Abstract
Background
Few data exist in Latin America concerning the association between organophosphate (OP) urinary metabolites and the consumption of fruits and vegetables and other exposure risk variables in schoolchildren.
Methods
We collected samples of urine from 190 Chilean children aged 6-12 years, fruits and vegetables, water and soil from schools and homes, and sociodemographic data through a questionnaire. We measured urinary dialkylphosphate (DAP) OP metabolites and OP pesticide residues in food consumed by these 190 children during two seasons: December 2010 (summer) and May 2011(fall). We analyzed the relationship between urinary DAP concentrations and pesticide residues in food, home pesticide use, and residential location.
Results
Diethylalkylphosphates (DEAP) and dimethylalkylphosphates (DMAP) were detected in urine in 76% and 27% of samples, respectively. Factors associated with urinary DEAP included chlorpyrifos in consumed fruits (p<0.0001), urinary creatinine (p<0.0001), rural residence (p=0.02) and age less than 9 years (p=0.004). Factors associated with urinary DMAP included the presence of phosmet residues in fruits (p<0.0001), close proximity to a farm (p=0.002), home fenitrothion use (p=0.009), and season (p<0.0001).
Conclusions
Urinary DAP levels in Chilean school children were high compared previously reported studies. The presence of chlorpyrifos and phosmet residues in fruits was the major factor predicting urinary DAP metabolite concentrations in children.
Keywords: Dialkylphosphate metabolites, organophosphate pesticides residues, schoolchildren
Introduction
Organophosphates (OP) are a group of synthetic pesticides used to control various insects on crops and in homes (Levine, 2007; Tadeo et al, 2008), and are widely used in agriculture. These pesticides are highly toxic cholinesterase-inhibiting compounds, but tend to degrade in the environment with exposure to sunlight and water. The lack of knowledge about how OPs should be applied and poor monitoring of their use have been associated with various human health problems, and environmental pollution by OP residues has accumulated over time (CDC, 2009; Levine, 2007; MINSAL, 2007; WHO, 2005).
There is ample evidence of adverse health outcomes associated with OP pesticide exposure in children and adults (Alavanja et al, 2004; Jurewicz & Hanke, 2008, 2006; Rosas & Eskenazi, 2008). Studies have linked OP pesticide exposure with adverse physiologic effects, increased frequency of cancer, neurobehavioral and cognitive abnormalities, teratogenicity, endocrine modulation and immunotoxicity (Alavanja et al, 2004; Bouchard et al, 2011; Bouchard et al, 2010; Engel et al, 2011; Handal et al, 2007; Jurewicz & Hanke, 2008, 2006; Marks et al, 2010; Rauth et al, 2006; Rosas & Eskenazi, 2008).
Human exposure to OP insecticides is widespread. Occupational use of OP insecticides, primarily in agriculture, represents the largest class of exposure. However, many populations, including children, have been shown to be widely exposed to OP insecticides from their diet, residential use, by living close to farms and from paraoccupational exposures via parents who work in agriculture (Barr et al, 2004; Bradman et al, 2005; Coronado et al, 2006; Kissel et al, 2005; Koch et al, 2002; Lu et al, 2008; Lu et al, 2004; Naeher et al 2010; Valcke et al, 2006; Vida & Moretto, 2007).
In exposed populations, OP insecticides tend not to persist for extensive periods of time, with half-lives not exceeding one week in the human body (CDC, 2009; MINSAL, 2007). Therefore, short-term OP pesticide exposure has often been assessed by measuring urinary metabolites of OP pesticides (Egeghy et al., 2011). Most biomonitoring studies have focused on measuring six dialkylphosphate (DAP) metabolites that are common to most OP insecticides currently in use (Wessels et al., 2003). Other more selective metabolites for assessing exposure to specific OP insecticides have also been used; however, they provide information for only a limited number of OP pesticides (Wessels et al., 2003). Although there are limitations associated with measuring these six non-specific metabolites (e.g., exposure to preformed metabolites and exposure to multiple OPs giving rise to the DAP), they have been useful tools in estimating exposure to OP insecticides as a class.
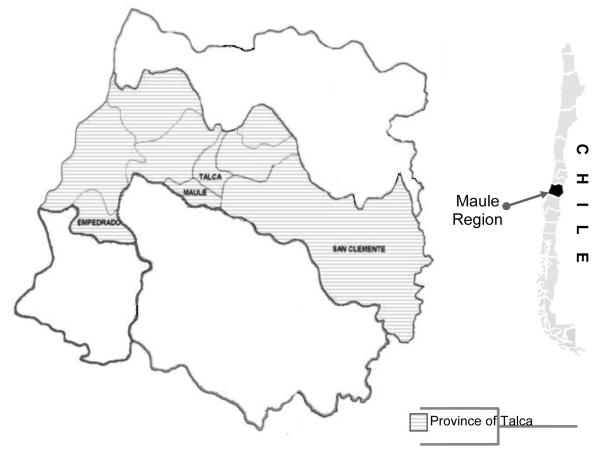
To date, almost no research has been conducted in South America investigating pesticide exposure and potential predictors of these exposures. Chile, a country located on the western coastline of South America, is a large producer of agricultural crops. The region that has the highest percentage of rural population engaged in agriculture and livestock husbandry is the Maule Region, with 34% of people living in rural areas (INE, 2002). The Maule Region, is located 254 kilometers south of capital Santiago in central Chile, and is divided into 4 Provinces: Talca, Linares, Curico and Cauquenes. The Province of Talca, has 10 counties. 4 counties were included in this research: San Clemente, Talca, Maule and Empedrado (see Figure 1). According to 2008 sales data, Maule used over 5 million kg of pesticides, making it the third highest pesticide-consuming Region in Chile, representing about 10% of all national sales (SAG, 2008). OP pesticides comprise 30% of all insecticide, acaricide and rodenticide use in Maule, surpassing the national rate (25.5%). The widespread use of OP insecticides in agriculture, their largely unrestricted sale, and insufficient knowledge of their proper application and risks has resulted in acute intoxications in Chile (Concha, 2010; MINSAL, 2010a, 2007), primarily in the occupational setting.
Figure 1.
Map of Chile with the Maule Region enlarged shows the participating counties from the Province of Talca. The shaded areas indicate the Talca Province and the individual participating counties are labeled.
We have conducted a pesticide exposure assessment study involving children in urban and rural Regions in Talca Province, Chile. We used biological and environmental monitoring coupled with questionnaire data, as has been done in others studies (Melnyck, 2012), to help us ascertain the predominant pathways of exposures to children in these areas. To date, no data exist on OP pesticide exposures in Chile and only a few exist in Latin America, but none that have examined several potential pathways for exposure in the general population.
