Abstract
Both the serotonergic and endocannabinoid systems modulate frontocortical glutamate release; thus they are well positioned to participate in the pathogenesis of psychiatric disorders. With the help of fluorescent and confocal microscopy, we localized the CB1 cannabinoid receptor (CB1R) in VGLUT1- and 2- (i.e. glutamatergic) and serotonin transporter- (i.e. serotonergic) -positive fibers and nerve terminals in the mouse and rat frontal cortex.
CB1R activation by the synthetic agonists, WIN55212-2 (1 microM) and R-methanandamide (1 microM) inhibited the simultaneously measured evoked Ca2+-dependent release of [14C]glutamate and [3H]serotonin from frontocortical nerve terminals of Wistar rats, in a fashion sensitive to the CB1R antagonists, O-2050 (500 nM) and LY320135 (5 microM). CB1R agonists also inhibited the evoked release of [14C]glutamate in C57BL/6J mice in a reversible fashion upon washout.
Interestingly, the evoked release of [14C]glutamate and [3H]serotonin was significantly greater in the CB1R knockout CD-1 mice. Furthermore, CB1R binding revealed similar frontocortical CB1R density in the rat and the CD-1 mouse. Still, the evoked release of [3H]serotonin was modulated by neither CB1R agonists nor antagonists in wild-type CD-1 or C57BL/6J mice.
Altogether, this is the first study to demonstrate functional presynaptic CB1Rs in frontocortical glutamatergic and serotonergic terminals, revealing species differences.
Keywords: serotonin, glutamate, CB1 cannabinoid receptor, frontal cortex, rat, mouse, knockout
1. Introduction
The CB1 cannabinoid receptor (CB1R) is a presynaptic metabotropic receptor of high density in the vertebrate CNS. CB1Rs control the growth, maturation and function of both inhibitory and excitatory neurons and their synapses in the brain (Harkany et al., 2008; Mackie et al., 2008). In the post-synaptic dendritic compartments apposing these terminals, the endocannabinoids, anandamide and 2-arachidonoyl-glycerol (2-AG) are released upon Ca2+ entry and/or metabotropic receptor stimulation, and then quickly traverse the synaptic cleft to activate presynaptic CB1Rs (Harkany et al., 2008; Di Marzo, 2009; Kano et al., 2009; Haj-Dahmane and Shen, 2011).
The serotonergic system of the brain primarily originates from the raphe nuclei of the brain stem which give rise to a robust, mostly non-synaptic innervation diffusely throughout the brain to control a myriad of physiological functions, including synaptic plasticity, through a large number of distinct metabotropic serotonin receptors (Geyer and Vollenweider, 2008; Daubert and Condron, 2010).
Neurological and psychiatric disorders are often associated with long-term impairment of serotonergic and endocannabinoid control of synaptic plasticity in the prefrontal cortex (van der Stelt and Di Marzo, 2003; Haller et al., 2007; Köfalvi and Fritzsche, 2008; Daubert and Condron, 2010; Forbes and Grafman, 2010; Ashton and Moore, 2011). This is partially rationalized by 1) the highly overlapping distribution pattern of the CB1 cannabinoid receptor (CB1R) and serotonin receptors in the brain (Hermann et al., 2002); by 2) that both neuromodulator systems control glutamatergic neurotransmission in the prefrontal cortex (Auclair et al., 2000; Andrade, 2011); and by 3) that both serotonin and endocannabinoids can shape the connections and the maturation of the neocortical circuitry (Harkany et al., 2008; Daubert and Condron, 2010). Last but not least, exogenous (botanical and fungal) activators of both neuromodulator systems, i.e. cannabis and LSD, are popular recreational drugs with profound effects on mood, memory, thought and perception (O’Connor and Roth, 2005) – processes involving the frontal cortex (Forbes and Grafman, 2010) and subject to persistent impairment in some psychiatric disorders.
Due to the similarities in their many functions, one might expect the serotonergic and the endocannabinoid systems to efficiently cross-control the activity of each other. Indeed, it has been found that CB1R activation can influence the excitability of the raphe nuclei by modulating their excitatory and inhibitory inputs (Andrade, 2011; Haj-Dahmane and Shen, 2011), or – since CB1Rs have been found in the raphe serotonergic neurons (Häring et al., 2007) – by direct inhibition of the excitability of these latter neurons (Tzavara et al., 2003). CB1Rs can even alter the expression and the function of serotonin receptors (Haj-Dahmane and Shen, 2011), while serotonin receptor activation my induce endocannabinoid release (Best and Regehr, 2008).
Interestingly, it has been shown that the modulation by cannabinoids of anxiety may be substantially different between the rat and the mice (Haller et al., 2007). This suggests that the effect of cannabinoid receptor activation on serotonergic neuromodulation may differ between the two species.
Altogether, these observations prompt the question if the endocannabinoid system is capable of presynaptically controlling the serotonergic system in the frontal cortex? In addition, are there species differences in these interactions?
To answer these, we investigated if CB1Rs are present in serotonin transporter- (SERT-) positive terminals, using fluorescent and confocal microscopy in the frontal cortex of the rat and the wild-type and the CB1R knockout mice. We also tested if CB1R activation affects stimulation-evoked, Ca2+-dependent serotonin release from frontocortical synaptoneurosomes of the rat and two mouse strains, to assess 1) ligand selectivity and 2) putative species differences.
Previously, functional CB1Rs has been reported in frontocortical glutamatergic terminals (Auclair et al., 2000; Barbara et al., 2003; Lafourcade et al., 2007). Thus, as a positive control for our immuno- and neurochemical experiments, we proved our assay showing the presence and functionality of CB1Rs in glutamatergic terminals.
Materials and methods
2.1. Subjects
All studies were conducted in accordance with the principles and procedures outlined in the EU guidelines (86/609/EEC) and by FELASA, and in accordance with the recommendations of the NC3Rs Reporting Guidelines Working Group (2010), and were approved by the local Animal Care Committee of the Institute. Animals were housed in an SPF facility, with 12 h light on/off cycles and ad libitum access to food and water. Male Wistar rats (180–240 g, 8–10-week old) and C57BL/6j mice (28–32 g, 8–10-week old) were purchased from Charles-River (Barcelona, Spain). CB1R null-mutant (knockout) male mice (Ledent et al., 2000 and their wild-type littermates on CD-1 background were genotyped by tail snips, housed as detailed above and sacrificed daily in pairs (one WT and one KO), until 16 weeks of age.
