Abstract
Inducible nitric oxide synthase (iNOS) upregulation and consequent NO formation are well-recognized neuroinflammatory responses associated with Parkinson’s disease (PD). These contribute to nitrosative protein modifications affecting neuronal injury and cell death. Indeed, a pathobiologic signature for PD is Lewy body formation containing misfolded and aggregated forms of alpha-synuclein (α-syn). Moreover, nitration of α-syn promotes protein aggregation in disease. To model such pathological events, we constructed controllable iNOS and bicistronic α-syn-IRES-tTA adeno-associated virus (AAV) expression vectors. Transduction of iNOS and α-syn AAV constructs led to nitration of α-syn in neurons and overexpression of iNOS promoted protein aggregation. We posit that this AAV system mimics critical protein misfolding events associated with the pathogenesis of PD.
Keywords: Adeno-associated virus, alpha-synuclein, Parkinson’s disease, nitration, atomic force microscopy
Introduction
Inducible nitric oxide synthase (iNOS) and NO are linked to neuroinflammatory responses operative in Parkinson’s disease (PD) and other neurodegenerative disorders [2, 9, 10]. For the former, NO contributes to the tyrosine nitration of α-synuclein (α-syn) in Lewy bodies [1,5]. Importantly, nitration increases the propensity of α-syn to aggregate [6, 14]. Indeed, others demonstrated that nitrated α-syn inclusions are common features of synucleinopathies, including PD [4]. NO is a pleiotropic molecule that functions in intercellular signaling and killing of phagocytosed foreign pathogens. Under normal conditions, excess NO diffuses out of cells rapidly and is scavenged by oxyhemoglobin [3]. However excess NO can also react with superoxide to form the oxidant peroxynitrite (ONOO−), which in turn, serves to nitrate tyrosine residues. Since iNOS is induced by glial activation coincidental with aggregated α-syn, iNOS may contribute to α-syn nitration as observed in PD. Such events are important in the pathophysiology of disease as nitrated α-syn can induce robust innate and adaptive immune responses implicated in neuronal dysfunction [1, 11, 12]. Thus, we sought to mimic these events using adeno-associated viral (AAV) vectors to study the role of iNOS in α-syn nitration and aggregation.
Materials and methods
AAV gene construction
We have constructed an AAV vector containing the tetracycline-response element/minimal cytomegalovirus (CMV) promoter (TRE), multiple cloning site (MCS), and woodchuck hepatitis post-transcriptional regulatory element (pAAV2-TRE-MCS-WPRE, see supplementary methods). Briefly, for pAAV2-TRE-iNOS-WPRE construction, a PCR fragment was amplified from Nos2 (Open Biosystems) digested with Xho I – Hind III and inserted into the MCS of pAAV2-TRE-MCS-WPRE. For construction of the bicistronic α-syn vectors, a PCR fragment of the human α-syn (HS)-internal ribosomal entry site (IRES) sequence was PCR amplified from pCSIGW (a generous gift from Dr. David Standaert [15]) and tetracycline-controlled transactivator (tTA, tet Repressor-VP16 fusion protein [5]) was separately amplified from pUHD15-1. These two amplicons were purified, digested with Mlu I, ligated to obtain an Xho I-Mlu I-Nhe I fragment containing HS-IRES-tTA, followed by insertion into the corresponding restriction sites of of pCSIGW [containing the CMV immediate early enhancer and chicken β-actin (CAG) promoter] to produce pAAV2-CAG-HS-IRES-tTA-WPRE. To make pAAV2-CAG-mouse-α-syn-IRES-tTA-WPRE, HS was replaced with mouse α-syn (MS) in pAAV2-CAG-HS-IRES-tTA-WPRE. For complete details of construction and AAV production see supplementary methods.