Materials and Methods
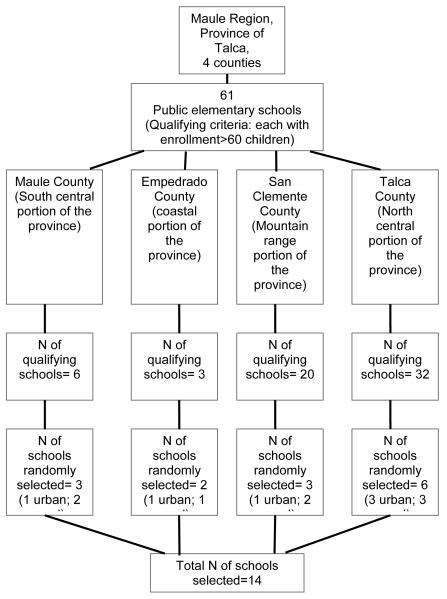
We collected longitudinal data during two seasons (summer and fall) from children living in four counties (Empedrado, Talca, Maule, and San Clemente) in Talca Province, in south central Chile. These counties were selected to represent a range of geographic locations and demographic conditions. Within these counties, 14 public elementary schools with enrollment ≥ 60 students were selected through a stratified random sampling methodology assuring both rural and urban schools were represented in each county (see Figure 2). Children from these schools were randomly selected for enrollment in the study. From a total of 2254 children attending the selected schools in Talca County, 180 (8%) were invited to a meeting to inform them about the study. We invited similar percentages of schoolchildren to participate from the remaining three counties: in Empedrado, 60 of 517 children (8%); in Maule, 90 of 918 children (10%); in San Clemente, 75 of 592 children (12%). We invited a total of 405 parents to meetings in the schools to explain the study and request for the informed consent. Two hundred and fourteen parents finally attended the meetings and 100% agreed to participate. This study was approved by the ethics committee on human research of the Faculty of Medicine at the University of Chile, the ethics committee of the Catholic University of Maule, and the Institutional Review Board of Emory University. Informed consent was obtained from the parent of each minor child enrolled.
Figure 2.
Random sampling method of selection of schools from the Province of Talca
Data collection occurred during two seasons: once in December 2010 (summer) during the peak agricultural season and once in May 2011 (fall), the low agricultural production season. Questionnaires were administered during each season to collect information on general sociodemographic characteristics, proximity of residence to agricultural fields, home use of pesticides, and consumption of fresh fruit and vegetables over the prior four days. Additionally, in the fall, questions were included about the purveyor of the produce since no locally grown produce was available for consumption.
In Chile, free meals are given to children of low-income public schools; all schools in this study were low-income schools. In general, children consumed raw fruit supplied to them at school, usually one or two pieces of fruit per week. During the peak agricultural season of summer in Chile (December), we collected 2 kg of produce (orange or apples, based on the survey about what the children consumed) from the schools on Monday of the sampling week.
From the questionnaire information obtained in the summer, we found that children also ate produce at their homes on weekends. Therefore, during the fall (May) sampling, we identified the food suppliers through questionnaires (primarily a large market in Talca Province), and we collected 2 kg of produce (including either kiwi, lettuce, apple, orange, tangerine, pear, cabbage or tomatoes) from those suppliers on Tuesday of the sampling week and also 2 kg from the school on Monday of the sampling week. All produce was bagged and refrigerated at 4°C, then transported by land for analysis the day after collection by the Laboratory Andes Control in Santiago, Chile. This laboratory operated under ISO/IEC 17025 accreditation. Both positive and negative controls were included in the analytic scheme. Sampling requirements for accurate measurement were that residues had to be measured in 2 kg of produce collected randomly, kept in good physical condition, avoiding exposure to high temperatures and sent for analysis within two days of collection.
More than 200 pesticides, including about 40 OP insecticides, were quantified in produce samples using the QuEChERS method for pesticide extraction (Lehotay et al, 2005; Schenck & Hobbs, 2004). Briefly, produce samples were homogenized separately in a blender then a 15-g aliquot was taken and spiked with surrogate internal standards. The produce was extracted with acetonitrile and dried with MgSO4 to remove residual water. The extract was cleaned using dispersive primary secondary amine sorbent along with anhydrous MgSO4. The extracts were concentrated and analyzed by gas chromatography-mass spectrometry. The limits of detection (LOD) for the pesticides were 0.01 mg/kg produce.
In addition, 2-L samples of drinking water that children consumed at schools and homes, and 2-kg samples of soil from the schools and public places within 500 m of the children’s homes were collected during both easons and were analyzed at the Laboratory Andes Control in Santiago, Chile, following the procedures described above for food analysis.
Spot urine samples were collected from each child in 100-mL sterile prepackaged bottles on the Tuesday of each sampling week during both December and May. Samples were kept frozen in a plastic container at −20°C, and shipped to Pacific Toxicology Laboratories (Los Angeles, California, USA) for analysis of the six DAP metabolites of most OP insecticides: dimethylphosphate (DMP); dimethylthiophosphate (DMTP); dimethyldithiophosphate (DMDTP); diethylphosphate (DEP); diethylthiophosphate (DETP); and diethyldithiophosphate (DEDTP). Freeze-dried urine samples were derivatized with a benzyltolytriazine reagent to produce benzyl derivatives of alkylphosphate metabolites. A saturated salt solution was added to the tubes and the benzyl derivatives were extracted with cyclohexane and analyzed by gas chromatography with flame photometric detection. The quality control sample was made in-house by spiking a urine sample with two levels of OP metabolites. The assay was run with a reagent water blank and a urine blank. The recovery rate ranged from 80 to 120% of the expected value. The LOD of the method was 5 ng/mL for all metabolites except DEDTP and DMDTP, which had an LOD of 10 ng/mL. To assess the precision of the method, 20 duplicate analyses of unknown urine samples were performed. Further quality assurance measures included participation in proficiency testing of the German E-QUAS program.
Creatinine was measured in the urine samples by a colorimetric method (Creatinine Procedure No 555; Sigma Diagnostics, St Louis, Mo). Its measurement was used to adjust results of OP metabolites (μg metabolite / g creatinine) to avoid the variable dilution caused by differing hydration states of the sample donor.
STATA 11.0 (StataCorp LP, College Station, TX) was used for all statistical analyses. Data from both December and May were combined in the analysis, and Generalized Estimated Equations (GEE) were used to account for the repeated measures on the same children during two seasons. DAP concentrations below the LOD were assigned a value equal to the LOD/√ 2 (Hornung & Reed, 1990). Concentrations were converted to SI units to create molar concentrations and all DEAP and DMAP metabolites were summed to create an aggregate exposure term (i.e., ΣDEAP and ΣDMAP) (Barr et al, 2004).
