patency. There have been surprisingly few longitudinal in vivo studies of graft remodeling associated with detailed hemodynamic computations [22, 23]. Unfortunately, many in-vivo studies correlating shear stress to intimal hyperplasia [5, 6, 12, 14, 15] have used simplified relationships for computing wall shear stresses (i.e. Poiseuille’s law). Transitional and/or recirculating flows, both critical on the development of intimal hyperplasia [9], are not well described with such simplistic fluid mechanical theories.
We have conducted a longitudinal retrospective case study of a revised autogenous bypass graft over 16 months after a patch angioplasty. We have reported previously on the methodology followed here and of the hemodynamics of this case [24]. However, new analyses and new conclusions with relevance to clinical practice are presented here. We combine hemodynamic computational simulations in the three patient-specific geometries reconstructed from 3D ultrasound at 1, 6 and 16 months after surgery. We correlate the observed in-vivo evolution of the vessel wall remodeling with the computational wall shear stresses, with the goal of determining the role of mechanical stresses on the changes in the lumen.
Methods
Patient History
A femoral to above-knee popliteal artery reversed saphenous vein graft was studied under a protocol approved by the University of Washington Human Subjects Division. Our study begins with a revision performed seven years after initial graft placement, due to stenosis formation at a venous valve site. The revision consisted of an endarterectomy and polytetrafluoroethylene (PTFE) patch angioplasty. Post-operative ultrasound scans of the graft were performed at 1, 6, and 16 months after the revision procedure. Twenty months after the revision, the graft segment was replaced by an interposition graft due to restenosis accompanied by symptoms of increasing claudication.
Ultrasound Imaging and 3D Reconstruction
Ultrasound imaging was performed with a custom three-dimensional system, as described previously [26]. Briefly, a magnetic tracking system (Flock of Birds, Ascension Technology, Burlington, VT) provides the location and orientation of the ultrasound scanhead during the examination. The ultrasound imager (HDI 5000, Philips Medical Systems, Bothell, WA) and magnetic tracking system are interfaced with a personal computer using custom software for simultaneous acquisition of the ultrasound images and location data. Data acquisition is gated to an ECG signal. Cross-sectional images were obtained at intervals of 1–2mm along the graft segment of interest. The ECG trigger was set to ensure that the data were gathered when velocity is maximal (200ms after ECG systole). Doppler spectral waveforms were also recorded at 5–20mm intervals along the length of the revised segment. All ultrasound measurements were performed with the patient in a supine position. The cross-sectional images of the entire length of the graft took between 6 and 7 minutes to acquire.
Custom MATLAB (The MathWorks, Natick, MA, USA) scripts are used to reconstruct 3D surfaces from the US images [26, 11]. Automatic color segmentation defines the boundary of the lumen on each 2D cross-sectional power Doppler view of the vessel. The contours are transformed to locations in the 3D coordinate system by use of the position and orientation information associated with each image plane. Registration of data sets across visits is accomplished by correlation of cross-sectional area profiles computed from the reconstructed 3D surfaces [11]. The 3D surface reconstructions of the three scans registered in a common coordinate system are shown in Figure 1. The cross-sectional area measurements from those scans are shown in Figure 2.
Figure 1.
Surface reconstructions of the three bypass grafts registered in a common coordinate system. Reconstructions are chronological from top to bottom. Times of acquisition are one month (a), six months (b), and sixteen months (c) after a PTFE patch angioplasty. The patch is approximately located between x = −30mm to x = +10mm and covers about one third of the vessel circumference.
Figure 2.
Cross-sectional area measurements vs. graft axis for three surface reconstructions. Coordinate system is the same as that in figure 1.
Computational Methods
The flow equations are solved with a Computational Fluid Dynamics software package (ANSYS® FLUENT® 12.1, ANSYS Inc., Cannonsburg, PA.), calculating the velocity and pressure on the points of a computational mesh created by GAMBIT® (ANSYS, Inc., Cannonsburg, PA.). Well-accepted assumptions on blood rheology, rigid vessel wall conditions, and blood flow profile at the inlet were used in the simulation, as described in McGah et al. [24]
Errors in the measurement of the cross sectional area of the vessel lumen were estimated to be within ±14% [11], while errors in the flow rate measurements are estimated to be ±13% [27]. Previous analysis places the error of the simulation itself at less than ±6% [24].
