Abstract
Cystic Fibrosis (CF) is accompanied with heightened inflammation worsened by drug resistant Burkholderia cenocepacia. Human CF macrophage responses to B. cenocepacia are poorly characterized and variable in the literature. Therefore, we examined human macrophage responses to the epidemic B. cenocepacia J2315 strain in order to identify novel anti-inflammatory targets. Peripheral blood monocyte derived macrophages were obtained from 23 CF and 27 non-CF donors. Macrophages were infected with B. cenocepacia J2315 and analyzed for cytokines, cytotoxicity, and microscopy. CF macrophages demonstrated significant increases in IL-1β, IL-10, MCP-1, and IFN-γ production in comparison to non-CF controls. CF patients on prednisone exhibited globally diminished cytokines compared to controls and other CF patients. CF macrophages also displayed increased bacterial burden and cell death. In conclusion, CF macrophages demonstrate exaggerated IL-1β, IL-10, MCP-1, and IFN-γ production and cell death during B. cenocepacia infection. Treatment with corticosteroids acutely suppressed cytokine responses.
Keywords: cystic fibrosis, burkholderia, macrophage, IL-1β, corticosteroids
INTRODUCTION
Cystic Fibrosis (CF) is the most common life-limiting autosomal recessive disease among people of European descent, affecting over 70,000 people worldwide [1]. CF causes a wide range of systemic symptoms due to viscous mucus plugging in epithelial lined organs, but is most recognized for chronic, progressive respiratory infections which contribute to over 85% of patient deaths [2]. One of the major contributors to the chronic respiratory symptoms of CF patients is an exaggerated systemic and lung inflammatory state with sustained pro-inflammatory mediators present in CF patients from an early age in response to infection [3], as well as neutrophilic lower airway inflammation independent of infection [4]. An area of investigation into the sustained pro-inflammation in CF has been the role of polymorphisms in IL-1β genes that are thought to help modulate CF lung disease, as patients with similar genotypes often manifest different disease phenotypes based on their particular polymorphism [5]. CF patients identified with two particular IL-1β gene single nucleotide polymorphisms demonstrated increased long term disease severity, underscoring the importance of IL-1β in CF and implicating a contribution of genetic variation in IL-1β in the overall pathogenesis of lung function decline in CF [5].
CF patients are affected by numerous bacterial pathogens, but a particularly pathogenic bacterium among CF patients is Burkholderia cenocepacia. This microorganism is a member of the Burkholderia Cepacia Complex, which is known to cause exaggerated inflammation in CF patients and is rapidly transmissible from patient to patient [6,7,8]. Clinical disease can range from a severe and often fatal sepsis state termed ‘cepacia syndrome’, to a chronic progressive deterioration of CF lung disease associated with worsened outcomes for CF patients [9]. Due to poor lung transplant post-operative outcomes and high antibiotic resistance, infection with B. cenocepacia is usually an exclusion criterion from lung transplant eligibility in most centers, thereby offering little hope to infected CF patients for prolonged survival [10,11]. B. cenocepacia can also infect patients with chronic granulomatous disease (CGD), a disorder characterized by defective microbial phagocytosis and increased caspase-1 dependent IL-1β production in the absence of reactive oxygen species [12].
Because of the refractory nature and sustained inflammation of B. cenocepacia infections in CF, we are investigating inflammatory pathways to better understand responses to CF pathogens and uncover new therapeutic targets. Recently, we demonstrated that murine CF macrophages had increased IL-1β release in response to B. cenocepacia infection in comparison to normal controls [13]. Murine models also demonstrated a caspase-1 dependent IL-1β production similar to that seen in CGD [13]. However, human CF studies are varying in the reported impact of IL-1β production, and incomplete in characterization of responses to B. cenocepacia infection. Longitudinal studies have shown very little change in IL-1β production over the first years of life [14], whereas others have shown increased IL-1β within subsets of CF patients infected with viruses or various bacterial pathogens [3]. In addition, non CF human macrophages have demonstrated increased IL-1β in response to B. cenocepacia [15], but it is unclear how this response compares to CF macrophages and the overall impact on inflammation in CF. In this study, using the virulent B. cenocepacia J2315 strain, we found increased levels of IL-1β, IL-10, MCP-1, and IFN-γ in infected human CF macrophages, along with increases in cell death and bacterial survival compared to non CF macrophages
MATERIALS AND METHODS
Bacterial strains and culture
Burkholderia cenocepacia strain J2315 was originally isolated from a CF patient of the prototypic epidemic ET12 lineage [16]. Strain J2315 was the index strain from which patient-to-patient spread of this lineage was first reported in Edinburgh, Scotland [17]. The bacterial strain was grown in Luria-Bertani (LB) broth at 37°C overnight with high amplitude shaking.