The frequency of detection (FOD) of at least one DEAP metabolites among children was 80%. The sum of DEAP metabolites was treated as a continuous variable after logarithmic transformation (the data were normalized by transformation, and data were considered normal by applying a studentized residual analysis). This variable was used as the outcome in a linear regression using GEE. The FOD of DMAP metabolites was much lower (25%) for any DMAP metabolite, and hence for the sum. Thus the sum of DMAP metabolites was dichotomized into two categories (i.e., below the LOD, above the LOD), and a logistic regression GEE was performed.
We first tested full models including all possible exposure variables and demographic covariates. Variables assessed for possible inclusion in the models were: pesticides in measured produce (present, not present); proximity of residence to farm (≤500 m, > 500 m); home use of fenitrothion insecticide (yes, no); location (urban, rural) according to the Chilean Census (26); season (summer -- December 2010, fall -- May 2011); creatinine concentration (continuous); county (Talca, Empedrado, Maule, and San Clemente); parental occupation (agriculture, non-agriculture); parental education (≤ 8 years of study, > 8 years of study); age (continuous); and sex (male, female). All variables with a p-value ≤ 0.10 in the full model were retained in final models.
Results
Reliability testing to ensure precise urinary DAP measurement demonstrated good agreement among duplicate samples. Among the 20 duplicate pairs tested, a high correlation was found between duplicates of DEP metabolites (r = 0.99, p < 0.0001), DETP (r = 0.97, p < 0.0001), DMP (r = 0.86, p < 0.0001). DMDTP and DEDTP were consistently below the LOD so could not be evaluated.
Of the 214 children initially enrolled in the study, the mean age was 8.6 ± 1.7 years with almost equal numbers of males (N=109) and females (N=105). Twenty-four children were withdrawn from the study because they could not provide a urine sample (N=22) or a urine sample was of insufficient volume for laboratory analysis (N=2). The final sample size for the summer season (December 2010) was 190 children with a mean age of 8.6 ± 1.6 years. For the fall season (May 2011), we evaluated 181 children, since 9 children were lost to follow up, six moved residences and three declined to participate. The demographic characteristics of the participating children are shown in Table 1.
Table 1.
Sociodemographic and exposure characteristics of 190 schoolchildren (aged 6-12 years) in Talca Province, Chile in December 2010 (summer) and May 2011 (fall).
| Variable | December 2010 | May 2011 | p value | ||
|---|---|---|---|---|---|
|
|
|||||
| N | % | N | % | ||
| County | 1 | ||||
| - Empedrado | 28 | 14.7 | 28 | 15.5 | |
| - Talca | 68 | 35.8 | 64 | 35.4 | |
| - San Clemente | 53 | 27.9 | 49 | 27.0 | |
| - Maule | 41 | 21.6 | 40 | 22.1 | |
| Location | 0.98 | ||||
| - Urban | 69 | 36.3 | 66 | 36.5 | |
| - Rural | 121 | 63.7 | 115 | 63.5 | |
| Gender | 0.79 | ||||
| - Female | 94 | 49.5 | 92 | 50.8 | |
| - Male | 96 | 50.5 | 89 | 49.2 | |
| Age of children | 0.001a | ||||
| - 6 - 8 years old. | 89 | 46.8 | 46 | 25.4 | |
| - 9 -12 years old. | 101 | 53.2 | 135 | 74.6 | |
| Father's years of education | 0.91 | ||||
| - <8 years. | 55 | 32.2 | 53 | 32.7 | |
| - ≥8 years. | 116 | 67.8 | 109 | 67.3 | |
| Mother's years of education | 0.95 | ||||
| - < 8 years. | 53 | 27.9 | 50 | 27.6 | |
| - ≥8 years. | 137 | 72.1 | 131 | 72.4 | |
| Father's occupation | 0.33 | ||||
| - Farmworker | 84 | 44.2 | 71 | 39.2 | |
| - Non-farmworker | 106 | 55.8 | 110 | 60.8 | |
| Mother’s occupation | 0.047b | ||||
| - Farmworker | 31 | 16.3 | 17 | 9.4 | |
| - Non-farmworker | 159 | 83.7 | 164 | 90.6 | |
| Distance of housing to farm | 0.94 | ||||
| - ≤ 500m | 111 | 58.4 | 105 | 58.0 | |
| - > 500m | 79 | 41.6 | 76 | 42.0 | |
| Use of OP pesticide fenitrothion at home | 0.20 | ||||
| - Yes | 17 | 9.0 | 10 | 5.5 | |
| - No | 171 | 91.0 | 171 | 94.5 | |
The significant change in this sociodemographic variable is expectable since the children aged from one time point to the next in data collection.
The mother’s occupation likely achieves significance because more women typically work summer seasonal farmworker jobs in Chile.
The majority of the demographic data remained consistent across seasons. Parental occupation changed in 14% of fathers and 18% of the mothers between the two seasons. More than half (58%) of the children lived within 500 m of a farm and less than 10% of the families used fenitrothion in the home.
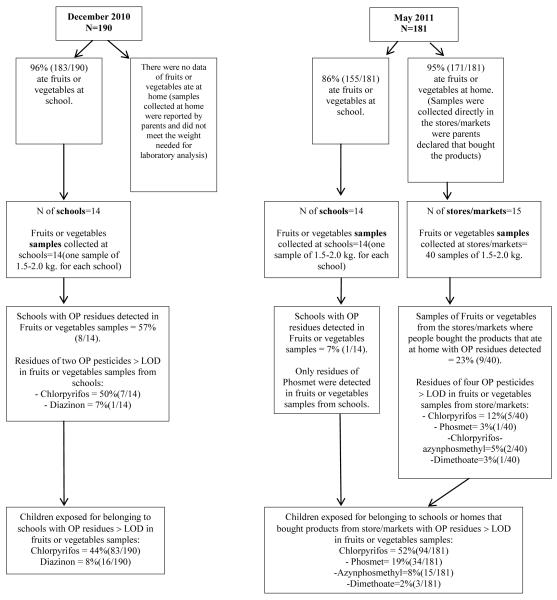
Figure 3 depicts the pesticide residues above the LOD in fruits or vegetables and the number of children exposed to these residues. OP pesticide residues were detected in produce samples taken during both summer and fall. Chlorpyrifos and diazinon were detected in 50% (n=7) and 7% (n=1), respectively, of 14 produce samples collected in December 2010. Based on the number of children who consumed produce in each school with detectable residues, 44% (n=83) were exposed to chlorpyrifos residues and 8% (n=16) were exposed to diazinon residues during the summer of 2010. Of the produce collected in May 2011, only phosmet was detected in 7% (n=1) of 14 samples collected from school. Of the 40 samples collected from the local markets, chlorpyrifos was detected in 12 % of the samples (n=5), azinphos methyl was detected in 5% of the samples (n=2); phosmet was detected in 8% of the samples (n=3) and dimethoate was detected in 3% of the samples (n=1). Multiple residues (i.e., chlorpyrifos and azinphos methyl) were detected in 5% of the samples tested (n=2). During the fall of 2011, 52% of the children (n=94) were exposed to chlorpyrifos residues, 19% of the children (n=34) were exposed to phosmet residues, 8% of the children (n=15) were exposed to azinphos methyl residues and 2% of the children (n=3) were exposed to dimethoate.