2.2. Microscopy sections
Under deep sodium pentobarbital anaesthesia (100 mg/kg body weight, i.p.), male Wistar rats, CB1R null-mutant mice of the CD-1 strain and their wild-type littermates were transcardially perfused with fixative (4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4). The brains were removed and immersed in the same fixative overnight and then kept in 30% sucrose in physiological saline (0.9% NaCl) for at least 48 h before sectioning. Forty micron-thick sections from the mouse brains and 30-μm-thick sections from the rat brains were cut using a Cryostat microtome (Leica) and collected into 0.1 M PBS containing 0.1% sodium azide.
2.3. Immunohistochemistry
Sagittal brain sections containing the frontal cortex of the animals were selected. Whether rat and mice have a prefrontal cortex and, if yes, what are its borders is contentious (Preuss, 1995; Seamans et al., 2008). Thus, we focused our study on the area identified as frontal associative cortex in the rat and mouse (Paxinos and Franklin, 1997; Paxinos and Watson, 1998). We will use the term frontal cortex hereafter. Free floating sections were blocked in 10% normal goat serum (Vector Laboratories, CA, USA)/5% BSA/0.3% Triton X-100 for 40 min and incubated overnight in a primary antibody cocktail of L-15 rabbit anti-CB1R (1:1000; Bodor et al., 2005), and mouse monoclonal [4A2.2] anti-SERT (1:250; Abcam, UK), or guinea pig polyclonal anti-VGLUT1 (1:200; Synaptic Systems, Germany), or guinea pig polyclonal anti-VGLUT2 (1:200; Synaptic Systems). Sections were then washed in phosphate buffer (PB; 0.1 M) and incubated with a secondary antibody cocktail containing DyLight 594 goat anti-guinea pig or anti-rabbit as well as and DyLight 488 goat anti-mouse (all at 1:200; Kirkegaard & Perry Laboratories, Inc, USA), for 2 h. After washing in PB 0.1 M, the sections were mounted and coverslipped using Vectashield Hardest Mounting Medium (Vector Laboratories). Low magnification images were taken on a Zeiss Axiovert 200M microscope equipped with AxioVision software and MosaiX module. Confocal images were taken using a Zeiss LSM510 META confocal microscope.
2.4. Quality control for CB1R immunoreactivity
Before acquiring high resolution laser scanning images, settings were tested for possible autofluorescence in a control slice without primary and secondary antibody treatment. Parameters were set to obtain the representative image seen in Figure S1A of the Supplemental Material. Next, we observed that slices treated solely with secondary antibodies (DyLight 488 with DyLight 594) also failed to produce detectable staining (Figure S1B). In conventional Western blotting, the L-15 antibody recognized a band at ~55 kDa in the RIPA-buffer protein extract from the wild-type but not from the CB1R knockout mouse cortex (Figure S1C). CB1R staining in the rat and in the wild-type mouse was virtually the same as with the guinea pig anti-CB1R (1:1000; Frontier Science, Hokkaido, Japan; licensed by Dr. Masahiko Watanabe) at any resolution tested (Duarte et al., 2011). No immunostaining was detected in the CB1R knockout mouse brain sections at any resolution (Figure S1D1).
2.5. Dual-label [3H]serotonin/[14C]glutamate release assay from frontocortical nerve terminals
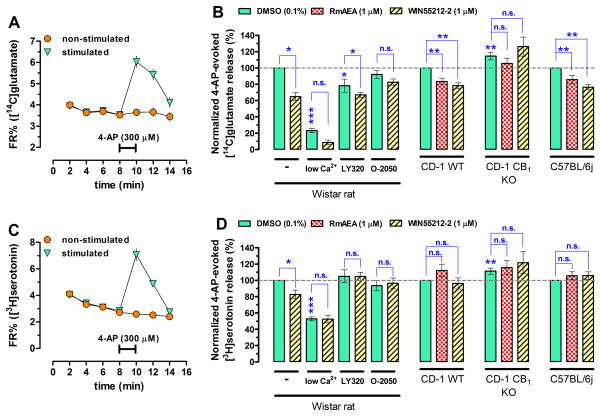
Experiments were carried out as before (Ferreira et al., 2009). The animals were decapitated under halothane anesthesia, and their brains were quickly removed into ice-cold sucrose solution (0.32 M, containing 5 mM HEPES, pH 7.4). Two minutes later, frontal cortices were quickly removed into 2 ml ice-cold sucrose solution, were homogenized instantly with a Teflon homogenizer, and centrifuged at 5,000 g for 5 min. The supernatant was centrifuged at 13,000 g for 10 min to obtain the P2 crude synaptosomal fraction. Synaptosomes were then diluted to 0.5 ml with Krebs’ solution (in mM: NaCl 113, KCl 3, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5, NaHCO3 25, glucose 10, HEPES 15, pH 7.4, 37°C), containing reboxetine (30 nM; Tocris Bioscience, UK) and GBR12783 (100 nM; Tocris Bioscience) to prevent the uptake of [3H]serotonin into noradrenergic and dopaminergic terminals. All assay medium also contained the MAO-B inhibitor, pargyline (10 μM) to prevent [3H]serotonin degradation, and the glutamate decarboxylase inhibitor, aminooxyacetic acid (100 μM) to prevent [14C]glutamate metabolism. Under these conditions, synaptosomes were incubated with both hydroxytryptamine 5-[1,2-3H] creatinine sulfate (American Radiolabeled Chemicals, Inc; Saint Louis, MO 63146 USA; final concentration, 300 nM) and [14C]-U-glutamate (PerkinElmer, USA; 20 μM) for 10 min. A 16-microvolume chamber perfusion setup was filled with the preloaded synaptosomes which were trapped by layers of Whatman GF/C filters and superfused continuously at a rate of 0.8 ml/min until the end of the experiment at 37°C. Upon termination of the 10-min washout, 2-min samples were collected for liquid scintillation assay, and the filters were also harvested to obtain the total radioactivity content. After collecting four 2-min samples as baseline, the evoked release of the transmitters was stimulated once with 4-aminopyridine (4-AP) for 2 min or 3 times with high K+ (25 mM, for 1 min) isomolar substitution of NaCl with KCl). Vehicle (0.1% DMSO if agonist was tested alone and 0.2% DMSO when agonist and antagonist were combined) and drugs were added 4 min before the stimulation. In each experiment, treatments were applied in duplicate (i.e. eight conditions or treatments in duplicate, each averaged as n = 1). For the single stimulation experiments with 4-AP, there was an unstimulated vehicle/drug baseline control that was then subtracted from the results obtained with stimulation to establish the effect of the stimulation (see Figure 3A,C). In this way we measured pure drug effects on the evoked release free from both the putative drug/vehicle effect on the baseline and the putative vehicle effect on the evoked release. In fact, there was neither significant drug/vehicle effect per se on the baseline or vehicle DMSO effect on the evoked release of either [3H]serotonin or [14C]glutamate (data not shown). As for the triple high-K+ stimulation, we always used the first peak as an internal control, and the second for testing the drug effect versus the vehicle, and the last stimulation was again a control to test if drug effects were persistent or transient.