Results
The entire graft underwent appreciable remodeling over the course of the three clinical visits. This remodeling was concentrated in two distinct regions. First, a stenosis developed at the proximal end of the patch. This can be seen in the cross sectional area reduction at the x=−30mm location in Figures 1 and 2. The second region of significant vessel wall remodeling is at the distal end of the patch where a post-stenotic dilatation is observed at the x=0 location in Figures 1 and 2.
A time-dependent velocity within the graft is obtained from the maximum velocity of the Doppler ultrasound spectral waveforms applied at the centerline velocity of the vessel [28]. This is converted to a flow rate and imposed as the condition driving the flow in the simulations. Flow rates are shown in Figure 3. The time-averaged flow rate is 130 ml/min, with a peak of 510 ml/min for the 1 and 6 month examinations. At 16 months, the time-averaged and maximum flow rates are 86.4 ml/min and 410 ml/min, respectively. These values correspond to mean Reynolds numbers (the key non-dimensional parameter that controls transition from laminar to turbulent flow) of 176 at 1 and 6 months and 110 at 16 months. The ECG-measured patient heart rate was 56–61 beats-per-minute (bpm) at one month, 54–58 bpm at six months, and 54–61 bpm at sixteen months. We used a representative value of 60 bpm in all simulations.
Figure 3.
Volumetric flow rates applied at domain inlet (mL / min) vs. time. The time has been normalized with the cardiac cycle period. Solid line is the flow rate used for one and six months. Dashed line is the flow rate used at sixteen months.
First, we examine the detached flow in light of the hypothesized connection of low shear stress to intimal hyperplasia. Second, we examine high frequency chaotic flow fluctuations as a cause of the post-stenotic dilatation.
Stenotic Region
The proximal end of the patch changed from no stenosis at 1 month, 15% diameter (25% area) reduction at 6 months, and 60% diameter (85% area) reduction at 16 months after revision. Proximal to the patch anastomosis, the simulated flow is laminar for all cases. The simulated time-averaged wall shear stresses at the inflow segment of the revision are 1.61Pa, 1.82Pa, and 1.02Pa at 1, 6, and 16 months, respectively, which is in agreement with previously published results of normal shear stress in femoral bypass grafts [6]. Flow separation occurs in the simulations distal to the start of the patch due to a sudden increase in the cross-sectional area of the lumen at x=−30mm. Figure 4 shows the simulated velocity at two cross sectional planes downstream from the location of the minimum cross section. We observe flow recirculation (blue region on the top right side of Fig. 4a) and flow instability; the flow rolls into a pair of vortices that can be seen as a “crescent moon” filling the top half of the vessel, in Fig. 4b. This alternate pattern of high speed flow in part of the vessel cross-section and low or retrograde flow in the rest of the lumen, associated with the chaotic nature of this flow, leads to large spatio-temporal fluctuations of shear stress.
Figure 4.
Simulated instantaneous streamwise velocity contours of the one month scan at systolic deceleration (t/T = 0.3); Subfigures A and B depict the out-of-plane velocity at two planes located at x = −27mm and −5mm, respectively.
Doppler ultrasound velocimetry was performed at multiple locations along the graft. The 3D tracker was not activated during the collection of Doppler spectral waveforms (which were captured as part of the patient’s standard clinical examination), so the exact location at which the Doppler spectra were taken cannot be quantitatively determined. However, this location can be estimated by examining the B-mode images that accompany each Doppler waveform. This allows us to compare the Doppler measurements from one and six months with the velocity measurements from the simulations at the same location. We observe spectral broadening, indicative of transitional/chaotic flow [11], in the in vivo measurements (data not shown). This observation is consistent with the simulation results: flow separation, jet formation, and recirculating vortical flow (as seen in figure 4). Figure 5a shows a 3D visualization of the flow transition in the patched segment for the visit at one month.
Figure 5.
Visualization of the flow transition at one month (subfig. a) and 16 months (subfig. b) time points. Note that the value of the velocity surface in (b) is twice that of (a). Flow is from left to right in both cases.
(a) Surface of constant velocity at one month during mid-systolic deceleration (t/T = 0.3). Value of velocity surface is 0.25 m/s. The velocity surface is smooth on the left-hand-side of the figure but becomes less regular on the right hand side due to the passage of flow vortices.