Figure 3.
Diagram of the results of OP residues on vegetables/fruits in summer (December 2010) and fall (May 2011) samplings, depicting the number of children that ate produce and the samples of food taken, the pesticide residues above the LOD on produce and the number of children estimated to be exposed to these residues.
Soil and drinking water were tested for the same OP pesticides residues. No pesticide residues were detected in any of the 36 drinking water or 28 soil samples taken.
Urinary DAP metabolite data are shown in Table 2. DEAP metabolites concentrations above the LOD were found in a greater proportion of children than those observed for DMAP metabolites both in December 2010 (72,6% versus 31,6%) and in May 2011 (80% versus 18,6%).
Table 2.
Descriptive statistics of five organophosphate metabolites (OP) in the urine of schoolchildren (geometric mean, minimum and maximum values of metabolites concentration, and percentage of children above the limit of detection (LOD) for December 2010(summer) and May 2011(fall).
| Metabolite | December 2010 (summer) | May 2011 (fall) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Geometric Mean and geometric SD |
Min – max | % > LOD |
Geometric Mean and geometric SD |
Min – max | % > LOD |
|
| Creatinine g/L | 1.07 (1.03) | 0.26 – 2.70 | 100 | 0.96 (1.04) | 0.12 – 2.70 | 100 |
| DEP μg/L | 9.1(1.10) | 3.5 – 417.1 | 40.5 | 19.5(1.09) | 3.5 – 383.8 | 80.1 |
| DEP μg/g cr | 8.7(1.09) | 3.5 – 268.0 | 18.6(1.09) | 3.5 – 237.0 | ||
| DEP nmol/L* | 59.3(1.10) | 22.9 –2708.4 | 126.4(1.09) | 0.0 – 2492.0 | ||
| DETP μg/L | 8.3 (1.09) | 3.5 – 691.4 | 43.2 | 3.7 (1.01) | 3. 5 – 25.1 | 2.8 |
| DETP μg/g cr | 7.7 (1.08) | 3.5 – 843.0 | 3.7 (1.01) | 3. 5 – 24.0 | ||
| DETP nmol/L* | 48.9 (1.09) | 20.8 – 4067.1 | 21.8 (1.02) | 0. 0 – 147.6 | ||
| SUM DEAP nmol/L | 155.8 (1.08) | 43.8 - 4090.5 | 72.6 | 160.7 (1.07) | 43.8 - 2609.0 | 80.0 |
| DMP μg/L | 5.2 (1.06) | 3.5 – 115.8 | 20.5 | 4.5 (1.05) | 3.5 – 72.6 | 14.9 |
| DMP μg/g cr | 5.1 (1.06) | 3.5 – 146.0 | 4.4 (1.05) | 3.5 – 74.0 | ||
| DMP nmol/L* | 41.3 (1.06) | 28.1 – 919.1 | 36.0 (1.05) | 0.0 – 576.2 | ||
| DMTP μg/L | 5.5 (1.06) | 3.5 – 94.6 | 25.8 | 4.5 (1.05) | 3.5 – 139.2 | 13.8 |
| DMTP μg/g cr | 5.3 (1.06) | 3.5 – 121.0 | 4.4 (1.06) | 3.5 – 120.0 | ||
| DMTP nmol/L* | 38.8 (1.06) | 24.9 – 666.2 | 32.0 (1.06) | 0.0 – 980.3 | ||
| DMDTP μg/L | 8.9 (1.04) | 7.1 – 146.4 | 16.3 | 7.1 (1.0) | 7.1 – 7.1 | 0.0 |
| DMDTP μg/g cr | 8.7 (1.04) | 7.1 – 126.0 | 7.1 (1.0) | 7.1 – 7.1 | ||
| DMDTP nmol/L* | 56.1 (1.04) | 44.8 – 926.6 | 44.75 (1.0) | 0.0 – 44.8 | ||
| SUM DMAP nmol/L | 147.7 (1.10) | 97.8-1662.5 | 31.6 | 120.5 (1.08) | 97.7-1195.7 | 18.6 |
DEP = diethylphosphate; DETP = diethylthiophosphate; DEAP = diethyl alkylphosphates; DMP = dimethylphosphate; DMTP =dimethyldithiophosphate; DMDTP = dimethyldithiophosphate; DMAP = dimethylalkylphosphates; LOD = limit of detection
*Samples below the LOD were defined as LOD///2
Table 3 shows the final GEE model for the sum of DEAP metabolites. According to the model, children who ate fruits or vegetables (at school or at home) with residues of chlorpyrifos above the LOD (either in December or May) had 2.8 (=exp1.03) times higher levels of DEAP metabolites than children who did not eat produce with chlorpyrifos residues above the LOD. Children with higher creatinine, children living in urban areas, children living in Talca County, and younger children had significantly higher levels of DEAP metabolites. Children who lived < 500 m from a farm also had higher levels of DEAP metabolites, but this variable showed only marginal significance (p = 0.074).
Table 3.
Linear regression model (GEE) for the logarithmic transformation of the sum of Diethyl-substituted metabolites (DEAP) in the urine of schoolchildren in Talca Province, Chile in December 2010 (summer) and May 2011 (fall) combined.
| DEAP* | Coef. | Std. Err. | P-value |
|---|---|---|---|
| Chlorpyrifos (a) | 1.03 | 0.088 | 0.000 |
| Creatinine (b) | 0.50 | 0.088 | 0.000 |
| Location (c) | -0.21 | 0.901 | 0.018 |
| Distance from farms (d) | 0.15 | 0.086 | 0.074 |
| Age (e) | -0.24 | 0.083 | 0.004 |
| Empedrado (f) | 0.00 | 0.135 | 0.984 |
| Talca (f) | 0.25 | 0.110 | 0.021 |
| San Clemente (f) | -0.04 | 0.118 | 0.712 |
| Constant | 4.1 | 0.170 | 0.000 |
n of observations=371; n of groups=190
Logarithmic transformation of the sum of DEAPs in schoolchildren urine.
Exposed =1, Unexposed=0, to chlorpyrifos residues in fruits/vegetables from either homes or schools.
Continuous measure of creatinine in urine of children (g/L).
Geographic location (urban=1, rural=0)
Distance of housing to farms that applied pesticides (Less than 500m=1, greater than 500 m=0).