Figure 3.
(A,C) Release diagrams showing the time-course of the release of [14C]glutamate and [3H]serotonin. (B) The synthetic non-selective CB1R agonist, WIN55212-2 (1 μM; hatched bars) and an anandamide analogue and selective CB1R agonists, R-methanandamide (RmAEA; 1 μM; checkerboard bars) both diminish the simultaneously measured 4-AP- (300 μM) evoked release of [14C]glutamate both in the rat and the CD-1 wild-type (WT) mice and in the C57BL/6 (WT) mice, but not in the CD-1 CB1R knockout (KO) mice. As expected, the effect of WIN55212-2 was sensitive to the CB1R antagonists/inverse agonists, LY320135 (5 μM) and O-2050 (1 μM) in the rat. (D) In contrast, the evoked release of [3H]serotonin was sensitive to WIN55212-2 only in the rat, and this effect of WIN55212-2 was also prevented by the CB1R antagonists/inverse agonists. In neither of the two mouse strains WIN55212-2 and RmAEA inhibited the evoked release of [3H]serotonin, suggesting species differences. The evoked release of both neurotransmitters was strongly Ca2+-dependent, which was measured employing 100 nM Ca2+ combined with 10 mM MgCl2. The Ca2+-independent fraction of the evoked release was already insensitive to CB1R activation. N ≥ 6, * p < 0.05; * p < 0.01; ***p < 0.001. Interestingly, the evoked release of [14C]glutamate and [3H]serotonin under control condition was greater in the CB1R global knockout mice. N = 15, * p < 0.05.
When the wild-type (WT) and CB1R KO mice were tested, one mouse of each strain was used simultaneously in the same experiment, i.e. 1 WT for 8 of the 16 channels and 1 KO for the other 8 channels.
The [14C] and [3H] content of each samples were counted by a dual-label protocol using a Tricarb β-counter (PerkinElmer), and DPM values were expressed as fractional release (FR%), i.e. the percent of actual content in the effluent as a function of the total synaptosomal content.
2.6. CB1R density measurement in frontocortical membranes of the rat and the CD-1 mouse with [3H]SR141716A binding
Membrane preparation was carried out according to Hartshorne and Catterall (1984), with slight modifications. Experiments were carried out with membrane preparations obtained from 4 rats and 4 WT and 4 CB1R KO mice in quintuplicates. CB1R binding with the selective CB1R antagonist/inverse agonist, [3H]SR141716A (American Radiolabeled Chemicals) was carried out with slight modifications to our previous protocol (Duarte et al., 2007). Membrane pellets were resuspended in 2 ml of ice-cold assay solution [Trish/HCl (50 mM), MgCl2 (3 mM), CaCl2 (1 μM), EDTA (2 mM) and protease inhibitor cocktail (Sigma, Saint Louis, Missouri, USA), pH = 7.4]. One uniplicate was made from 100 μl of assay solution containing AM251 (3 × 1 μM) or its vehicle DMSO (3 × 0.1%), plus 100 μl [3H]SR141716A (3 × 0.955 ± 0.086 nM) solution, and 100 μl of membrane suspension (3 × 0.1 mg protein). Hence, the final concentration of each constituent decreased to 1/3. Reactions were left at ambient temperature in Eppendorf tubes under agitation for 30 min, and then the reaction mixture was resuspended in 10 ml ice-cold assay solution in glass tubes and rapidly vacuum-filtered onto GF/B filters (Whatman, Sigma). Glass tubes were rinsed onto these filters once again. Filters were then collected into 20 ml scintillation vials, in 3 ml scintillation liquid, and counts were measured in two consecutive days until reaching stable DMP values. Protein quantity were determined with the BCA method for the different samples, and the specific binding values were expressed as fmole/mg protein.
2.7. Chemicals not listed above
WIN55212-2 mesylate and 4-aminopyridine were purchased from AscentScientific (UK). HEPES, sucrose, aminooxyacetic acid, tetrahydrolipstatin and pargyline were obtained from Sigma. R-methanandamide, O-2050, GBR12783, LY320135, and reboxetine mesylate were bought from Tocris Bioscience, UK. Non-water soluble substances were dissolved or reconstituted in DMSO (except R-methanandamide which came as an ethanol solution) and stored at − 20 °C until use.
2.7. Data treatment
All data represent mean ± SEM of n ≥ 4 observations (at least 6 animals in the release experiments and 4 animals for the binding experiment). Statistical significance was calculated on the raw data using repeated measures ANOVA with Bonferroni’s post-test for selected groups of data for the release and the binding experiments. Data then was normalized to everyday controls to visually enhance effect amplitudes. A p < 0.05 was accepted as significant difference.