(b) Surface on constant velocity in the post-stenotic jet at 16 months during mid-systolic deceleration (t/T = 0.3). Value of velocity surface is 0.50 m/s. The shape of the velocity surface is irregular on the right-hand-side of the figure, indicating the flow transition.
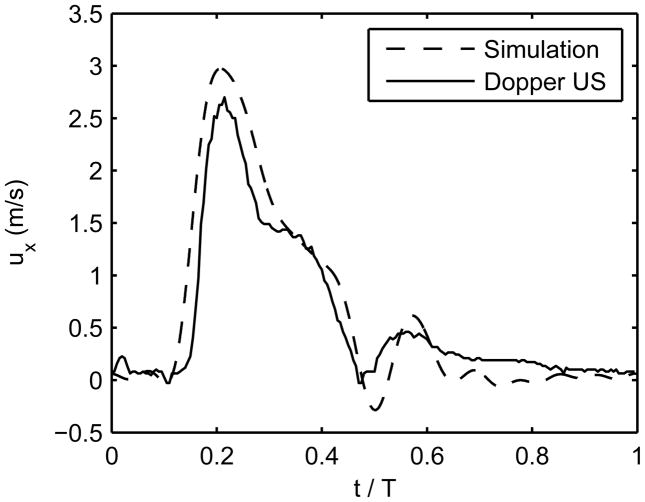
At 16 months, the hemodynamics in the graft are markedly different from the first visits. At 16 months, Doppler ultrasound recorded peak systolic velocities of approximately 0.54m/s proximal to the stenosis (x=−45 mm, not shown) with no signs of transitional flow. At the throat of the stenosis (x=−27 mm), recorded peak systolic velocities were between 2.35 and 2.71m/s. The simulation computed peak systolic velocities at this location were approximately 2.97m/s (see Figure 6 for a comparison between the in-vivo and in-silico stenosis velocities). This produces a post-stenotic jet that persists throughout systole (Figure 5b). The shear stress values computed at the stenosis throat, Figure 7 (bottom panel), are much greater than predicted by Poiseuille’s law. The time-averaged wall shear stress is above 30 Pa in this section of the graft, and the instantaneous wall shear stress reaches above 100 Pa during peak systole. This is a pathological level of shear stress and is expected to strongly influence endothelial function, considering that the peak systolic shear stresses computed at the stenotic segment is 6.33Pa and 7.05Pa at 1 and 6 months, respectively. The three panels in Figure 7 display a rendering of the time-averaged shear stress acting on the vessel lumen at the three visits, allowing us to observe the spatial and temporal patterns of wall shear stress as the vessel remodels.
Figure 6.
Simulated and in vivo velocity vs. time at the center of the stenosis for one cardiac cycle (examination at 16 months).
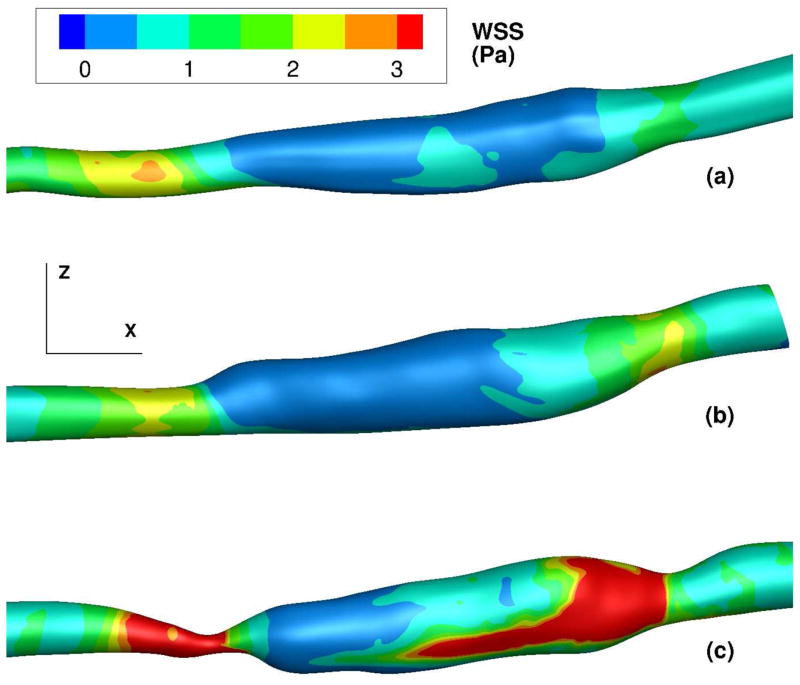
Figure 7.
