Abstract
This study recruited persons at increased risk of colon cancer to an intensive dietary intervention study that required biopsies of the colon by flexible sigmoidoscopy at baseline and after six months of intervention. A total of 1314 individuals contacted the study, and only 16 individuals indicated that the sigmoidoscopy procedure was an obstacle to study participation. A total of 270 individuals completed a screening visit and signed a screening consent form. Inquiries about the study tended to be fewer in the winter and late summer. Failure to return food records was the most common reason for exclusion. Dietary recall at enrollment indicated that subjects were consuming significantly more vegetables, lower sodium and a lower glycemic load on the day before starting the study versus during the eligibility phase which might have an impact on biomarker measures. This makes it important to capture dietary changes in the period between determination of eligibility and enrollment. Subjects (n=120) were randomized to follow a Healthy Eating or a Mediterranean Diet, each of which required substantial dietary record-keeping. The study completion rate was 78%, and subjects reported high satisfaction with study participation. Of the 93 individuals who completed the study, only one refused the flexible sigmoidoscopy at the final visit. These findings suggest that flexible sigmoidoscopy does not appear to be a barrier for recruitment of high-risk individuals to an intensive dietary intervention trial, but that completing food records can be.
Keywords: colon biopsy, recruitment, dietary intervention, cancer prevention
1. Introduction
Every person in the industrialized world has on average a one in twenty chance of developing colorectal cancer in their lifetime, and the incidence in the U.S. is amongst the highest in the world (1–3). Many epidemiological studies have investigated the role that diet may play in the increased rates observed in Western countries, and the available evidence points to a protective effect of Mediterranean diets (4–7). A Mediterranean diet encompasses low omega-6 fat intake and higher omega-3 fat intake, higher monounsaturated fat (MUFA) intake from olive oil, lower red meat, less refined carbohydrates and increased variety and quantity of fruits and vegetables (4). Despite the promising cancer-preventive properties of Mediterranean diets, there have been few intervention studies reported using this type of diet in American populations.
This study recruited subjects at increased colon cancer risk since this would be a likely target population for prevention (8). Recruitment to prevention trials is, however, difficult even though one would expect good interest from a high risk population. Typically large numbers of individuals need to be contacted, and often recruitment requires more time than envisioned (9, 10). This is especially true when invasive procedures are required since the optimal biomarkers for enhancing our knowledge of the links between diet and cancer are generally tissue-based (11, 12). Flexible sigmoidoscopy is a well-accepted procedure for colon cancer screening, and it can be used to obtain colon biopsies that are valuable for biomarker research (13). The present study required two flexible sigmoidoscopies of the colon, a substantial amount of dietary counseling and regular dietary record-keeping. The study used a screening consent to allow capture of information about the individuals who were and were not enrolled. This can help capture possible bias in study enrollment, which is important since it can affect the applicability and interpretation of the results (14). Here we describe recruitment and retention strategies, changes in diet before the flexible sigmoidoscopy procedure, and satisfaction with study participation. This provides guidance for future studies involving intensive dietary intervention and invasive procedures.
2. Methods
2.1. Eligibility
The Healthy Eating for Colon Cancer Prevention Study was approved by the University of Michigan Institutional Review Board (HUM00007622). The study was listed on the Clinical Trials website maintained by the National Institutes of Health (registration number NCT00475722). The study enrolled persons at increased risk for colon cancer in an effort to maximize the evaluation of the intervention effects on biomarkers of colon cancer risk.
The inclusion criteria for the study required an increased risk for colon cancer as defined by having either a strong family history of colon cancer or a prior adenomatous polyp or resected early (Dukes A, B or C) colon cancer. Individuals with a previous colon cancer were at least two years post treatment for cancer, with the exception of curative surgery for small lesions, such as endoscopically removed cancers. For other cancers, the eligibility criterion was to be at least five years post any type of cancer treatment. A strong family history was defined as having one first degree relative or two second degree relatives with colon cancer. Eligible subjects were in good general health and not expecting major lifestyle changes in the next 6 months. Dietary eligibility criteria included at least 23% of calories from fat with no more than 48% of fats as MUFA and no more than 5.5 servings/day of fruits and vegetables. This was determined from the average of two days of food records and one un-announced 24-hour recall. Subjects also had to have a telephone and speak English since the counseling was done largely by phone.
Exclusion criteria included being on a medically prescribed diet, taking dietary supplements or medications that would interfere with the dietary changes, being pregnant or lactating, age less than 25 since the recommended dietary allowances differ for young persons, and conditions that would interfere with biomarker measures in the colon such as colitis and hereditary polyposis. Persons with BMI <18.5 or >35 kg/m2 were excluded since low BMI could indicate eating disorders and high BMI values, above the midpoint of the obesity range, could indicate more prevalent health problems and these persons can be more difficult to counsel. Maximum allowed aspirin use was either 81 mg/day or 325 mg aspirin every other day for prevention of cardiovascular disease. Subjects were asked to take acetaminophen for occasional pain.
2.2. Recruitment Strategies
The recruitment plan included many different channels. A study flyer and brochure were created that included information on basic eligibility criteria and basic information on study design: there were two diets, study procedures could take up to 3 hours and payment for completing 6 months of study added up to $300. Information on flexible sigmoidoscopies was given when subjects contacted the study since this would be too complex to explain on a flyer. The study flyer was posted in the hospital and research complex common areas, and in multiple community locations: physicians’ offices, community bulletin boards, churches, exercise facilities, and libraries. An institutional website containing information on all studies at the University of Michigan also listed the study. Other non-specific recruitment strategies included a press release which resulted in newspaper stories about the study, brochure distribution at health fairs, and paid advertisements in local newspapers.