3. Results
3.1. Immunohistochemistry
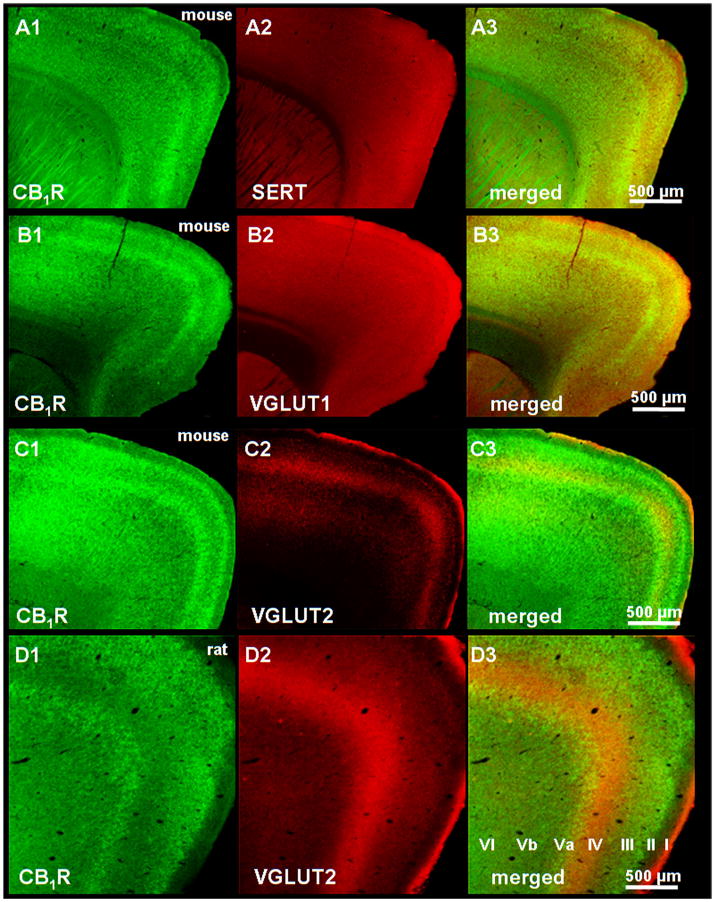
The intensity of CB1R staining varied across cortical layers of the rat and mice: layers II–III, Va and VI stained strongly with the CB1R antisera, intercalated with low (layers IV and Vb) or no CB1R staining (layer I) (Figure 1A1–C1, mouse, D1 rat). The serotonin transporter SERT and the vesicular glutamate transporter type-1 (VGLUT1) homogenously stained all layers of the cortex in the mouse (Figure 1A2,B2) and the rat (figure not shown), while VGLUT2 staining was complementary to CB1R-positive layers both in the mouse (Figure 1C2,3) and the rat (Figure 1D2,3), i.e. in layers IV and to a smaller extent, in layer Vb.
Figure 1.
Low magnification (5×) fluorescent microscope images showing the distribution of the CB1R immunoreactivity in sagittal frontocortical sections of the mouse (panels A1–C1) and the rat (panel D1). Among the cortical layers (indicated in panel D3), layers II–III, Va and VI stained for CB1Rs with the greatest intensity. Scale bars represent 1 mm.
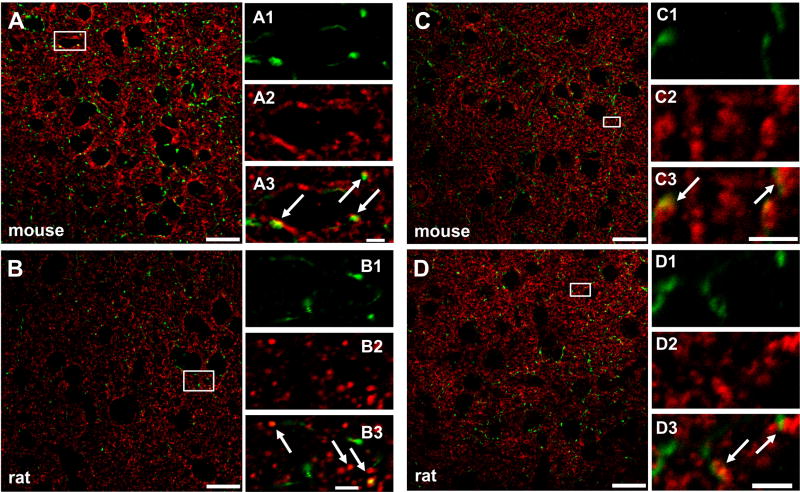
At the highest resolution of confocal microscopy, CB1R immunoreactivity revealed fiber-like and punctate-like structures, i.e. probable axons and nerve terminals. SERT, and VGLUT1 immunoreactivities, marking putative serotonergic and glutamatergic fibres and terminals, were found in co-localization with CB1R immunoreactivity (Figures 2A–D) both in the mouse and the rat. Since VGLUT2-positive glutamatergic terminals have low density in CB1R-rich fields, only occasional co-localization was detected (figure not shown).
Figure 2.
CB1R is present in VGLUT1-positive (glutamatergic) terminals in the mouse (A, A1–3) and the rat (B, B1–3) frontal cortex. These data serve as positive control and Supplemental Figure S1 as negative control. CB1R is also detected in SERT-positive (serotonergic) frontocortical terminals in the mouse (C, C1–3) and the rat (D, D1–3). Confocal microscopy images were taken with a 63×/1.40 ApoChromat objective, at 1 and 3× zoom and at 1024 dpi resolution. Co-localization was always verified in the respective orthogonal projections of the 380 nm-thick optical sections of the Z-stack images. Small rectangles mark the zones in images (A–D, 1× zoom) which are further magnified (3× zoomed) and cropped, resulting in images A1–3 - D1–3. Scale bars represent 20 μm (AD) and 2.5 μM (A1–3 - D1–3). White arrows point to co-localization.
3.2. Functional assays
CB1R activation decreases presynaptic transmitter release by inhibiting various Ca2+ channels (Twitchell et al., 1997), thus one needs a robust Ca2+-dependent release assay, such as the 4-AP or the high-K+ stimulation-evoked transmitter release assay to reliably assess CB1R function. To avoid polysynaptic effects and interference with the uptake systems, a synaptoneurosome preparation allows characterizing presynaptic functional neuromodulator receptors such as the CB1R (Köfalvi et al., 2005).
Thus, first we optimized the simultaneously measured 4-AP-evoked [14C]glutamate (Figure 3A,B) and [3H]serotonin (Figure 3C,D) release from rat frontocortical synaptosomes. We chose stimulation with 4-AP at the concentration of 300 μM based on the peak amplitude (Figure 3A,C) and reasonable Ca2+-dependency (Figure 3B,D). Notably, we used 100 nM Ca2+ combined with 10 mM MgCl2 instead of the total omission of Ca2+ to preserve membrane integrity and to diminish Na+ entry through open voltage-gated Ca2+ channels in the “low calcium” condition.
The synthetic CB1R agonist, WIN55212-2 (1 μM) inhibited the evoked release of [14C]glutamate by 35.0 ± 4.7% (n = 8; p < 0.05) and that of [3H]serotonin by 17.4 ± 5.3% (n = 8; p < 0.05) (Figure 3B,D). The evoked release of both [14C]glutamate and [3H]serotonin was not affected by WIN55212-2 under “low calcium” condition (n = 6, p > 0.05 for both, Figure 3B,D).
