Abstract
Objective
To determine if there are gender-based differences in the inflammatory phenotype of patients undergoing lower extremity bypass (LEB), and if they correlate with clinical outcomes.
Methods
Retrospective analysis of a prospective cohort study of 225 patients (161 men and 64 women) who underwent LEB using autogenous vein between February 2004 and May 2008. Fasting baseline blood samples were obtained prior to surgery and included the inflammatory biomarkers high-sensitivity C-reactive protein (CRP) and fibrinogen. All patients underwent ultrasound graft surveillance. CRP levels were dichotomized at 5mg/L, and fibrinogen levels were dichotomized at 600mg/dL.
Results
There were no significant differences in age, race, history of hypertension or diabetes mellitus, body mass index, and coronary artery disease between men and women. Men were more likely to be current smokers (p=0.02), have a history of hypercholesterolemia (p=0.02) and taking statins (p=0.02). Women were more likely to present with critical limb ischemia (p=0.03) and had higher baseline CRP levels (median:5.15 mg/L; IQR:1.51-18.62mg/L) compared to men (median:2.70; IQR:1.24-6.98mg/L) (p=0.02). The median follow-up was 893 days (IQR:539-1315 days). In a multivariable Cox proportional hazards model for primary vein graft patency, there was a significant interaction between gender and both CRP (p=0.03) fibrinogen (p=0.02). After adjustment for key covariates, primary vein graft patency was significantly less in women with CRP>5mg/L compared to women with CRP<5mg/L (p=0.02), while there was no such difference seen in men (p=0.95). Primary graft patency was also decreased in women with fibrinogen>600mg/dL compared to women with fibrinogen<600mg/dL (p=0.002), but again, this pattern was not evident in men (p=0.19).
Conclusions
Women undergoing LEB for advanced peripheral artery disease have a different inflammatory phenotype compared to men, and elevated baseline levels of CRP and fibrinogen are associated with inferior vein graft patency in women, but not in men. These findings indicate an important interaction between gender and inflammation in the healing response of lower extremity vein bypass grafts. Women with elevated pre-operative CRP and fibrinogen levels may benefit from more intensive post-operative graft surveillance protocols.
Introduction
Although women have lower overall rates of cardiovascular disease (CVD) than men until the seventh decade of life1, several population studies have demonstrated higher age-adjusted rates of peripheral artery disease (PAD) in women2-4. Also, women who undergo lower extremity bypass (LEB) procedures appear to have increased rates of wound complications and lower rates of graft patency compared to men5-7. The potential reasons for these poorer outcomes in women include smaller conduit and target vessels, more advanced disease, older age at presentation, and a difference in the underlying state of inflammation in women compared to men.
Clinical studies have established that chronic inflammation is a strong risk factor for future atherothrombotic disease. C-reactive protein (CRP) is an acute-phase protein that is elevated in persons at high risk for myocardial infarction8-10, stroke9,11, and cardiovascular death9. CRP levels are also elevated in individuals with PAD12,13, and higher CRP levels have been associated with both risk and progression of PAD14,15. Recent studies have also suggested that preoperative CRP levels are predictive of adverse outcomes following vascular procedures, including lower extremity bypass surgery16-18.
Fibrinogen, like CRP, is an acute phase reactant19. In addition, fibrinogen has important hemostatic properties, as it affects platelet and red cell aggregation as well as endothelial function19. As a result, high levels of fibrinogen in plasma might reduce blood flow, predispose to thrombosis, and enhance atherogenesis. Fibrinogen also plays a critical role in inflammation; in an ex-vivo study, fibrinogen was important in mediating leukocyte adhesion to human vein grafts20. Fibrinogen also predicts the severity of PAD17,21, and serves as a marker for the future development of PAD22,23. Moreover, some studies have also demonstrated a positive association between plasma fibrinogen levels and subsequent graft stenosis and occlusion24,25.
Several population studies have demonstrated higher levels of both CRP26,27 and fibrinogen28-30 in women compared to men, but the clinical significance of this is not known. Although many studies have evaluated the association between inflammatory markers and CVD outcomes, fewer have investigated the role of inflammation and graft patency after LEB procedures. Moreover, it is unclear whether the differences in the underlying inflammatory profiles between men and women are important in the outcome after LEB. To address this gap in research, our current study investigated whether there are gender-based differences in the baseline inflammatory markers of patients undergoing LEB, and if these biomarker levels are associated with primary vein graft patency.
Methods
Study design
This was a prospective cohort study sponsored by the National Institutes of Health, and conducted at three Boston academic medical centers. The study sought to determine the relationship between inflammation and outcomes following LEB using autogenous vein grafts. Enrollment began in February 2004 and ended in May 2008, and included a total of 225 patients. Details of patient selection, as well as inclusion and exclusion criteria, and operative procedure have been previously described17,31. Subjects were excluded if they were treated with a prosthetic or nonautologous vein graft, had a history of a hypercoagulable state, or had a concurrent significant event within 30 days prior to the index bypass operation (i.e. myocardial infarction, stroke, or major surgical procedure). Patients with deep space infections of the foot or active infection were excluded from the study. Although patients with small ulcers or those with dry gangrene were included in the study, those with ulceration or gangrene requiring operative debridement were excluded.