Specific strategies targeted at high risk individuals included announcements about the study during events for colon cancer awareness month each year, recommendations to patients made by a gastroenterologist, flyer posting in the medical gastroenterology suite, a study newsletter asking current study participants to refer anyone they may know and targeted mailings. The targeted mailings were done using electronic health records of five large local primary care physician’s offices to identify patients at increased risk for colorectal cancer. Letters from the patient’s primary physicians were then mailed by the study staff informing the patient about the study. Finally, the call center for the University of Michigan Comprehensive Cancer Center referred patients who expressed an interest about studies on colon cancer. All promotion of the study provided telephone, and email contact information for the study team.
2.3. Screening of Interested Participants
The study coordinator called all interested persons to confirm the study requirements and review the eligibility requirements. Subjects were told about the flexible sigmoidoscopy on the phone and were told that this procedure required less than 10 minutes and did not require colonic preparation, sedation, or pain medications. Persons still interested and eligible for the study then scheduled an in-person appointment to be further screened for eligibility. This appointment required about 30 minutes and was conducted in an outpatient clinical research facility either within the hospital or at an offsite medical office building. During the screening visits, interested persons were asked to sign a written informed consent to be screened for the study. This consent allowed for the capture of information about the individuals who were and were not enrolled. This method allows for capturing potential bias in the study enrollment process.
Subjects were asked to complete food records on a Sunday and a Monday and to return those in a postage paid envelope by mail. After the food record was returned to the research team, the subject was called for an unannounced 24-hour recall. Subjects were enrolled in the study on average 50 days after completing the 24-hour recall, mainly due to scheduling issues. The dietary eligibility requirements were confirmed using an average of those three days.
2.4. Study Design
The recruitment goal for the study was 120 high-risk subjects, and this was based on considerations of the changes that might be expected in biomarker endpoints in the colon. Participants were randomized within four strata: body mass index (BMI, normal weight or overweight/obese as defined by a BMI ≥25 kg/m2), regular aspirin use (Yes/No), gender (male/female), and risk status (personal history of cancer or not). These eligibility criteria were important since they could affect the colonic biomarkers of dietary compliance and of prostaglandin (PG) E2 formation. Higher BMI has been associated with higher n-6 and lower n-9 and n-3 fatty acids in adipose tissue, which are markers of dietary compliance, and BMI also can affect PGE2 levels, which is a biomarker of colon cancer risk (15, 16). Increased BMI is associated with increased oxidative stress and inflammation, which induces PGE2 formation (17). Low dose aspirin use would still allow for detection of further decreases in PGE2 levels in the colon with diet (18). The categorization of subjects by gender and whether or not they had colon cancer in the past was used to ensure equal representation in the two study arms. It is possible that plasma fatty acids and carotenoids can vary by these factors, making equal representation of subjects in these categories between the two study arms potentially important (19, 20).
The two diets in the study were a Healthy Eating diet that was based on Healthy People 2010 recommendations and a modified Mediterranean diet. Both study arms required weekly telephone counseling for one month, biweekly counseling for the next two months and monthly counseling for the final three months. All subjects received arm-specific bimonthly newsletters containing notes on study recruitment progress, seasonal cooking hints, a recipe and a “participants are saying” column to relay helpful discoveries or comments made by current study participants.
In the Healthy Eating arm, goals included consumption of 2 servings/day of fruit, 3 servings/day of vegetables with at least one of those servings being dark green or orange, 3 servings/day from whole grains, less than 10% of calories from saturated fat and less than 30% of calories from total fat. Subjects were asked to complete self-monitoring booklets to enumerate daily servings of fruits, vegetables, and whole grains and grams of saturated fat.
In the Mediterranean arm, dietary goals were based on an exchange list that classified foods into categories. There were daily goals from each category, giving individuals flexibility in food choices for meeting the study goals. This type of approach would be expected to maximize the long-term compliance that is required for reduction of cancer risk. The fat goal was to achieve a PUFA:SFA:MUFA ratio of 1:2:5. Consumption of foods high in omega 3 fatty acids was requested at least twice a week, and the whole grain goal was at least three servings/day. To ensure intake of fruit and vegetables near Cretan levels, the goal was for consuming 7–9 FDA servings per day, depending on total caloric intake (7/day for <1700 kcal/day, 8/day for 1700–2000 kcal/d and 9/day for >2000 kcal/d; 7 servings can weigh about 600 g). The fruit and vegetable exchange lists encouraged variety of intake with daily intake goals for specific types of vegetables (including at least one dark green vegetable or herb) and fruits, similar to what we used previously (21). This published exchange list was modified to include categories for high omega 3 foods and whole grains.
2.5. Study Procedures
Subjects were asked to arrive at the baseline and 6-month study visits in the fasting state. The study visits consisted of providing a blood sample, eight colon biopsies obtained by flexible sigmoidoscopy, questionnaires, a 24-hour dietary recall, and measures of height, weight, waist and hip circumference, and blood pressure. Study participants were provided with breakfast and a parking voucher. There was also a 3-month visit for checking body weight, filling out brief questionnaires and meeting with the study dietitian. Participants were paid $130 at 0 and 6 months and $40 at 3 months for their time and inconvenience in coming to the clinic, and for any increased food costs.