Time-averaged wall shear stress (WSS) contours on the graft lumen (in Pa). Images are one month (a), six months (b), and 16 months (c). Flow is from left to right. Shear patterns are approximately symmetrical on the reverse side of the lumen.
Post-Stenotic Dilatation
The post-stenotic jet becomes unstable and transitions into a chaotic state, particularly during systolic deceleration (the Reynolds number for the jet, based on the stenosis diameter and the peak velocity, is approximately 1500 at the 16-month scan). Figure 5(b) shows a 3D surface of constant velocity where the post-stenotic jet is observed to impinge on the graft wall several centimeters distal to the stenosis. The vortices impinging on the graft wall produce very high shear stresses and high frequency pressure fluctuations on the vessel wall.
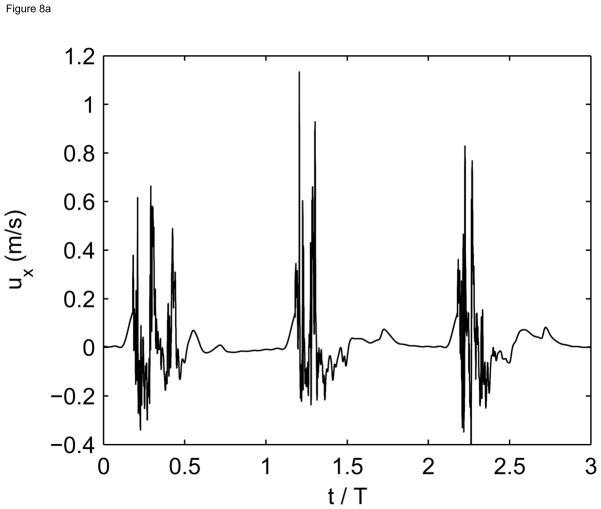
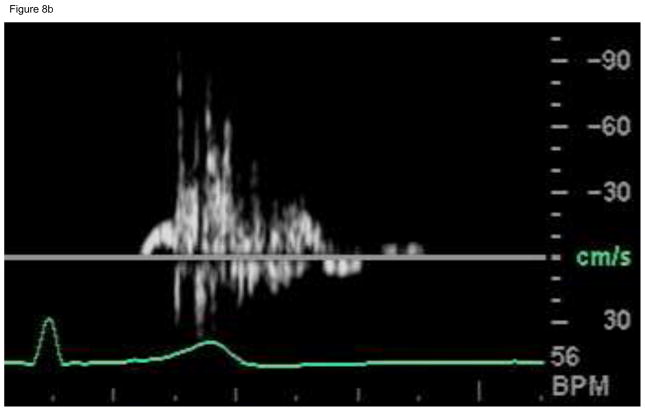
The in-vivo Doppler measurements made in the post-stenotic region at 16 months also show spectral broadening and reversed flow during systole (see Figure 8a), indicative of flow transition/turbulence in the fluid jet. For comparison, computed flow waveforms in the post-stenotic region also show high frequency fluctuations during systole and relatively quiescent motion during diastole (see Figure 8(b)).
Figure 8.
Simulated vs. in vivo velocimetry for the visit at 16 months.
(a) Simulated streamwise velocity vs. time for three cardiac cycles at a point located at x=0 in the center of the lumen.
(b) In vivo Doppler ultrasound velocimetry near the distal patch anastomosis for one cardiac cycle. Abscissa is the time (approximately 1s) and the ordinate is the velocity (cm/s).