Monocyte-derived Macrophages (MDMs) with Ethics Statement
Monocytes were isolated from heparinized blood of 23 CF and 27 non CF human donors aged 2–46 years (mean CF 23.4 years, non CF 23 years). Patient demographics are listed in Table 1. No patients were colonized with B. cenocepacia and subjects were excluded if on any chronic immunosuppressants. Non CF patients represented a mixed population of healthy volunteers and asthma exacerbations to represent a model of airway inflammation for comparison to CF. 5 CF and 3 non CF patients received 60mg of prednisone one time 16–20 hours prior to blood collection as part of their clinical care. Written informed consent was obtained from all subjects as approved by the Institutional Review Board of Nationwide Children’s Hospital. Written consent from legal guardians of minors was obtained as well as written assent from minors aged 9 to 17 years. Monocytes were separated from the whole blood using Ficol-Hypaque (Sigma). Isolate monocytes were re-suspended in RPMI (Gibco) plus 10% human AB serum (Lonza) to a concentration of 2 × 10^6 cells/mL and incubated for 5 days at 37°C. On day 5 macrophages were isolated and infected with J2315 at a multiplicity of infection (MOI) of 10.
Table 1.
Patient Demographics
| Cystic Fibrosis | Non Cystic Fibrosis | |
|---|---|---|
| Age (mean years) | 23.4 | 23.0 |
| Caucasion | 100% | 100% |
| Male | 50% | 48% |
| P. aeruginosa colonization | 62% | N/A |
| MRSA colonization | 33% | N/A |
| Average FEV1 % predicted | 50% +/− 23% | N/A |
Bioplex
MDMs were infected with J2315 for 24 hours and the culture supernatant was collected, centrifuged, and stored at −20°C until assayed for cytokine content. The concentration of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17, IFN-γ, MCP-1, GM-CSF, G-CSF, and MIP-1β in the supernatant was determined by Bioplex assay following the manufacturer’s protocol (BioRad, Hercules, CA).
Enzyme-Linked Immuno Sorbent Assay (ELISA)
MDMs were infected with J2315 for 24 hours and culture supernatants were centrifuged and stored at -20°C until assayed for cytokine content. The quantification of IL-1β, IL-8, IL-10, and TNF-a in the supernatant was determined by sandwich ELISA following the manufacturer’s protocol (R&D system Inc, Minneapolis, MN, USA) as previously described [18].
Cytotoxicity
MDMs were infected with J2315 for 24 hours and the culture supernatants were collected and centrifuged. Histone-associated DNA fragments were detected using the cytotoxicity detection photometric assay kit according to the manufacturer’s protocol (Roche Applied Science, Indianapolis, IN). Experiments were performed in triplicate.
Confocal Microscopy
2.0 × 105 MDMs cultured on 12 mm glass cover slips in 24 well tissue culture plates were infected synchronously with J2315 at an MOI of 10. Nuclei were stained with the nucleic acid dye 4′,6′-diamino-2-phenylindole (DAPI) and converted to green for pictures. Lysosomes were stained red with Lysotracker Red (L7528, Invitrogen, Carlsbad, CA). One hundred bacteria were scored for each condition, and scoring was verified by blinded reviewers. Experiments were performed in triplicate. Samples were analyzed with an Olympus FV10i Spectral Confocal microscope.
Transmission Electron Microscopy
MMDs were isolated and infected with J2315 at an MOI of 10 for 2 hours. Cells cultured on Permanox (Lab-Tek) chamber slides were fixed with 2.5% gluteraldehyde in 0.1M phosphate buffer with 0.1M sucrose. These were post fixed with 1% osmium tetroxide in phosphate buffer then en bloc stained with 2% uranyl acetate in 10% ethanol, dehydrated in a graded series of ethanols and embedded in Eponate 12 epoxy resin (Ted Pella Inc. USA). Ultrathin sections were cut on a Leica EM UC6 ultra microtome (Leica microsystems, Germany), collected on copper grids, and then stained with lead citrate and uranyl acetate. Images were acquired with an FEI Technai G2 Spirit transmission electron microscope (FEI, USA), Macrofire (Optronics) digital camera and AMT image capture Software.