Blood collection and biomarker measurement
Blood was collected while the patient was in the fasting state on the morning of the bypass procedure. All samples were immediately centrifuged at 3000 rpm and stored at −80 degrees until the time of analysis. All samples were analyzed in batches at a core lab in order to minimize variability. CRP levels were determined by a high-sensitivity immunoturbidimetric assay on a Hitachi 917 analyzer (Roche-Diagnostics, Indianapolis, IN) using reagents and calibrators from Denka Seiken (Niigata, Japan), with a sensitivity of 0.03mg/L. Day-to-day variations of the assay at differing concentrations are less than 3%. Fibrinogen was measured using an immunonephelometric technique on the Behring BNII analyzer (Dade Behring, Newark, DE). Other inflammatory and metabolic biomarkers were measured in the parent study and have been previously reported31.
Covariates
Race and gender were assessed by self-report. Hypertension was defined as systolic blood pressure >140mmHg, diastolic blood pressure > 90mmHg, or if the patient was taking prescription medications for hypertension. Both hypercholesterolemia and diabetes mellitus were present if subjects were taking prescribed medications or a self-report of the diagnosis. Active smoking was defined by a positive answer to the question “Do you now smoke cigarettes?” and a former smoker was defined as one who had smoked over 100 cigarettes in his/her life but had not smoked in 30 days. Chronic kidney disease was defined by an estimated glomerular filtration rate (eGFR) of <60 ml/min/1.73m2 using the Modification of Diet in Renal Disease Study equation32. Body mass index (BMI) was calculated as weight divided by height and expressed as kg/m2.
CRP levels were dichotomized at 5mg/L and fibrinogen levels at 600mg/dL. Although the American Heart Association and Center for Disease Control and Prevention (AHA/CDC) guidelines support measurement of CRP for risk stratification and considers CRP>3mg/L to be a high cardiovascular risk, the median value in our overall cohort was nearly 3mg/L, and the median value in women was over 5mg/L. We dichotomized at CRP>5mg/L as this value has previously been shown to represent a high risk subgroup in a stroke cohort33, and patients with CRP>5mg/L have been shown to have impaired early vein graft luminal remodeling34.
CRP>5mg/L also represented the upper limit of our core laboratory’s reference range.. With regards to fibrinogen, the standard reference values range from 150-450mg/dL19. The median value in our cohort was 480 mg/dL (IQR 401-585 mg/dL); we chose to dichotomize at 600 mg/dL, as this represented the top quartile of values.
Operative procedure and surveillance
All patients underwent LEB using autogenous vein, with ipsilateral greater saphenous vein (GSV) as the conduit of first choice. In the absence of suitable ipsilateral vein, the contralateral GSV or arm vein was used. Routine follow-up included a complete vascular examination by the attending surgeon as well as a standard duplex ultrasound examination of the bypass graft performed in an accredited vascular laboratory at 1, 3, 6, 9, and 12 months postoperatively. After 12 months, follow-up visits occurred every 6 months. All clinical and graft-related events were recorded prospectively. Although the decision to intervene on a failing graft was not standardized across all surgeons, as a general rule, all patients underwent graft intervention for all hemodynamically significant stenoses detected on surveillance ultrasound. The type of graft re-intervention was left to the discretion of the operating surgeon.
Definition of primary patency
Loss of primary patency included any type of graft revision (balloon angioplasty, patch angioplasty, or interposition graft) when a graft stenosis was detected by duplex ultrasound or other imaging modality. Loss of primary patency was also noted if there was documentation of occlusion of the vein graft without revision.
Statistical analysis
All statistical analyses were performed using STATA/SE version 10.1 (StataCorp, College Station, TX). The clinical characteristics and biomarker values of the cohort were compared for men and women and presented as proportions for categorical characteristics and as mean ± standard deviation (SD) or median and interquartile range (IQR) for continuous characteristics. Continuous variables between groups were compared with the Student’s t-test; if the distribution was not approximately normal, the Wilcoxon rank sum test was used. Proportions between groups were compared with the Pearson’s chi-square test. A p-value of <0.05 was considered statistically significant.
Variable selection for multivariable models
The bivariate associations between clinical variables, graft characteristics, and biomarker levels with primary graft patency were assessed for the overall cohort using a Cox proportional hazards model. Variable selection was based on this primary analysis. Any of the variables with a p-value of <0.30 was included in the subsequent multivariable models. Interactions between gender and biomarker levels, race and biomarker levels, gender and race, and gender and critical ischemia were also assessed and further analyzed if the p-value for interaction was <0.30. A multivariable Cox proportional hazards model was used to evaluate primary patency in men and women. We chose not to include both CRP and fibrinogen in the same multivariable models because it is scientifically plausible that CRP and fibrinogen are potential mediators for one another in the pathway to graft failure; hence, including both biomarkers in the same model would result in over-adjustment. Indeed, CRP and fibrinogen were highly correlated in our cohort (Pearson correlation coefficient=0.56, p<0.001).
Results
Among the 225 patients, the mean age was 68±11 years, 28% were women, and 8% were black. There were no significant differences in age, race, diabetes mellitus, BMI, eGFR, history of hypertension, and coronary artery disease between men and women (Table 1). Men were more likely to be current smokers (p=0.02), have a history of hypercholesterolemia (p=0.02), and be on statin therapy (p=0.02) compared to women (Table 1). Women were more likely to present with critical limb ischemia (p=0.03) and had significantly higher baseline CRP levels (p=0.02) compared with men (Table 1).