Discussion
Our simulations show that transitional or chaotic flow can easily occur in arterial conduits despite the flow conditions being well below the classical value of 2000 for the Reynolds number for laminar-to-turbulent transition in steady pipe flow [29]. There are two primary reasons for the early transition from laminar flow in this study: rapidly changing lumen cross section and flow pulsatility. In the patched region, the lumen cross section expands by nearly a factor of three. The flow must slow down approximately by a factor of three in order to satisfy conservation of mass giving rise to an adverse pressure gradient due to the Venturi effect (whereby the flow pressure downstream increases as the flow decelerates). The adverse pressure gradient “pushes back” onto the fluid near the vessel wall as it decelerates through the expansion. If the fluid’s inertia is not strong enough, the fluid can no longer move forward in a streamlined fashion and separation occurs [29]. The flow deceleration after peak systole enhances the adverse pressure gradient and therefore the incidence of separation. A third factor which alters the threshold for laminar transition is the curvature of the blood vessel. Vessel curvature can also enhance adverse pressure gradients and induce secondary flows.
The altered hemodynamics occurring in the region of flow separation creates localized low wall shear stress (<0.5 Pa) which is particularly pronounced at the proximal region of the patch at 1 month after revision. In subsequent scans, a significant stenosis develops at a location coincident with a localized flow transition. This is in agreement with the widespread hypothesis [9] that there is a feedback loop whereby localized flow instabilities induce altered hemodynamic stresses (such as low shear stress below 0.5Pa) and subsequently lead to endothelial dysfunction, misregulation of smooth muscle cells and fibroblasts, and ultimately intimal hyperplasia. This, in turn, might enhance the development of stenosis which feeds back into the flow instability [10, 14, 15].
Furthermore, we propose that there exists an optimal graft diameter following patch angioplasty that would maximize the likelihood of long term patency. The graft diameter should be large enough to ensure sufficient blood flow to the distal tissue but not so large such that flow separation and recirculation occur, which could accelerate intimal hyperplasia. Specification of the optimal diameter for a given patient-specific configuration is a topic for further study.
Certainly additional factors beyond hemodynamics are related to the generation of stenoses. We hypothesize that physiological factors such as a localized loss of endothelial function, pre-existing venous lesions, or endothelial denudation due to surgical injury are coadjuvant to the effect of low shear on intimal hyperplasia. Furthermore, it has long been speculated that the injury sustained during the surgery or the repeated stretching of the graft due to the compliance mismatch between the autogenous graft and the PTFE induces a proliferative cellular response in the intima [13]. However, it has also long been speculated that the endothelium mitigates the proliferative response when subjected to normal values of wall shear stress by releasing nitric oxide or prostacyclin[9,12]. Therefore, we conjecture that altered hemodynamic forces are likely one of many confluent factors influencing vein graft patency. The focal nature of vein graft stenosis may be explained by the co-localization of vessel injury and non-laminar flow shear stresses.
We found a post-stenotic dilatation in the graft region distal to the stenosis. We hypothesize that the jet formed at the stenosis transitions to turbulence and impinges on the graft wall inducing high frequency fluctuations in the mechanical stresses that elicit the dilatory response. Our simulations do not calculate wall stretch, but the high frequency flow fluctuations would induce bruits corresponding to the frequencies of flow fluctuations(50–200Hz). We speculate that both mechanical stretch due to pressure fluctuations, as well as flow shear stress, which is assumed to reduce muscular tone via NO [9], initiate a response in endothelial cells that stimulates the vessel dilation. However, future studies are needed to differentiate the role of stretch vs. shear in post-stenotic dilatation.
We acknowledge several limitations to this study. First, histological analysis of the failed vein graft segment was not conducted, as it was eventually bypassed by an interposition graft and not excised. Since it is impossible to know the distribution and extent of intimal hyperplasia within the graft, a quantitative correlation between low shear and intimal thickness cannot be made. Second, this case study involves only one patient. We can show an association between transitional flow and remodeling, but we cannot prove causality. Further case-control studies will be needed to validate this hypothesis. Third, more frequent monitoring of grafts in conjunction with hemodynamic simulations, for example at one month intervals during the first three months post-surgery when the wall remodeling occurs at its fastest rate [6, 11], are necessary to further elucidate the feedback process between changes in flow and lumen cross-section.
Conclusions
This study presents the application of ultrasound image-based computational modeling to the study of peripheral artery graft remodeling and failure. Simulations in a failing peripheral artery bypass graft allow us to quantify the hemodynamic stresses and to illustrate the interplay between flow and remodeling, and can serve as a compliment to standard in vivo ultrasound surveillance. The availability, ease of use, and temporal resolution of ultrasound provide it with certain advantages over other imaging modalities for biomechanics simulations. Computational simulations, coupled with longitudinal patient scans, have the potential to reveal complex hemodynamic features, their role in determining long-term morphological changes and, more importantly, clinical outcomes.