Statistical Analysis
Statistical Analysis was performed using GraphPad Prism software (version 5.0). Unpaired t-tests and ANOVA were used when appropriate. Statistical significance was determined with a two-tailed p < 0.05.
RESULTS
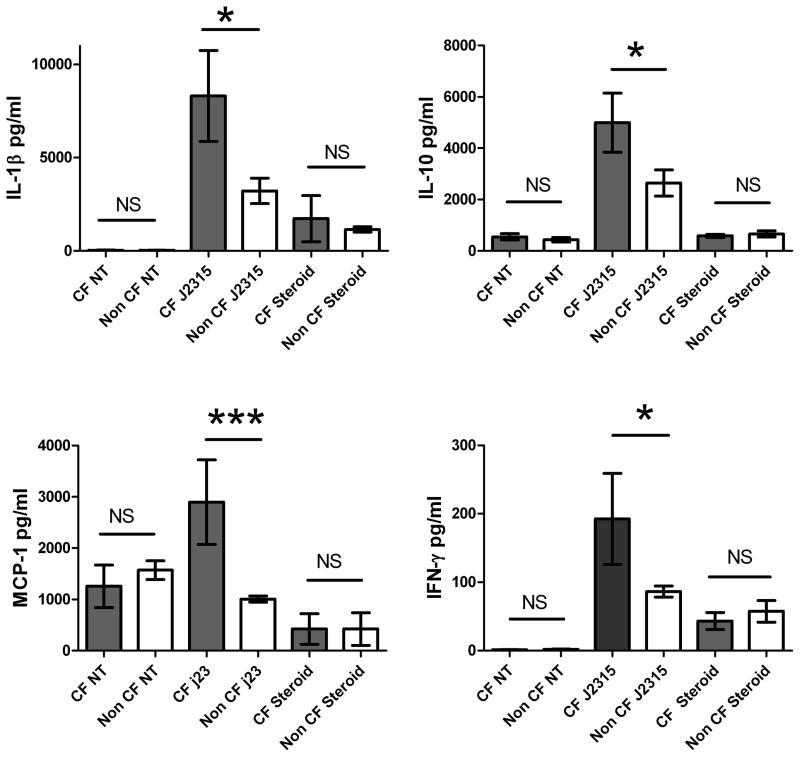
IL-1β, IL-10, MCP-1, and IFN-γ are increased in blood MDMs derived from CF patients infected with J2315
Previous murine models have demonstrated increased pro-inflammatory IL-1β production in CF macrophages after infection with B. cenocepacia K56–2, but studies in humans are controversial [19,20]. Therefore, we assessed multiple inflammatory cytokines to determine differential responses to the J2315 strain in CF patients. Human CF and non CF peripheral MDMs were infected for 24 hours with the J2315 strain of B. cenocepacia and supernatants of infected macrophages were collected and analyzed for IL-1β, IL-10, MCP-1, and IFN-γ release. IL-1β, IL-10, MCP-1, and IFN-γ production was significantly increased in macrophages derived from CF patients compared to non CF controls (Figure 1A–D). P values were 0.0105 (IL-1β), 0.0478 (IL-10), <0.001 (MCP-1) and 0.025 (IFN-γ). There was no significant difference in cytokine production between male and female patients (data not shown). There was also no difference when comparing CF patients to matched non CF asthmatic and healthy controls. These results demonstrate that both pro inflammatory IL-1β, MCP-1, and IFN-γ and anti-inflammatory IL-10 are increased in production in response to B. cenocepacia infection in macrophages from CF patients. There was no significant difference in IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-12, IL-13, IL-17, GM-CSF, G-CSF, MIP-1β, and TNF-α production between CF and non CF patients in response to J2315 infection (data not shown). These results indicate that although these cytokines may be important in other CF pathogen states, their production is not increased in response to B. cenocepacia infections in CF patients.
Figure 1. Cytokine concentrations.
(A) IL-1β, (B) IL-10, (C) MCP-1, and (D) IFN-γ levels for CF and non CF human MDMs infected with B. cenocepacia J2315 for 24 hours. CF patients on steroids were on 60 mg of prednisone daily at collection. Supernatant concentrations are in pg/mL.