Cell culture
AAV-293 (293) cells (Stratagene) were cultivated in tissue culture flasks or in multi-well tissue culture plates in complete DMEM [DMEM supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (P/S) (Invitrogen)] in the presence of 5% CO2. PC12 cells were maintained on poly-D-Lysine (Sigma) coated flasks in DMEM containing 10% horse serum, 5% FBS, 4 mM L-glutamine, and P/S in the presence of 10% CO2. For experiments, PC12 cells were seeded in 24-well tissue culture plates coated with poly-D-Lysine (Sigma) at 15 ×103 cells/1.5 ml/well. Cells were differentiated 24-48 hr after seeding by media change [DMEM supplemented with 2% FBS, 4 mM L-glutamine, P/S, and 100 ng/ml mouse nerve growth factor (NGF) (R&D Systems)] for 7 days prior to transduction. Primary cortical mouse neurons were cultivated at 4 × 105 cells/1.5 ml/well in poly-D-Lysine coated 24-well plates [7].
AAV2/1 transduction
Transduction of cells was accomplished by adding a reduced volume of fresh media (500 μl) containing viruses to each well and incubating for 90 min, followed by addition of 1 ml of fresh media to the cells (1.5 ml total volume). A 2/3 volume of media was exchanged on the following day and every 2 days following transduction. Efficiency of AAV2/1 was calculated using differentiated PC12 cells infected with TRE-eGFP and CAG-tTA. On days 3, 4, 5, 7, and 9 post-transduction, bright field and fluorescence images were taken from random fields of view of cells from 3 separately treated wells of cells per experiment. Total cells and eGFP+ cells were counted using ImageJ (NIH) to determine percent eGFP+ cells. Except where otherwise noted, cells were transduced with 1011 viral particles (VP)/ 2 × 105 cells. Post transduction neuronal viability was determined by CellTiter-Glo assay (Promega) using differentiated PC12 cells and primary neurons 10 days after AAV transduction.
Transfection
Cells from the 293 line were seeded at 2 × 105 cells per well of 24-well plates in complete media. The following day, media was changed to complete DMEM without antibiotics 3 hr prior to transfection to prevent toxicity. Endotoxin-free plasmid DNA was transfected using the calcium phosphate method (5 ng/well/per construct)[8]. Empty vector DNA was used to normalize DNA between treatment groups. Twenty-four hours following transfection the media was exchanged for complete DMEM containing P/S. Doxycycline (DOX) was used at 100 ng/ml to suppress gene expression driven by TRE promoter in 293 cells, PC12, and primary neurons. Cells were imaged with a 10× objective 48 hr post-transfection and mean fluorescence values, using the same exposure settings, were measured from random fields of view from 3 separately treated wells/experiment.
Western blot and immunofluorescence for α-syn
For western blot assays, 15 μg of protein from total cell lysates [in RIPA buffer (Pierce) containing protease inhibitor cocktail and PMSF (Sigma)] were electrophoretically separated on 4-20% Tris-glycine TGX gels and transferred to polyvinylidene difluoride membranes (BioRad). Membranes were blocked, incubated with primary antibodies, washed, incubated with HRP-conjugated secondary antibodies, washed, and visualized using West Pico chemiluminescent substrate (Thermo Scientific). For detection of nitrated α-syn, 100 μg of total cell lysates were diluted to 1 ml in PBS, pH 7.4 and incubated with α-syn antibody (Syn42, 2.5 μg, BD Biosciences) on an orbital rocker at 4°C overnight, followed by immunoprecipitation with Dynabeads Protein G (Invitrogen). For immunofluorescence, AAV-infected primary neurons were fixed with ice-cold 4% PFA in PBS, pH 7.4 for 10 min at room temperature (RT), permeabilized with 0.2% Triton X-100 for 10 min at RT, blocked with 5% BSA, incubated with α-syn antibody (1:200, Calbiochem), and incubated with a FITC-labeled secondary antibody. Hoechst 33342 was added to stain nuclei.
NO measurements
NO concentrations in cell culture supernatants were determined using the Total Nitric Oxide Assay Kit (Thermo Scientific) according to manufacturers instructions. Supernatants were centrifuged at 500 × g for 10 min to remove any potential cells and diluted 1:2 prior to use.