Biopsies of the colon were obtained by flexible sigmoidoscopy without any prior preparation to empty the intestinal tract. The preparation procedure for colonoscopy can be a detriment to accrual and may alter eicosanoid concentrations in the mucosa. Eight un-prepped biopsy samples of normal mucosa (about 5 mg each) were obtained so that enough tissue would be available for fatty acid analysis (1 biopsy), an eicosanoid profile (2 biopsies), carotenoid analysis (1 biopsy), proliferation/nuclear morphology (2 fixed biopsies) and two frozen biopsies for future use. The biopsies were obtained circumferentially 15 cm above the anus using biopsy forceps.
2.6. Statistical Analyses
Descriptive summary measures were obtained for the study participants. Characteristics of the screened subjects were compared between the study participants and the ones who did not go on the study by means of two-sample t-test for mean comparison and chi-square test of comparing proportions as appropriate. After randomization of the study participants, the two arms were again compared in a similar manner to investigate any imbalance across them. A similar comparison was carried out between the group of study completers (93 subjects) and the non-completers (27 subjects). Dietary intake was assessed for eligibility approximately one month before study enrollment and by 24-hour recall at the baseline visit. This was done to obtain data on recent diet since this might affect the serum and colon biomarker measures. The two measures of dietary intake were compared for any differences by means of paired t-test. All study completers also completed a questionnaire regarding study satisfaction that asked various questions regarding the study on a 5-point scale with 5 being “very much so” and 1 being “not at all”. The item responses were factor analyzed using the prinicipal component extraction methods with a varimax rotation. The factor scores were subsequently analyzed in a linear regression framework with the scores as (continuous) outcomes, and the study arm indicator as the primary factor. The models were controlled for age as a covariate. The regression models were further run using a weighted least squares approach, with weights calculated via the response propensity method. Specifically, the weights for the participants were calculated as the reciprocal of the probability of completing the study, with the probabilities being estimated from a logistic regression with completion or non-completion as outcome, age, baseline vegetable consumption, marital and smoking status being independent variables.
3. Results
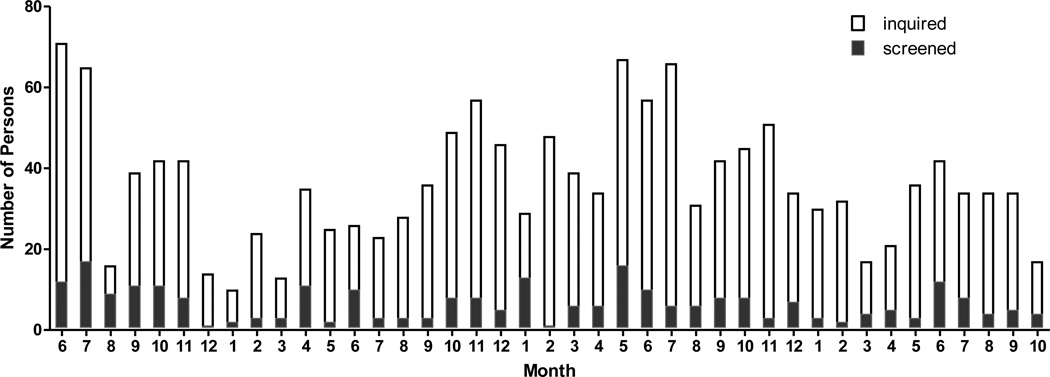
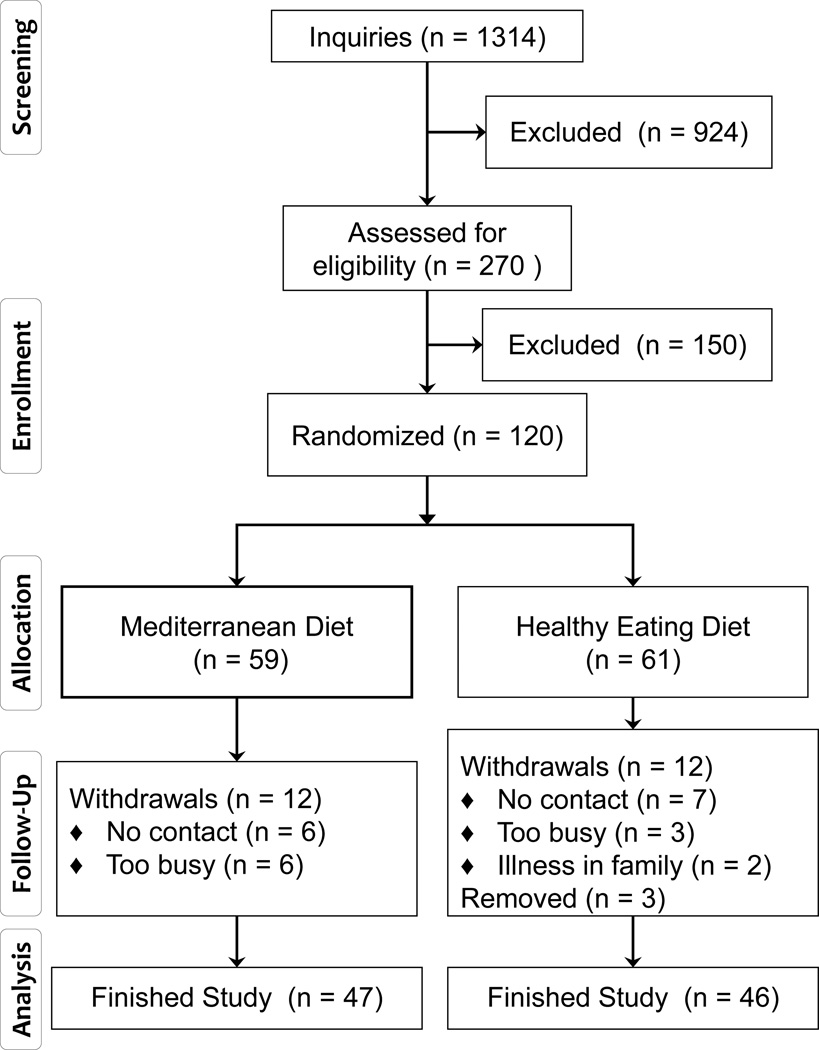
During the enrollment period from to July 1, 2007 to October 15, 2010, there were 1314 inquiries received about study participation (Table 1). Inquiries about the study, and screening visits, were most plentiful in the summer and fall, with lower numbers of inquiries in the winter and August (Figure 2). Most of the inquiries came from females, perhaps because women traditionally are more involved in making family meals. Telephone was the most often used method of contact, but a substantial number of inquiries were by email. Email was a useful method of contact as it required minimal staff time, but of the 488 individuals who responded via email, 307 did not reply after more information about the study was sent to them. Interestingly targeted recruitment methods such as physician referral or personal referral to individuals who might be eligible yielded only a minority of the inquiries (Table 1). Flyers and a posting on the university’s clinical trial website were the methods that yielded the largest number of inquiries.