Table 1.
Demographic characteristics of the cohort.
| Men | Women | P-value | |
|---|---|---|---|
|
| |||
| Number | 161 | 64 | |
| Age (years) | 67.0±10.4 | 69.3±12.0 | 0.14 |
| Black race | 9 (5.6%) | 8 (12.5%) | 0.09 |
| Current Smoker | 67 (41.6%) | 16 (25.0%) | 0.02 |
| Diabetes | 88 (54.7%) | 29 (45.3%) | 0.21 |
| BMI (kg/m2) | 27.9±5.4 | 29.6±8.6 | 0.08 |
| Hypertension | 133 (82.6%) | 57 (89.1%) | 0.23 |
| CAD | 90 (55.9%) | 28 (43.8%) | 0.10 |
| eGFR (ml/min/1.73m2) | 78.2±32.9 | 70.8±39.3 | 0.15 |
| Hypercholesterolemia | 123 (76.4%) | 39 (60.9%) | 0.02 |
| Statin therapy | 135 (83.9%) | 45 (70.3%) | 0.02 |
| Critical ischemia | 88 (54.7%) | 45 (70.3%) | 0.03 |
| Composite vein | 21 (13.0%) | 14 (21.9%) | 0.10 |
| CRP (mg/L) * | 2.70 (1.24,6.98) | 5.15 (1.51,18.62) | 0.02 |
| CRP>5 mg/L | 56 (34.8%) | 34 (53.1%) | 0.01 |
| Fibrinogen (mg/dL) | 498±159 | 536±211 | 0.16 |
| Fibrinogen>600 mg/dL | 38 (23.6%) | 21 (32.8%) | 0.16 |
Median (IQR)
The indication for bypass graft surgery was critical limb ischemia in 133 (59%) patients, and 73 (32%) patients had evidence of distal tissue loss. One hundred ninety (84%) patients underwent bypass graft placement with a single-segment GSV, whereas the remainder underwent construction of bypass graft using composite GSV or arm vein. Twenty two (10%) patients had a previous ipsilateral bypass graft that failed. There were no differences in the type of conduit used, primary vs. re-do bypass, or type of outflow vessel (popliteal, tibial, or pedal artery) in women compared with men.
The median CRP level in the entire cohort was 2.98 mg/L (IQR: 1.28, 12.25). Women (5.15 mg/L; IQR 1.51,18.62) had a higher median CRP level compared to men (2.70 mg/L; IQR 1.24, 6.98) (p=0.02, Table 1). The mean fibrinogen level in the cohort was 509±176 mg/dL. The mean level of fibrinogen was not significantly different in women (536±211 mg/dL) compared with men (498±159 mg/dL) (p=0.16, Table 1). In a multivariable analysis that included gender, race, age, BMI, coronary artery disease, diabetes, statin use, critical ischemia, current smoking, and eGFR, both gender (p=0.04) and critical ischemia (p<0.001) were independently associated with baseline CRP level >5mg/L. In a similar model for fibrinogen, diabetes (p=0.008) and critical ischemia (p=0.03) were associated with baseline fibrinogen levels >600mg/dL.
The median follow-up was 893 days (IQR:539-1315 days). There were 78 (35%) primary graft failures; this occurred in 27/64 (42%) women and 51/161 (32%) men (p=0.14) Of the 78 primary patency events, 26 (33%) were graft thromboses and 52 (67%) were revisions for graft stenosis. Men and women had similar profiles for loss of primary patency, with 10/27 (37%) thrombotic events in women and 16/51 (31%) thrombotic events in men (p=0.61). There was no significant difference in the type of primary patency event (stenosis vs. thrombosis) based on CRP levels (p=0.20) or fibrinogen values (p=0.48) in the overall cohort, or when analyzed separately by gender. There were 35 secondary graft failures in this cohort, 11/64 (17%) in women and 24/161 (15%) in men (p=0.67).
The bivariate associations between all of the variables listed in Table 1 and primary graft patency were evaluated in the entire cohort, and then separately for men and women (Table 2). In the overall cohort, black race (p=0.002), diabetes mellitus (p=0.07), critical ischemia (p=0.009), composite vein graft (p=0.06), CRP>5mg/L (p=0.02), and fibrinogen>600mg/dL (p=0.17) met the criteria (p<0.30) for inclusion into the multivariable models. When these bivariate associations between predictors and primary graft failure were stratified by gender, the presence of diabetes mellitus (p=0.05) and critical ischemia (p=0.02) were significantly associated with primary graft failure in men. In women, black race (p=0.03), CRP>5mg/L (p=0.02), and fibrinogen>600mg/dL (p=0.01) were associated with primary graft failure (Table 2).
Table 2.