Both the low-potency CB1R inverse agonist, LY320135 (5 μM) and the highly potent and selective neutral CB1R antagonist, O-2050 (1 μM) abolished the effect of WIN55212-2 on the evoked release of [3H]serotonin (Figure 3B). O-2050 in the same experiments also prevented the inhibitory action of WIN55212-2 on the evoked release of [14C]glutamate (Figure 3B). However, LY320135 itself had a 21.8 ± 8.2% inhibitory action alone, which is probably due to its weak muscarinic agonist activity (Felder et al., 1998). Although the inhibitory action of WIN55212-2 was largely decreased by LY320135 it still remained statistically significant versus LY320135 alone (Figure 3B).
The amplitude of the evoked [14C]glutamate release was 14.6 ±7.7 greater (n = 15, p = 0.008) in the CB1R KO mice than in the wild-type (WT of the CD-1 strain) (Figure 3B). Additionally, WIN55212-2 (1 μM; n = 9) and the metabolically stable endocannabinoid analogue R-methanandamide (RmAEA; 1 μM; n = 7) inhibited the evoked [14C]glutamate release both in the CD-1 and the C57bl/6 WT mice which effect was absent in the CB1R KO mouse on the CD-1 strain (Figure 3B).
The evoked release of [3H]serotonin was 11.3 ± 3.7% (n = 15; p = 0.009) greater in the CB1R KO mouse. However and to our greatest surprise, neither WIN55212-2 nor RmAEA inhibited of evoked release of [3H]serotonin in the CD-1 or the C57bl/6 WT mice.
These data brings about several different explanations:
CB1Rs are tonically negatively coupled to the release of glutamate and serotonin in the synaptosomes of CD-1 mice, and there is no room for further inhibition by exogenous ligands on the release of serotonin the mice; and/or
stimulation with 4-AP does not recruit CB1R-coupled Ca2+ channels in the serotonergic terminals in the mice; and/or
there is a difference in cortical CB1R density between the rat and the mouse; and/or
a developmental alteration appears in the CB1R knockout resulting in a generally increased release probability.
To test the first hypothesis, we measured the evoked release of both neurotransmitters from the synaptosomes of the C57BL/6J strain under the blockade of the CB1Rs by O-2050 (0.5 μM). O-2050 failed to affect the evoked release of [14C]glutamate (102.4 ± 2.7% of CTRL; n = 6; p = 0.42) and [3H]serotonin (102.2 ± 7.5% of CTRL; n = 6; p = 0.78). Similarly, the other CB1R antagonist LY320135 (5 μM) and the endocannabinoid synthesis inhibitor, tetrahydrolipstatin (10 μM) both failed to change the evoked release of [14C]glutamate and [3H]serotonin in the CD-1 WT mice (n = 3, data not shown).
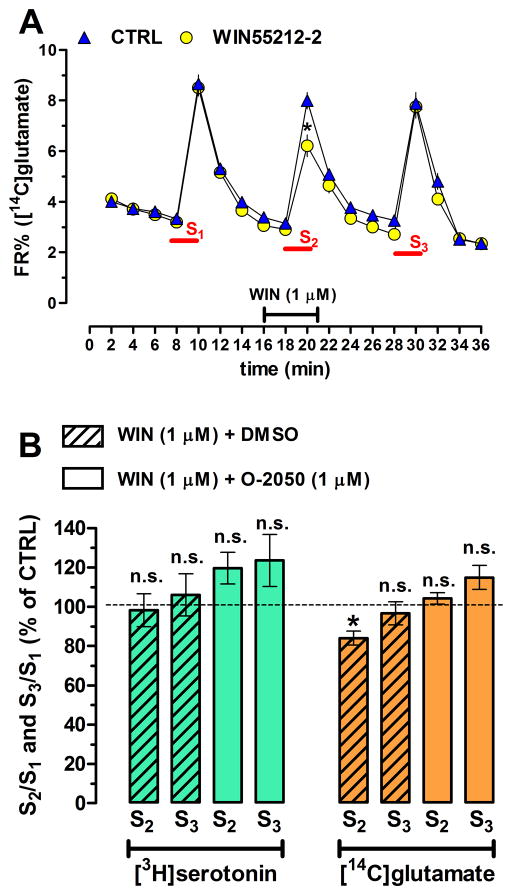
One may assume that the 4-AP stimulation does not activate those Ca2+ channels in serotonergic terminals which are coupled to neurotransmitter release in the mice. Thus, we determined the effect of WIN55212-2 on the transmitter release evoked by the triple high-K+ (25 mM) stimulation in the C57BL/6J. The three stimuli evoked repeatable release of [14C]glutamate (Figure 4A) and of [3H]serotonin (figure not shown). Interestingly, and as expected, the first 1-min high-K+ stimulation evoked greater [14C]glutamate release (S1KCl, 7.27 ± 0.44 FR%, n = 6 in octuplicate) than the 2-min 4-AP stimulation in the other experiments (S14AP, 3.83 ± 0.35 FR%, n = 6, p < 0.001). Oddly enough, the converse occurred for the evoked release of [3H]serotonin (S1KCl, 4.34 ± 0.32 FR%, n = 6 in octuplicate) vs. (S14AP, 9.97 ± 1.23 FR%, n = 6, p < 0.001).
Figure 4.
To test if the lack of cannabinoid effect on serotonin release in mice is associated with 4-AP stimulation, we performed a triple high-K+- (25 mM) stimulation paradigm. WIN55212-2 (1 μM; hatched bars) inhibited the second stimulus-evoked release of [14C]glutamate in a reversible manner (i.e., the 3rd peak, S3 was no longer significantly smaller than the S1 control peak), and sensitive to the selective CB1R antagonists, O-2050 (1 μM). However, WIN55212-2 did not affect the high-K+-evoked release of [3H]serotonin (B) in the C57BL/6 mice. N = 6, * p < 0.05.
WIN55212-2 (1 μM) or the vehicle DMSO (0.1%) was added 4 min before the second stimulus (S2) and continued until the end of S2. WIN55212-2 inhibited the evoked [14C]glutamate (Figure 4A,B) but not [3H]serotonin (Figure 4B) release. After the 8-min washout period, this inhibitory action of WIN55212-2 on glutamate release was no longer present. The inhibitory action of WIN55212-2 was also prevented by the co-administration of the CB1R antagonist, O-2050.