Atomic Force Microscopy (AFM)
Cells from the 293 line were seeded at 3 × 105 cells/well of 6-well plates in 3 ml complete DMEM and co-transfected 24 hr later with tTA and TRE-iNOS plasmid DNAs (1 μg of each construct). Twenty-four hours after transfection recombinant α-syn (1 μM) or nitrated and aggregated recombinant α-syn (1 μM) was added to supernatants of cells in culture for an additional 48 hr. For AFM measurements, α-syn containing supernatants were diluted in PBS and deposited on 1-(3-aminopropyl)silatrane (APS) mica coated glass slides and dried under argon gas flow. Images were taken using a Molecular Force Probe 3D controller (Asylum Research Inc). Recombinant α-syn was prepared and separate aliquots nitrated and aggregated as previously described [1].
Statistical Analyses
Analyses were performed by one-way ANOVA with Bonferroni post hoc test using GraphPad Prism version 4.0c for Macintosh (GraphPad Software). Linear regression was also done using this software.
Results
AAV2/1-mediated α-syn and iNOS expression
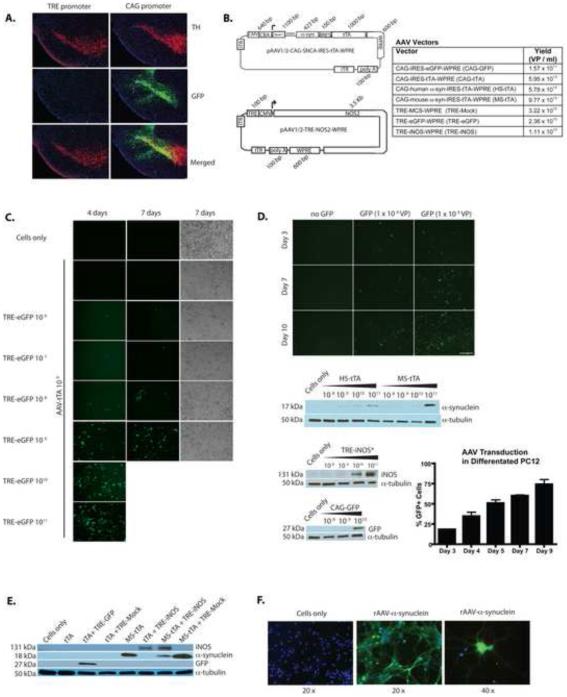
We sought to control both α-syn and iNOS expression using the Tet-Off system that utilizes the TRE promoter consisting of several Tet-Operator sites coupled to a miniCMV promoter, and DOX. To assess if the system would be operative in disease associated brain subregions, AAV-TRE-eGFP was stereotaxically injected (supplemental methods) into the substantia nigra pars compacta (SNpc) of transgenic mice that express tTA driven by the CaMKIIa promoter. However, eGFP reporter expression was not observed at 4 weeks after injection, suggesting lack of robust CaMKIIa activity in the SNpc (Fig. 1A). Thus, we employed a CAG promoter system that provided robust expression of eGFP in the tyrosine hydroxylase-positive (TH+) SNpc neurons (Fig. 1A). Currently, no transgenic tTA mice are available that express tTA in the SNpc and overexpression of AAV-mediated α-syn elicits pathology after several months [15]. Thus, we used α-syn expression driven by CAG promoter for these studies. To create an expression system where iNOS expression is controlled with DOX, we constructed bicistronic α-syn overexpression vectors that included IRES and tTA gene sequence downstream of the α-syn gene (Fig. 1B). This allows TRE-driven Nos2 gene expression to be controlled by DOX in cells overexpressing α-syn. The expression of TRE-driven eGFP with tTA co-transduction increased in a dose and time-dependent manner (Fig. 1C and D). This was also seen for CAG driven human (HS-tTA) and mouse (MS-tTA) α-syn genes and for TRE-iNOS (Fig. 1D and data not shown). Differentiated PC12 neurons were transduced with TRE-eGFP and tTA, and the percentage of cells expressing eGFP over time was determined. The results showed that > 74% of cells were transduced after 9 days (Fig. 1D). In addition, vector-mediated eGFP, α-syn, and iNOS were expressed by primary cortical mouse neurons in the presence of tTA (Fig. 1E). Fluorescence microscopy revealed that AAV delivered α-syn has a widespread somatodendritic distribution easily observed over endogenous levels of α-syn in primary neurons (Fig. 1F).