Hazard ratios for bivariate associations between predictors and primary patency, stratified by gender.
| Men | P-value | Women | P-value | |
|---|---|---|---|---|
| Age | 1.00 (0.98,1.03) | 0.72 | 1.00 (0.97,1.04) | 0.90 |
| Black race | 2.39 (0.95,6.06) | 0.07 | 2.80 (1.11,7.06) | 0.03 |
| Smoking | 1.06 (0.60,1.85) | 0.85 | 0.94 (0.39,2.25) | 0.89 |
| Diabetes | 1.80 (1.01,3.21) | 0.05 | 1.17 (0.53,2.57) | 0.70 |
| BMI | 0.97 (0.91,1.02) | 0.22 | 1.01 (0.97,1.06) | 0.57 |
| Hypertension | 1.01 (0.49,2.08) | 0.97 | 3.18 (0.43,23.54) | 0.26 |
| CAD | 1.08 (0.62,1.88) | 0.79 | 1.64 (0.75,3.61) | 0.22 |
| Hypercholesterolemia | 0.84 (0.44,1.57) | 0.58 | 2.36 (0.94,5.91) | 0.07 |
| Statin therapy | 0.68 (0.35,1.32) | 0.25 | 1.07 (0.45,2.56) | 0.88 |
| eGFR | 1.00 (0.99,1.01) | 0.66 | 1.00 (0.99,1.01) | 0.74 |
| Critical ischemia | 2.02 (1.14,3.63) | 0.02 | 1.42 (0.59,3.40) | 0.43 |
| Composite vein | 1.41 (0.67,3.03) | 0.36 | 1.93 (0.85,4.36) | 0.12 |
| CRP>5 mg/L | 1.35 (0.76,2.39) | 0.30 | 2.64 (1.16, 6.02) | 0.02 |
| Fibrinogen>600 mg/dL | 0.94 (0.48, 1.84) | 0.87 | 2.87 (1.29, 6.38) | 0.01 |
In a multivariable Cox proportional hazards model for primary graft patency for the entire cohort, there was no significant difference in primary vein graft patency in those individuals with CRP>5mg/L compared to those with CRP<5mg/L (p= 0.11) (Figure 1a). However, there was a statistically significant interaction between gender and CRP levels (p=0.03). Women with CRP>5mg/L were significantly more likely to lose primary vein graft patency compared to women with CRP<5mg/L (HR 2.63 [1.15,6.02], p=0.02), but there was no such difference seen in men (HR 0.98 [0.52,1.85], p=0.95) (Figures 1b, 1c). The findings were similar with respect to secondary graft patency. Women with CRP>5mg/L were significantly more likely to lose secondary graft patency compared to women with CRP<5mg/L (p=0.05), but no such difference was seen in men (p=0.78).
Figure 1a.
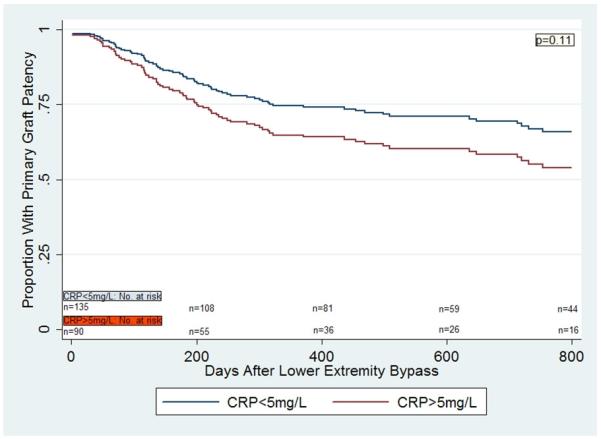
Multivariable Cox regression curves for primary graft patency in patients undergoing lower extremity bypass surgery with autogenous vein, based on CRP values.
Figure 1b.
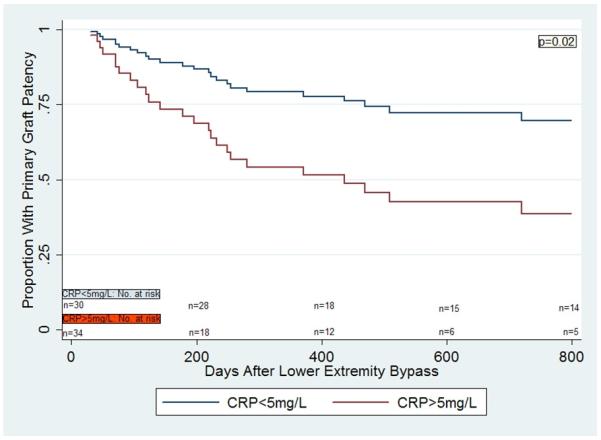
Multivariable Cox regression curves for primary graft patency in women, based on CRP values.
Figure 1c.
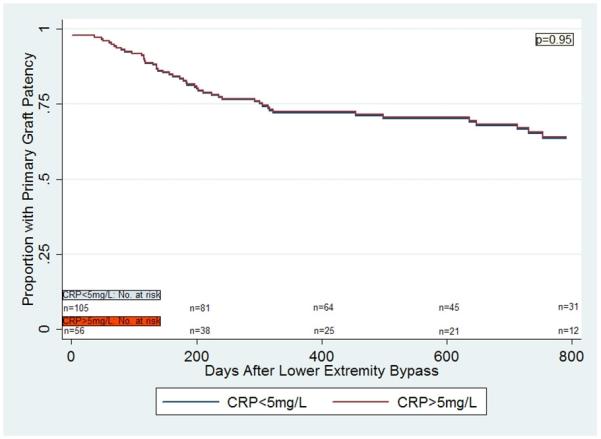
Multivariable Cox regression curves for primary graft patency in men, based on CRP values.