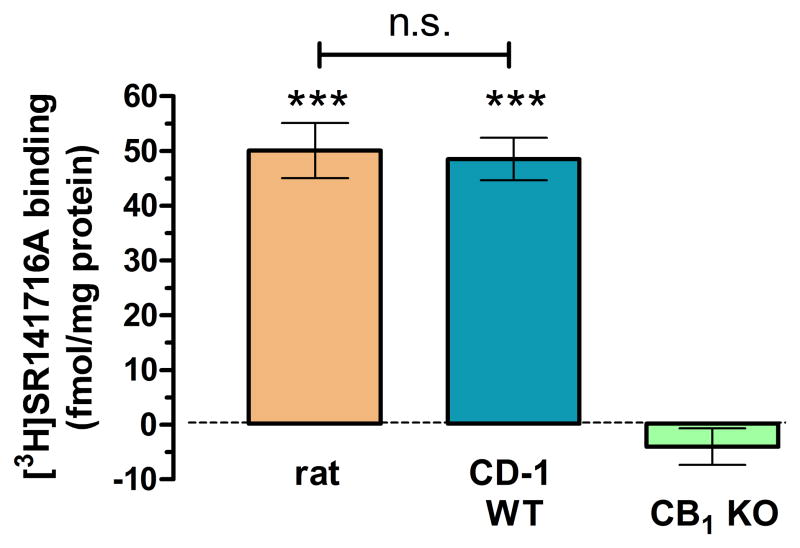
Finally, we measured frontocortical CB1R density with the help of the CB1R antagonist/inverse agonist [3H]SR141716A as radioligand and another CB1R antagonist/inverse agonist, AM251 (1 μM) to displace specific binding. While AM251 failed to displace the radioligand in the CB1R KO mice it displaced equal amount of [3H]SR141716A both in the rat and the CB1R KO mouse frontocortical membrane preparations (Figure 5), indicating the lack of density differences.
Figure 5.
CB1R binding experiments reveal no different frontocortical CB1R density between the rat and the CD-1 mouse (n = 4 animals of each type in quintuplicate). A ~100 μg protein quantity of Wistar rats, CD-1 WT and KO mice, respectively, was incubated with ~1 nM [3H]SR141716A, in the presence of the CB1R antagonist/inverse agonist AM251 (1 μM) or its vehicle, DMSO (0.1%). There was no specific binding found in the CB1R KO mice. N = 4, ***p < 0.001 vs. KO.
4. Discussion
Previous studies reported bidirectional interactions between the endocannabinoid and the serotonergic systems, involving various direct and indirect mechanisms and brain areas (for review see Haj-Dahmane and Shen, 2011). To our knowledge, this study is the first showing the presence of presynaptic functional CB1Rs in serotonergic nerve terminals of the rat frontal cortex. We showed here that CB1R activation decreases the Ca2+-dependent release of [3H]serotonin from frontocortical nerve terminals of the rat. Previous electrophysiological studies have found that CB1R activation inhibits some glutamatergic transmission in the frontal cortex of the rat and mouse (Auclair et al., 2000; Barbara et al., 2003; Lafourcade et al., 2007). Interestingly, it was never mapped in the frontal cortex if CB1Rs are present in VGLUT1- or VGLUT2-positive terminals, or both. We found co-localization mostly between VGLUT1 and CB1R, because glutamatergic fibers of subcortical origin containing VGLUT2 terminate in fields poorly innervated with CB1R-posive fibres. A recent study however found CB1R mRNA in subcortical VLGUT2-positive nuclei and tracts (Hrabovszky et al., 2012) indicating that certain VGLUT2-positive terminals may be subject to endocannabinoid control.
Importantly, the distribution of CB1R immunoreactivity in the sagittal slices both at low and high resolution was essentially the same as in previous reports using either autoradiography (Herkenham et al., 1991) or immunohistochemistry with light microscopy (Tsou et al., 1998) and fluorescent/confocal microscopy (Bodor et al., 2005). Although the anatomical description of the endocannabinoid system follows a generally consistent pattern throughout studies and assays, this is not quite true for the physiology and pharmacology. In fact, it is more the exception than the rule when cannabinoid pharmacology is consistent across species; due to the increasing number of potential cannabinoid receptors and issues of ligand selectivity (Köfalvi et al., 2008). The neutral CB1R antagonist, O-2050 abolished the effect of WIN55212-2 without having an effect per se on serotonin or glutamate release. The CB1R inverse agonist, LY320135 did the same for the release of serotonin, but already inhibited per se the release of glutamate and competitively antagonized the effect of WIN55212-2. This latter finding might be the result of LY320135 binding to muscarinic and serotonin receptors in the low micromolar range (Felder et al., 1998).
In contrast, CB1R agonists did not affect serotonin release either in the CD-1 or the C57BL/6 WT mice. This was not because of already constitutively active CB1Rs leaving no further room for modulation by exogenous ligands: Both nerve terminal types in the rat and the mice strains were free from an endogenous cannabinoid tone since O-2050, LY320135 and tetrahydrolipstatin did not augment the evoked release – as one would expect in superfused synaptosomes.
The contrasting observations in the mice are not likely a result of failing to activate CB1R-coupled Ca2+ channels either, and additionally, no difference in frontocortical CB1R density was observed between species. Intriguingly, the genetic ablation of the CB1R stepped up the evoked glutamate and serotonin release in the CD-1 KO mice. This we can not explain with anything else but a developmental alteration resulting in greater response to stimuli. In concert with this, increased glutamatergic excitotoxicity is observed in the forebrain CB1R knockout mice (Marsicano et al., 2003).
Currently it is not known if CB1Rs fail to presynaptically control release of serotonin in mice, or if these receptors need additional in situ circumstances (e.g. an activation of another receptor) to function, or if they are chronically negatively coupled to serotonin release in mice. But then again, understanding that the majority of serotonergic terminals are actually varicosities and the serotonergic communication is rather non-synaptic, the origin and identity of endocannabinoids acting at these terminals/varicosities are not expected to be the same as for a glutamatergic synapse. We assume that, at least in the rat, the origin of endocannabinoids to activate CB1Rs in serotonergic varicosities might occur in an autocrine manner. Importantly, in vivo data suggests that presynaptic CB1Rs can inhibit medial frontocortical serotonin release, as assessed by microdialysis (Tzavara et al., 2003). Additionally, the serotonergic neurons of the raphe nuclei (the origin of the serotonergic fibers throughout the brain) express CB1R mRNA (Häring et al., 2007) and endocannabinoid release machinery (Haj-Dahmane and Shen, 2009). Thus, theoretically the necessary components are present allowing a serotonergic neuron to release endocannabinoids and activate its own CB1Rs.