Age of children categorized as 6 - 8 years old (=1) or 9 – 12 years old (=0).
County (reference = Maule)
After running the GEE model with covariates we noted that the values of the DEAP metabolites within the same child were not correlated with each other across time periods. Hence the GEE results were equivalent to a simple linear regression. Running such a regression with the variable log ΣDEAP resulted in R2 = 0.42. Chlorpyrifos residues were the biggest predictor, accounting alone for 33% of the variance. There was an interaction (p <.05) in the model with the variable season (summer/fall) for the covariate chlorpyrifos (β = 0.490, p = 0.008, 95% CI = 0.130 - 0.851). In reviewing the relationship of chlorpyrifos with the log of ΣDEAP separately for December 2010(summer) and May 2011(fall), we found a coefficient for chlorpyrifos of 0.56 in summer and 1.29 in fall, indicating that the presence of chlorpyrifos residues in produce had a more pronounced effect on DEAP metabolite levels for the second collection period, that is when we collected samples both schools and homes.
Table 4 shows the final model for the DMAP metabolites. According to the model, children who ate produce that had detectable levels of phosmet were 60.6 (95% CI: 19.2-191.4) times more likely to have detectable urinary DMAP concentrations than those who did not. Children who lived closer to farms were 2.5 times more likely to have higher urinary DMAP concentrations than those who did not. Children whose homes were treated with fenitrothion were 3.5 times more likely to have higher urinary DMAPs than those whose homes were not. Finally, children living in Empedrado were 2.4 times more likely to have higher urinary DMAP concentrations than children living in Maule. Among all variables in the model, phosmet residues in fruit was the strongest contributor to the overall likelihood.
Table 4.
Logistic regression (GEE) model of the variable sum of dimethyl-substituted metabolites (DMAP) below vs. above the limit of detection in the urine of schoolchildren in Talca Province Chile in December 2010 (summer) and May 2011 (fall).
| DMAP* | Coef. | Std. Error | Odds Ratio | [95% Conf. Interval] | p-value | |
|---|---|---|---|---|---|---|
| Phosmet (a) | 4.12 | 0.59 | 60.61 | 19.19 | 191.37 | <0.0001 |
| Distance from farms (b) | 0.9 | 0.29 | 2.48 | 1.38 | 4.45 | 0.002 |
| Pesticide at home(c) | 1.44 | 0.48 | 3.48 | 1.37 | 8.85 | 0.009 |
| Empedrado (d) | 0.87 | 0.42 | 2.37 | 1.04 | 5.39 | 0.04 |
| Talca (d) | −0.38 | 0.37 | 0.66 | 0.31 | 1.36 | 0.26 |
| San Clemente (d) | −0.45 | 0.41 | 0.64 | 0.29 | 1.41 | 0.27 |
| Time (e) | −2.16 | 0.43 | 0.12 | 0.05 | 0.27 | <0.0001 |
| Constant | 0.74 | 0.59 | ||||
n of observations=369; n of groups=190
Sum of Dimethyl-substituted metabolites (DMAP) in the urine of schoolchildren dichotomized as below vs. above the Limit of Detection.
Variable related with exposure/not exposure to eating of vegetables/fruits with phosmet residues,
Distance of housing to farms that apply pesticides (Less or equal to 500m).
Use of OP pesticide fenitrothion at home.
County (reference=Maule).
Summer =1 (December 2010) or fall=0 (May 2011)
Discussion
We sought to evaluate pesticide exposure in schoolchildren living in Talca Province, Chile and to understand the factors predictive of these exposures. We collected samples in May and December to understand seasonal variation in pesticide exposure.
We found that, of the variables evaluated, dietary exposure was the predominant contributor to OP metabolites in urine during both high and low agricultural seasons. Because no pesticide residues were detected in drinking water or soil, these were not believed to be pathways of potential OP pesticide exposure.
We found that the major route of exposure to OP metabolites that we evaluated in the schoolchildren was ingestion, both for the period of greatest local agricultural productivity (summer) and for the period of lowest local agricultural productivity (fall), most likely due to consumption of fruits containing residues of OP insecticides. The origin of the vegetables consumed was primarily from a large market in the city of Talca, which provides agricultural products for the whole Province of Talca. Produce from this large market is normally derived locally during the summer but is transferred from farms in northern Chile during the fall months. In the summer 70%, and in the fall 77% of children, consumed vegetables from that market.
Urinary DAP concentrations were compared with those in other studies reported in the literature (Table 5). Neither of the two studies evaluating urinary DAP concentrations in Latin America reported actual urinary DAP concentrations for the populations studied; only frequencies of detection were given. The study of farm children in El Salvador had considerably lower frequency of detection than in our study but that is likely because of their higher LODs in this older work. The study by Grandjean et al (2006) in Ecuador had higher frequencies of detection than most metabolites in our study, however, we cannot directly compare the magnitude of concentrations. Participants in this study had levels of urinary metabolites higher than those reporting actual metabolites concentrations in other research studies conducted in the United States. The laboratory which analyzed the urine samples have relatively higher LOD than the other studies mentioned in Table 5, which could explain a slight increase in the geometric means of the metabolites in this study compared with others, but these LOD differences are not greater than 5 μg/L for DEAP and 8 μg/L for DMAP. Despite this consideration, OP metabolite concentrations in Chilean children are still much higher than American children studied previously.
Table 5.
Comparison of urinary concentrations of dialkylphosphates with the results of other similar studies.