Fig. 1. Expression of rAAV delivered genes and vector design.
(A) Representative 30 μm coronal brain images showing that AAV2/1-TRE-eGFP-WPRE delivered to the mouse SNpc of CaMKIIa-Tg mice (left half of panel) showed no expression in SNpc due to lack of promoter activity, but AAV2/1-CAG-eGFP-WPRE delivery resulted in robust eGFP expression (green) and co-localization with TH+ neurons (red) in the SNpc of WT mice (right half of panel) at 4-weeks post injection. (B) Design of vectors used to circumvent Tg mouse requirement. Table indicates typical yields [viral particles (VP)] of vectors produced. (C) Titration of TRE-eGFP in 293 cells (co-transduced with AAV2/1-CMV-tTA-WPRE at 109 VP/ 2 × 105 cells) showing increasing expression with increasing infective dose. (D) TRE-eGFP transduced 293 cells (co-transduced with AAV2/1-CMV-tTA-WPRE at 109 VP/ 2 × 105 cells) show increased eGFP expression over the course of 10 days. Western blots show expression of HS-tTA, MS-tTA, and CAG-eGFP delivered genes resulting in dose dependent protein expression at 5 days post transduction in 293 cells. *Indicates cells were also transduced with AAV2/1-CAG-IRES-tTA-WPRE at 1010 VP/ 2 ×105 cells. Graph shows percent of cells visibly expressing GFP on days after treatment with AAV-GFP (TRE-eGFP) in differentiated PC12 neurons (co-infected with AAV2/1-CAG-IRES-tTA-WPRE). Data represent mean percentages (± SEM) using fluorescence and light microscopy images from 3 separate wells of cells (r2 = 0.93). (E) AAV delivered genes show robust expression in primary cortical neurons from embryonic day 18 (E18) mice when used alone or in combination by western blot. (F) Immunofluorescence staining in primary neuron cultures showing expression and distribution of α-syn 12 days post-transduction with MS-tTA. Data are representative of 3 independent experiments.
AAV delivers functional Tet-off controllable iNOS
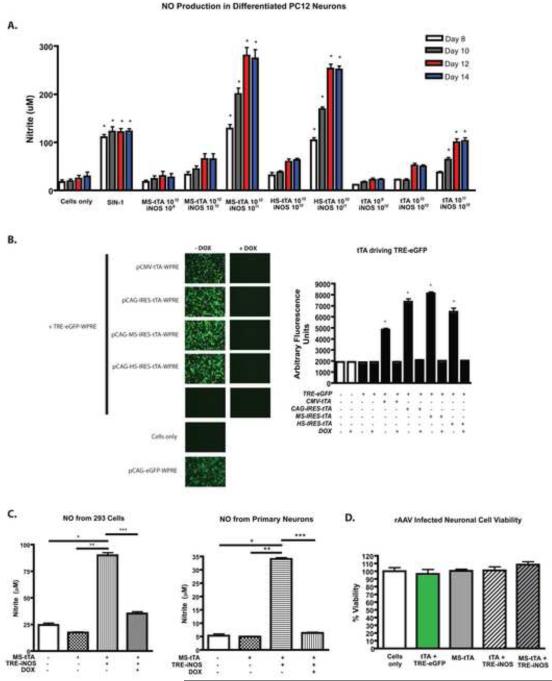
Since the TRE-iNOS construct approaches the packaging limitation of AAV, truncation of the iNOS gene with diminished NO production remained possible. To test the function of the AAV-delivered iNOS protein, we quantified the amount of NO produced from differentiated PC12 neurons (Fig. 2A) and MES23.5 cells (data not shown) every 2 days following transduction. As a positive control, 100 μM 3-morpholinosydnonimine HCl (SIN-1) (Invitrogen) was added to cell supernatant fluids at each media exchange. The NO concentration was significantly higher in cells transduced with TRE-iNOS (Fig. 2A). NO production by TRE-iNOS could be driven by CAG-IRES-tTA (tTA) as well as CAG-MS-tTA (MS-tTA) and CAG-HS-tTA (HS-tTA), demonstrating bicistronic effects of the tTA vectors. Furthermore, NO production increased