The difference between the rat and mouse does not come as a full surprise knowing that there are overt differences even among mouse substrains in behavioral assays that involve the serotonergic system (Matsuo et al., 2010). Furthermore, there are considerable differences among rodent species, including the rat and the mouse in the pharmacology and function of their serotonergic system (Limberger et al., 1991; Fernández-Guasti et al., 1992; Takahashi et al., 2001; Setola and Roth, 2005).
Interestingly, Haller and co-workers have shown that the effect of WIN55212-2 is anxiogenic in the Wistar rats and anxiolytic in the CD-1 mice (Haller et al., 2007). Since anxiety is a paradigm heavily relying on serotonergic neuromodulation (Takahashi et al., 2001; Haller et al., 2007; Durant et al., 2010) it might be predicted from the results of Haller and co-workers that CB1R activation will inhibit serotonin release in rats but not in CD-1 mice.
Of note, endocannabinoids do not necessarily need to activate putative CB1Rs in raphe serotonergic neurons (and in their terminals) to inhibit serotonergic activity: Endocannabinoids acting at presynaptic CB1Rs in excitatory afferents of other neurons can indirectly inhibit the activity of serotonergic neurons. For instance, Haj-Dahmane and Shen (2009) showed that glutamate induces endocannabinoid release from dorsal raphe serotonergic neurons, which in turn decreases further glutamate release. This process is termed depolarization-induced inhibition of excitation or DSE. Thus, CB1R activation decreases the excitatory input of serotonergic neurons and thereby indirectly decreases serotonin release in the brain. Notably, local glutamate release onto serotonergic fibers (Balázsa et al., 2008) can be also suppressed by CB1R activation which further contributes to indirect inhibition of serotonergic activity by endocannabinoids. Such an indirect mechanism could have also contributed to the inhibition by CB1Rs of the electrically stimulated [3H]serotonin release from cortical slices (Nakazi et al., 2000). Balázsa et al. (2008) elegantly showed that NMDA channel blockade is necessary to reveal CB1R-mediated direct inhibition of depolarization-induced [3H]serotonin release in the hippocampal slice.
Altogether, the interaction between the endocannabinoid and serotonergic neuromodulator systems is multifaceted, and an imbalance in one of them would affect the other. This explains why the two systems are so intricately involved in several physiological and pathological mechanisms. To better understand psychiatric disorders (Forbes and Grafman), additional efforts dissecting the complex interaction between serotonin and endocannabinoids inside and outside the frontal cortex are needed.
Supplementary Material
Highlights.
CB1Rs have high density in three layers of the frontal cortex of the rat and mouse.
CB1Rs are present in frontocortical glutamatergic and serotonergic nerve terminals.
CB1R activation decreases evoked glutamate release in rat and mouse synaptosomes.
CB1R activation decreases serotonin release in rat frontocortical synaptosomes.
Evoked glutamate and serotonin release is greater in the CB1R KO versus WT mice.
Acknowledgments
This work was supported by the PTDC/SAU-OSM/105663/2008 grant (A.K.; F.M.T), a SFRH/BD/33467/2008 fellowship (S.G.F.) of the FCT, and the NIH DA011322 & DA021696 (K.M.).
Abbreviations
- 2-AG
2-arachidonoyl-glycerol
- 4-AP
4-aminopyridine
- CB1R
cannabinoid receptor type 1
- DMSO
dimethylsulfoxide
- FELASA
Federation for Laboratory Animal Science Associations
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- PB
phosphate buffer
- PBS
phosphate buffered saline
- RIPA
radioimmunoprecipitation assay
- SERT
serotonin transporter
- VGLUT1
2, vesicular glutamate transporter types 1 and 2
- WIN
WIN55212-2 mesylate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrade R. Serotonergic regulation of neuronal excitability in the prefrontal cortex. Neuropharmacology. 2011;61:382–386. doi: 10.1016/j.neuropharm.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton CH, Moore PB. Endocannabinoid system dysfunction in mood and related disorders. Acta Psychiatr Scand. 2011;124:250–261. doi: 10.1111/j.1600-0447.2011.01687.x. [DOI] [PubMed] [Google Scholar]
- Auclair N, Otani S, Soubrie P, Crepel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol. 2000;83:3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- Balázsa T, Bíró J, Gullai N, Ledent C, Sperlágh B. CB1-cannabinoid receptors are involved in the modulation of non-synaptic [3H]serotonin release from the rat hippocampus. Neurochem Int. 2008;52:95–102. doi: 10.1016/j.neuint.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Barbara JG, Auclair N, Roisin MP, Otani S, Valjent E, Caboche J, Soubrie P, Crepel F. Direct and indirect interactions between cannabinoid CB1 receptor and group II metabotropic glutamate receptor signalling in layer V pyramidal neurons from the rat prefrontal cortex. Eur J Neurosci. 2003;17:981–990. doi: 10.1046/j.1460-9568.2003.02533.x. [DOI] [PubMed] [Google Scholar]
- Best AR, Regehr WG. Serotonin evokes endocannabinoid release and retrogradely suppresses excitatory synapses. J Neurosci. 2008;28:6508–6515. doi: 10.1523/JNEUROSCI.0678-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyíri G, Mackie K, Ledent C, Hájos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubert EA, Condron BG. Serotonin: a regulator of neuronal morphology and circuitry. Trends Neurosci. 2010;33:424–434. doi: 10.1016/j.tins.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Duarte JM, Ferreira SG, Carvalho RA, Cunha RA, Köfalvi A. CB1 receptor activation inhibits neuronal and astrocytic intermediary metabolism in the rat hippocampus. Neurochem Int. 2011;60:1–8. doi: 10.1016/j.neuint.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Duarte JM, Nogueira C, Mackie K, Oliveira CR, Cunha RA, Köfalvi A. Increase of cannabinoid CB1 receptor density in the hippocampus of streptozotocin-induced diabetic rats. Exp Neurol. 2007;204:479–484. doi: 10.1016/j.expneurol.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Durant C, Christmas D, Nutt D. The pharmacology of anxiety. Curr Top Behav Neurosci. 2010;2:303–330. doi: 10.1007/7854_2009_8. [DOI] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Glass M, Mackie KP, Fahey KJ, Cullinan GJ, Hunden DC, Johnson DW, Chaney MO, Koppel GA, Brownstein M. LY320135, a novel cannabinoid CB1 receptor antagonist, unmasks coupling of the CB1 receptor to stimulation of cAMP accumulation. J Pharmacol Exp Ther. 1998;284:291–297. [PubMed] [Google Scholar]