| Diethylphosphate metabolitesa | Dimethylphosphate metabolites | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||
| No. subjects | >LODbDEP | >LODbDEPT | >LODb Sum DEAP | >LODb DMP | >LODb DMTP | >LODb DMDTP | >LODb Sum DMAP | |||||||||
| (Area) | Age | (%) | (nmol/l)c | (%) | (nmol/l)c | (%) | nmol/ld | (%) | (nmol/l)c | (%) | (nmol/l)c | (%) | (nmol/l)c | (%) | nmol/ld | |
| Current Chilean | ||||||||||||||||
| Study | ||||||||||||||||
| Dec 2010 samples | 190 | 6-11 | 40.5 | 59.3 | 43.2 | 48.9 | 72.6 | 155.8 | 20.5 | 41.3 | 25.8 | 38.8 | 16.3 | 56.0 | 31.6 | 147.7 |
| (spray month) | (Talca, Chile) | (49.1-75.5) | (41.4-57.6) | (133.4-182.0) | (36.6-46.5) | (34.5-43.7) | (51.6-60.8) | (133.3-163.6) | ||||||||
| May 2011 samples | 181 | 126.4 | 21.8 | 160.7 | 36.0 | 32.0 | 44.7 | 120.5 | ||||||||
| (nonspray month) | (Talca, Chile) | 7-12 | 80.1 | (107.1-149.2) | 2.8 | (20.9-22.7) | 80.0 | (140.1-184.4) | 14.9 | (32.9-39.4) | 13.8 | (28.8-35.6) | <LOD | (44.7-44.7) | 18.6 | (111.7-129.9) |
| Comparison studies | ||||||||||||||||
| Azaroff et al. 1999k | 136 (El Salvador, farm children) |
8-17 | -- | -- | -- | 14 | -- | -- | -- | -- | -- | -- | 35 | -- | ||
| Koch et al. 2002f(16) | 44 (Seattle) |
2-5 | - | - | 53.0 | - | 0.03(1.56)g | - | - | 73.0 | - | - | - | - | 0.080(2.51) g | |
| Barr et al. 2004 h(25) | 471 (US population) |
6-11 | - | 1.73 h (1.06-2.83) |
- | - | - | 17.4h (11.1-27-3) |
- | - | - | 3.08 h (1.9-4.9) |
- | - | - | 72.8 h (54.3-97.5) |
| Grandjean et al. 2006 k | 72 (Ecuador) |
5-9 | 82 | -- | 58 | -- | 89 | -- | 85 | -- | 17 | -- | 28 | -- | 85 | -- |
| Marks et al. 2010i, j(14) | 290 | 3.5 | - | - | - | - | - | 7.0 | - | - | - | - | - | - | - | 62.5 (52.5-74.7) |
| 320 (Salinas Valley, California) |
5 | - | - | - | - | - | 7.2 (6.0-8.7) |
- | - | - | - | - | - | - | 72.4 (61.0-86.0) |
|
| Bouchard et al. 2010i, j(15) | 1139 (US population) |
8-15 | 53.1 | 4.7 (0.9-28.1) |
57.2 | 2.0 (0.4-7.6) |
77.8 | 11.0 (2.1-35.0) |
49 | 10.7 (2.8-39.0) |
64.3 | 13.7 (1.9-58.8) |
41.7 | 1.7 (0.4-7.3) |
81.7 | 41.3 (10.1-130.7) |
DEP = diethylphosphate; DETP = diethylthiophosphate; DEAP = diethyl alkylphosphates; DMP = dimethylphosphate; DMTP =dimethyldithiophosphate; DMDTP = dimethyldithiophosphate; DMAP = dimethylalkylphosphates; LOD = limit of detection
Diethyldithiophosphate (DEDTP) are not presented in this table because its values were lower than the LOD in almost all the children.
Samples below the LOD were assigned a value of LOD/V2 (Hornung and Reed, 1990). The limit of detection was 10.0 μg/L for dimethyl dithiophosphate (DMDTP) and 5.0 μg/L for all other alkylphosphate metabolites measured.
Values correspond to geometric means and confidence interval(95%).
Diethyl and dimethyl phosphate metabolites were summed as nmol/l. Values correspond to geometric mean and confidence interval.
Double dash (−) indicates LOD % or analyte not reported.
LOD are 7.4(μg/l) for DMP, 1.1 for DMTP, 0.7 for DMDTP, 6.6 for DEP, 1.2 for DETP, and 1.1 for DEDTP.
Geometric mean and SD.
LOD were 0.58(μg/l) for DMP, 0.18(μg/l) for DMTP, 0.08 (μg/l) for DMDTP, 0.2 (μg/l) for DEP, 0.09 (μg/l) for DETP, and 0.05 (|μg/l) for DEDTP. Geometric means were adjusted for age, sex, race/ethnicity, and concentrations of serum cotinine and urinary creatinine. GMs were calculated for metabolites with detection frequencies of ≥ 60%.
Values for LOD of metabolites were not reported.
Neither of these two studies evaluating urinary DAP concentrations in Latin America reported actual urinary DAP concentrations for the populations studied; only frequencies of detection were given.
Phosmet residues were the biggest contributor to DMAPs metabolites. However, the association was found at only in May 2011(fall). In December 2010(summer), we did not find phosmet residues in fruits/vegetables measured in the school. However the fruit eaten at home was not measured in the summer, which is a limitation of the study.
The presence of DEAP metabolites in the urine of 80% of the schoolchildren is associated with dietary exposure to chlorpyrifos through fruits and vegetables. Chlorpyrifos is the OP pesticide most commonly sold in Chile (SAG, 2008). In both the Province of Talca, and throughout Chile, this pesticide is widely used because it is inexpensive and is easily accessible to the public. The pesticide phosmet, which produces DMAPs, is another best-selling pesticide in the Province of Talca, but only in the spring and summer season. However, in northern Chile, phosmet is sold in the fall due to better climatic conditions for agriculture. According to interviews, the fruits/vegetables eaten at school often originate not from local produce but from areas farther north than the Region of Maule.
Long-term health complications (e.g. neurological effects, cancer) have been associated with the use of the pesticide chlorpyrifos (Alavanja et al, 2004; Bouchard et al, 2011; Bouchard et al, 2010; Engel et al, 2011; Handal et al, 2007; Jurewicz & Hanke, 2008, 2006; Marks et al, 2010; Rauth et al, 2006; Rosas & Eskenazi, 2008). In the United States and Europe strict regulations govern their use and sale (CDC, 2009). The results presented here suggest the need for a review of policies to regulate the sale and application of chlorpyrifos in Chile, as well as some of the other harmful OP pesticides currently in use. We observed that children living in urban areas have greater levels of diethyl-substitued OP metabolites than those who come from rural areas. This is seen also in some recent studies (Bradman et al, 2005; Lu et al, 2004) and can be explained because children in rural areas are more likely to consume fruits from their own orchards where no pesticides are used, in contrast to urban children who access produce purchased in markets or stores which may have higher levels of OP residues. The highest urine concentrations of DEAP metabolites were observed in Talca, where fruits and vegetables are provided by a large produce market. On the other hand, both diethyl- and dimethyl-substituted OP metabolites were increased for childen living close to farms, suggesting that there is also a component of exposure from local agriculture. Furthermore, children had higher DMAP metabolite levels in urine in December 2010 than in May 2011, which may be associated with increased local application of DMAP-producing pesticides in the summer (SAG, 2008). Even though food that children ate at home was not collected in the first time point, which is a limitation of this study, it is noteworthy that the presence of DMAP metabolites in December 2010 was associated to the distance of farms (OR=1.9; p=0.046; IC=1.0 - 3.8) and to the use of pesticides at home (OR=3.7; p=0.014; IC=1.3 - 10.4). There were no differences in pesticide use in the homes between urban and rural children, or in proximity to farms in both periods (p value ≥ 0.05).