Table 1.
Study recruitment to the Healthy Eating Study from July 1, 2007 through October 15, 2010.
| Inquiries | Number |
|---|---|
| Total | 1314 |
| Gender | |
| Male | 372 |
| Female | 890 |
| Unknown | 52 |
| Type of inquiry | |
| 488 | |
| Phone | 826 |
| Source of inquiries | |
| Posted Flyer | 277 |
| Clinical Trials Websitea | 257 |
| Newspaper Articles about Study | 70 |
| Word of Mouth | 58 |
| Physician Referralb | 32 |
| Otherc | 85 |
| Reasons for lack of follow-up | |
| Ineligibled | 625 |
| No responsee | 347 |
| Live too far away | 17 |
| Did not want flexible sigmoidoscopy | 16 |
| Want to lose weight | 9 |
| Taking high dose NSAIDs or supplements | 8 |
| Other reasons | 22 |
| Completed study screening visit | 270 |
The University of Michigan supported a clinical trials website for the community called “Engage” that listed the study and the basic eligibility requirements.
Physician referral was obtained through physicians in the Department of Family Medicine. Providers identified potentially eligible patients, and the study then sent a letter and study brochure to 280 subjects, 32 of whom responded by contacting the study.
Other sources of recruitment were listing of the study in a University of Michigan alumni electronic newsletter, the hospital website, and referral from the call center.
Ineligible individuals lacked colon cancer risk factors (n=282) or were ineligible for other reasons such as medical conditions or being above the upper BMI limit.
Email inquiries accounted for 307 of the “no response” category, with the remaining 40 resulting from not being able to return a call from either a call center message or answering machine.
Figure 2.
Number of persons who inquired about the study or were screened, shown by calendar month from 2007 to 2010.
Of the 1314 persons who inquired about the study, about half were not eligible (625) and only 16 indicated that they were not interested because of the flexible sigmoidoscopy procedure. A total of 270 subjects completed a study screening visit. The screening visit served to verify eligibility factors in person and to provide instructions for completing food records so that dietary eligibility could be verified. Of the 245 individuals who were asked to keep food records, 69 did not return a food record. Keeping food records can be tedious, but having the ability to complete food records was an important aspect of eligibility since complying with the dietary goals of the study required substantial amounts of dietary recordkeeping. Only 35 (20%) of the returned food records indicated a diet that was not eligible, and the most common reason was an intake of fruits and vegetables that was too high. The demographic characteristics of subjects who were or were not enrolled did not differ significantly, and about 8% had both a previous adenoma and a strong family history of colon cancer (Table 2).
Table 2.
Characteristics of screened subjects given as mean and SD, or number and percent.
| Characteristic | On Study, n=120 | Not on study, n=150a |
|---|---|---|
| Age, years | 52, 12 (range 22–82) | 51, 12 (range 21–85) |
| Female | 86, 72% | 97, 65% |
| Body mass index, kg/m2 | 27, 4 (range 19–35) | 28, 4 (range 19–41) |
| Regular aspirin userd | 24, 20% | 28, 19% |
| Previous adenoma | 33, 28% | 29, 19% |
| Family history of colon cancerb | 87, 76% | 97, 65% |
| Both risk factorsc | 10, 8% | 12, 8% |
Reasons subjects did not go on study were: did not meet eligibility criteria (n = 25), did not return food records (n = 69 of 245 potentially eligible persons), dietary intake not eligible (n = 35), dietary issues that would interfere with counseling (such as eats out too much or food record inadequate, n = 15), and not interested (n = 6).
One person who enrolled in the study with a family history of cancer also had a personal history of colon cancer. Three people who did not enroll had a personal history of colon cancer but no family history or previous adenomas. At the screening visit, it was determined that an additional nine people who were not enrolled did not fulfill the family history or prior adenoma requirement.