Corticosteroids decrease inflammatory and anti-inflammatory cytokine production from CF monocyte-derived macrophages
Although control of heightened inflammation during B. cenocepacia infection is extremely difficult, corticosteroids have been shown to be acutely beneficial in case reports of Burkholderia cepacia sepsis [21]. We therefore analyzed cytokine production in five CF patients (mean age 25.8 years) and three non CF patients (mean age 17 years) on corticosteroid treatment. CF and non CF patients were given one dose of 60 mg prednisone as part of their standard treatment 16–20 hours prior to blood isolation. Isolated MDMs were infected with J2315 for 24 hours and supernatants were analyzed. There was a significant decrease in IL-1β, IL-10, MCP-1, and IFN-γ production in the CF patients on prednisone in comparison to CF patients not on steroids (Figure 1A–D). Therefore, exaggerated pro and anti-inflammatory responses in CF patients to B. cenocepacia infection may be substantially reduced with corticosteroid treatment.
B. cenocepacia avoids lysosomal degradation in CF macrophages
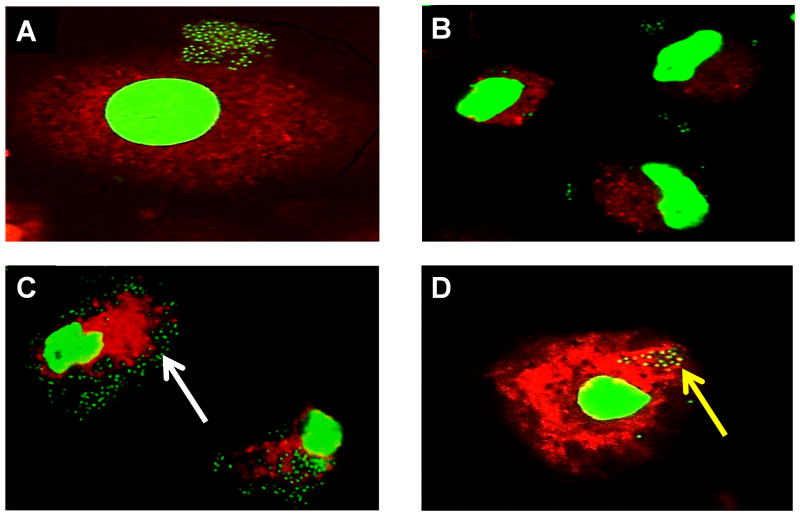
Defective macrophage clearance of B. cenocepacia has been reported previously as a potential contributor to B. cenocepacia virulence in CF [22,23,24]. We used confocal microscopy to determine co-localization of J2315 to the lysosome where bacterial degradation would take place to determine any differences in bacterial trafficking between CF and non CF MDMs that could contribute to the increased inflammation seen in CF MDMs. CF and non CF MDMs were infected with J2315 for 30 minutes and 1 hour and then a fluorescent lysotracker label was added. Lysotracker label is weakly basic and concentrates in the acidic lysosome. First, we scored the presence of bacteria associated per 100 macrophages. After 30 minutes of infection there was no significant difference in mean bacteria present between CF and non CF macrophages (p value 0.4548), but at one hour post infection there was a significant increase in bacteria present in CF macrophages compared to non CF macrophages (Figure 2A–D, 3A–B, p value <0.0060). Second, we evaluated the percent of bacteria localized with lysotracker red. After 30 minutes of infection there was a significant increase in lysosomal co-localization of bacteria observed in non CF macrophages (p value 0.010) with a continued significant increase in lysosomal co-localization of bacteria after one hour in non CF macrophages (Figures 3C–D, p value <0.0001). Taken together, these data indicate that B. cenocepacia avoids trafficking to the lysosome in CF macrophages leading to increased bacterial survival.
Figure 2.
Macrophage derived blood monocytes from CF (A, C) and non CF patients (B, D) were infected with J2315 for 30 minutes (A, B) and 1 hour (C,D) and examined by confocal microscopy. The white arrow highlights increased bacteria. The nucleus is bright green, lysosome stained red, and bacteria displayed in dark green. Co-localization of bacteria in the lysosome is indicated in yellow and increased co-localization in non CF macrophages is marked by a yellow arrow.
Figure 3. Confocal microscopy scoring.