The presence of DEAP metabolites was also associated with younger age and higher creatinine levels, likely because of differences in the metabolism of younger children compared to older (Bouchard et al, 2010; Valcke et al, 2006), hygiene habits, such as inadequate washing of fruit or hands (Vida & Moretto, 2007), mouthing activity, greater ingestion of produce on a body weight basis (Cohen-Hubal et al., 2000).
Few families apply OP pesticides in the home (Table 1). The only OP pesticide compound used in houses is fenitrothion. Children whose households applied that pesticide had higher levels of DMAP metabolites, which is consistent with the home application of this pesticide (CDC, 2009).
We found no association between schoolchildren of parents who work in agriculture and OP metabolites, and no association between the years of education of parents and metabolite levels. This differs from other studies, where employment in farm work and low level of education of parents were correlated with high levels of OP metabolites (Barr et al 2004; Bouchard et al, 2011; Bouchard et al, 2010; Bradman et al, 2005; Coronado et al, 2006; Engel et al, 2011; Kissel et al, 2005; Koch et al, 2002; Lu et al, 2008; Lu et al, 2004; Marks et al, 2010; Naeher et al, 2010; Valcke et al, 2006; Vida & Moretto, 2007).
Our study has several associated limitations. The absence of pesticide residue data from the summer prevents us from fully understanding the pathways of exposure during that time period. Further, the produce sampling was taken from what the children were expected to consume but did not represent their actual dietary intake. We had similar limitations with our urinary metabolites. The DAPs measured are non-specific metabolites and can be derived from a multitude of OP pesticides or their preformed metabolites. Given the importance of chlorpyrifos exposure in our study, the measurement of a more specific metabolite, namely 3,5,6-trichloropyridinol (TCPy) may have provided more a compound-specific exposure assessment. However, since our initial goal was to evaluate class exposure and we had no a priori evidence that chlorpyrifos would be such an important pesticide to assess, we did not measure TCPy. Clearly, given the evidence produced in this study, future studies should include the measurement of TCPy.
The findings of this study provide some of the first data on pesticide exposure in children in Latin America and their potential exposure risk factors. The consumption of produce, both in school and at home, and living close to farms were the main predictors of pesticide exposure in children. Our data show higher levels of urinary OP pesticide metabolites in children than that found in other studies. It is notable that the levels of OP pesticide residues found in vegetables meet the standards required by regulatories agencies in Chile (MINSAL), which are regulated under the Codex Alimentarius (MINSAL, 2010b). However, several studies suggest that exposure to OP residues in low doses over a long period of time can lead to health consequences such as those mentioned above, plus attention span difficulties, behavioral and cognitive deficits (Bouchard et al, 2011; Bouchard et al, 2010; Engel et al 2011; Handal et al 2007; Marks et al, 2010).
We generated evidence that in the province of Talca, Chile, there is continual exposure for schoolchildren to low doses of OP pesticides, mainly through the dietary pathway. A suggested use of these data is the reconsideration of regulations of pesticide food tolerances, since little control exists over the sale or use of OP pesticides rendering them one of the most widely used groups of pesticide in Chile.
Highlights.
-
-
Chilean children had higher levels of OP metabolites than most published studies.
-
-
Exposure during both summer and fall regardless of regional peaks in agriculture.
-
-
OP residues in fruits was the major predictor of urinary DAP metabolites.
-
-
First data on OP metabolites and dietary exposure in children of Latin America.
Acknowledgments
Funding: This work was supported by NIH Fogarty grant D43 TW 05746-02, USA, and through the National Commission for Scientific and Technological Investigation (CONICYT) of the Chilean government through a FONIS grant SA10I20001
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alavanja M, Hoppin J, Kamel F. Health effects of chronic pesticide exposure: cancer and neurotoxicity. Annual Review of Public Health. 2004;25:155–197. doi: 10.1146/annurev.publhealth.25.101802.123020. [DOI] [PubMed] [Google Scholar]
- Azaroff LS. Biomarkers of exposure to organophosphorous insecticides among farmers’ families in rural El Salvador: factors associated with exposure. Environ Res. 1999;80(2 Pt 1):138–47. doi: 10.1006/enrs.1998.3877. [DOI] [PubMed] [Google Scholar]
- Barr D, Bravo R, Weerasekera G, Caltabiano L, Whitehead R. Concentrations of dialkylphosphate metabolites of organophosphorus pesticides in the U.S. population. Environ Health Perspect. 2004;112:186–200. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M, Chevrier J, Harley K, Kogut K, Vedar M, Calderon N, Trujillo C, Johson C, Bradman A, Barr D, Eskenazi B. Prenatal exposure to organophosphates pesticides and IQ in 7-year-old children. Environ Health Perspect. 2011;119(8):1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M, Bellinger D, Wright R, Weisskopf M. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 2010;125:e1270. doi: 10.1542/peds.2009-3058. http://pediatrics.aappublications.org/content/125/6/e1270.full.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Eskenazi B, Barr D, Bravo R, Castorina R, Chevrier J, Kogurt K, Harnly M, McKone T. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ Health Perspect. 2005;113(12):1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Fourth National report on human exposure to environmental chemicals. Atlanta, USA: 2009. http://www.cdc.gov/exposurereport/pdf/FourthReport.pdf. [Google Scholar]
- Cohen Hubal E, Sheldon L, Burke J, McCurdy T, Berry M, Rigas M, Zartarian V, Freeman N. Childreńs exposure assessment: A review of factors influencing childreńs exposure and the data available to characterize and assess that exposure. Environ Health Perspect. 2000;108(6):475–486. doi: 10.1289/ehp.108-1638158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha C. Informe intoxicaciones agudas por plaguicidas Séptima Región. Ministerio de Salud de Chile; Talca: 2010. [Google Scholar]