There was also a significant interaction between gender and fibrinogen (p=0.02). In a multivariable Cox proportional hazards model for primary graft patency, there was no significant difference in primary vein graft patency in those with fibrinogen>600mg/dL compared to those with fibrinogen<600mg/dL in the overall cohort (p=0.48, Figure 2a). However, women with fibrinogen>600mg/dL were significantly more likely to lose primary vein graft patency compared to women with fibrinogen<600mg/dL (HR 4.09 [1.66,10.05], p=0.002, Figure 2b), but again, no such difference was demonstrated in men (HR 0.62 [0.30,1.27], p=0.19, Figure 2c). These findings were similar when secondary graft patency was evaluated. Women with fibrinogen>600mg/dL were significantly more likely to lose secondary vein graft patency compared to women with fibrinogen<600mg/dL (p=0.03), but no difference was demonstrated in men (p=0.45).
Figure 2a.
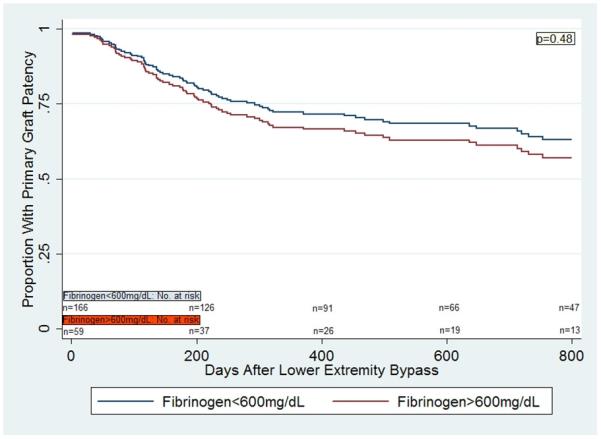
Multivariable Cox regression curves for primary graft patency in patients undergoing lower extremity bypass surgery with autogenous vein, based on fibrinogen levels.
Figure 2b.

Multivariable Cox regression curves for primary graft patency in women, based on fibrinogen levels.
Figure 2c.
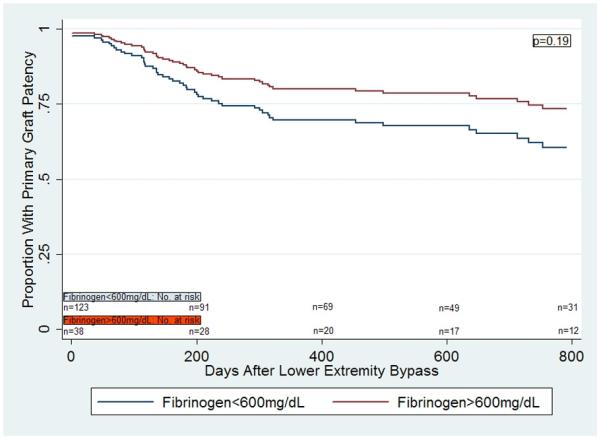
Multivariable Cox regression curves for primary graft patency in men, based on fibrinogen levels.
The hazard ratios for loss of primary graft patency, stratified by gender, and in separate multivariable models of CRP and fibrinogen are shown in Tables 3 and 4, respectively. In the CRP multivariable model for primary graft patency, black race was significantly associated with primary graft loss in men (HR 2.68 [1.04,6.86], p=0.04), whereas both black race (HR 3.04 [1.14,8.10], p=0.03) and CRP>5mg/L (HR 2.63 [1.15,6.02, p=0.02) were significantly associated with primary graft loss in women (Table 3). In the multivariable model with fibrinogen, black race (HR 2.80 [1.09,7.22], p=0.03) and critical ischemia (HR 1.87 [1.00,3.50], p=0.05) were significantly associated with primary graft loss in men, whereas in women, black race (HR 4.22 [1.53,11.64], p=0.005) and fibrinogen>600mg/dL (HR 4.09 [1.66,10.05], p=0.002) were significantly associated with primary graft loss (Table 4).
Table 3.
Hazard ratios for Multivariable Cox Proportional Hazards Model for primary graft patency including CRP, stratified by gender.
| Men | P-value | Women | P-value | |
|---|---|---|---|---|
| Black race | 2.68 (1.04,6.86) | 0.04 | 3.04 (1.14,8.10) | 0.03 |
| Diabetes | 1.64 (0.88,3.04) | 0.12 | 1.10 (0.47,2.57) | 0.82 |
| Composite vein | 1.21 (0.56,2.64) | 0.63 | 1.85 (0.78,4.39) | 0.16 |
| Critical ischemia | 1.72 (0.88,3.34) | 0.11 | 1.16 (0.48,2.83) | 0.74 |
| CRP >5 mg/L | 0.98 (0.52,1.85) | 0.95 | 2.63 (1.15,6.02) | 0.02 |
Table 4.
Hazard ratios for Multivariable Cox Proportional Hazards model for primary graft patency including fibrinogen, stratified by gender.
| Men | P-value | Women | P-value | |
|---|---|---|---|---|
| Black race | 2.80 (1.09,7.22) | 0.03 | 4.22 (1.53,11.64) | 0.005 |
| Diabetes | 1.85 (0.98,3.50) | 0.06 | 0.92 (0.40,2.10) | 0.84 |
| Composite vein | 1.13 (0.51,2.46) | 0.77 | 2.11 (0.92,4.83) | 0.08 |
| Critical ischemia | 1.87 (1.00,3.50) | 0.05 | 0.84 (0.33,2.14) | 0.71 |
| Fibrinogen>600 mg/dL | 0.62 (0.30,1.27) | 0.19 | 4.09 (1.66,10.05) | 0.002 |
Discussion
This prospective study of patients with advanced PAD undergoing LEB with autogenous vein demonstrated that elevated baseline levels of CRP and fibrinogen were each associated with graft failure in women but not in men. We found a significant interaction between gender and both CRP and fibrinogen in the primary patency rate of lower extremity vein bypass grafts, suggesting a different underlying inflammatory profile in men and women who present with severe PAD.