CF and non CF macrophages infected with B. cenocepacia J2315. Mean bacteria presence per 100 macrophages after 30 minutes (A) and 1 hour (B). Percent co-localization of bacteria to the lysosome after 30 minute infection (C) and 1 hour (D).
B. cenocepacia bacterial burden is increased in CF macrophages
Given that B. cenocepacia avoids trafficking to the lysosome in CF MDMs, transmission electron microscopy was used to further characterize the macrophage interaction with J2315 in CF and non CF patients. Macrophages were infected with J2315 for 2 hours and transmission electron microscopy images were obtained as described in the methods. CF macrophages infected with J2315 demonstrated an increased presence of active bacteria as indicated by lipid droplets, with more than twice the amount of active bacteria present in the CF macrophage compared to the non CF macrophage (Figure 4–AB). There was also an increased number of bacteria with signs of degradation in the non CF macrophages (Figure 4B). Bacteria were noted to be in well defined vacuoles in the non CF macrophages (Figure 4B). These observations suggest that CF macrophages exhibit an increased bacterial burden during B. cenocepacia infection.
Figure 4. Electron microscopy data.
Macrophages derived from blood monocytes of CF (A) and non CF (B) patients were infected with B. cenocepacia for 2 hrs, and then processed for electron microscopy. The black arrow indicates degraded bacteria and the white arrow indicates active bacteria.
Cell death is increased in macrophages derived from CF patients
To determine the correlation between increased inflammatory cytokine production and cell injury, an LDH assay was performed to assess cell death. MDMs were infected for 24 hours with J2315 and supernatants were assessed for LDH release. There was a significant increase in cell death in CF macrophages compared to non CF macrophages after 24 hours of infection with J2315 as measured by percent LDH release (not shown, p value 0.043). No difference in basal cell death between the two groups in untreated macrophages was observed. Therefore, increased cell death in CF patients is attributable to B. cenocepacia infection and is not a baseline derangement in CF macrophages.
DISCUSSION
Cystic Fibrosis patients endure exaggerated inflammation that is further increased during B. cenocepacia infections leading to poor clinical outcomes and may result in rapid death [8,9]. While ongoing research into potential curative therapies for CF is promising, patients currently infected with B. cenocepacia continue to suffer from poor outcomes and pose serious risks for transmitting infection to other patients due to a lack of effective therapeutics. It is therefore vital to develop new medications to help combat the heightened inflammatory cascade observed in these infections.
While an increased IL-1β inflammatory response to B. Cenocepacia has been previously demonstrated in murine models of CF [19,20], human CF macrophage responses to B. cenocepacia are still poorly characterized with controversial findings. Previous studies have shown normal IL-1β levels in young CF patients not infected with B. cenocepacia [14,25], elevated levels in adult CF alveolar macrophages not infected with B. cenocepacia [26], and increased IL-1β in non CF macrophages in response to B. cenocepacia [15]. Therefore, it is not clear if IL-1B is specifically increased in CF macrophages upon B. cenocepacia infection. Here we demonstrate exaggerated human macrophage IL-1β responses to B. cenocepacia J2315. Given the findings in this study, in addition to the availability of anti-IL-1β monoclonal antibodies which have proven efficacy in clinical syndromes characterized by excessive IL-1β production [27], IL-1β could prove to be an interesting target for acute and chronic B. cenocepacia infections in CF patients.
Additionally, we also demonstrate evidence of increased MCP-1, IFN-γ and IL-10 production in CF patients. These findings may be related to the need for more effective macrophage bacterial clearance and antigen presentation during infection as evidenced by the increased bacterial burden in CF patients seen using microscopic analysis. Interestingly, IFN-γ has been shown to be an effective therapy in CGD, the other clinical entity affected by B. cenocepacia. In CGD, IFN-γ works by improving the phagocytic clearance of apoptotic cells [28] and therefore could be another target of similar clinical application in CF patients. Previous studies have also shown a protective role for MCP-1 in Burkholderia mallei infections and a close relationship with IFN- in order to clear pulmonary infection [29]. IL-10’s role in CF is less clear, but decreases in IL-10 production are thought to contribute to airway inflammation present during acute and chronic infections [30]. However, the acute increase in IL-10 seen in this study must be taken with caution, as elevated IL-10 has been linked to immunoparalysis and poor outcomes during sepsis states [31], which would need to be considered in the treatment of ‘cepacia syndrome’. All together these findings lend further support to our theory that the aberrations in the cytokines observed in this study may be appropriate targets for future therapeutic interventions.