- Coronado G, Vigoren E, Thompson B, Griffith W, Faustman E. Organophosphate pesticide exposure and work in pome fruit: Evindence for the tak-home pesticide pathway. Environ Health Perspect. 2006;114(7):999–1006. doi: 10.1289/ehp.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeghy P, Cohen Hubal E, Tulve N, Melnyck L, Morgan M, Fortmann R, Sheldon L. Review of pesticide urinary biomarker measurements from selected US EPA childreńs observational exposure studies. Int. J. Environ. Res. Public Health. 2011;8:1727–1754. doi: 10.3390/ijerph8051727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel S, Wetmur J, Chen J, Zhum C, Barr D, Canfield R, Wolff M. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect. 2011;119(8):1182–1188. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Harari R, Barr DB, Debes F. Pesticide exposure and stunting as independent predictors of neurobehavioral deficits in Ecuadorian school children. Pediatrics. 2006;117(3):e546–56. doi: 10.1542/peds.2005-1781. [DOI] [PubMed] [Google Scholar]
- Handal A, Lozoff B, Breilh J, Siobán H. Effect of community of residence on neurobehavioral development in infants and young children in a flower-growing Region of Ecuador. Environ Health Perspect. 2007;115(1):128–133. doi: 10.1289/ehp.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl Occup Environ Hyg. 1990;5(1):46–51. [Google Scholar]
- INE (Instituto Nacional de Estadísticas) Censo 2002, síntesis de resultados. Instituto de Nacional de Estadísticas de Chile; Santiago: 2002. http://www.ine.cl/cd2002/sintesiscensal.pdf. [Google Scholar]
- Jurewicz J, Hanke W. Prenatal and childhood exposure to pesticides and neurobehavioral development: Review of epidemiological studies. Int J Occup Med Environ Health. 2008;21(2):121–132. doi: 10.2478/v10001-008-0014-z. [DOI] [PubMed] [Google Scholar]
- Jurewicz J, Hanke W. Exposure to pesticides and childhood cancer risk: has there been any progress in epidemiological studies? Int J Occup Med Environ Health. 2006;19(3):152–169. doi: 10.2478/v10001-006-0024-7. [DOI] [PubMed] [Google Scholar]
- Kissel J, Curl C, Kedan G, Lu C, Griffith W, Barr D, Needham L, Fenske R. Comparison of organophosphorus pesticides metabolite levels in single and multiple daily urine samples collected from preschool children in Washington State. J Expo Anal Environ Epidemiol. 2005;15(2):164–171. doi: 10.1038/sj.jea.7500384. [DOI] [PubMed] [Google Scholar]
- Koch L, Fisker-Andersen J, Jolley L, Fenske RA. Temporal association of children’s pesticide exposure and agricultural spraying: report of a longitudinal monitoring study. Environ Health Perspect. 2002;110:829–833. doi: 10.1289/ehp.02110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehotay SJ, de Kok A, Hiemstra M, Van Bodegraven P. Validation of a fast and easy method for the determination of residues from 229 pesticides in fruits and vegetables using gas and liquid chromatography and mass spectrometric detection. JAOAC Int. 2005;88(2):595–614. [PubMed] [Google Scholar]
- Levine M. Pesticides: a toxic time bomb in our midst. Praeger; USA: 2007. [Google Scholar]
- Lu C, Barr D, Pearson M, Waller L. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ Health Perspect. 2008;116(4):537–542. doi: 10.1289/ehp.10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Kedan G, Fisker-Andersen J, Kissel J, Fenske R. Multipathway organophosphorus pesticide exposures of preschool children living in agricultural and noagricultural communities. Environ Res. 2004;96(3):283–289. doi: 10.1016/j.envres.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Marks A, Harley K, Bradman A, Kogut K, Barr D, Johnson C, Calderon N, Eskenazi B. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environ Health Perspect. 2010;118(12):1768–1774. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyck L, McCombs M, Brown G, Raymer J, Nishioka M, Buehler S, Freeman N, Michael L. Community duplicate diet methodology: a new tool for estimating dietary exposures to pesticides. J Environ Monit. 2012;14(1):85–93. doi: 10.1039/c1em10611b. [DOI] [PubMed] [Google Scholar]
- MINSAL (Ministerio de Salud de Chile) Norma de vigilancia de intoxicaciones agudas por plaguicidas REVEP. Ministerio de Salud de Chile; Santiago: 2007. http://epi.minsal.cl/epi/html/normas/normaREVEP.pdf. [Google Scholar]
- MINSAL Intoxicaciones agudas por plaguicidas (IAP) situaciones epidemiológicas Enero a Diciembre de 2010. 2010a http://epi.minsal.cl/epi/html/AtlasInteractivos/AB_90/Revep.htm.
- MINSAL . Resolución exenta n°33. Ministerio de Salud de Chile; Santiago: 2010b. Fija tolerancias máximas de residuos de plaguicidas en alimentos y deja sin efecto la resolución exenta n°581, de 1999, y sus modificaciones. http://webhosting.redsalud.gov.cl/transparencia/public/minsal/normativa_a7c-2.html. [Google Scholar]
- Naeher L, Tulve N, Egeghy P, Barr D, Adetona O, Fortmann R, Needham L, Bozeman E, Hilliard A, Sheldon L. Organophosphorus and pyrethroid insecticide urinary metabolite concentrations in young children living in a southeastern United State city. Sci Total Environ. 2010;408:1145–1153. doi: 10.1016/j.scitotenv.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Rauth V, Garfinkel R, Perera F, Andrews H, Hoepner L, Barr D. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the firts 3 years of life among inner-city children. Pediatrics. 2006;118(6):1845–1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas L, Eskenazi B. Pesticides and child neurodevelopment. Curr Opin Pediatr. 2008;20(2):191–197. doi: 10.1097/MOP.0b013e3282f60a7d. [DOI] [PubMed] [Google Scholar]
- SAG (Servicio Agrícola Ganadero) Declaración de ventas de plaguicidas año 2008. Servicio Agrícola Ganadero; Santiago de Chile: 2008. http://www.sag.cl/common/asp/pagAtachadorVisualizador.asp?argCryptedData=GP1TkTX dhRJAS2Wp3v88hMLZj9ZYIK9qAaTC9s9%2FJWY%3D&argModo=&argOrigen=BD&argFlagYaGrabados=&argArchivoId=40626. [Google Scholar]
- Schenck FJ, Hobbs JE. Evaluation of the quick, easy, cheap, effective, rugged, and safe (QuEChERS) approach to pesticide residue analysis. Bull Environ Contam Toxicol. 2004;73(1):24–30. doi: 10.1007/s00128-004-0388-y. [DOI] [PubMed] [Google Scholar]
- Tadeo J, Sánchez-Brunete C, González L. Pesticides: Classification and properties,in: Tadeo, J. (Eds), Analysis of pesticides in food and environmental samples. CRC Press; New York: 2008. pp. 1–34. [Google Scholar]
- Valcke M, Samuel O, Bouchard M, Dumas P, Belleville D, Tremblay C. Biological monitoring of exposure to organophosphate pesticides in children living in peri-urban areas of the Province of Quebec, Canada. Int Arch Occup Environ Health. 2006;79:568–577. doi: 10.1007/s00420-006-0085-8. [DOI] [PubMed] [Google Scholar]
- Vida P, Moretto A. Pesticide exposure pathways among children of agricultural workers. J Public Health. 2007;15:289–299. [Google Scholar]
- Wessels D, Barr D, Mendola P. Use of biomarkers to indicate exposure of children to organophosphate pesticides: Implications for a Longitudinal Study of Childreńs environmental health. Environ Health Perspect. 2003;111(16):1939–1946. doi: 10.1289/ehp.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization) The WHO recommended classification of pesticides by hazard and guidelines to classification: 2004. World Health Organization; Switzerland: 2005. [Google Scholar]