Persons who were not sure if their colon polyps were adenomas were not included as having adenomas. This was true for six persons who enrolled in the study and nine persons who did not enroll. All of these individuals fulfilled the family history requirement of one primary relative or two secondary relatives with colon cancer.
Regular aspirin users were individuals taking low dose aspirin (typically either 81 mg/day or 325 mg every other day) for prevention of cardiovascular disease.
Subjects enrolled in the study were stratified by four factors that could affect the outcome measures: gender, BMI, aspirin use and whether or not they had colon cancer in the past. This resulted in 61 subjects being randomized to the Healthy arm and 59 to the Mediterranean arm. Only one subject had colon cancer in the past. Table 3 shows that the randomization was effective for equalizing demographic characteristics of the subjects on each arm. The subjects tended to be Caucasian and well educated. The largest group of subjects were from Ann Arbor, MI (n=44) but most of the subjects were from nearby communities including Detroit suburbs (n=29), Ypsilanti (n=16), Grand Rapids and Lansing area (n=7), and several small communities within a 30 mile radius of Ann Arbor (n=24). About 20% were regular aspirin users and 15% were smokers.
Table 3.
Characteristics of study subjects in each study arm given as mean and SD or number and percent.
| Characteristic | Healthy Eating Diet n = 61 |
Mediterranean Diet n = 59 |
|---|---|---|
| Age, years | 50, 14 (range 22–72) | 55, 10 (30–82) |
| Female gender | 43, 70% | 43, 73% |
| BMI, kg/m2 | 27, 4 (range 19–34) | 28, 4 (range 20–35) |
| Regular aspirin user | 11, 18% | 13, 22% |
| Caucasian racea | 55, 90% | 50, 85% |
| Married/committed | 41, 67% | 35, 59% |
| College graduate | 47, 77% | 45, 76% |
| Tobacco userb | 7, 11% | 10, 17% |
| History of adenomas | 16, 26% | 17, 28% |
| Family history of colon cancer | 41, 67% | 36, 61% |
| Both risk factors | 4, 7% | 6, 10% |
Persons of other racial categories enrolled in the study were 9 African Americans, 1 mixed, 3 Asian and 1 Native American.
There were five smokers and two smokeless tobacco users in the Healthy Eating arm and ten smokers in the Mediterranean arm.
Dietary intake was assessed for eligibility approximately one month before study enrollment and by 24-hour recall at the baseline visit. This was done to obtain data on recent diet since this might affect the serum and colon biomarker measures more than intake at eligibility determination. The analysis of dietary intake indicated better quality diets, on average, for the recall done at study enrollment. This was statistically significant for sodium, glycemic load and vegetable intake, but differences were not large (Table 4). On average, total fat intake in this study population was about 31% of calories, which is just below the average intake in the United States from the National Health and Nutrition Examination Survey 2007–2008 (NHANES) of about 36% of calories from fat for Caucasian adults (22). Other aspects of study subjects’ diets were also closer to recommendation versus usual American intakes including higher fruit, vegetable and fiber intakes (22).
Table 4.
Dietary Intake during eligibility determination and on the day before study enrollment given as mean and SD for all 120 enrolled subjects.
| Nutrient or Food Group | Eligibility | At Study Enrollment a |
|---|---|---|
| Energy, kcal/day | 2123, 58 | 2167, 185 |
| Saturated Fat, % of energy | 11.8, 0.3 | 11.6, 0.4 |
| Monounsaturated Fat, % of energy | 12.7, 0.3 | 12.6, 0.4 |
| Polyunsaturated Fat, % of energy | 7.1, 0.2 | 6.7, 0.3 |
| Sodium, g/day | 3368, 69 | 3030, 140b |
| Calcium, mg/day | 871, 24 | 912, 81 |
| Fiber, g/day | 22, 1 | 24, 2 |
| Glycemic Load, vs. bread | 200, 7 | 183, 8b |
| Fruit, servings/day | 1.7, 0.1 | 1.7, 0.2 |
| Vegetables, servings/day | 2.8, 0.1 | 3.4, 0.2b |
| Whole Grains, servings/day | 1.9, 0.1 | 1.7, 0.2 |
A flexible sigmoidoscopy was performed at the study enrollment visit.
Significantly different than intake at eligibility by the paired t-test.
The percentage of subjects remaining on study after 6 months was 78%, which is consistnet with that observed in other intensive dietary studies (23, 24). The demographic characteristics of subjects who did or did not complete the study were similar with the following exceptions. Subjects who completed the study had higher baseline vegetable intakes and higher age at baseline. Non-completers were somewhat more likely to be smokers (p=0.06) and un-married (p=0.07). Most of the drop-outs occurred before the 3-month visit; only two subjects left the study after the 3-month time point.
Despite the fact that this was an intensive dietary intervention study, with a total of 12 planned contacts over 6 months, satisfaction with study participation was high (Table 6). Most subjects thought they were able to follow the diet to which they were randomized, it was not too burdensome to be in the study, would recommend the diet and wanted to keep following the diet. An average answer of 4.5 would indicate answers of “very much so” or “somewhat” on a 5-point scale. The average answer was neutral when asked whether or not the diet takes more time than their usual diet. The similarity in responses between subjects randomized to the two diets was surprising since the Mediterranean group had a much larger number of dietary goals. When asked the question “what was the hardest part of being in the study”, responses in the two study arms were again similar with the following categories of answers (some subjects gave more than one answer, 5 subjects gave no answer): aspects of buying food/eating/cooking n=48, recordkeeping n=21, nothing n=17, flexible sigmoidoscopy n=5 and other n=5. When asked “what was the easiest part of being in the study”, the categories of answers were: aspects of healthy eating n=57, interacting with the dietitian n=10, recordkeeping n=7, learning n=3 and other n=6.