In addition to potential specific cytokine directed therapies, there have been reports of cases of B. cepacia sepsis alleviated by corticosteroids [21], but the exact influence of broad immunomodulators such as corticosteroids acutely and chronically remains to be elucidated in CF patients with B. cenocepacia infection. The markedly diminished inflammatory responses observed in the subset of CF patients who had been placed on corticosteroids prior to infection, suggests the possibility of acutely blocking the intense inflammatory response elicited by B. cenocepacia through broad immunosuppression. However, the exact timing and duration of steroid therapy needs to be clarified in future studies examining human macrophage responses as the patients in this study received corticosteroids prior to infecting their macrophages.
In summary, CF macrophages have exaggerated inflammatory responses to B. cenocepacia infection along with host trafficking defects that are potential targets of future studies and therapeutic interventions.
CF macrophages produce excess IL-1β, IL-10, MCP-1, and IFN-γ during B. cenocepacia infection
B. cenocepacia avoids lysosomal degradation in CF macrophages
Corticosteroids may blunt exaggerated inflammatory responses to B. cenocepacia infection
Acknowledgments
Transmission Electron Microscopy images used in this article were generated at the Campus Microscopy and Imaging Facility, the Ohio State University. Many thanks to Miguel Valvano for kindly donating the J23 bacterial strain. B.K. is supported by an NIH loan repayment award and Nationwide Children’s Hospital Intramural Grant #244810. A.A. is supported by NIH grants R01HL094586, R21Al083871 and the American Lung Association. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- CF
Cystic fibrosis
- CGD
Chronic granulomatous disease
- IL-
1βInterleukin 1β
- IL-10
Interleukin 10
- IFN-γ
Interferon gamma
- LDH
Lactate dehydrogenase
- MCP-1
Monocyte Chemotactic Protein 1
- MDM
Monocyte derived macrophage
Footnotes
AUTHORSHIP
B.K. designed, performed, analyzed results and wrote the manuscript. B.A., A.A., S.K., A.K., M.T. and K.C contributed to the performance of the experiments and editing of the manuscript. R.M. generated the transmission electron microscopy images. K.M. edited and analyzed the manuscript. A.O.A. helped design experiments, analyze results, and write the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cystic Fibrosis Foundation Patient Registry. 2009 Annual data report. Bethesda, Maryland: 2010. [Google Scholar]
- 2.Bondong A, Kopp B, Dreger P, Ho A. Case management in allogeneic stem cell transplantation: the Heidelberg experience. Bone Marrow Transplantation. 2006;37:S291–S292. [Google Scholar]
- 3.Armstrong DS, Hook SM, Jamsen KM, Nixon GM, Carzino R, Carlin JB, Robertson CF, Grimwood K. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr Pulmonol. 2005;40:500–510. doi: 10.1002/ppul.20294. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld M, Gibson RL, McNamara S, Emerson J, Burns JL, Castile R, Hiatt P, McCoy K, Wilson CB, Inglis A, Smith A, Martin TR, Ramsey BW. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol. 2001;32:356–366. doi: 10.1002/ppul.1144. [DOI] [PubMed] [Google Scholar]
- 5.Levy H, Murphy A, Zou F, Gerard C, Klanderman B, Schuemann B, Lazarus R, Garcia KC, Celedon JC, Drumm M, Dahmer M, Quasney M, Schneck K, Reske M, Knowles MR, Pier GB, Lange C, Weiss ST. IL1B polymorphisms modulate cystic fibrosis lung disease. Pediatr Pulmonol. 2009;44:580–593. doi: 10.1002/ppul.21026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LiPuma JJ, Dasen SE, Nielson DW, Stern RC, Stull TL. Person-to-person transmission of Pseudomonas cepacia between patients with cystic fibrosis. Lancet. 1990;336:1094–1096. doi: 10.1016/0140-6736(90)92571-x. [DOI] [PubMed] [Google Scholar]