Acknowledgments
This work has been supported by an R21 grant from NIDDK (DK08-1823)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50(1):54–60. doi: 10.1016/j.jvs.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 2.Mills JL, Bandyk DF, Gahtan V, Esses GE. The origin of infrainguinal vein graft stenoses: A prospective study based on duplex surveillance. J Vasc Surg. 1995;21(1):16–25. doi: 10.1016/s0741-5214(95)70240-7. [DOI] [PubMed] [Google Scholar]
- 3.Watson HR, Buth J, Schroeder TV, Simms MH, Horrocks M. Incidence of stenosis in femorodistal bypass vein grafts in a mulicentre study. Eur J Vasc Endovasc Surg. 2000;20:67–71. doi: 10.1053/ejvs.2000.1135. [DOI] [PubMed] [Google Scholar]
- 4.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of prevent III: A multicenter, randomized trial of edifolidge for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43(4):742–751. doi: 10.1016/j.jvs.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 5.Fillinger MF, Cronenwett JL, Besso S, Walsh DB, Zwolak RM. Vein adaption to the hemodynamic environment of infrainguinal grafts. J Vasc Surg. 1994;19:970–978. doi: 10.1016/s0741-5214(94)70208-x. [DOI] [PubMed] [Google Scholar]
- 6.Owens CD, Wake N, Jacot JG, Gerhard-Herman M, Gaccionce P, Belkin M, et al. Early biomechanical changes in lower extremity vein grafts-distinct temporal phases of remodeling and wall stiffness. J Vasc Surg. 2006;44(4):740–746. doi: 10.1016/j.jvs.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Dobrin PB. Mechanical factors associated with the development of intimal and medial thickening in vein grafts subjected to arterial pressure. Hypertension. 1995;26:38–43. doi: 10.1161/01.hyp.26.1.38. [DOI] [PubMed] [Google Scholar]
- 8.Galt SW, Zwolak RM, Wagner RJ, Gillbertson JJ. Differential response of arteries and vein grafts to blood flow reduction. J Vasc Surg. 1993;17(3):563–570. [PubMed] [Google Scholar]
- 9.Owens CD. Adaptive changes in autogenous vein grafts for arterial reconstruction: Clinical implications. J Vasc Surg. 2010;51(3):736–746. doi: 10.1016/j.jvs.2009.07.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karla M, Miller VM. Early remodeling of saphenous vein grafts: Proliferation, migration, and apoptosis of adventitial and medial cells occur simultaneously with changes in graft diameter and blood flow. J Vasc Res. 2000;37:576–584. doi: 10.1159/000054091. [DOI] [PubMed] [Google Scholar]
- 11.Leotta DF, Primozich JF, Beach KW, Bergelin RO, Zierler RE, Strandness DE., Jr Remodeling in peripheral vein graft revisions: Serial study with three-dimensional ultrasound imaging. J Vasc Surg. 2003;37(4):798–807. doi: 10.1067/mva.2003.137. [DOI] [PubMed] [Google Scholar]
- 12.Kohler TR, Kirkman TR, Kraiss LW, Zierler BK, Clowes AW. Increased blood flow inhibits neointimal hyperplasia in endothelialized vascular grafts. Circulation Research. 1991;69:1557–1565. doi: 10.1161/01.res.69.6.1557. [DOI] [PubMed] [Google Scholar]
- 13.Bassiouny HS, White S, Glagov S, Choi E, Giddens DP, Zarins CK. Anastomotic intimal hyperplasia: Mechanical injury or flow induced. J Vasc Surg. 1992;15(4):708–717. doi: 10.1067/mva.1992.33849. [DOI] [PubMed] [Google Scholar]
- 14.Meyerson SL, Skelly CL, Curi MA, Shakur UM, Vosicky JE, Glagov S, et al. The effects of extremely low shear stress on cellular proliferation and neointimal thickening in the failing bypass graft. J Vasc Surg. 2001;34(1):90–97. doi: 10.1067/mva.2001.114819. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Z, Wu L, Miller BL, Goldman DR, Fernandez CM, Abouhamze ZS, et al. A novel vein graft model: Adaption to differential flow environments. Am J Physiol: Heart Circ Physiol. 2003;286:240–245. doi: 10.1152/ajpheart.00760.2003. [DOI] [PubMed] [Google Scholar]