In our study, women had significantly higher baseline levels of CRP compared to men. This is not surprising, as several large cohort studies have demonstrated differences in CRP levels by both gender and race. The Multiethnic Study of Atherosclerosis (MESA) cohort of 6814 men and women between the ages of 45 to 84 years found substantially higher median CRP levels in women compared to men, despite accounting for estrogen use, BMI, and other confounding variables26. This gender difference was maintained across all ethnic subgroups. In the Dallas Heart Study, women had significantly higher CRP levels than men, and black subjects had significantly higher levels than white subjects27. These findings remained significant after adjustment for BMI and traditional cardiovascular risk factors, as well as after exclusion of subjects taking statins and estrogens. Our study population is limited with regards to racial diversity, therefore we were unable to examine the potential synergies between gender, race, and inflammation. Although men and women differed in several baseline factors, it is noteworthy that female gender was independently correlated with CRP level even after adjustment for multiple covariates.
Previous studies have demonstrated CRP to independently predict future CVD events in both men and women8-10. However, subgroup observations from both the Cardiovascular Health Study (CHS) and the Rural Health Promotion Project demonstrated the risks of vascular disease associated with CRP to be greater for women than for men35. Similarly, in the Women’s Health Study, the adjusted relative risk for either MI or stroke in women with the highest quartile of CRP was 5.5 compared with 2.8 in men participating in the Physicians’ Health Study8,9. Although CRP is a strong and independent risk factor for adverse cardiovascular outcomes in men and women, these findings suggest that women with elevated CRP may be at relatively higher risk for a cardiovascular event compared with men. Few studies have been performed examining the association between CRP and lower extremity bypass graft patency16-18, and none of these examined results by gender.
Recent work suggests there may be an important relationship between systemic inflammation and the early remodeling changes in lower extremity vein grafts; specifically, the early (one month) venous dilation response to arterialization appears to be impaired in patients with elevated baseline CRP levels34. This suggests a possible uncoupling between hemodynamic stress and vessel remodeling. If true, such impaired early remodeling may likely have greater clinical impact in the setting of smaller conduit vessels. In this study, we found that the divergence of loss of primary patency, by pre-operative biomarker level, primarily occurred within the first year, further supporting this idea. This hypothesis will need to be formally examined in future and ongoing studies.
Fibrinogen levels also differ by gender and race, and have been reported to be higher in women than men28-30, and in blacks compared to whites28-30. Fibrinogen levels increase with age, smoking, body size, diabetes, fasting serum insulin, low density lipoprotein cholesterol, and menopause28-30. A meta-analysis of fibrinogen as a CVD risk factor identified six prospective epidemiologic studies, all of which demonstrated that fibrinogen was associated with subsequent MI or stroke36. Although fibrinogen appears to be a strong CVD risk factor in both men and women, it is unclear from any of these previous studies whether there is a differential association in women compared to men.
We found a strong interaction between gender and fibrinogen with regards to vein graft patency in our cohort. A high fibrinogen level was significantly associated with graft failure in women, but not in men. Fibrinogen plays an important role in patients with PAD. In the CHS cohort, ankle-brachial index levels were inversely correlated with fibrinogen levels37. Plasma fibrinogen has also been shown to be a predictor of the development22,23 and severity of PAD17,21. In studies in PAD patients, fibrinogen levels were found to be the strongest independent predictor of death from coronary disease38 and all-cause cardiovascular mortality39. There are fewer data reporting the association between fibrinogen level and outcomes after lower extremity revascularization. Two reports evaluating patients with lower extremity vein grafts demonstrated elevated fibrinogen levels to be associated with both vein graft stenoses24 and graft occlusion25. In contrast, a study that evaluated 57 patients undergoing infrainguinal arterial reconstruction with saphenous vein narrowly failed to demonstrate a statistically significant association between fibrinogen levels and graft failure40. It is possible that a type II error occurred, or perhaps that an unidentified interaction contributed to the overall negative results of the study. To our knowledge, there are no studies that evaluate the association between fibrinogen and lower extremity bypass graft patency separately for women and men.
There are several studies that suggest that women have higher rates of graft failure and increased rates of wound complications following lower extremity bypass graft procedures, but clear predisposing factors have not been identified5-7. It is possible that women who present with advanced PAD have an underlying inflammatory phenotype that puts them at disproportionately higher risk for subsequent graft failure compared to men. Measurement of both CRP and plasma fibrinogen in women before revascularization may identify those who are at highest risk for graft failure, and could potentially improve results either by aggressive risk factor modification or a more intensive graft surveillance schedule. Prospective studies are needed to validate the use of these biomarkers for risk prediction in this population.