- 7.De Soyza A, Ellis CD, Khan CM, Corris PA, Demarco de Hormaeche R. Burkholderia cenocepacia lipopolysaccharide, lipid A, and proinflammatory activity. Am J Respir Crit Care Med. 2004;170:70–77. doi: 10.1164/rccm.200304-592OC. [DOI] [PubMed] [Google Scholar]
- 8.Isles A, Maclusky I, Corey M, Gold R, Prober C, Fleming P, Levison H. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984;104:206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- 9.Jones AM, Dodd ME, Govan JR, Barcus V, Doherty CJ, Morris J, Webb AK. Burkholderia cenocepacia and Burkholderia multivorans: influence on survival in cystic fibrosis. Thorax. 2004;59:948–951. doi: 10.1136/thx.2003.017210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olland A, Falcoz PE, Kessler R, Massard G. Should cystic fibrosis patients infected with Burkholderia cepacia complex be listed for lung transplantation? Interact Cardiovasc Thorac Surg. 2011 doi: 10.1510/icvts.2011.271874. [DOI] [PubMed] [Google Scholar]
- 11.De Soyza A, Meachery G, Hester KL, Nicholson A, Parry G, Tocewicz K, Pillay T, Clark S, Lordan JL, Schueler S, Fisher AJ, Dark JH, Gould FK, Corris PA. Lung transplantation for patients with cystic fibrosis and Burkholderia cepacia complex infection: a single-center experience. J Heart Lung Transplant. 2010;29:1395–1404. doi: 10.1016/j.healun.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 12.van de Veerdonk FL, Smeekens SP, Joosten LA, Kullberg BJ, Dinarello CA, van der Meer JW, Netea MG. Reactive oxygen species-independent activation of the IL-1beta inflammasome in cells from patients with chronic granulomatous disease. Proc Natl Acad Sci U S A. 2010;107:3030–3033. doi: 10.1073/pnas.0914795107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotrange S, Kopp B, Akhter A, Abdelaziz D, Abu Khweek A, Caution K, Abdulrahman B, Wewers MD, Mccoy K, Marsh C, Loutet SA, Ortega X, Valvano MA, Amer AO. Burkholderia cenocepacia O polysaccharide chain contributes to caspase-1-dependent IL-1 beta production in macrophages. Journal of Leukocyte Biology. 2011;89:481–488. doi: 10.1189/jlb.0910513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranganathan SC, Parsons F, Gangell C, Brennan S, Stick SM, Sly PD. Evolution of pulmonary inflammation and nutritional status in infants and young children with cystic fibrosis. Thorax. 2011;66:408–413. doi: 10.1136/thx.2010.139493. [DOI] [PubMed] [Google Scholar]
- 15.McKeon S, McClean S, Callaghan M. Macrophage responses to CF pathogens: JNK MAP kinase signaling by Burkholderia cepacia complex lipopolysaccharide. FEMS Immunol Med Microbiol. 2010;60:36–43. doi: 10.1111/j.1574-695X.2010.00712.x. [DOI] [PubMed] [Google Scholar]
- 16.Holden MT, Seth-Smith HM, Crossman LC, Sebaihia M, Bentley SD, Cerdeno-Tarraga AM, Thomson NR, Bason N, Quail MA, Sharp S, Cherevach I, Churcher C, Goodhead I, Hauser H, Holroyd N, Mungall K, Scott P, Walker D, White B, Rose H, Iversen P, Mil-Homens D, Rocha EP, Fialho AM, Baldwin A, Dowson C, Barrell BG, Govan JR, Vandamme P, Hart CA, Mahenthiralingam E, Parkhill J. The genome of Burkholderia cenocepacia J2315, an epidemic pathogen of cystic fibrosis patients. J Bacteriol. 2009;191:261–277. doi: 10.1128/JB.01230-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govan JR, Brown PH, Maddison J, Doherty CJ, Nelson JW, Dodd M, Greening AP, Webb AK. Evidence for transmission of Pseudomonas cepacia by social contact in cystic fibrosis. Lancet. 1993;342:15–19. doi: 10.1016/0140-6736(93)91881-l. [DOI] [PubMed] [Google Scholar]