over time, peaking at 12 days post-transduction. This demonstrated time and dose dependency of both TRE-iNOS and the tTA AAV vectors. To assess the control of genes expressed under the TRE promoter, 293 cells were transfected with tTA plasmids for AAVs and pTRE-eGFP-WPRE. Robust eGFP expression was observed when driven by any of the tTA constructs as well as a pCMV-tTA-WPRE construct used as an additional positive control. Importantly, eGFP expression was completely inhibited in the presence of DOX demonstrating the Tet-Off controllability of TRE-driven genes (Fig. 2B). DOX inhibition of TRE-driven iNOS expression from AAVs was also demonstrated by a decrease in NO in supernatants of both primary neurons and 293 cell cultures (Fig. 2C). Notably, AAV-mediated gene delivery itself caused minimal or no loss of cell viability (Fig. 2D, Supplemental Fig. 1A, and data not shown).
Fig. 2. AAV delivered iNOS results in NO production in a dose and time-dependent manner.
(A) Total NO production by TRE-iNOS-transduced differentiated PC12 neurons. NO is produced from TRE-iNOS transduced cells when co-infected with tTA containing vectors dependent upon the infective doses of both TRE-iNOS and the tTA containing vector. NO production increases with time as protein production increases (days 8-14 shown). Data are means (± SEM) from 4 separate wells per group from 2 separate assays. *p < 0.001 compared to cells only at same time point. Nitrite is a stable breakdown product of NO. (B) Regulation of TRE-driven genes by DOX. TRE-eGFP is used as a reporter in 293 cells demonstrating the ability of tTA viruses to drive expression of TRE-delivered genes as well as the inhibitory effect of DOX on TRE-controlled gene expression. A CMV construct is also used as a control promoter for tTA expression (pCMV-tTA-WPRE). No expression is observed in the absence of tTA. Data represent mean fluorescence values (± SEM) using fluorescence and light microscopy images from 3 separate wells of cells per group. *p < 0.001 compared to cells only. (C) NO concentration (nitrite) in 293 cells or primary neurons transduced with TRE-iNOS and MS-tTA showing inhibitory effect of DOX on NO production. Data represent mean NO concentrations (+/- SEM) from 4 separate wells per group. *p < 0.001 compared to cells only, **p < 0.001 compared to addition of MS-tTA, ***p < 0.001 compared to addition of MS-tTA+TRE-iNOS (- DOX) (D) CellTiter-Glo assay showing viability of differentiated PC12 cells transduced with AAVs. Data represent mean percent viability measurements based upon quantitation of ATP (± SEM) from 4 separate wells per group. Data are representative of 3 independent experiments. No significant differences in viability were detected among groups.