Table 6.
Satisfaction with study participation from a questionnaire administered at the final visit. Data shown are mean and SD for answers on a scale of 1 to 5 with 5 being “very much so” and 1 being “not at all”.
| Characteristic | Healthy Eating Diet | Mediterranean Diet |
|---|---|---|
| I feel good about being in the study | 4.93, 0.25 | 4.96, 0.20 |
| The diet was easy to follow | 4.47, 0.63 | 4.43, 0.54 |
| I have time to follow diet | 4.74, 0.43 | 4.64, 0.61 |
| The diet takes more time than my usual diet | 3.02, 1.34 | 3.28, 1.23 |
| Others at home make it difficult to follow the diet | 1.69, 0.90 | 2.02, 1.22 |
| Being in the study was stressful | 1.58, 0.78 | 1.65, 0.97 |
| Finishing is like a weight being lifted from my shoulders | 2.20, 1.07 | 1.98, 1.03 |
| I like phone calls to minimize appointments | 4.44, 0.88 | 4.41, 0.83 |
| I would recommend this diet to my family and friends | 4.84, 0.37 | 4.89, 0.31 |
| I want to keep following this diet after the study is over | 4.91, 0.29 | 4.85, 0.36 |
| Factor 1a | −0.018(1.17), 0.31(0.95) | 0.017(0.83), 0.16(1.29) |
| Factor 2a | −0.06(1.14), 0.28(1.86) | 0.055(1.12), 0.07(1.12) |
| Factor 3a | −0.06(0.86), 0.22(1.16) | 0.06(1.12), 0.44(1.41) |
The item responses were factor analyzed using the principal component extraction methods with a varimax rotation using age as a covariate. Factor 1 contained four items: “feel good about being the study”, “diet was easy to follow”, “have time to follow diet”, “recommend diet to family and friends”. Factor 2 contained “diet takes more time than usual diet”, “being in the study was stressful”, and “finishing is like a weight being lifted”. Factor 3 contained “would recommend diet to family and friends”, “want to keep following the diet”, and “would like phone calls to minimize appointments”. Data shown are mean (SD), median (inter-quartile range). Based on the linear regression analysis, none of these factors were significantly different across the two diet arms.
A factor analysis of the ten satisfaction survey item responses revealed existence of three factors. All factors had associated eigenvalues larger than 1 and collectively the factors accounted for more than 50% variability. The first factor (Factor 1) had four items loaded highly on it with loading coefficients larger than 0.48 in magnitude. The items were “feel good about being the study”, “diet was easy to follow”, “have time to follow diet”, “recommend diet to family and friends”. This factor clearly represented a group of subjects that were satisfied with the most aspects of the study. By contrast, Factor 2 had three items loaded highly all of which can be associated with stress and difficulty experienced by the subjects on the study. These three items were “diet takes more time than usual diet”, “being in the study was stressful”, and “finishing is like a weight being lifted”. The third factor (Factor 3) also comprised of subjects with positive impression who would “recommend diet to family and friends”, “want to keep following the diet”, and “would like phone calls to minimize appointments”. One item, namely “others at home make it difficult to follow diet” did not load sufficiently highly on any of the factors. Based on the linear regression analysis, however, none of these factors were significantly different across the two diet arms, indicating that the satisfaction (or lack of it) was not particularly a function of the specific diet regime, and was probably more a reflection of the general attitude. It is interesting to note that age exhibited a significantly positive association (p < .001) with Factor 1 indicating that older subjects had a higher overall satisfaction level. The weighted analysis did not reveal anything different.
4. Discussion
This study recruited individuals at increased risk of colon cancer to an intensive dietary intervention study. In order to recruit individuals with either a prior adenomatous polyp or a strong family history of colon cancer, generalized recruitment with flyers and a website listing proved to yield the highest number of inquiries about the study (Table 1). Interestingly advertisements in local newspapers, which likely reach a large number of individuals, were not as successful. We do not have data on which flyer posting location yield the most inquiries, but flyers at the medical center and the clinical trials website are likely viewed by individuals who are familiar with the medical center. It might be the case that individuals familiar with the medical center might be more inclined to volunteer for a study being conducted there. It was, however, surprising that letters mailed to patients of physicians affiliated with the medical center only yielded an 11% response rate.
The flyers for the study mentioned that study procedures can take some time but the flyers did not indicate that flexible sigmoidoscopy was required. It was felt that explaining this procedure would be complicated and that individuals might confuse it with colonoscopy, which is much more involved and requires preparation of the bowels. When individuals called or emailed to inquire about the study, they were then told about the procedure, what it entails and how long it takes. After being told about flexible sigmoidoscopy, very few individuals indicated that they would not participate because of this, although we cannot exclude that this might have been a reason for at least some of the individuals who did not reply or further pursue participation (Table 1). Individuals at increased risk for colon cancer likely have had at least one colonoscopy, with the exception of very young individuals, and in comparison un-prepped flexible sigmoidoscopy is much easier to tolerate than full colonoscopy with preparation. After six months of study participation, one subject did refuse the flexible sigmoidoscopy, however, and five of 93 subjects who completed the study indicated that this was the hardest part of the study.