This study has several limitations. Our series of patients had advanced PAD, including a large proportion with critical limb ischemia. Also, all patients were treated with a vein conduit. Therefore, our results may not be generalizable to those with more mild disease or in those who undergo lower extremity bypass with a prosthetic graft. We dichotomized CRP at 5mg/L and fibrinogen at 600mg/dL, either or both of which may not be the ideal cutpoints. CRP and fibrinogen were not included together in the same multivariable Cox model, and hence we could not assess whether each biomarker was independently associated with graft failure. In addition, only preoperative levels of inflammatory markers were available in this study, and hence we could not evaluate whether changes in CRP and fibrinogen levels after LEB were associated with graft failure. The modest size and diversity of the study population also limit its generalizability. The findings should be considered hypothesis-generating and provide impetus for further studies examining the interaction of gender and inflammation in peripheral revascularization.
Acknowledgments
Funding support:
This project was supported by NIH/NCRR/OD UCSF-CTSI Grant Number KL2 RR024130 (JSH) and NHLBI K23 HL 92163 (CDO). This work was also supported in part by grants from the National Heart, Lung and Blood Institute (HL 75771 to CDO, MAC, MSC). Its contents are the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 26th annual meeting of the Western Vascular Society, September 18, 2011, Kauai, HI
References
- 1.Mosca L, Manson JE, Sutherland SE, Langer RD, Manolio T, Barrett-Connor E, Writing Group Cardiovascular disease in women: a statement for healthcare professionals from the American Heart Association. Circulation. 1997 Oct 7;96(7):2468–2482. doi: 10.1161/01.cir.96.7.2468. [DOI] [PubMed] [Google Scholar]
- 2.Kullo IJ, Bailey KR, Kardia SL, Mosley TH, Jr., Boerwinkle E, Turner ST. Ethnic differences in peripheral arterial disease in the NHLBI Genetic Epidemiology Network of Arteriopathy (GENOA) study. Vasc Med. 2003 Nov;8(4):237–242. doi: 10.1191/1358863x03vm511oa. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004 Aug 10;110(6):738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 4.Zheng ZJ, Rosamond WD, Chambless LE, et al. Lower extremity arterial disease assessed by ankle-brachial index in a middle-aged population of African Americans and whites: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Prev Med. 2005 Dec;29(5 Suppl 1):42–49. doi: 10.1016/j.amepre.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen LL, Hevelone N, Rogers SO, et al. Disparity in outcomes of surgical revascularization for limb salvage: race and gender are synergistic determinants of vein graft failure and limb loss. Circulation. 2009 Jan 6;119(1):123–130. doi: 10.1161/CIRCULATIONAHA.108.810341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen LL, Brahmanandam S, Bandyk DF, et al. Female gender and oral anticoagulants are associated with wound complications in lower extremity vein bypass: an analysis of 1404 operations for critical limb ischemia. J Vasc Surg. 2007 Dec;46(6):1191–1197. doi: 10.1016/j.jvs.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enzler MA, Ruoss M, Seifert B, Berger M. The influence of gender on the outcome of arterial procedures in the lower extremity. Eur J Vasc Endovasc Surg. 1996 May;11(4):446–452. doi: 10.1016/s1078-5884(96)80180-8. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998 Aug 25;98(8):731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997 Apr 3;336(14):973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 10.Koenig W, Sund M, Frohlich M, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999 Jan 19;99(2):237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 11.Chei CL, Yamagishi K, Kitamura A, et al. C-reactive protein levels and risk of stroke and its subtype in Japanese: The Circulatory Risk in Communities Study (CIRCS) Atherosclerosis. 2011 Jul;217(1):187–193. doi: 10.1016/j.atherosclerosis.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Beckman JA, Preis O, Ridker PM, Gerhard-Herman M. Comparison of usefulness of inflammatory markers in patients with versus without peripheral arterial disease in predicting adverse cardiovascular outcomes (myocardial infarction, stroke, and death) Am J Cardiol. 2005 Nov 15;96(10):1374–1378. doi: 10.1016/j.amjcard.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 13.McDermott MM, Guralnik JM, Corsi A, et al. Patterns of inflammation associated with peripheral arterial disease: the InCHIANTI study. Am Heart J. 2005 Aug;150(2):276–281. doi: 10.1016/j.ahj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation. 2005 Aug 16;112(7):976–983. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998 Feb 10;97(5):425–428. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 16.Biancari F, Alback A, Kantonen I, Luther M, Lepantalo M. Predictive factors for adverse outcome of pedal bypasses. Eur J Vasc Endovasc Surg. 1999 Aug;18(2):138–143. doi: 10.1053/ejvs.1999.0875. [DOI] [PubMed] [Google Scholar]
- 17.Owens CD, Ridker PM, Belkin M, et al. Elevated C-reactive protein levels are associated with postoperative events in patients undergoing lower extremity vein bypass surgery. J Vasc Surg. 2007 Jan;45(1):2–9. doi: 10.1016/j.jvs.2006.08.048. discussion 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matzke S, Biancari F, Ihlberg L, et al. Increased preoperative c-reactive protein level as a prognostic factor for postoperative amputation after femoropopliteal bypass surgery for CLI. Ann Chir Gynaecol. 2001;90(1):19–22. [PubMed] [Google Scholar]