iNOS overexpression increases nitration and aggregation of α-syn
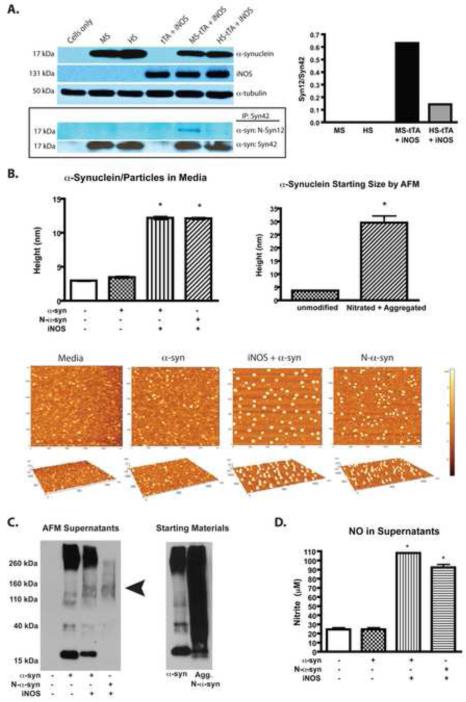
Finally, we tested whether iNOS overexpression modifies α-syn to affect protein misfolding and aggregation. Differentiated PC12 neurons were transduced using AAVs expressing α-syn with iNOS and the soluble fraction of cellular lysates 12 days following transduction were subjected to immunoprecipitation with α-syn antibody (syn42). Immunodetection using nitrated α-syn antibody (nsyn12) showed that α-syn was nitrated through iNOS expression (Fig. 3A). Nitrated mouse α-syn was detected > 4 times that observed with nitrated human α-syn using nsyn12. To determine the effect of iNOS overexpression on α-syn aggregation, recombinant mouse α-syn was added to the media of 293 cells or 293 cells overexpressing iNOS. This was used as no secreted or aggregated intracellular α-syn was detected by western blot in prior determinations (Supplemental Fig. 1B and data not shown). Supernatants were collected and analyzed by AFM after 48 hours. The height of α-syn/particles in the absence of iNOS expression showed no difference compared to media alone (Fig. 3B). However, when iNOS was expressed, aggregated proteins were larger in size, similar to recombinant α-syn that was nitrated and aggregated before addition to the supernatant (Fig. 3B). Separate aliquots of recombinant α-syn used here were diluted in PBS (media-free) and measured by AFM before adding it to supernatants to determine the expected sizes of native and aggregated α-syn. The mean height of aggregated N-α-syn decreased when added to cells in culture media, likely due to the largest particles remaining on the bottom of the wells (top 2/3 of media/well collected) or to cellular uptake. Recombinant protein evaluated before adding to cells in culture was dissolved in PBS and vortexed vigorously prior to adding to slides for AFM measurements allowing measurement of highly insoluble species as well. To further evaluate α-syn aggregates, supernatants were subjected to western blot using anti-α-syn antibody (Fig. 3C). Overexpression of iNOS resulted in decreased α-syn monomers and an increase in higher molecular weight species (>30 kD) compared to α-syn in media cultured with cells not expressing iNOS (ratio of oligomers/monomers was 6 times greater with iNOS overexpression). We could not detect iNOS in these supernatants by western blot (data not shown). In a separate AFM experiment, we also observed no difference in particle size in media cultured with non-iNOS expressing cells and media from cells overexpressing iNOS, suggesting that the increased aggregates were not secreted iNOS (data not shown). NO was increased only in culture supernatants from whence aggregated α-syn originated (Fig. 3D). Taken together these data demonstrate the functionality of AAV vectors that can be used in combination to study the role of iNOS expression on α-syn modification.
Fig. 3. Overexpression of iNOS results in nitration of α-synuclein and aggregation in vitro.
(A) Western blot showing differentiated PC12 neuron lysates (15 μg/well) after infection with AAVs before immunoprecipitation (IP) probed with antibodies against α-syn, iNOS, or α-tubulin (top panel) and after IP with an anti-α-syn antibody (syn42) probed with a nitrated α-syn specific (Nsyn12) antibody (bottom boxed panel). The IP membrane was then reprobed with syn42 to verify IP. Graph shows densitometric analysis (Image J) as ratio of N-α-syn/total α-syn monomers (Nsyn12/syn42). (B) AFM measurements from supernatants from 293 cell cultures with recombinant α-syn added to the supernatants for 48 hrs (Left graph) +/- iNOS expression. N-α-syn control was aggregated in vitro prior to addition to media. Data represent the mean ± SEM of 52 measurements/treatment group. *p < 0.001 compared to cells in the absence or presence of α-syn. The right graph is the AFM measurements from recombinant α-syn starting materials (before addition to cell cultures) either unmodified or nitrated and aggregated. *p < 0.001 compared to height of unmodified α-syn. Representative images of α-syn/particles in supernatants are shown. (C) Western blots showing supernatants (25 μl/ lane) from the AFM experiment detected with syn42 α-syn antibody (Left blot). Increased presence of oligomers indicated by arrowhead. Also shown is detection of starting materials (recombinant proteins, 15 μg/well) with syn42 antibody. (D) NO concentration (nitrite) in the 293 supernatants taken for AFM. Data represent the mean ± SEM of 4 measurements/treatment group. *p < 0.001 compared to cells only.