The study screening procedure included a face-to-face visit, and a screening consent form was required. This screening consent proved to be valuable since it allowed for capture of information about screened subjects. A recent review of the literature on bias arising from recruitment and retention issues has indicated that very few studies described the screened population and reported drop-out rates (14). Here we were able to show that the demographic characteristics of screened and enrolled subjects were not significantly different (Table 2). Randomization entailed 16 strata, and this was effective for equalizing the demographic characteristics across the two study arms, but it did result in slightly uneven number of subjects in the two arms (59 vs. 61). Subjects in the Mediterranean arm were, on average, slightly older than in the Healthy Eating arm, but this was not significant (Table 3). Subjects who did or did not complete the study also had similar demographic characteristics, and this should minimize bias in the interpretation of the results (Table 5)
Table 5.
Characteristics of study completers and non-completers given as means and SD or number and percent.
| Characteristic | Completer n = 93 |
Non-completer n=27 |
|---|---|---|
| Age, yearsa | 54, 11 (range 24–82) | 48, 14 (range 22–71) |
| Female gender | 67, 72% | 19, 70% |
| BMI, kg/m2 | 27, 4 (range 19–35) | 28, 3 (range 22–35) |
| Regular aspirin user | 20, 21% | 4, 15% |
| Caucasian race | 83, 89% | 22, 81% |
| Married/committed | 63, 68% | 13, 48% |
| College graduate | 73, 78% | 19 70%, |
| Tobacco user | 10, 11% | 7, 26% |
| History of adenomas | 27, 29% | 6, 22% |
| Family history of colon cancer | 58, 62% | 19, 70% |
| Both risk factors | 8, 9% | 2, 7% |
| Vegetable intake, servings/daya | 2.99, 1.25 | 2.44, 1.25 |
The differences between completers and non-completers were significant using the two-sample t-test for age at study entry (p=0.04) and dietary intake of vegetables (p=0.05). The other dietary variables listed in Table 4 were evaluated as well and none of these differed significantly by study completion status.
One important consideration for this study was whether or not recent diet would affect the biomarkers being measured, especially in the colon. In addition, dietary analyses for determining study eligibility were typically completed 6 weeks prior to starting on study. The comparison of dietary intakes used for eligibility determination and the 24-hour recall taken at the baseline visit indicated that subjects had slightly better quality diets the day before starting in the study. This may be due to the interest of the subjects in healthy diets and the anticipation of embarking on a dietary study might have made them more aware of their eating patterns. The differences were small, although statistically significant, and point to the potential importance of capturing recent diet.
Of the subjects who finished study participation, satisfaction with being in the study was high (Table 6). It is interesting to note that when asked about what was the hardest and easiest part of the study, by far most of the replies indicated some aspect of the dietary changes. It therefore appears that changing one’s diet can be difficult, but that the perceived benefit from eating healthier foods is substantial. It is especially encouraging that subjects would recommend the diet they were assigned to and intend to keep following it after the study is over.
Overall, the findings in this study indicate that flexible sigmoidoscopy does not appear to be a barrier for recruitment of high risk individuals but that completing food records can be. In addition, individuals may change their diet when they are about to start on a dietary intervention study, which could affect diet-responsive biomarkers. Although the intensive dietary changes requested in this study were difficult to make, subjects had high satisfaction with making those changes. Mediterranean diets are popular, but the recruitment of subjects at high risk for colon cancer required an intensive effort to enroll 120 subjects over three years. Future studies should budget staff time and marketing costs to accommodate this need. Once subjects were enrolled, however, retention of subjects over six months was reasonably good and satisfaction with the study was high for study completers.
Figure 1.
Recruitment and retention in the Healthy Eating Study. Three individuals were removed from study either due to initiation of supplement use (fish oils, high level thiamine) or diet change (increased sodium intake recommended by a physician).
Acknowledgments
We thank all the individuals who volunteered their time for the Healthy Eating Study and the students who helped with data verification and organization: Megan Rook, Gary Schneider, Shony Reuven, Perry Ahn, Nancy Wener, Tiffany Yang and Ofra Duchin. This study was supported by NIH grants RO1 CA120381 and P30 CA046592.