- 19.Kamath S, Lip GY. Fibrinogen: biochemistry, epidemiology and determinants. QJM. 2003 Oct;96(10):711–729. doi: 10.1093/qjmed/hcg129. [DOI] [PubMed] [Google Scholar]
- 20.Eslami MH, Gangadharan SP, Belkin M, Donaldson MC, Whittemore AD, Conte MS. Monocyte adhesion to human vein grafts: a marker for occult intraoperative injury? J Vasc Surg. 2001 Nov;34(5):923–929. doi: 10.1067/mva.2001.118590. [DOI] [PubMed] [Google Scholar]
- 21.Lassila R, Peltonen S, Lepantalo M, Saarinen O, Kauhanen P, Manninen V. Severity of peripheral atherosclerosis is associated with fibrinogen and degradation of cross-linked fibrin. Arterioscler Thromb. 1993 Dec;13(12):1738–1742. doi: 10.1161/01.atv.13.12.1738. [DOI] [PubMed] [Google Scholar]
- 22.Smith FB, Lee AJ, Hau CM, Rumley A, Lowe GD, Fowkes FG. Plasma fibrinogen, haemostatic factors and prediction of peripheral arterial disease in the Edinburgh Artery Study. Blood Coagul Fibrinolysis. 2000 Jan;11(1):43–50. [PubMed] [Google Scholar]
- 23.Wattanakit K, Folsom AR, Selvin E, et al. Risk factors for peripheral arterial disease incidence in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2005 Jun;180(2):389–397. doi: 10.1016/j.atherosclerosis.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 24.Hicks RC, Ellis M, Mir-Hasseine R, et al. The influence of fibrinogen concentration on the development of vein graft stenoses. Eur J Vasc Endovasc Surg. 1995 May;9(4):415–420. doi: 10.1016/s1078-5884(05)80009-7. [DOI] [PubMed] [Google Scholar]
- 25.Wiseman S, Kenchington G, Dain R, et al. Influence of smoking and plasma factors on patency of femoropopliteal vein grafts. BMJ. 1989 Sep 9;299(6700):643–646. doi: 10.1136/bmj.299.6700.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakoski SG, Cushman M, Criqui M, et al. Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am Heart J. 2006 Sep;152(3):593–598. doi: 10.1016/j.ahj.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Khera A, McGuire DK, Murphy SA, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005 Aug 2;46(3):464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 28.Folsom AR, Qamhieh HT, Flack JM, et al. Plasma fibrinogen: levels and correlates in young adults. The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1993 Dec 15;138(12):1023–1036. doi: 10.1093/oxfordjournals.aje.a116821. [DOI] [PubMed] [Google Scholar]
- 29.Folsom AR, Wu KK, Davis CE, Conlan MG, Sorlie PD, Szklo M. Population correlates of plasma fibrinogen and factor VII, putative cardiovascular risk factors. Atherosclerosis. 1991 Dec;91(3):191–205. doi: 10.1016/0021-9150(91)90167-2. [DOI] [PubMed] [Google Scholar]
- 30.Kaptoge S, White IR, Thompson SG, et al. Associations of plasma fibrinogen levels with established cardiovascular disease risk factors, inflammatory markers, and other characteristics: individual participant meta-analysis of 154,211 adults in 31 prospective studies: the fibrinogen studies collaboration. Am J Epidemiol. 2007 Oct 15;166(8):867–879. doi: 10.1093/aje/kwm191. [DOI] [PubMed] [Google Scholar]
- 31.Owens CD, Kim JM, Hevelone ND, et al. Novel adipokines, high molecular weight adiponectin and resistin, are associated with outcomes following lower extremity revascularization with autogenous vein. J Vasc Surg. 2010 May;51(5):1152–1159. doi: 10.1016/j.jvs.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 33.Kabagambe EK, Judd SE, Howard VJ, et al. Inflammation biomarkers and risk of all-cause mortality in the reasons for geographic and racial differences in stroke cohort. Am J Epidemiol. 2011 Aug 1;174(3):284–292. doi: 10.1093/aje/kwr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owens CD, Rybicki FJ, Wake N, et al. Early remodeling of lower extremity vein grafts: inflammation influences biomechanical adaptation. J Vasc Surg. 2008 Jun;47(6):1235–1242. doi: 10.1016/j.jvs.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tracy RP, Lemaitre RN, Psaty BM, et al. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997 Jun;17(6):1121–1127. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 36.Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: a meta-analysis and review of the literature. Ann Intern Med. 1993 Jun 15;118(12):956–963. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]
- 37.Newman AB, Siscovick DS, Manolio TA, et al. Cardiovascular Heart Study (CHS) Collaborative Research Group Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Circulation. 1993 Sep;88(3):837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 38.Fowkes FG, Lowe GD, Housley E, et al. Cross-linked fibrin degradation products, progression of peripheral arterial disease, and risk of coronary heart disease. Lancet. 1993 Jul 10;342(8863):84–86. doi: 10.1016/0140-6736(93)91288-w. [DOI] [PubMed] [Google Scholar]
- 39.Doweik L, Maca T, Schillinger M, Budinsky A, Sabeti S, Minar E. Fibrinogen predicts mortality in high risk patients with peripheral artery disease. Eur J Vasc Endovasc Surg. 2003 Oct;26(4):381–386. doi: 10.1016/s1078-5884(03)00340-x. [DOI] [PubMed] [Google Scholar]
- 40.Beattie DK, Sian M, Greenhalgh RM, Davies AH. Influence of systemic factors on pre-existing intimal hyperplasia and their effect on the outcome of infrainguinal arterial reconstruction with vein. Br J Surg. 1999 Nov;86(11):1441–1447. doi: 10.1046/j.1365-2168.1999.01259.x. [DOI] [PubMed] [Google Scholar]