Discussion
The availability of chronic progressive models of PD is important for understanding the pathobiology and testing future interdictive therapeutic strategies. The production of vectors used to assess the interplay between different genes associated with PD and thought to play a role in disease can provide more realistic animal models that better recapitulate disease etiology or progression. AAV has several advantages as a gene delivery vector, including no known pathology associated with the virus itself; safety, as all viral genes are removed in construction of the vectors; and stable, long-term expression of delivered genes. We posit that during PD, activated glia produce NO, which forms ONOO−and results in aberrant protein nitration and aggregation of neuronal proteins such as α-syn. Having the ability to control the expression level of iNOS in cells also overexpressing α-syn now allows the evaluation of threshold NO levels necessary to overwhelm biological defenses and create pathology, or nitrate α-syn to the extent whereby immunological tolerance is broken or evaded [1, 12]. These models may also be used to evaluate possible critical periods such as in the aged mouse where immunological and antioxidant homeostasis breaks down, reversibility of protein modification/clearance, and adaptation mechanisms. We anticipate that these vectors will be useful for creation of a chronic progressive mouse model of PD, and may be adapted to explore the role of iNOS in other neurodegenerative disorders such as Alzheimer’s disease.
Evaluation of α-syn nitration and aggregation in vitro using these vectors is limited by amount of virus, number of cells, and prolonged culture necessary to produce sufficient quantities of protein to detect nitration of α-syn. Long-term in vivo studies are needed to determine the ultimate effect of iNOS expression on α-syn and any potential neurotoxicity. Nitration may target α-syn for destruction or repair and make it less soluble. Indeed, we observed less α-syn when nitrated and aggregated; and did not detect intracellular aggregates, possibly due to decreased solubility, destruction, or low abundance. The observation of decreased soluble intracellular α-syn monomers with concomitant iNOS expression delivered by AAVs could be reflective of competition for cellular protein expression machinery, limitations of infectivity, formation of insoluble species, protein degradation, or an unknown mechanism. We also observed less nitration of human α-syn as compared to mouse α-syn in cell culture, which we speculate may be due to one less nitratable tyrosine residue in the human protein, limitations of the antibodies used, or decreased solubility. While these vectors had limited toxicity on neurons in vitro, previous studies demonstrated that aggregated N-α-syn stimulates microglia to produce neurotoxic secretions [11, 13]. If aggregated N-α-syn accumulates and is released from neurons using these vectors in vivo, microglia responses will be key to a neurodegenerative response.
Conclusions
We show that AAV2/1-CAG-α-syn-IRES-tTA-WPRE and AAV2/1-TRE-iNOS-WPRE used in combination direct iNOS expression and α-syn protein modification. The use of AAV2/1-TRE-iNOS-WPRE with a tTA AAV2/1 vector results in robust dose-dependent NO production that increases with time and is controlled by DOX. Importantly, iNOS expression leads to nitration and aggregation of α-syn and as such serves to mimic a pivotal component of the pathobiology of PD.
Supplementary Material
Highlights.
AAV2/1 vectors were constructed that express iNOS and α-synuclein.
These permit DOX control of iNOS expression in a dose dependent manner.
AAV2/1 delivered iNOS leads to robust NO production.
iNOS overexpression induces nitration and aggregation of α-synuclein.