- Fernández-Guasti A, Hong E, López-Rubalcava C. Species differences in the mechanism through which the serotonergic agonists indorenate and ipsapirone produce their anxiolytic action. Psychopharmacology (Berl) 1992;107:61–68. doi: 10.1007/BF02244966. [DOI] [PubMed] [Google Scholar]
- Ferreira SG, Lomaglio T, Avelino A, Cruz F, Oliveira CR, Cunha RA, Köfalvi A. N-acyldopamines control striatal input terminals via novel ligand-gated cation channels. Neuropharmacology. 2009;56:676–683. doi: 10.1016/j.neuropharm.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Forbes CE, Grafman J. The role of the human prefrontal cortex in social cognition and moral judgment. Annu Rev Neurosci. 2010;33:299–324. doi: 10.1146/annurev-neuro-060909-153230. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Vollenweider FX. Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29:445–453. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol Psychiatry. 2010;67:199–207. doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY. Endocannabinoids suppress excitatory synaptic transmission to dorsal raphe serotonin neurons through the activation of presynaptic CB1 receptors. J Pharmacol Exp Ther. 2009;331:186–196. doi: 10.1124/jpet.109.153858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY. Modulation of the serotonin system by endocannabinoid signaling. Neuropharmacology. 2011;61:414–420. doi: 10.1016/j.neuropharm.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Mátyás F, Soproni K, Varga B, Barsy B, Németh B, Mikics E, Freund TF, Hájos N. Correlated species differences in the effects of cannabinoid ligands on anxiety and on GABAergic and glutamatergic synaptic transmission. Eur J Neurosci. 2007;25:2445–2456. doi: 10.1111/j.1460-9568.2007.05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häring M, Marsicano G, Lutz B, Monory K. Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience. 2007;146:1212–1219. doi: 10.1016/j.neuroscience.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Harkany T, Mackie K, Doherty P. Wiring and firing neuronal networks: endocannabinoids take center stage. Curr Opin Neurobiol. 2008;18:338–345. doi: 10.1016/j.conb.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne RP, Catterall WA. The sodium channel from rat brain. Purification and subunit composition. J Biol Chem. 1984;259:1667–1675. [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann H, Marsicano G, Lutz B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience. 2002;109:451–460. doi: 10.1016/s0306-4522(01)00509-7. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Wittmann G, Kalló I, Füzesi T, Fekete C, Liposits Z. Distribution of type 1 cannabinoid receptor-expressing neurons in the septal-hypothalamic region of the mouse: colocalization with GABAergic and glutamatergic markers. J Comp Neurol. 2012;520:1005–1020. doi: 10.1002/cne.22766. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Köfalvi A. Alternative Interacting Sites and Novel Receptors for Cannabinoid Ligands. In: Köfalvi A, editor. Cannabinoids and the Brain. Springer; US: 2008. pp. 131–160. [Google Scholar]
- Köfalvi A, Fritzsche M. The Endocannabinoid System is a Major Player in Schizophrenia. In: Köfalvi A, editor. Cannabinoids and the Brain. Springer; US: 2008. pp. 485–528. [Google Scholar]
- Köfalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, Sperlágh B. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade M, Elezgarai I, Mato S, Bakiri Y, Grandes P, Manzoni OJ. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS One. 2007;2:e709. doi: 10.1371/journal.pone.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Böhme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Limberger N, Deicher R, Starke K. Species differences in presynaptic serotonin autoreceptors: mainly 5-HT1B but possibly in addition 5-HT1D in the rat, 5-HT1D in the rabbit and guinea-pig brain cortex. Naunyn Schmiedebergs Arch Pharmacol. 1991;343:353–364. doi: 10.1007/BF00179039. [DOI] [PubMed] [Google Scholar]
- Mackie K. Signaling via CNS cannabinoid receptors. Mol Cell Endocrinol. 2008;286:S60–65. doi: 10.1016/j.mce.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutiérrez SO, van der Stelt M, López-Rodriguez ML, Casanova E, Schütz G, Zieglgänsberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Takao K, Nakanishi K, Yamasaki N, Tanda K, Miyakawa T. Behavioral profiles of three C57BL/6 substrains. Front Behav Neurosci. 2010;4:29. doi: 10.3389/fnbeh.2010.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazi M, Bauer U, Nickel T, Kathmann M, Schlicker E. Inhibition of serotonin release in the mouse brain via presynaptic cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:19–24. doi: 10.1007/s002109900147. [DOI] [PubMed] [Google Scholar]
- NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. J Physiol. 2010;588:2519–2521. doi: 10.1113/jphysiol.2010.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor KA, Roth BL. Screening the receptorome for plant-based psychoactive compounds. Life Sci. 2005;78:506–511. doi: 10.1016/j.lfs.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2 1997. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. 1998. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Do Rats Have Prefrontal Cortex? The Rose-Woolsey-Akert Program Reconsidered. J Cogn Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res. 2008;14:249–262. doi: 10.1007/BF03033814. [DOI] [PubMed] [Google Scholar]
- Setola V, Roth BL. Why mice are neither miniature humans nor small rats: a cautionary tale involving 5-hydroxytryptamine-6 serotonin receptor species variants. Mol Pharmacol. 2003;64:1277–1278. doi: 10.1124/mol.64.6.1277. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Berton O, Mormède P, Chaouloff F. Strain-dependent effects of diazepam and the 5-HT2B/2C receptor antagonist SB 206553 in spontaneously hypertensive and Lewis rats tested in the elevated plus-maze. Braz J Med Biol Res. 2001;34:675–682. doi: 10.1590/s0100-879x2001000500017. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Davis RJ, Perry KW, Li X, Salhoff C, Bymaster FP, Witkin JM, Nomikos GG. The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Br J Pharmacol. 2003;138:544–553. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V. The endocannabinoid system in the basal ganglia and in the mesolimbic reward system: implications for neurological and psychiatric disorders. Eur J Pharmacol. 2003;480:133–150. doi: 10.1016/j.ejphar.2003.08.101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.