- 16.Lei M, Archie JP, Kleinsteuer C. Computational design of a bypass graft that minimizes wall shear stress gradients in the region of the distal anastomosis. J Vasc Surg. 1997;25(4):637–646. doi: 10.1016/s0741-5214(97)70289-1. [DOI] [PubMed] [Google Scholar]
- 17.Lei M, Giddens DP, Jones SA, Loth F, Bassiouny HS. Pulsatile flow in and end-to-side vascular graft model: Comparison of computations with experimental data. J Biomech Engr. 2001;123:80–87. doi: 10.1115/1.1336145. [DOI] [PubMed] [Google Scholar]
- 18.Walsh MT, Kavanagh EG, O’Brien T, Grace PA, McGloughlin T. On the existence of an optimum end-to-side junctional geometry in peripheral bypass surgery- a computer generated study. Eur J Vasc Endovasc Surg. 2003;26:649–656. doi: 10.1016/j.ejvs.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien TP, Grace P, Walsh M, Burke P, McGloughlin T. Computational investigations of a new prosthetic femoral-popliteal bypass graft design. J Vasc Surg. 2005;42:1169–1175. doi: 10.1016/j.jvs.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Pousset Y, Lermusiaux P, Berton G, Gouez JL, Leroy R. Numerical model study of flow dynamics through an end-to-side anastomosis: Choice of anastamosis angle and prosthesis diameter. Ann Vasc Surg. 2006;20:773–779. doi: 10.1007/s10016-006-9125-9. [DOI] [PubMed] [Google Scholar]
- 21.Moore JA, Steinman DA, Prakash S, Johnston KW, Ethier CR. A numerical study of blood flow patterns in anatomically realistic and simplified end-to-side anastomoses. J Biomech Engr. 1999;121:265–272. doi: 10.1115/1.2798319. [DOI] [PubMed] [Google Scholar]
- 22.Giordana S, Sherwin SJ, Peiro J, Doorly DJ, Crane JS, Lee KE, et al. Local and global geometric influence on steady flow in distal anastomoses of peripheral bypass grafts. J Biomech Engr. 2005;127:1087–1098. doi: 10.1115/1.2073507. [DOI] [PubMed] [Google Scholar]
- 23.Jackson M, Wood NB, Zhao S, Augst A, Wolfe JH, Gedroyc WM, et al. Low wall shear stress predicts subsequent development of wall hypertrophy in lower limb bypass grafts. Artery Research. 2009;3:32–38. doi: 10.1016/j.artres.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGah PM, Leotta DF, Beach KW, Riley JJ, Aliseda A. A longitudinal study of remodeling in a revised peripheral artery bypass graft using 3D ultrasound and computational hemodynamics. J Biomech Engr. 2011;133(4):041008. doi: 10.1115/1.4003622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loth F, Fischer PF, Bassiouny HS. Blood flow in end-to-side anastomoses. Ann Rev Fluid Mech. 2008;40:367–393. [Google Scholar]
- 26.Leotta DF, Primozich JF, Beach KW, Bergelin RO, Strandness DE., Jr Serial measurement of cross-sectional area in peripheral vein grafts using three-dimensional ultrasound. Ultrasound Med Biol. 2001;27(1):61–68. doi: 10.1016/s0301-5629(00)00296-9. [DOI] [PubMed] [Google Scholar]
- 27.Zierler BK, Kirkman TR, Kraiss LW, Reiss WF, Horn JR, Bauer LA, et al. Accuracy of duplex scanning for measurement of arterial volume flow. J Vasc Surg. 1992;16(4):520–526. [PubMed] [Google Scholar]
- 28.Womersley JR. Method for the calculation of velocity, rate of flow and viscous drag in arteries when the pressure gradient is known. J Physiology. 1955;127:553–563. doi: 10.1113/jphysiol.1955.sp005276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kundu PK, Cohen IM. Fluid Mechanics. 3. Elsevier Academic Press; San Diego: 2000. [Google Scholar]