Acknowledgments
The authors would like to thank Dr. Lyudmila Shlyakhtenko for assistance with AFM measurements; Dr. Stephen Bonasera, and Dr. Daniel Murman for their advice; and Dr. David G. Standaert and Dr. Ashley Harms for help with stereotaxic microinjections. We would also like to thank Alex Knesevic and Dr. Kathy Estes for their technical assistance. This work was supported by the UNMC Graduate Studies Fellowship (to D.K.S.), National Institutes of Health grants 5R01NS070190-02 (to R.L.M.), 1P01 DA028555, 2R01 NS034239, 2R37 NS36126, P01 NS31492, P20RR 15635, P30 MH062261, P01MH64570 and P01 NS43985 and the Carol Swarts Emerging Neuroscience Fund(to H.E.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Benner EJ, Banerjee R, Reynolds AD, Sherman S, Pisarev VM, Tsiperson V, Nemachek C, Ciborowski P, Przedborski S, Mosley RL, Gendelman HE. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS One. 2008;3:e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Broom L, Marinova-Mutafchieva L, Sadeghian M, Davis JB, Medhurst AD, Dexter DT. Neuroprotection by the selective iNOS inhibitor GW274150 in a model of Parkinson disease. Free Radic Biol Med. 2011;50:633–640. doi: 10.1016/j.freeradbiomed.2010.12.026. [DOI] [PubMed] [Google Scholar]
- [3].Butler AR, Megson IL, Wright PG. Diffusion of nitric oxide and scavenging by blood in the vasculature. Biochim Biophys Acta. 1998;1425:168–176. doi: 10.1016/s0304-4165(98)00065-8. [DOI] [PubMed] [Google Scholar]
- [4].Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- [5].Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hodara R, Norris EH, Giasson BI, Mishizen-Eberz AJ, Lynch DR, Lee VM, Ischiropoulos H. Functional consequences of alpha-synuclein tyrosine nitration: diminished binding to lipid vesicles and increased fibril formation. J Biol Chem. 2004;279:47746–47753. doi: 10.1074/jbc.M408906200. [DOI] [PubMed] [Google Scholar]
- [7].Huang X, Stone DK, Yu F, Zeng Y, Gendelman HE. Functional proteomic analysis for regulatory T cell surveillance of the HIV-1-infected macrophage. J Proteome Res. 2010;9:6759–6773. doi: 10.1021/pr1009178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jordan M, Schallhorn A, Wurm FM. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 1996;24:596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Knott C, Stern G, Wilkin GP. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci. 2000;16:724–739. doi: 10.1006/mcne.2000.0914. [DOI] [PubMed] [Google Scholar]
- [10].Kummer MP, Hermes M, Delekarte A, Hammerschmidt T, Kumar S, Terwel D, Walter J, Pape HC, Konig S, Roeber S, Jessen F, Klockgether T, Korte M, Heneka MT. Nitration of tyrosine 10 critically enhances amyloid beta aggregation and plaque formation. Neuron. 2011;71:833–844. doi: 10.1016/j.neuron.2011.07.001. [DOI] [PubMed] [Google Scholar]
- [11].Reynolds AD, Glanzer JG, Kadiu I, Ricardo-Dukelow M, Chaudhuri A, Ciborowski P, Cerny R, Gelman B, Thomas MP, Mosley RL, Gendelman HE. Nitrated alpha-synuclein-activated microglial profiling for Parkinson’s disease. J Neurochem. 2008;104:1504–1525. doi: 10.1111/j.1471-4159.2007.05087.x. [DOI] [PubMed] [Google Scholar]
- [12].Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE. Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J Immunol. 2010;184:2261–2271. doi: 10.4049/jimmunol.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Reynolds AD, Stone DK, Mosley RL, Gendelman HE. Proteomic studies of nitrated alpha-synuclein microglia regulation by CD4+CD25+ T cells. J Proteome Res. 2009;8:3497–3511. doi: 10.1021/pr9001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Souza JM, Giasson BI, Chen Q, Lee VM, Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha -synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem. 2000;275:18344–18349. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- [15].St Martin JL, Klucken J, Outeiro TF, Nguyen P, Keller-McGandy C, Cantuti-Castelvetri I, Grammatopoulos TN, Standaert DG, Hyman BT, McLean PJ. Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. J Neurochem. 2007;100:1449–1457. doi: 10.1111/j.1471-4159.2006.04310.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.