- 18.Akhter A, Gavrilin MA, Frantz L, Washington S, Ditty C, Limoli D, Day C, Sarkar A, Newland C, Butchar J, Marsh CB, Wewers MD, Tridandapani S, Kanneganti TD, Amer AO. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 2009;5:e1000361. doi: 10.1371/journal.ppat.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotrange S, Kopp B, Akhter A, Abdelaziz D, Abu Khweek A, Caution K, Abdulrahman B, Wewers MD, McCoy K, Marsh C, Loutet SA, Ortega X, Valvano MA, Amer AO. Burkholderia cenocepacia O polysaccharide chain contributes to caspase-1-dependent IL-1{beta} production in macrophages. J Leukoc Biol. 2010 doi: 10.1189/jlb.0910513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdulrahman BA, Khweek AA, Akhter A, Caution K, Kotrange S, Abdelaziz DH, Newland C, Roberto Rosales-Reyes R, Kopp B, McCoy K, Montione R, Schlesinger LS, Gavrilin MA, Wewers MD, Valvano MA, Amer A. Autophagy stimulation by rapamycin suppresses lung inflammation and infection by Burkholderia cenocepacia in a model of cystic fibrosis. Autophagy. 2011;7 doi: 10.4161/auto.7.11.17660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kazachkov M, Lager J, LiPuma J, Barker PM. Survival following Burkholderia cepacia sepsis in a patient with cystic fibrosis treated with corticosteroids. Pediatr Pulmonol. 2001;32:338–340. doi: 10.1002/ppul.1127. [DOI] [PubMed] [Google Scholar]
- 22.Lamothe J, Valvano MA. Burkholderia cenocepacia-induced delay of acidification and phagolysosomal fusion in cystic fibrosis transmembrane conductance regulator (CFTR)-defective macrophages. Microbiology. 2008;154:3825–3834. doi: 10.1099/mic.0.2008/023200-0. [DOI] [PubMed] [Google Scholar]
- 23.Saldias MS, Ortega X, Valvano MA. Burkholderia cenocepacia O antigen lipopolysaccharide prevents phagocytosis by macrophages and adhesion to epithelial cells. J Med Microbiol. 2009;58:1542–1548. doi: 10.1099/jmm.0.013235-0. [DOI] [PubMed] [Google Scholar]
- 24.Keith KE, Hynes DW, Sholdice JE, Valvano MA. Delayed association of the NADPH oxidase complex with macrophage vacuoles containing the opportunistic pathogen Burkholderia cenocepacia. Microbiology. 2009;155:1004–1015. doi: 10.1099/mic.0.026781-0. [DOI] [PubMed] [Google Scholar]
- 25.Pillarisetti N, Williamson E, Linnane B, Skoric B, Robertson CF, Robinson P, Massie J, Hall GL, Sly P, Stick S, Ranganathan S. Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med. 2011;184:75–81. doi: 10.1164/rccm.201011-1892OC. [DOI] [PubMed] [Google Scholar]
- 26.Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152:2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 27.Kuemmerle-Deschner JB, Ramos E, Blank N, Roesler J, Felix SD, Jung T, Stricker K, Chakraborty A, Tannenbaum S, Wright AM, Rordorf C. Canakinumab (ACZ885, a fully human IgG1 anti-IL-1beta mAb) induces sustained remission in pediatric patients with cryopyrin-associated periodic syndrome (CAPS) Arthritis Res Ther. 2011;13:R34. doi: 10.1186/ar3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez-Boyanapalli R, McPhillips KA, Frasch SC, Janssen WJ, Dinauer MC, Riches DW, Henson PM, Byrne A, Bratton DL. Impaired phagocytosis of apoptotic cells by macrophages in chronic granulomatous disease is reversed by IFN-gamma in a nitric oxide-dependent manner. J Immunol. 2010;185:4030–4041. doi: 10.4049/jimmunol.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodyear A, Jones A, Troyer R, Bielefeldt-Ohmann H, Dow S. Critical protective role for MCP-1 in pneumonic Burkholderia mallei infection. J Immunol. 2010;184:1445–1454. doi: 10.4049/jimmunol.0900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonfield TL, Konstan MW, Burfeind P, Panuska JR, Hilliard JB, Berger M. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol. 1995;13:257–261. doi: 10.1165/ajrcmb.13.3.7544594. [DOI] [PubMed] [Google Scholar]
- 31.Abe R, Hirasawa H, Oda S, Sadahiro T, Nakamura M, Watanabe E, Nakada TA, Hatano M, Tokuhisa T. Up-regulation of interleukin-10 mRNA expression in peripheral leukocytes predicts poor outcome and diminished human leukocyte antigen-DR expression on monocytes in septic patients. J Surg Res. 2008;147:1–8. doi: 10.1016/j.jss.2007.07.009. [DOI] [PubMed] [Google Scholar]