Abbreviations
- BMI
body mass index
- MUFA
monounsaturated fat
- PUFA
polyunsaturated fat
- PGE2
prostaglandin E2
- SFA
saturated fat
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Colorectal Cancer Facts and Figures - Special Edition 2005. Atlanta, GA: American Cancer Society; 2005. [Google Scholar]
- 2.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition and Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research; 1997. [DOI] [PubMed] [Google Scholar]
- 3.Schottenfeld D, Jr, JFF . Cancer Epidemiology and Prevention. 2nd ed. New York: Oxford; 1996. [Google Scholar]
- 4.Simopoulos AP. The traditional diet of Greece and cancer. Eur J Cancer Prev. 2004;13(3):219–230. doi: 10.1097/01.cej.0000130011.99148.07. [DOI] [PubMed] [Google Scholar]
- 5.Kontou N, Psaltopoulou T, Panagiotakos D, Dimopoulos MA, Linos A. The Mediterranean Diet in Cancer Prevention: A Review. J Med Food. 2011 doi: 10.1089/jmf.2010.0244. [DOI] [PubMed] [Google Scholar]
- 6.Verberne L, Bach-Faig A, Buckland G, Serra-Majem L. Association between the Mediterranean diet and cancer risk: a review of observational studies. Nutr Cancer. 2010;62(7):860–870. doi: 10.1080/01635581.2010.509834. [DOI] [PubMed] [Google Scholar]
- 7.Frenandez E, Vecchia CL, Gonzales JR, Lucchini F, Negri E, Levi F. Coverging patterns of colorectal cancer mortality in Europe. European Journal of Cancer. 2005;41:430–437. doi: 10.1016/j.ejca.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan K, Ruffin MT, Normolle D, Shureiqi I, Burney K, Bailey J, et al. Colonic mucosal prostaglandin E2 and cyclooxygenase expression before and after low aspirin doses in subjects at high risk or at normal risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10(5):447–453. [PubMed] [Google Scholar]
- 9.Tworoger SS, Yasui Y, Ulrich CM, Nakamura H, LaCroix K, Johnston R, et al. Mailing strategies and recruitment into an intervention trial of the exercise effect on breast cancer biomarkers. Cancer Epidemiol Biomarkers Prev. 2002;11(1):73–77. [PubMed] [Google Scholar]
- 10.Goodman GE, Valanis B, Meyskens FL, Jr, Williams JH, Jr, Metch BJ, Thornquist MD, et al. Strategies for recruitment to a population-based lung cancer prevention trial: the CARET experience with heavy smokers. Beta-Carotene and Retinol Efficacy Trial. Cancer Epidemiol Biomarkers Prev. 1998;7(5):405–412. [PubMed] [Google Scholar]
- 11.Long Q, Zhang X, Bostick RM. Semiparametric estimation for joint modeling of colorectal cancer risk and functional biomarkers measured with errors. Biom J. 2011;53(3):393–410. doi: 10.1002/bimj.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenwald P. Cancer risk factors for selecting cohorts for large-scale chemoprevention trials. J Cell Biochem Suppl. 1996;25:29–36. [PubMed] [Google Scholar]
- 13.Kelloff GJ, Boone CW, Crowell JA, Nayfield SG, Hawk E, Steele VE, et al. Strategies for phase II cancer chemoprevention trials: cervix, endometrium, and ovary. J Cell Biochem Suppl. 1995;23:1–9. doi: 10.1002/jcb.240590902. [DOI] [PubMed] [Google Scholar]
- 14.Demark-Wahnefried W, Bowen DJ, Jabson JM, Paskett ED. Scientific bias arising from sampling, selective recruitment, and attrition: the case for improved reporting. Cancer Epidemiol Biomarkers Prev. 2011;20(3):415–418. doi: 10.1158/1055-9965.EPI-10-1169. [DOI] [PubMed] [Google Scholar]
- 15.Garaulet M, Perez-Llamas F, Perez-Ayala M, Martinez P, de Medina FS, Tebar FJ, et al. Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am J Clin Nutr. 2001;74(5):585–591. doi: 10.1093/ajcn/74.5.585. [DOI] [PubMed] [Google Scholar]
- 16.Martinez ME, Heddens D, Earnest DL, Bogert CL, Roe D, Einspahr J, et al. Physical activity, body mass index, and prostaglandin E2 levels in rectal mucosa. J Natl Cancer Inst. 1999;91(11):950–953. doi: 10.1093/jnci/91.11.950. [DOI] [PubMed] [Google Scholar]
- 17.Ferroni P, Basili S, Falco A, Davi G. Inflammation, insulin resistance, and obesity. Curr Atheroscler Rep. 2004;6(6):424–431. doi: 10.1007/s11883-004-0082-x. [DOI] [PubMed] [Google Scholar]
- 18.Ruffin M, Krishnan K, Rock C. Suppression of human colorectal mucosal prostaglandins: determining the lowest effective aspirine dose. Journal of the National Cancer Institute. 1997;89(15):1152–1160. doi: 10.1093/jnci/89.15.1152. [DOI] [PubMed] [Google Scholar]
- 19.Jiang J, Suzuki S, Xiang J, Kuriki K, Hosono A, Arakawa K, et al. Plasma carotenoid, alpha-tocopherol and retinol concentrations and risk of colorectal adenomas: A case-control study in Japan. Cancer Lett. 2005 doi: 10.1016/j.canlet.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 20.Steck-Scott S, Forman MR, Sowell A, Borkowf CB, Albert PS, Slattery M, et al. Carotenoids, vitamin A and risk of adenomatous polyp recurrence in the polyp prevention trial. Int J Cancer. 2004;112(2):295–305. doi: 10.1002/ijc.20364. [DOI] [PubMed] [Google Scholar]
- 21.Djuric Z, VanLoon G, Radakovich K, DiLaura NM, Heilbrun LK, Sen A. Design of a Mediterranean Exchange List Diet That Can Be Implemented by Telephone Counseling. J. Amer. Diet. Assoc. 2008 doi: 10.1016/j.jada.2008.09.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Service AR, editor. What We Eat in America, NHANES 2007–2008, Individuals age 2 and older, day 1 dietary intake data. Beltsville, MD: U.S.D.A.; 2010. [Google Scholar]
- 23.Djuric Z, Chen G, Ren J, Venkatramanamoorthy R, Covington CY, Kucuk O, et al. Effects of high fruit-vegetable and/or low-fat intervention on breast nipple aspirate fluid micronutrient levels. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1393–1399. doi: 10.1158/1055-9965.EPI-06-0766. [DOI] [PubMed] [Google Scholar]
- 24.Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, Brill C, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153(3):147–157. doi: 10.1059/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]